Abaristophora sachalinensis Michailovskaya (Diptera: Phoridae) representing genitalic affinities with the genus Borophaga Enderlein
Abstract
Abaristophora sachalinensis Michailovskaya is reviewed based on Japanese materials. Its male genitalia are compared with those of the genus Borophaga, which is a genus in a group of the related genera, the Borophaga subgroup. A synapomorphic character of the Borophaga subgroup including Abaristophora, the left flattened arm derived from the posterodorsal margin of the hypandrium being broadened at the base, is confirmed in A. sachalinensis. Morphology of the aedeagus in A. sachalinensis is complex and extremely asymmetric, and very similar to that of species of the genus Borophaga, but the characters observed in this study are not regarded as synapomorphic for the Borophaga subgroup.
INTRODUCTION
The genus Abaristophora Schmitz, 1927, is a member of the Borophaga group proposed by Brown (1992). The Borophaga group was further divided into two subgroups by Brown (1992): the Borophaga subgroup comprising Borophaga Enderlein, 1924, GodavariaBrown, 1992, LatiborophagaBrown, 1992, Abaristophora and AntipodiphoraSchmitz, 1939; and the Stichillus subgroup comprising Stichillus Enderlein, 1924, Peromitra Enderlein, 1924 and Trineurocephala Schmitz, 1923. There was, however, no convincing synapomorphy to unite the Borophaga subgroup, whereas the tube-shaped aedeagus was considered to be one of the synapomorphic characters of the Stichillus subgroup. In our previous paper (Nakayama & Shima 2005), we found that the left flattened arm derived from the posterodorsal margin of the hypandrium is broadened at the base in genera of the Borophaga subgroup. We further suggested that this state is synapomorphic for the Borophaga subgroup. The aedeagus, however, is poorly investigated except in the genus Borophaga, and even characters in the aedeagus suggesting relationships or similarities in the genera of the Borophaga subgroup are unknown.
The genus Abaristophora comprises two subgenera, Abaristophora s. str. and AntipodiphoraSchmitz, 1939 (Disney & Ross 1997). Abaristophora s. str. comprises three described species distributed in the Far East and North America and an undescribed species recognized from Venezuela (Brown 1992). The subgenus Antipodiphora consists of six described species occurring in New Zealand and a species described from Nepal (Disney & Ross 1997). Disney (in Disney & Ross 1997) described a fossil species from Dominican amber. The genus Abaristophora is currently characterized by the following combination of external characters: frons lacking supra-antennal bristles; male first flagellomere globular basally, elongated dorsally into a slender arista-like process bearing long hairs; female proboscis geniculate; hind tibia with two hair seams. Abaristophora s. str. is distinguished from Antipodiphora by the absence of the arista on the antenna in the male and a more sinuous wing vein CuA1. Flies of the genus Abaristophora are tiny, only 1–2 mm long. Their life history is as yet unknown.
Antipodiphora was described from New Zealand as a subgenus in Abaristophora by Schmitz (1939). Borgmeier (1963) raised it to the generic rank without providing a reason. Brown (1992) treated Antipodiphora as a genus, but he was suspicious of its generic status because it is characterized by plesiomorphic characters, and later Disney and Ross (1997) returned Antipodiphora to the status of a subgenus in the genus Abaristophora. Monophyly of Antipodiphora is not currently suggested, and its systematic position in the Borophaga subgroup deserves further intensive study.
In Japan, Abaristophora sachalinensisMichailovskaya, 1988, belonging to Abaristophora s. str., has been recorded from Iturup (Etorofu) Island (Michailovskaya 1998). No further information on the genus Abaristophora in Japan is available. We have recently examined several male specimens of this genus collected in Japan, which can be identified as being A. sachalinensis. Based on these specimens, A. sachalinensis is revised, and the state in the basal part of the flattened arms, a synapomorphic character in the Borophaga subgroup as mentioned above, is described in detail. We further provide basic morphological information for the male genitalia, especially of the aedeagus, and briefly review the relationships of A. sachalinensis to the genus Borophaga, because Borophaga is the only genus in the Borophaga subgroup that is well investigated for the male genitalia, especially the aedeagus.
MATERIALS AND METHODS
Specimens used in this study were air-dried and glued on micropins. Two specimens were slide-mounted with Euparal (Chroma Geselschaft Schmidt, Köngen, Germany) following the method of Disney (1994). For examining the male genitalia, the terminal abdominal segments were detached from the body and placed in a 10% solution of KOH at 48°C for 8 h, then placed in 8% solution of CH3COOH for 30 min, and finally transferred to distilled water for dissection. Observations were carried out under both stereoscopic dissecting and compound light microscopes.
Terminology mostly follows Peterson (1987) as in our previous papers (Nakayama & Shima 2005), while differing terminology and interpretations are suggested by Disney (1994, 1998, 2001). Measurements were carried out using air-dried specimens. Body length was measured from the head (excluding antennae) to the tip of abdominal segment VI. Measurements of the wings followed Disney (1994). The following indices were used in the description, in accordance with Gotô (1984): costal index = length of costa/length of wing; costal sector index = length of first costal sector/length of second costal sector.
The materials used in the present paper are deposited in the collection of the Biosystematics Laboratory, Graduate School of Social and Cultural Studies, Kyushu University, Fukuoka, Japan.
DESCRIPTION
Abaristophora sachalinensis Michailovskaya 1-3–7)

Male antenna of Abaristophora sachalinensis. Left antenna from the right side. The arrow indicates the small branch on the pre-apical portion of the first flagellomere. Scale line: 0.1 mm.
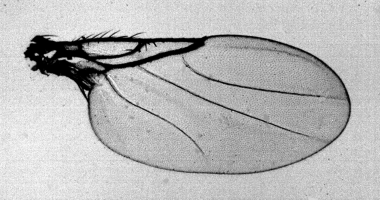
Male wing of Abaristophora sachalinensis.
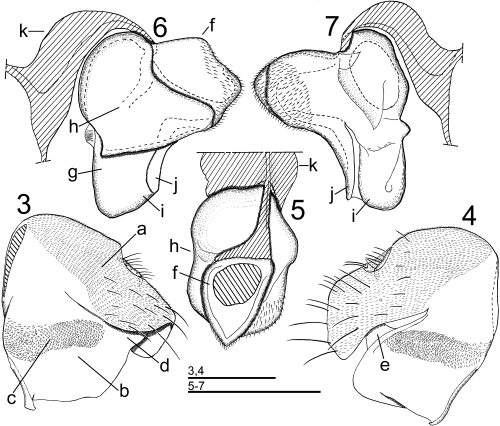
Male genitalia of Abaristophora sachalinensis. 3,4 Epandrium and hypandrium (3 left lateral view, 4 right lateral view); and 5–7 aedeagus (with aedeagal apodeme; 5 dorsal view, 6 left lateral view, 7 right lateral view). (a) epandrium; (b) hypandrium; (c) membranous region of hypandrium; (d) base of left flattened arm; (e) base of right flattened arm; (f) basal ring; (g) center plate; (h) left plate; (i) anterior basal plate of center plate; (j) posterior thin plate of center plate; and (k) aedeagal apodeme.
Abaristophora sachalinensis Michailovskaya, 1988: 470; Michailovskaya (2004a): 45; Michailovskaya (2004b): 13.
Undescribed species of Abaristophora from Japan: Nakayama and Shima (2005): 289.
Male. Head: Frons and vertex subshining, brown to black, with fine hairs; frons wider than long, without median furrow; vertex forming a weak ridge along posterior margin. Frons and vertex with three transverse rows of bristles, each consisting of four bristles; front row convex anteriorly, lower interfrontal bristle lower than lower fronto-orbital bristle; middle row convex anteriorly, upper interfrontal bristle lower than upper fronto-orbital bristle. Lower interfrontal bristles close together. Supra-antennal bristles absent. Clypeus brownish black, thin. Antenna (Fig. 1): First flagellomere brown, longer than eye height, globular basally, elongated dorsally into a slender arista-like process with a preapical small branch on posterior face, bearing long hairs; arista absent. Palpus brown to yellow, long and slender, longer than basal globular part of first flagellomere, with six or seven bristles. Proboscis brown, short.
Thorax: Scutum dark brown, finely haired, with a pair of dorsocentral bristles; scutellum dark brown, with a pair of long posterior bristles and a pair of anterior short and fine hairs; pleura brown; anepisternum with some fine hairs on anterior portion of upper part.
Wing (Fig. 2) hyaline, 1.36–1.69 mm long; vein Rs not forked (R2+3 absent), without a series of hairs; costal index 0.50–0.53; costal sector index 0.94–1.41; vein M1 curved at base, nearly straight on distal half, weaker at the apex but complete; vein M2 nearly straight, weaker at the apex but complete; vein CuA1 sinuate as S-form, incomplete at the apex; vein A1 + CuA2 present but weak. Halter black.
Legs: Coxae brown, tibiae and tarsi yellowish brown. Fore tibia lacking bristle, with a row of 13–14 anterodorsal spines below proximal one-fifth along tibial length. Mid-tibia with a dorsal hair seam, which is a little deflected anteriorly on proximal half, and palisade-like hairs on distal half of anterior face; a pair of bristles present near proximal one-fourth, one of the pair dorsal and the other anterior, dorsal one a little higher than anterior one, and an anterior preapical bristle present; a long ventral spur present at distal end, which is approximately threefold as long as preapical anterior bristle. Hind tibia with two dorsal hair seams, and palisade-like hairs on distal end of posterior face; two weak anterodorsal bristles present at proximal one-fourth and at preapical portion, the preapical one approximately half of the proximal one, two unequal robust ventral spurs present at distal end, the longer one more than twice as long as the shorter one. Apical fore tarsomere not distinctly broadened.
Abdomen dark brown, oval in dorsal view, widest at posterior margin of tergite II; long and fine hairs present on posterolateral portion of tergite I and upper part of lateral portion of tergite II.
Genitalia (Figs 3–7): Epandrium finely pubescent, epandrial lobe elongated posteroventrally, right side more expanded posteroventrally than left side, both sides fused with each other on posterior portion; sparse hairs present on posterior half of epandrium, longer on posterior margin; anal tube short; cerci hairy, with minute pubescence; hypoproct small, with a few hairs. Hypandrium without pubescence, transverse membranous region with flattened, rounded spinuli present. Aedeagal apodeme curved to arch-shape in lateral view, arising interiorly from fused portion of both sides of hypandrium and attached to anterior margin of basal ring of aedeagus; flattened arms arising bilaterally from dorsal edge of hypandrium and attached to posterior portion of basal ring of aedeagus, left arm shifted to posterior margin of hypandrium and distinctly broadened at base. Aedeagus extremely asymmetric, spiral to some extent, consisting of basal ring and two major sclerites, center plate and left plate; basal ring present on dorsal (proximal) portion of aedeagus, with fine hair-like spines on its posterior portion; center plate fused with basal ring and extended distally (ventrally), forming anterior basal plate and posterior thin plate on distal end; left plate arising from right side of center plate, surrounding basal ring and proximal half of center plate through anterodorsal portion and extending to left side.
Body length: 1.2–1.5 mm.
Female. Unknown.
Specimens examined. Hokkaido: 3♂, Gensei-kaen, Sarobetsu, Soya, 8.ix.1977, M. Yamamoto; 1♂, Lake Toro, Shibecha, 5.ix.1977, K. Ohara. Honshu: 1♂, Mount Odake, Hakkodasan, Aomori, 13.viii.1980, T. Gotô. Kyushu: 1♂, Top of Mount Ofuna, Kuju, Oita, 19.vii.1978, K. Maeto.
Distribution. Japan (Etorofu (Iturup) Island, Hokkaido, Honshu, Kyushu); Sakhalin, Paramushir Island.
Remarks. This species is distinguished from other species of Abaristophora s. str. by the absence of tibial bristle on the fore tibia.
The membranous region with spinuli in the hypandrium of this species is transversely elongated, smaller than in Borophaga, Peromitra and Stichillus, and not expanded as a lobe or sack as in these genera.
DISCUSSION
Morphology of the left flattened arm derived from the hypandrium
Brown (1992) proposed that the Borophaga group comprises Borophaga and its related genera, and further classified the group into two subgroups: the Borophaga subgroup consisting of six genera, and the Stichillus subgroup consisting of three genera, as mentioned above. There was, however, no convincing synapomorphy for the Borophaga subgroup.
Nakayama and Shima (2005) described the male genitalia of two Borophaga species and found that the left flattened arm derived from the posterodorsal margin of the hypandrium was broadened at the base, and further suggested that this state would be synapomorphic for the Borophaga subgroup. It was confirmed in the present study that the left flattened arm of the hypandrium was shifted to the posterior margin and broadened at the base in A. sachalinensis (Fig. 3d). The right flattened arm (Fig. 4e) is a little wider than that of Borophaga (Nakayama & Shima 2005; fig. 5f), but it is not shifted to the posterior margin of the hypandrium as on the left side. Conspicuous bilateral asymmetry is observed with respect to the morphology of the arms. Brown (1992; fig. 15g,f) illustrated the left side of the male genitalia of an undescribed species of Abaristophora s. str. from Venezuela and another undescribed species of Antipodiphora from New Zealand. These species also have the left flattened arm broadened at the base. Such an extremely asymmetric character of the flattened arm could be common to the species of Abaristophora s. str. and Antipodiphora. As suggested by Nakayama and Shima (2005), this distinct character would be shared by members of Borophaga subgroup.
Morphology of the aedeagus
Morphology of the aedeagus in A. sachalinensis is interesting, with a complex and asymmetric structure, similar to that of Borophaga presented by Nakayama and Shima (2005), but dissimilar to the tube-shaped aedeagus of Peromitra or Stichillus in the Stichillus subgroup. Three major sclerites of the aedeagus, the basal ring, center plate and left plate, in A. sachalinensis are apparently homologous with those in the genus Borophaga. Interesting similarity is also observed in more minute structures. The distal end of the center plate is composed of two sclerites. The anterior basal plate (Figs 6i,7i) in A. sachalinensis should be homologous with the ventral plate of the posterior hook of Borophaga clandestinaNakayama and Shima, 2005 (Nakayama & Shima 2005; figs 6k,7k) and the saw-blade area of Borophaga femorata Meigen, 1830 (Nakayama & Shima 2005; fig. 12g). The posterior thin plate (Figs 6j,7j) should be homologous with the dorsal plate of the posterior hook of B. clandestina (Nakayama & Shima 2005; figs 6l,7l) and hood-like upper process of B. femorata (Nakayama & Shima 2005; figs 11h,12h).
Separation of the left plate from the center plate in the aedeagus seems to be plesiomorphic in comparison with the tube-shape observed in the Stichillus subgroup. Features in the distal end of the center plate observed in A. sachalinensis and Borophaga also would not be synapomorphic for the Borophaga subgroup, because a similar structure is observed in the genera Stichillus and Peromitra in the Stichillus subgroup. The general feature of the aedeagus of A. sachalinensis is very similar to that of Borophaga, but no synapomorphic character to relate A. sachalinensis with Borophaga was found in the characters investigated in this study.
ACKNOWLEDGMENT
We thank Dr T. Gotô for the gift of materials.




