A systematic study of the pulchra species group of Archips occurring in Japan (Lepidoptera, Tortricidae)
Abstract
This paper reviews the pulchra species group of Archips, describes a new species, Archips stellata, and redescribes A. pulchra (Butler) and A. abiephaga (Yasuda). Although long confused with each other, these three species are distinguishable by differences in the wing markings, abdominal dorsal pits, and genitalia. To position the pulchra group systematically within the genus Archips, earlier related studies are reviewed and the phylogenetic relevance and polarities of traits are discussed. The forewing pattern, consisting of transverse black and glossy gray fasciae, is presumed to be the only synapomorphy of this group; affinity between the pulchra group and other groups of Archips in some traits may reflect symplesiomorphy.
INTRODUCTION
The genus Archips Hübner, 1822, is a large genus of the tribe Archipini. The genus consists of 102 species distributed in the Holarctic and Oriental regions (Razowski 1977, 1993, 2000; Liu 1987; Tuck 1990; Brown 2005). Twenty species have been recognized in Japan (Yasuda 1975; Kawabe 1982; Razowski 1993). Although this genus remains undefined by a synapomorphy, most members can be recognized by pairs of “dorsal pits” on the terga, particularly terga II and III (Razowski 1977). The crescent-shaped coremata is also regarded as a possible synapomorphy of this genus (Jinbo 2000).
Archips pulchra Butler, 1879, and the allied species form a distinct species group defined by characteristic longitudinal fasciae on the forewing. Originally, this group was recognized as the subgenus PararchipsKuznetzov, 1970; of the genus Archips. Razowski (1977) lowered the subgenus designation to a synonym of Archips, but retained it as a species group, namely the pulchra group. However, the phylogeny of Archips and the systematic position of the pulchra group within the genus have been only partly discussed (Razowski 1977; Jinbo 2000; Kruse & Sperling 2002). Two species of this species group, A. pulchra and A. abiephagaYasuda, 1975, have been recorded from Japan (Yasuda 1975; Kawabe 1982). They are well-known pests of Abies sachalinensis (Pinaceae) plantations in Hokkaido (Suzuki & Kamijo 1967; Suzuki & Komai 1984; Komai 1990; Furuta & Kobayashi 1991). However, taxonomic study of Japanese Tortricinae by the author has revealed the presence of a previously undescribed species. This species and the two known allied species have long been confused with one another. This paper describes the three Japanese species in light of the discovery of the new species and discusses their systematic position within the genus.
MATERIALS AND METHODS
This study was based on dried specimens collected by the author and other entomologists, as well as specimens borrowed from or deposited at the following institutions or individual collections: National Science Museum, Tokyo (NSMT); Osaka Prefecture University (OPU); Dr Yoshitsugu Nasu’s collection (YNC); and Dr Toshio Oku’s collection (TOC). Specimens without abbreviations are held privately by the author. The following abbreviations are used for collectors of specimens: SI (S. Issiki), UJ (U. Jinbo), TK (T. Kodama), HK (H. Kogi), SM (S. Moriuti), HN (H. Nakajima), MY (M. Yamamoto), and TY (T. Yasuda).
Male abdomen and genitalia drawings are based on samples displayed on temporary slides mounted in glycerin or permanent slides in Canada balsam. The drawings of female genitalia are based on samples placed on a Petri dish filled with 70% ethyl alcohol.
SYSTEMATICS
The pulchra group of Archips
Diagnosis. Medium to large moths of the tribe Archipini. Forewing length 9–12 mm. This group is characterized by the forewing having glossy gray longitudinal fasciae and by the absence of a costal fold.
Male. Antenna ciliate. Forewing (1–6, 7–11) elongate with rounded apex; costal fold absent; forewing with 13 veins; Sc terminating near R1; R2 to R4 parallel and spaced equally; R4 to costa; R5 to termen; M3 and CuA1 curved towards wing apex; CuA2 originating at 3/5 of discal cell; chorda atrophied. Hindwing with Sc + R1 terminating at 3/4 of costa; Rs to costa; M1 to termen; M2 near M3; M3 and CuA1 approximated toward base; CuA2 originating beyond middle of cell.
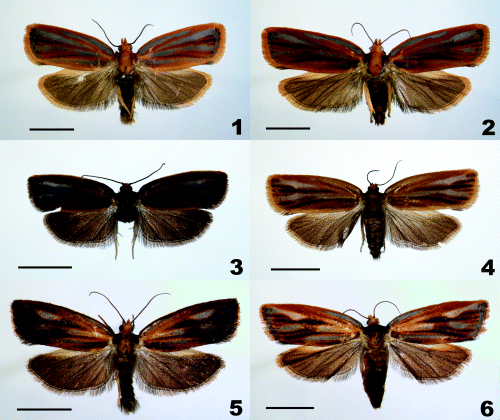
Adults of the Archips pulchra group. 1,2 Archips pulchra (1♂ and 2♀); 3, 4 Archips abiephaga (3♂ and 4♀); and 5,6 Archips stellata sp. nov. (5♂ holotype and 6♀ paratype). Scale bars: 5 mm.
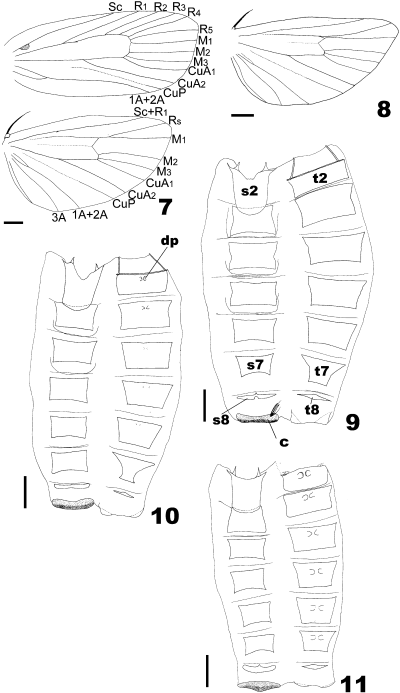
Males of the Archips pulchra group. 7,8 Wing venation (7 A. pulchra and 8 A. stellata sp. nov., paratype); and 9–11 abdomen (9 A. pulchra, 10 A. abiephaga and 11 A. stellata sp. nov., paratype). s2, Sternum II; s7, sternum VII; s8, sternum VIII; c, coremata; t2, tergum II; t7, tergum VII; t8, tergum VIII; dp, dorsal pits. Scale bars: 1 mm.
Abdomen (Figs 9–11): Terga II–III rectangular; terga IV–VII trapezoidal; tergum VIII represented by narrow anterior plate; sternum II elongate with pair of apodemes on apical margin; sterna III–VI rectangular; sternum VII trapezoidal; sternum VIII represented by narrow anterior plate with median process on anterior edge; coremata large, consisting of crescent-shaped area bearing sockets with long scales.
Male genitalia (Figs 12–17): Uncus long; socius small; gnathos with simple arms; tegumen slender, its median portion with long suture; transtilla wide, flat, concave medially; pulvinus well developed, membranous; juxta large; vinculum simple; valva rounded, membranous and plicate except for delicately sclerotized medial portion; costa atrophied; sacculus strong, broad, occupying proximal third of valva, protruded medially with small free termination; aedeagus rather long, weakly arched basally, left side of its subapical portion having series of minute processes; coecum penis strong, occupying 2/5 of aedeagus; vesica with some deciduous cornuti; ductus ejaculatorius long.
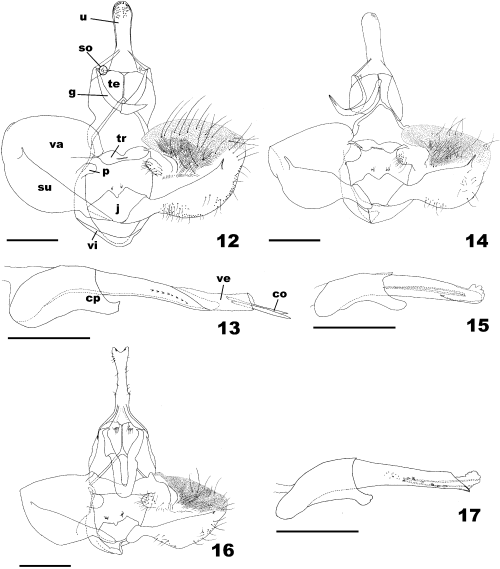
Male genitalia of Archips pulchra group (12,14,16 ventral view, removed aedeagus and 13,15,17 aedeagus). 12,13 A. pulchra; 14,15 A. abiephaga, paratype; and 16,17 A. stellata sp. nov., holotype. u, Uncus; so, socius; g, gnathos; te, tegumen; tr, transtilla; j, juxta; vi, vinculum; p, pulvinus; va, valva; su, sacculus; cp, coecum penis; ve, vesica; co, cornuti. Scale bars: 0.5 mm.
Female. Larger than the male. Antenna piliform. Forewing (Figs 2,4,6) similar to that of the male but with brighter ground color and stronger markings. Abdomen without terminal tuft; sternum VIII simple with weakly concave posterior edge.
Female genitalia (Figs 18–20): Papillae anales typical of tortricid species; apophyses posteriores as long as apophyses anteriores; sterigma large, its posterior portion having a pair of patches of dense scales and proximal part strong and cup-shaped; antrum well-sclerotized; ductus seminalis narrow, arising from proximal end of antrum; bulla seminalis ovate, slightly smaller than corpus bursae; membranous portion of ductus bursae long and sinuate with long cestum represented by sclerotized rib; corpus bursae ovate, signum a long sclerite with beak-shaped inner process and capitulum.
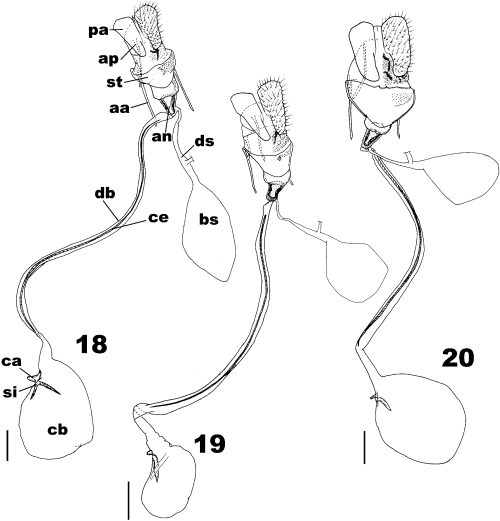
Female genitalia of the Archips pulchra group, ventral view. 18 A. pulchra; 19 A. abiephaga; and 20 A. stellata sp. nov., paratype. pa, Papillae anales; ap, apophyses posteriores; aa, apophyses anteriores; st, sterigma; an, antrum; ds, ductus seminalis; bs, bulla seminalis; db, ductus bursae; ce, cestum; cb, corpus bursae; si, signum; ca, capitulum of signum. Scale bars: 0.5 mm.
Remarks. The pulchra group consists of three species (Kuznetzov 2001), A. pulchra, A. abiephaga, and A. myrrhophanes (Meyrick, 1931; =A. sayonaeKawabe, 1985; see Brown 2005), the last of which is known from mainland China and Taiwan (Razowski 1977; Kawabe 1985; Kuznetzov 2001). Two Chinese species, A. kellerianaLiu, 1987, and A. strigopteraLiu, 1987, have similar markings according to the descriptions (Liu 1987). They can probably be included in this group, although the present author has not examined specimens of these species. Further study is necessary to confirm the systematic position of these Chinese species. The distribution of this species group is restricted to the eastern part of the Palearctic (Japan, Korea, northern mainland China and Russia) and Oriental (southern mainland China and Taiwan) regions. Known host plants of the pulchra group are limited to conifers.
Archips pulchra (Butler) (1–6-18–20)
-
Ariola pulchra Butler, 1879: 19, pl. 44: 6. Lectotype: male, “Japan, 77-9,” designated by Razowski (1977), deposited at the British Museum (Natural History).
-
Tortrix pulchra: Walsingham (1900): 458; Issiki (1922): 285; Matsumura (1931): 1077; Issiki (1932): 1453.
-
Cacoecia pulchra: Meyrick (1912): 19; Meyrick (1913): 24.
-
Ptycholoma pulchra: Obraztsov (1955): 220.
-
Ariola pulchra: Issiki (1957): 81, pl. 14: 427; Okano (1959): 265, pl. 177: 14; Issiki and Mutuura (1961): 34; Issiki and Mutuura (1962): 3.
-
Archippus pulchra: Suzuki and Kamijo (1967): 18; Yasuda (1969): 102, pl. 49: 195.
-
Archips (Pararchips) pulchra: Kuznetzov (1970): 448.
-
Archippus (Pararchips) pulchra: Yasuda (1972): 92; Yasuda (1975): 108, figs 57,58,394,578.
-
Archips pulcher: Razowski (1977): 105, figs 62,63,181; Kawabe (1982): 66 (part.), pl. 15: 16, pl. 288: 5; Suzuki and Komai (1984): 94; Komai (1990): 4, fig. 6 (left), 7; Razowski (1993): 677; Oku (2003): 89.
-
Archips pulchra: Byun et al. (1998): 22, p. 215: fig. 16, p. 225: fig. 16, p. 265: fig. 16; Brown (2005): 129.
-
Archips (Pararchips) pulcher: Kuznetzov (2001): 139, fig. 75: 1, 89: 1.
Diagnosis. This is a medium- to rather large-sized species characterized by the broad forewing with four glossy, purplish-gray, longitudinal fasciae and a series of black dots along the termen. Sexual dimorphism of the wing shape and markings is slight. Abdominal dorsal pits are vestigial or absent.
Male. Forewing length 9.0–10.5 mm. Head: Antenna filiform. Frons and vertex covered with brownish-orange scales. Labial palpus twice as long as diameter of compound eye and covered with brownish-orange scales.
Thorax: Tegula and patagium covered with dark brown scales. Forewing uniformly broad with rounded apex; ground color reddish-brown; markings represented by four glossy, purplish-gray longitudinal fasciae running from basal area, first along costa to 3/5; second to below apex; third to middle of termen, sometimes indistinct basally; and fourth to tornus; indistinct fuscous longitudinal fasciae on portions between each fascia; termen with some black dots and glossy gray portion connected with second and third fasciae; dorsum margin narrow, orange; cilia orange, except for fuscous portion at tornus. Hindwing grayish-ochre; cilia grayish-ochre with orange scales at upper half; Rs and M1 stalked.
Abdomen: Glossy, brownish-gray with grayish-ochre terminal tuft; dorsal pits vestigial, sometimes appearing on tergum II or completely atrophied; terga and sterna as in description of the species group; posterior edge of sternum VIII strongly invaginated medially.
Genitalia: Uncus long, slender. Gnathos strong, rather slender. Transtilla medially constricted band with strong proximal edge. Juxta heart-shaped. Valva short, costa gently curved, apex obtuse; sacculus broad and rounded with slender free termination. Aedeagus typical of the species group, broad at base, with row of minute processes subapically.
Female. Forewing length 9.0–12.0 mm. Very similar to male, but differing by somewhat longer forewing and by absence of dorsal pits on abdomen.
Genitalia: Papillae anales slender and subtriangular. Sterigma large, its posterior portion convex laterally with pair of scale patches. Ante-ostial part of sterigma large, cup-shaped, slightly expanded proximally. Antrum small, constricted medially, tapering proximally. Ductus bursae very long and sinuate with cestum. Corpus bursae ovate, signum with slender capitulum and rather bold beak-shaped process.
Specimens examined. Japan: Hokkaido: Kamisarufutsu, 1♀, 29.viii.1992, HK; Kunbetsu, 1♂, 22.viii.1998, HK; Miwa, 1♀, 24.vii.1975, 1♂, 15.vii.1992, 1♀, 17.viii.1993, 1♂, 14.vii.1994, S. Kawahara; Ikutabara, 3♂, 20.vii.1995, H. Ono; Nishisekinai, 1♀, 16.viii.1992, HK; Sapporo, 1♂ (OPU), emerged (em.) 8.vii.1958, SI, ex Picea abies; Jozankei, 1♂ (OPU), 18.viii.1917, SI; Shirogane, Ishikari, 1♀ (OPU), 15.vi.1958, TY; Shioya, Otaru-shi, 1♀, 31.vii.1997, K. Sugisima; Takaoka, Tomakomai-shi, 1♀, 27.vii.1996, HK; Tomuraushi, 1♂, 24.vii.1991, 1♂, 29.vii.1995, HK; Yamabe, Ishikari, 2♂5♀, em. 26.vi−1.vii.1958, TK, ex Abies sachalinensis (OPU); same locality (loc.), 1♀, em. 29.vi.1958, 2♂3♀, em.17–27.vi.1959, TK, ex Abies homolepis (OPU). Honshu: Iwate: Nishimatsuzono, Morioka, 1♀, 4.ix.1992, M. Tanaka. Gunma: Kawafuru-onsen, 1♀ (NSMT) 1.vii.1967, T. Ebato; Kitakaruizawa, 1♂ (NSMT), 12–18.vii.1970, T. Okada; Minakami, 1♀ (NSMT), 22.vii.1931 (S. Asahina); Mount Akagi, 1♂ (NSMT), 19–21.vii.1964, A. Kawabe; Tanigawa Onsen, 1♀ (NSMT), 4.x.1975, HN. Saitama: Bushi, Iruma, 1♀ (NSMT), 20.vi.1978, H. Inoue. Tokyo: Asakawa, 4♂6♀ (OPU), em. 20.v−17.vi.1958, 1♀ (OPU), em. 5.vi.1959, TK, ex Abies firma; Mount Takao, 1♂ (NSMT), 19.vii.1960, T. Ebato; 7♂, 2.vi.1998, 1♂, 19.vi.1998, 1♂, 15.viii.1998, 3♂, 23.vi.1999, UJ; Kôtokuji, Akiruno, 2♂3♀, 30.vi.1984, 1♂, 29.ix.1984, HN; Yanoiri, Hinode, 1♂, 4.vi.1994, 1♂, 9.vii.1994, UJ; Ônita, Ôme, 1♂, 10.vi.1995, 1♂, 15.vi.1996, UJ; same loc., 3♂4♀, em. 28.v.−4.vi.2003, 1♀, em. 1.ix.2003, UJ, ex Abies firma; Mount Mitake, 1♂, 24.vii.1999, UJ; Nippara, Okutama, 1♀ (NSMT), 2.x.1961, T. Ebato. Kanagawa: Sekiryôzan, 460 m, Tsukui, 1♂, 5.vii.1986, HN; Hôkizawa, Yamakita, 1♂, 9–10.viii.1977, HN; Fudakake, 1♂, 5.vii.1978, 3♂, 21.vi.1980, 4♂, 27.vi.1981, 1♂1♀, 3.vii.1981, 1♂, 29.viii.1981, HN; Karasawa, Kiyokawa, 1♂, 3.vii.1981, HN; Dôdaira, Kiyokawa, 1♂, 17.vii.1982, 1♂, 16.vii.1993, HN; Daikanzan, Hakone, 1♀, 29.vii.1988, HN; Sengokuhara, Hakone, 4♂, 1.vii. 2000, HN. Nagano: Naranoki-zaka, 1500 m, Norikura, 1♀, 1–4.viii.2000; UJ. Hirukubo-tôge, 1680 m, 1♀, 4.viii.1997, UJ; Kisojihara, 1♂ (OPU), 28.vii.2001, T. Hirowatari, B. W. Lee and N. H. Ahn; Urugi-mura, 1♂, 30.vii.1983, M. Ihara; Murakage, Anan, 1♂, 13.vii.1983, M. Ihara; Himemiya, Iida, 1♂, 12.vii.1996, M. Ihara. Gifu: Takayama, Hida, 1♂ (OPU), 25.vii.1954, TY. Aichi: Kurokura, Shitara, 1♀, 19.vii.1991, T. Mano. Shiga: Hieisan, 2♂ (OPU), 10.vii.1956, TK. Nara: Kasugayama, 1♀ (OPU), 20.vi.1955, 1♂ (OPU), 1.vii.1957, S. Moriuti; 4♂3♀ (OPU), em. 27.v.-6.vi.1958, 1♂ (OPU), em. 5.vi.1959, TK, ex Abies firma; Mount Obako-dake, 1♀ (OPU), 14–18.vii.1976, A. Tatara and K. Yasuda; Mount Wasamata, Kamikitayama, 1♂ (OPU), 31.vii.1990, T. Hirowatari and T. Ueda; Shiratani, Tokusawa, 1♂ (OPU), 16.ix.1969, S. Moriuti. Osaka: Kawachinagano, 1♂1♀ (OPU), 28.vi.1963, M. Takahama; Mount Iwawaki, 1♀(OPU), 23.vi.1950, A. Mutuura, 1♂ (OPU), 28.vi.1954, TY, 1♂ (OPU), 26.vi.1980, T. Tanabe. Wakayama: Ryujin, 1♂ (OPU), 29.ix.1971, F. Komai; Mount Kasatou, Ryujin, 1♀ (OPU), 22.vi.1998, T. Ohno; Osugidani, Mount Ôtô, 1♀ (NSMT), 1–4.vii.1978, M. Owada and Y. Nishi. Hyogo: Haga, 2♂ (OPU), 30–31.vii.1965, S. Moriuti. Shikoku: Ehime: Mount Takanawa, 1♀ (OPU), 18.viii.1955, M. Okada; Ochide, Iyo, 1♂ (OPU), 23.vii.1959, M. Okada; Omogokei, 1♂ (OPU), 9.vii.1971, F. Komai. Kyushu: Fukuoka: Hikosan, Buzen, 1♀ (OPU), 9.vii.1963, 1♀ (OPU), 22.vi.1959, H. Kuroko. Miyazaki: Kirei-tôge, 850 m, 1♂, 9.vii.1992, Y. Yanagita. Kagoshima: Mount Kirishima, 2♂ (OPU), em. 25.v.1958, SI & TY, ex Abies firma. Korea: Mount Gaebang, Kanwon-do, 1♂ (YNC), 19.vii. 2000, Y. Nasu.
Distribution. Japan (Hokkaido, Honshu, Shikoku, Kyushu); Russia (Primorye, Kuznetzov 2001), Korea.
Host plants. Abies firma, A. homolepis, A. sachalinensis, Picea abies (Pinaceae), and Taxus cuspidata (Taxaceae).
Remarks. Butler (1879) described this species from “Yokohama” and illustrated an adult in color, although its type specimen was not designated. Razowski (1977) examined specimens of this species deposited at the British Museum (Natural History) and designated the lectotype based on a male labeled “Japan.” The genitalia of the lectotype have not been examined as the abdomen is missing. The present author identified this species on the basis of the illustration from the original description, information on the lectotype, and by examining the same series of bred specimens from which the male genitalia were figured and described by Razowski; these specimens are held in the OPU collection.
In Japan, this species is widely distributed in lowlands but is sometimes collected in mountainous areas below approximately 1800 m a.s.l. (e.g. collected at the Hirukubo-tôge Pass, 1780 m a.s.l., Nagano Prefecture). Adults are attracted to light. This species has one generation per year in colder areas and two generations in warmer areas, and hibernates in the young larval stage.
Other authors have described the immature stages and life history of A. pulchra (Issiki 1961; Yasuda 1969, 1975; Komai 1990), which is one of the most serious pests in Abies sachalinensis plantations. Young trees of this conifer species are particularly susceptible to damage by the larvae (Suzuki & Kamijo 1967). The host range is rather wide; the larvae feed not only on Abies species but also on Picea and Taxus. However, in lowlands and montane areas of Honshu, A. pulchra strongly depends on Abies firma. This easily collected and recognizable moth is a useful indicator species of fauna that depends on A. firma.
Liu et al. (1983), Liu (1987) and Liu and Li (2002) recorded “A. pulcher” from Heilongjiang Province in north-eastern China. However, the figures in their references seem to indicate the species discussed below. Records of this species from China remain uncertain.
The name of this species has frequently been spelled as A. pulcher, following Razowski (1977). There are two opinions about the gender of the name Archips (i.e. whether it is masculine or feminine; see the synonym list). The present author regards the gender as feminine, following the original description and the latest checklist (Brown 2005).
Archips abiephaga (Yasuda) (1–6-18–20)
-
Archippus (Pararchips) abiephagaYasuda, 1975: 109 (part., abiephage[sic]), fig. 51. Holotype, male, “Japan, Hokkaido, Yamabe; bred from Abies concolor, 12.vi.1959, T. Kodama,” deposited at the OPU (missing).
-
Ariola abiephaga: Issiki and Mutuura (1962): 3 (nomen nudum).
-
Archippus sp.: Suzuki and Kamijo (1967): 18.
-
Archips pulcher: Kawabe (1982): 66 (part.), pl. 15: 15, pl. 279: 10 (nec Butler 1879).
-
Ariola pulchra: Liu et al. (1983): 32, pl. 7: 157 (nec Butler 1879).
-
Archips pulcher: Liu (1987): 127, figs 6,44, pl. I: 10; Liu and Li (2002): 164, pl. XXIII: 215, pl. LXX: 215, pl. CXII: 215 (nec Butler 1879).
-
Archips abiephagus: Suzuki and Komai (1984); 94; Komai (1990): 5, figs 6 (right), 7; Razowski (1993): 677.
-
Archips abiephage[sic]: Brown (2005): 124.
Diagnosis. Archips abiephaga is similar to A. pulchra, but is distinguished from the latter by the more slender forewing having darker coloration and usually a lack of the lower longitudinal fasciae, and by the presence of distinct small abdominal dorsal pits. Compared with A. pulchra, the male genitalia have a broader uncus, a less rounded valva, and a weakly curved aedeagus with more numerous apical minute processes. Using the female genitalia, this species can be distinguished from A. pulchra by the smaller sterigma with a less expanded posterior lateral portion and by the smaller ostium. The male has a darker forewing than the female.
Male. Forewing length 8.0–11.0 mm. Head: Antenna filiform. Frons and vertex covered with brownish-orange scales. Labial palpus approximately 1.7 times longer than diameter of compound eye and covered with brownish-orange scales, darkened apically.
Thorax: Tegula covered with dark brown scales. Patagium covered with brownish-orange scales, darkened laterally. Forewing more slender than that of A. pulchra; ground color chestnut- to dark brown; markings glossy purplish-gray; basal area with three longitudinal fasciae from basal portion, first running to basal 2/5 along costa; second to below wing apex along upper vein of cell with basal half usually atrophied; and third running to half of termen along lower vein of cell, indistinct basally and sometimes interrupted at middle; portion between second and third fasciae and portion below third fasciae bearing fuscous longitudinal fasciae; termen margin wide, fuscous mixed with chestnut-brown; dorsum margin brownish-orange; cilia fuscous. Hindwing and cilia fuscous; Rs and M1 stalked.
Abdomen: Glossy fuscous with dark brown terminal tuft. Dorsal pits small; distinct pits present on terga II–III, atrophied pits on terga IV–VI; sclerites of terga and sterna similar to those of A. pulchra, but posterior invagination of sternum VIII much weaker than that of A. pulchra.
Genitalia: Essentially similar to A. pulchra. Uncus long, rather broad. Transtilla medially constricted band with strong proximal edge. Juxta heart-shaped. Valva short, costa slightly curved, apex protruded; sacculus broad and rounded with free termination variable in shape. Aedeagus typical of the species group, broad at base with some rows of minute processes apically.
Female. Forewing length 9.0–11.0 mm. Similar to male. Antenna simple. Forewing longer and broader than in male; ground color dull reddish-brown to purplish-brown; gray longitudinal fasciae more developed than in male, sometimes with additional fascia running along dorsum.
Genitalia: Papillae anales slender and subtriangular. Sterigma similar to A. pulchra, ante-ostial portion only slightly expanded laterally with pair of scale patches, its proximal cup-shaped portion short and not distinctly expanded. Antrum rather short. Ductus bursae slightly shorter than in A. pulchra. Corpus bursae ovate, signum bearing rather short capitulum and slender beak-shaped process.
Specimens examined. Japan: Hokkaido: Mount Poroshiri-dake, 1♀ (NSMT), 27.vii.1971, Y. Watanabe; Yamabe, Ishikari, 1♂ (OPU), em. 15.vi.1959, 1♂ (OPU), em. 16.vi.1959, 2♂1♀ (OPU, paratypes), em. 17.vi.1959, TK, ex Abies concolor; 1♂ (OPU), em. 17.vi.1959, TK, ex Abies koreana; Manji, Kurisawa, 1♀, 10.vii. 2001, HK; Mount Tarumae-zan, Tomakomai, 1♂, 15.vii.1994, HK; Otonrui, 1♂, 7.vii.1995, HK; Shikaribetsu, 1♂, 12.vii.1997, HK. Honshu: Kanto Mountains, Manza, 2♂4♀ (OPU), em. 26.vii.1959, S. Moriuti, ex Abies veitchii. Chichibu Mountains, Ôdarumi-tôge, 2000 m, 1♂, 21.vii.2001, UJ; Ôdarumi-tôge, 2000 m, Kawakami-mura, 1♂, 20.vii.2001, UJ. Yatsugadake Mountains, Kiyosato, 1300 m, 1♂ (NSMT), 20–25.viii.1967, (A. Kawabe); Nagasawazawa, Yachiho, 1♀, 22.viii.1997, M. Yamamoto; Mugikusa-tôge, 2120 m, 3♂2♀, 4.viii.1997, 5♂, 22.viii.1997, 1♂1♀, 22.viii.1988, M. Yamamoto; 5♂2♀, 23.viii.1989, 1♂, 24.viii.1989, HN, Shirokoma-ike, 1♂2♀, 23.viii., 1993, 1♀, 9.viii.1994, 2♂, 17–18.viii.1995, 1♂, 31.vii−1.viii.1999, UJ Shirokoma-shindô, 2♂2♀, 31.vii−1.viii.1999, UJ. Tateshina: Okutateshina, 1♀ (OPU), 24.vii.1994, T. Ueda. Mikuni Mountains, Shiga-kôgen, 1♀ (OPU), em. 23.V.1959, TK, ex Abies firma, 1♂1♀ (OPU), 12–14.vi.1959, A. Mutuura. Hida Range: Tokusawa, 1♂ (OPU), 25.vii.1955, A. Mutuura. Mount Norikura: Hirukubo-tôge, 1680 m, 1♀, 4.viii.1997, UJ: Sanbondaki, 1810 m, 1♀, 3.viii.2000, UJ; Reizen-goya, 2130 m, 1♀, 3.viii.2000, UJ; Kuraigahara-sansô, 2300 m, 1♂, 1.viii.2000, UJ; Norikura-kôgen, 1♀, 5.viii.1983, T. Mano. Mount Ontake: Ontakesan, 1♂ (OPU), 19.viii.1954, A. Mutuura. Kiso Range: Mount Anpeiji-san, 2138 m, 2♂, 25.vii.2001, K. Shikata. Mount Fuji: Yamanakako, 2♂ (YNC), 2.viii.1998, Y. Nasu; Subashiri-5 gome, 2♂, 5.viii.1995, K. Jinbo. Akaishi Range: near Senmaigoya, 2600 m, 1♂ (NSMT), 7.viii.1991, HN; Sensui-tôge, 2264 m, 1♀, 16.viii.2001, K. Eda; Kitazawa-tôge, 1♂, 14.viii.1992, 1♀, 15.viii.1992, UJ; 1♂1♀, 9–10.viii.2001, A. Sasaki; Higashimata, near Niken-goya, 7♂, 17.vii.1999, UJ; Nishimata, near Niken-goya, 3♂2♀, 18–19.vii.1998, UJ.
Host plants. Abies sachalinensis, A. veitchii, A. concolor, A. koreana, A. firma, and Picea abies (Pinaceae).
Distribution. Japan (Hokkaido, Honshu); China (Heilongjiang Province).
Remarks. My study revealed that the Archips pulchra group in Japan consists of three species, including one previously unknown species, and the three species have been confused with each other in the past. By examination of A. abiephaga specimens, including the type series deposited in OPU, the author found that the paratypes contained two species and that the description by Yasuda (1975) of A. abiephaga relied on both species; the description of the male, except for the genitalia, was based on one species, whereas the description of the male genitalia and the female was based on another species. Hence, it is essential to confirm which is the correct A. abiephaga. The holotype of A. abiephaga, labeled as “Holotype ♂, Japan, Hokkaido, Yamabe; bred from Abies concolor, 12.vi.1959, T. Kodama,” has been lost, but Yasuda (1975) provided a photograph of the holotype. Although the two species are similar to each other, specimens in good condition are easily distinguished. The photograph of the A. abiephaga holotype has fine enough resolution to distinguish which species the specimen belongs to. The present author determined that the holotype belongs to the species corresponding to the description of the male (except the genitalia) provided by Yasuda (1975). The remaining species is new to science and is described below. The present author also examined specimens, including the paratypes, bred along with the holotype (at Yamabe, Hokkaido). On the basis of the shape of wing patterns, dorsal pits and genitalia, all were clearly conspecific with the holotype. The figures of the male genitalia in the present paper are based on one of the paratypes of this series. To date, only the true A. abiephaga is known from Hokkaido.
My examination revealed that the paratypes of Archips abiephaga comprised two species. The list of the paratypes provided by Yasuda (1975) is as follows: “Paratypes. 2♂, Japan, Hokkaido, Yamabe; bred from Abies concolor, 17.vi.1959, T. Kodama. 1♀, Japan, Hokkaido, Yamabe; bred from Abies concolor, 17.vi.1959, T. Kodama. s♂[sic, probably a typographic error of “5”], 2♀, Japan, Honshu, Sinano, Manza, 27.vii.1958; 1♂ 1♀, Okutatesina, Nagano, 23.viii.1968, A. Kawabe; 1♂, Honshu, Hachimantai, Iwate Pref. 27.vii.1970, Se. Yamane.” All examined paratypes of A. abiephaga are in good condition. The present author concluded that all the paratypes that were bred with the holotype are a single species, A. abiephaga, whereas all the remaining paratypes collected in Honshu were identified as another species described below. Yasuda (1975) noted that the holotype was not dissected and thus he has not examined the genitalia of the holotype; the incorrect description of male genitalia was not based on the holotype.
Yasuda (1975) originally described this species as “Archippus (Pararchips) abiephage[sic] sp. n,” but the name “abiephaga” was used in the remainder of his description, including the figure captions (pp. 204, 208, 211). Brown (2005) referred to this species as “Archips abiephage.” However, this situation may be a case of “multiple original spellings” (ICZN article 19.3; International Commission on Zoological Nomenclature 2000), and a correct spelling should have been determined by the “First Reviser” (ICZN article 24.2.3). Razowski (1977) quoted both spellings, “abiephage” and “abiephaga,” and adopted “abiephagus” as the valid name (p. 106). Hence, Razowski can be regarded as the First Reviser, and the name abiephaga (or abiephagus) can be viewed as the correct species name. Razowski changed the ending of the species name to agree with the gender of the genus, because he regarded Archips as masculine.
In Hokkaido, A. abiephaga and A. pulchra can be found in plantation forests of Abies sachalinensis. In Honshu, A. abiephaga occurs only in mountainous and subalpine forests higher than 1300 m a.s.l. (e.g. Kiyosato, 1300 m a.s.l., Nagano Prefecture, Honshu), whereas A. pulchra is distributed in lower areas. Distribution ranges of the two species overlap at altitudes between approximately 1300 and 1800 m a.s.l. For example, at Hirukubo-tôge Pass (1780 m a.s.l., Nagano Prefecture) both A. abiephaga and A. pulchra were collected on the same day. This species has never been collected in the lowlands of Honshu where Abies firma is abundant and Archips pulchra is distributed; however, a specimen of Archips abiephaga was bred from an Abies firma tree. Adults are attracted to light.
Various authors have described the larva and life history of Archips abiephaga (Suzuki & Kamijo 1967; Yasuda 1969, 1975; Komai 1990). These studies were carried out in Hokkaido, where the species described below has not been found. The present author examined some reared specimens reported in these papers, and confirmed that all of them are true A. abiephaga (included in the specimens examined). Larvae of A. abiephaga are indistinguishable from those of A. pulchra, but pupae of the two species can be differentiated from each other by the shape of the dorsal pits. The life history of A. abiephaga is also similar to that of A. pulchra; however, in Hokkaido, larvae of A. abiephaga grow more rapidly than those of A. pulchra (Suzuki & Kamijo 1967). This species has one generation per year and hibernates in the young larval stage.
Archips stellata sp. nov. (1–6-18–20)
-
Archippus (Pararchips) abiephaga: Yasuda (1975): 109 (part., abiephage[sic]), figs 52, 396, 579 (nec Yasuda 1975).
-
Archips abiephagus: Razowski (1977): 106, figs 64, 65, 182; Kawabe (1982): 66, pl. 15: 17–18, pl. 279: 11, pl. 288: 6 (nec Yasuda 1975).
-
Archippus (Pararchips) abiephagus: Kuznetzov (2001): 139, fig. 89: 2–3 (nec Yasuda 1975).
-
Archips sp.: Oku (2003): 89.
Diagnosis. This species is characterized by the presence of two “shooting star-shaped” blotches on the outer area of the forewing and strong sexual dimorphism in markings. The male has a blackish forewing similar in appearance to that of A. abiephaga; worn specimens of the two species are sometimes indistinguishable from each other. Archips stellata can be easily separated from A. abiephaga by the male genitalia, which have a very long and bifurcated uncus and larger dorsal pits. The female genitalia of A. stellata are characterized by the very large and subtriangular sterigma.
Male. Forewing length 9.5–10.0 mm. Head: Antenna filiform. Frons and vertex covered with brownish-orange scales. Labial palpus about twice as long as diameter of compound eye and covered with brownish-orange scales.
Thorax: Tegula covered with dark brown scales. Patagium covered with brownish-orange scales scattered with dark brown scales, darkened laterally. Forewing broadest among the three species of the pulchra group; ground color dark brown, except basal 1/3 which is brownish-ochre; basal area with three longitudinal, glossy, purplish-gray fasciae with wide brownish-orange margin; first fascia running from base to basal 1/3 along costa; second positioned below first, running from basal 1/4 to before midpoint and somewhat weakened medially; and third running from base to beyond midpoint along dorsum; outer portion with two purplish glossy blotches, first blotch near costa, longitudinally elongate, with distinct brownish-orange margin; second smaller than first one, positioned at middle; termen margin wide, glossy, purplish-gray; cilia fuscous, with orange margin. Hindwing and cilia fuscous; Rs and M1 separate.
Abdomen: Covered with glossy fuscous scales with brownish-gray terminal tuft; dorsal pits distinct and large, present on terga II–VI and sometimes also on VII, moderate; sternum VIII more developed than in A. abiephaga with barely concave posterior edge.
Genitalia: Uncus elongate and constricted at middle with apex shallowly bifurcated. Gnathos broad with rounded apex. Transtilla a broad band gently constricted medially. Valva relatively slender, costa slightly curved, apex pointed; sacculus rather narrow and rounded with a small free termination. Aedeagus slender with some rows of minute processes subapically; cornuti long.
Female. Forewing length 9.5–11.0 mm. Antenna simple. Forewing with dull orange ground color; some longitudinal, short, black lines present; basal area with three longitudinal, glossy, purplish-gray fasciae more developed than in male; two purplish, glossy blotches on outer portion of forewing more developed, elongate; termen with glossy, purplish-gray margin, which usually connects to the two blotches; cilia yellowish-orange except for a dark portion near tornus. Hindwing paler than in male; cilia yellowish-orange in upper half, fuscous in lower half. Abdomen covered with glossy fuscous scales.
Genitalia: Papillae anales elliptic with inner edge concave proximally. Sterigma very large, subtriangular, its posterior portion with a pair of large, weakly convex areas bearing patches of scales. Ante-ostial portion of sterigma very short. Antrum well developed, delicately tapering proximally. Ductus bursae rather short and sinuate with a cestum. Corpus bursae ovate, signum bearing a small capitulum and a short beak-shaped process.
Holotype. “Kawakami-makioka rindô, 1600 m, Kawakami-mura, Nagano, ♂, 21.vii. 2001, U. Jinbo,” genitalia slide No. UJ-774, deposited at the National Science Museum, Tokyo.
Paratypes. Japan: Honshu: Mount Hachimantai: Hachimandai, 1♂ (OPU, paratype of A. abiephaga), 27.vii.1970, Se. Yamane; Mount Nakakura, Matsuo-mura, 2♂1♀ (OKC), 7.viii.1999, N. Doi. Mount Hayachine: Odagoe, 1♀(OKC), 5.ix.1975, T. Oku. Kanto Mountains, Manza, 1♂2♀ (OPU, paratypes of A. abiephaga), 27.vii.1958, SI and TY. Chichibu Mountains, Kabagoya, 1♀ (NSMT), 24.vii.1933, (S. Asahina); Shikanoyu, 1♂ (NSMT) 27.vii.1933, (S. Asahina); Ôdarumi-tôge, 2000 m, 3♂3♀, 20.vii. 2001, UJ. Yatsugadake Mountains, Mugikusa-tôge, 2100 m, 1♂, 22.viii.1988, M. Yamamoto; 3♀, 23.viii.1989, HN; Shirokoma-ike, 1♀, 23.viii.1993, 1♂2♀, 24.viii.1993, UJ, Shirokoma-shindô, 5♂4♀, 31.vii−1.viii.1999, UJ. Mount Tateshina: Okutateshina, 1♂1♀ (OPU, paratypes of A. abiephaga), 23.viii.1963, A. Kawabe; 1♀ (NSMT), 23.viii.1963, A. Kawabe; 1♂ (OPU), 24.vii.1994, T. Hirowatari; 4♂ (OPU), 24.vii.1994, T. Ueda. Nagano: Akasaka, Hiraya, 1♀, 30.viii.1984, M. Ihara; Aokuzure, Minami-shinano, 1♀, 6.vii.1991, M. Ihara; Kamasawa, Ôshika, 1♀, 19.ix.1987, M. Ihara; Meiji-Onsen, 1♂ (OPU), 9.viii.1970, 1♀ (OPU), 23.viii.1971, F. Komai. Kiso Range: Mount Kisokomagatake, 1♂ (OPU), 18.viii.1992, T. Hirowatari. Mount Fuji: Subashiri-5 gome, 1♂, 5.viii.1995, K. Jinbo. Akaishi Range: Sensuitôge, 1♂, 16.viii. 2001, K. Eda; Nishimata, near Niken-goya, 1♂1♀, 18–19.vii.1996, UJ; Kitazawa-tôge, 7♂2♀, 9–10.viii. 2001, A. Sasaki. Aichi: Dandouradani, Kita-shitara, 1♂, 15.vii.1989, T. Mano.
Distribution. Japan (Honshu).
Host plants. Unknown.
Etymology. The species name stellata refers to the markings like falling stars on the forewing (stella: star +ta).
Remarks. As mentioned above, A. stellata has long been confused with A. abiephaga. All the examined paratypes of A. abiephaga collected on Honshu were this species. The author examined specimens recorded as an unidentified species of Archips by Oku (2003) and found that it corresponded to A. stellata.
Archips stellata has been collected only from mountainous and subalpine coniferous forests of central and north-eastern Honshu, Japan. In north-eastern Honshu, this species is distributed in subalpine areas of the Hachimantai Plateau and Mount Hayachine. In central Honshu, A. stellata occurs in forest at altitudes above approximately 900 m. Sympatric distributions with A. abiephaga are only known to occur in subalpine areas of central Honshu. Adults have been collected from July to October. The immature stages of this species remain unknown, but its life history and host plants can be assumed to be similar to those of A. abiephaga.
DISCUSSION
The three Japanese species of the pulchra group of Archips, A. pulchra, A. abiephaga, and the new species, A. stellata, have long been confused with one another. Yasuda (1975) erroneously described A. stellata male genitalia and an A. stellata female as belonging to A. abiephaga. Razowski (1977) figured the male and female genitalia of A. abiephaga paratypes collected at Manza (Gumma Prefecture, Honshu); however, these samples actually belong to A. stellata. Kawabe (1982) misidentified a male of A. abiephaga as a color variation of A. pulchra and the male genitalia of A. abiephaga as those of A. pulchra, and A. abiephaga sensu Kawabe corresponds to A. stellata. The image file of an adult A. abiephaga from a CD-ROM published by Meijerman and Ulenberg (2000) is that of A. pulchra, and these authors also called upon drawings of A. abiephaga genitalia from Razowski (1977), but those drawings were actually of A. stellata. Similarly, Kuznetzov (2001) misidentified the male genitalia of A. stellata as being those of A. abiephaga. The Chinese specimens of pulchra described by Liu et al. (1983), Liu (1987) and Liu and Li (2002) seem to indicate A. abiephaga, although the author has not yet had the opportunity to examine the Chinese material. Komai (1990) correctly identified an adult of A. abiephaga collected in Hokkaido.
Archips pulchra was described by Butler (1879) under the genus Ariola Walker, 1858, currently placed in Chloephorinae, Nolidae (sensu lato). Walsingham (1900) classified this species under Tortrix in Tortricidae. Subsequently, the species was included in various genera of this family (see the synonym list). Currently, the pulchra species group is placed broadly in Archips because members have genitalia similar to the type species of the genus, A. piceana Linnaeus, 1758 (=A. oporana Linnaeus, 1758), including a crescent-shaped coremata that is a presumed synapomorphy in Archips (Jinbo 2000).
Three main opinions exist on the systematic placement of this species group within the genus Archips.
- 1
The pulchra group should be separated as the subgenus Pararchips of Archips (Kuznetzov 1970, 2001). Kuznetzov (1970) erected the monotypic subgenus Pararchips based on A. pulchra. The subgenus was defined by the forewing without a costal fold, a pattern consisting of some longitudinal fasciae, and weak sexual dimorphism in pattern and shape.
- 2
Archips should be divided into two genera, Archips (sensu stricto) and ArchippusFreeman, 1958; the pulchra group should be included in the subgenus Pararchips of the latter (Yasuda 1972). Archippus was determined based on a Nearctic species, Tortrix packardiana Fernald, 1886, defined by the male genitalia having a uniformly broad uncus and a narrow and strong sacculus. Many species of Archips were once transferred to this genus (Freeman 1958; Kawabe 1965; Suzuki & Kamijo 1967). Yasuda (1972) redefined Archippus on the basis of its long labial palpus, the shape of the costal fold (if present), hibernation in the larval stage, and two or more generations. He transferred Pararchips from Archips to Archippus on the basis of the generation number and recognized the stalked Rs and M1 of the hindwing as additional diagnoses of this subgenus.
- 3
The pulchra group should be recognized as a species group of Archips (Razowski 1977). Razowski regarded both Pararchips and Archippus as synonyms of Archips. He defined six species groups, including the pulchra group, in this genus. At the same time, he illustrated the phylogenetic relationships among the species groups and presumed that Archippus sensu Yasuda, including the pulchra group (=Pararchips), was monophyletic.
The above three opinions agree that Archips pulchra and its allies form a distinct species group of the genus, defined by gray longitudinal fasciae on the forewings. Similar wing markings are observed in some archipine species (e.g. Pandemis ignescana Kuznetzov, 1976, and Archips striana Fernald, 1905; see Freeman 1958; Kuznetzov 2001; Jinbo 2002). They have a brownish forewing with black transverse fasciae but without glossy gray ones. Moreover, the conifer-feeding species of Naragodes Hampson, 1910 (Chloephorinae, Nolidae sensu lato), also have similar markings represented by a red-brownish forewing with silver or white transverse fasciae (Sugi 1990). Such wing patterns are considered to be cryptic patterns that may have evolved among several conifer-feeding groups independently (Kuznetzov 1970; Jinbo 2000). However, two other proposed diagnoses of the pulchra group are based on characters that are neither unique nor constant among its members. Both Kuznetzov (1970) and Yasuda (1972) regarded the absence of a costal fold on the forewing as being diagnostic of this species group, but the same state has also been observed in some Nearctic Archips species placed in other species groups (Razowski 1977; Kruse & Sperling 2002). Yasuda (1972) proposed the stalked veins Rs and M1 of the hindwing as another diagnostic feature of this group. Though Archips pulchra and A. abiephaga have this vein configuration, A. stellata has separate veins. In addition, similar stalked veins have been noted in at least two Japanese Archips species not included in the pulchra group: Archips nigricaudana and Archips insulana (Jinbo 2000).
The monophyly of Archippus sensuYasuda (1972), including the pulchra group, is not supported. Yasuda (1972) used hibernation at the mid-instar larval stage as a diagnostic feature of Archippus and hibernation at the egg stage as being distinctive of Archips. However, hibernation in the larval stage may be plesiomorphic because members of Choristoneura Lederer, 1859, presumed by Jinbo (2000) to be a sister group, also hibernate in the larval stage. The polarity of the evolution of generation number, another determinant proposed by Yasuda (1972) to distinguish Archippus, is also unclear because there are species having one or two generation(s) in both Archips, including the pulchra group, and Choristoneura.
The separation of the pulchra group as a subgenus is problematic from a phylogenetic standpoint, although the species group can be defined by the characteristic forewing markings with the gray transverse fasciae unique among archipine species. The nominotypical subgenus of Archips sensu Kuznetzov, including all remaining species of Archips except the pulchra group, is unsupported by synapomorphies. Similarly, the monophyly of Archippus sensu Yasuda is unsupported as discussed above. Thus, until the phylogeny of the genus Archips can be detailed, the opinion of Razowski (1977), that is, that the pulchra group should be regarded as a species group, is more reasonable than the viewpoint that the pulchra group should be separate as a subgenus.
ACKNOWLEDGMENTS
I wish to express my sincere gratitude to Drs M. Owada, Y. Kobayashi, H. Inoue, A. Shimizu, and T. Yamasaki for constant encouragement and critical comments on an early draft of this paper. The author is also indebted to Drs T. Hirowatari, M. Ishii, and T. Yasuda for access to specimens deposited at Osaka Prefecture University, and to Drs F. Komai, Y. Nasu, T. Oku and J. Razowski, and Mr S. Sugi for continuous guidance, valuable comments, and assistance with procuring literature and specimens. Thanks also to the following entomologists for loaning or donating valuable specimens and their multifaceted help: Mr K. Eda, Mr K. Fukuzumi, Mr N. Hirano, Dr H. Hara, Mr M. Ihara, Mr N. Iizuka, the late Mr K. Jinbo, Mr T. Kaneko, the late Mr A. Kawabe, Mr S. Kawahara, Mr H. Kogi, Mr T. Mano, Dr M. Maruyama, Mr A. Miyano, Dr H. Nakajima, Mr S. Niitsu, Mr A. Sasaki, Mr K. Shikata, Mr K. Sugisima, Mr M. Tanaka, Dr K. Ueda, Mr M. Yamamoto, Dr H. Yamanaka, Dr T. Yamauchi, and Mr Y. Yanagita.




