Japanese oak silkmoth feeding preference for and performance on upper-crown and lower-crown leaves
Abstract
We quantified differences in leaf traits between upper and lower crowns of a deciduous oak, Quercus acutissima, and examined feeding preference, consumption and performance of the Japanese oak silkmoth, Antheraea yamamai, for those leaves. Upper-crown leaves had significantly smaller area, larger dry mass per area, greater thickness, lower water content, higher nitrogen content and a higher N/C ratio than lower-crown leaves. When simultaneously offered upper-crown and lower-crown leaves, moth larvae consumed a significantly larger amount of the former. However, when fed with either upper-crown or lower-crown leaves (no choice), they consumed a significantly larger amount of the latter. Female larvae reared on upper-crown leaves had a significantly smaller fresh weight, but attained a significantly larger pupal fresh and dry weight, with a significantly higher relative growth rate than those on lower-crown leaves. Although, like female larvae, male larvae had a significantly smaller fresh weight when reared on upper-crown leaves, they had a significantly larger value only for pupal dry weight. These results suggest that: (i) larvae ingest a greater amount of lower-crown leaves to compensate for the lower nitrogen content of the foliage, resulting in having an excess of water because of the higher water content of the foliage; (ii) feeding preference for upper-crown leaves accords with better performance (with respect to dry pupal weight and relative growth rate) on the foliage; (iii) better performance is explained by a higher nitrogen content and N/C ratio of the upper-crown foliage; and (iv) the effects of leaf quality on performance differ between sexes.
INTRODUCTION
Upper-crown and lower-crown leaves have recently received much attention from the viewpoint of relationships between leaf quality, folivore performance and folivore distribution within tree crowns (Suomela et al. 1995; Fortin & Mauffette 2002; Yamasaki & Kikuzawa 2003). Upper-crown leaves grow under conditions of high solar irradiance, and thus have sun-leaf properties such as smaller area, greater thickness and higher nitrogen content. In contrast, lower-crown leaves are shaded by other branches and grow under conditions of low irradiance, and thus have shade-leaf properties such as larger area, less thickness and lower nitrogen content (Mooney & Ehleringer 1997; Le Corff & Marquis 1999). Sun leaves generally have higher light-saturated rates of photosynthesis (Morecroft & Roberts 1999; Terashima et al. 2001), which are closely related to leaf nitrogen content (Field & Mooney 1986). Leaf nitrogen content is generally considered to be one of the most important factors in determining the performance of insect folivores (Schoonhoven et al. 1998; Chown & Nicolson 2004). In fact, Fortin and Mauffette (2002) have shown that upper-crown leaves of the sugar maple (Acer saccharum Marsh) have a higher nitrogen content than lower-crown leaves, and that the performance of the forest tent caterpillar (Malacosoma disstria Hübner), especially with respect to pupal weight and the number of eggs of adult females, is better when larvae are fed with upper-crown leaves. Several studies have also shown that larvae fed with upper-crown leaves or sun leaves attain significantly heavier larval and pupal weights, and have a shorter developmental time and/or higher relative growth rates (Harrison 1987; Jensen 1988; Watt 1992; Suomela et al. 1995). Hence, Fortin and Mauffette (2002) suggested that heterogeneity in leaf quality along a vertical stratification within forests might influence the distribution of folivorous insects.
However, larvae fed with upper-crown leaves or sun leaves do not necessarily perform better than those fed with lower-crown leaves or shade leaves. Some studies have shown the opposite results or insignificant differences (Futuyma & Saks 1981; Jansen & Stamp 1997; Henriksson et al. 2003). Inconsistency among these studies could be attributed to several problematic experimental designs. First, although natural populations of folivores vary in size (Stamp & Bowers 1990; Hemming & Lindroth 2000), some studies failed to minimize size and other genetic effects on the feeding performance of folivores because they did not use strains or sibs (Futuyma & Saks 1981; Harrison 1987; Suomela et al. 1995). Second, although male and female larvae generally differ in size (Bauce et al. 1994; Pöykkö & Hyvärinen 2003), some studies did not distinguish larval sexes because of the difficulty in sexing larvae (Harrison 1987; Jensen 1988; Watt 1992; Jansen & Stamp 1997; Henriksson et al. 2003). Third, although pupal weight is related positively to adult size (Stamp & Bowers 1990; Scheirs et al. 2002), some studies focused on larval growth (Harrison 1987; Jensen 1988; Watt 1992; Jansen & Stamp 1997; Henriksson et al. 2003). Fourth, although heavy fresh weight does not necessarily lead to heavy dry weight (Jensen 1988; Bauce et al. 1994), some studies calculated the dry weights of larvae from their fresh weights using conversion formulae obtained in other studies (Suomela et al. 1995; Henriksson et al. 2003).
Furthermore, two further reasons for the inconsistencies in the results of these studies could be added in the context of the effects of other chemical traits of leaves on folivore performance (Schoonhoven et al. 1998; Awmack & Leather 2002; Chown & Nicolson 2004). First, because the nitrogen content of leaves tends to correlate with the water content, which is another substantial factor in determining folivore performance, assessment of the importance of leaf nitrogen is complicated. Second, dilution of nitrogen by carbohydrates has generally negative effects on folivore performance.
In the present study, we first investigated the nitrogen content, water content and N/C ratio of upper-crown and lower-crown leaves of a deciduous oak (Quercus acutissima Carruthers). Second, we examined the larval feeding preference of the Japanese oak silkmoth, Antheraea yamamai (Guérin-Méneville), for the two categories of leaves. Third, we compared the performance and leaf consumption of A. yamamai on those leaves. To obtain reliable data on performance, we measured larval development time, larval fresh weight, and pupal fresh and dry weights for each sex using strains. Thereby, we discuss the effects of nutritional differences between upper-crown and lower-crown leaves on folivore performance.
MATERIALS AND METHODS
Study organism
Antheraea yamamai is widespread in Japan. It is polyphagous, feeding on oaks and chestnuts of Fagaceae and cherry and apple trees of Rosaceae. The adults fly in September–October, and the females lay their eggs on the twigs of their food trees. The eggs overwinter and hatch in mid- to late April. The larvae feed for approximately 2 months, and pupate in spun leaves (Inoue 1982; N. Teramoto, unpubl. data, 1994–1999). A deciduous oak, Quercus acutissima, is one of the most suitable food plants for larval growth of A. yamamai (Teramoto 2001).
Strains of A. yamamai have been established and maintained for their silk in sericulture research stations. For each of the present experiments, we used larvae hatched from eggs laid by a single female of a strain reared in Shiga Prefecture Agricultural General Center to minimize the effects of genetic variability on the results.
Leaf sampling site
Leaves of Q. acutissima were sampled in a secondary forest of Kasuga Hill in Nara City (380 m a.s.l.; 34°41′N, 135°51′E). The forest is dominated by two deciduous oaks, Q. serrata and Q. acutissima, which are 12–16 m in height. Larvae of A. yamamai are occasionally found on these oaks.
Leaf traits
Three or five trees of Q. acutissima were selected monthly from May to August 2000 (13 May, 24 June, 19 July, and 26 August). We haphazardly sampled 30 undamaged leaves from each of the upper (12–16 m above the ground) and lower (4–6 m above the ground) parts of the individual tree crowns. These leaves were brought to the laboratory in an insulated box filled with ice. For 20 leaves the outline was scanned using an image scanner (GT-4000; Epson, Suwa, Japan), and the area was measured by using free image analysis software (LIA32 for Windows 95, version 0373; Yamamoto 1997). After being weighed, the leaves were dried at 80°C for 48 h to determine their dry weight. The leaf dry mass was then calculated as dry weight per unit area (g/cm2) and the water content (%) as ((wet weight − dry weight)/wet weight) × 100. Afterwards, the leaves were ground together into fine powder. The sample was analyzed two or three times for the nitrogen and carbon contents using an NC analyzer (Sumigraph NC-90 A; Shimazu, Kyoto, Japan).
The other ten leaves were cross-sectioned at the middle of the mid-vein with a razor by hand, and the thickness was measured using a microscope with an ocular micrometer.
Feeding preference of A. yamamai larvae
Eggs deposited by a single A. yamamai female of Shiga SG strain were exposed to an incubator (25°C and 0 h light : 24 h dark (LD 0:24)). After 3 days, the light condition was changed to LD 24:0 for induction of hatching. Twenty-three hatchlings that emerged within 3 days after induction (2–4 July 2000) were used for the experiment. They were individually placed in a transparent plastic box (100 mm × 100 mm × 25 mm) that contained a sheet of dry filter paper on the bottom and moistened sanitary cotton on the inside of the lid, and were kept in an incubator maintained at 23 ± 1°C with a photoperiod of LD 14:10. They were reared on primary young or secondly flushed young leaves of Q. acutissima except during the following trials. On the fourth day after the third moulting (26–28 July) and the fifth day after the fourth moulting (5–10 August), larvae were offered a set of four or six expanded undamaged leaves, half of which were from the upper crown and half of which were from the lower crown. The area of each leaf was measured in advance so that areas of leaves from the upper and lower crowns were almost equal. After 6 h, the area of each leaf was measured again, and the total area consumed by the larva was determined. The dry weight consumed was calculated as area consumed × dry mass, where dry mass was the mean value obtained in the leaf trait investigation mentioned previously.
Leaf consumption and performance on upper-crown and lower-crown leaves
Offspring deposited by a single female of Shiga YG strain were used for the experiment. A total of 160 hatchlings that emerged during 28–30 May 2001 were individually reared on young leaves until the second instar (12–17 June) by using the same method as described above. After being weighed on the first day of the second moulting, they were divided into two groups with the same mean and variance in fresh weight. The larvae of one group were individually reared on expanded undamaged leaves collected from the upper crown, whereas those of the other group were reared on leaves from the lower crown. The larvae were checked daily and were given new undamaged leaves every 1–2 days. Rearing boxes were randomized daily within the incubator to avoid position effects.
Immediately after the fifth molting stage, individual larvae were offered four to six expanded undamaged leaves, the areas of which were measured in advance. After 24 h, the area of each leaf was measured again to determine the total leaf area consumed by the larva. Leaf wet and dry weights consumed by individual larvae were calculated as area consumed × wet or dry mass, where wet and dry masses were the mean values obtained from 15 upper-crown leaves (wet mass: 19.1 × 10−3 g/cm2; dry mass: 9.44 × 10−3 g/cm2) or 15 lower-crown leaves (wet mass: 15.1 × 10−3 g/cm2; dry mass: 6.56 × 10−3 g/cm2).
For each larva, we measured fresh weights at apolysis phases of the third, fourth and fifth instars and on the 14th day of the sixth instar, developmental time from the third instar to the start of spinning for pupation, and fresh weight of the pupa on the 16th day after spinning. Pupae were dried at 80°C for 48 h, and their dry weight was measured. They were sexed on the basis of the morphology of the antenna.
The relative growth rate (RGR) of each individual was calculated using the formula (Radford 1967):
RGR = (logeWP − logeWL3)/T
where WP is the dry weight of the pupa, WL3 is the dry weight of the second moulting larva, and T is the time (days) from third instar to spinning for pupation. WL3 was represented by a mean value from 11 larvae (i.e. 28.1 × 10−3 g).
Statistical analyses
Differences in leaf area, leaf dry mass, thickness and water content between upper-crown and lower-crown leaves were investigated using split-plot analyses of variance (anova). Sampling date and leaf were treated as fixed factors, and tree was nested within sampling date and was considered to be a random factor. In order to meet the assumptions of normality, leaf areas were loge transformed and water content data were arcsine transformed before analyses.
Differences in nitrogen content and N/C ratio between upper-crown and lower-crown leaves were examined by mixed factorial anova. Sampling date and leaf were treated as fixed factors, and tree was considered as a random factor. Nitrogen content data were arcsine transformed before analysis.
The preference of larvae for upper-crown and lower-crown leaves (variables: leaf dry weight) was examined using mixed factorial anova. Leaf and larval instar were fixed factors, whereas larva was a random factor.
Differences in larval fresh weight between upper-crown and lower-crown leaves were investigated using repeated-measures anova. Individual larvae were subjects, leaf was the between-subject factor, and instar was the within-subject factor.
The statistical analyses were carried out using SPSS version 7.5 (SPSS 1997).
RESULTS
Leaf traits
Budburst occurred in mid-April in both upper and lower crowns. Leaf area was significantly larger in the lower crown than upper crown (Fig. 1A). Leaf dry mass and leaf thickness gradually or somewhat increased until August, being significantly greater in the upper crown than lower crown (Fig. 1B,C). Leaf water content gradually decreased until August, being significantly higher in the lower crown than upper crown (Fig. 1D). Leaf nitrogen content and N/C ratio decreased until late June, afterwards remaining almost constant (Fig. 1E,F). These parameters were slightly but significantly higher in the upper crown than lower crown.
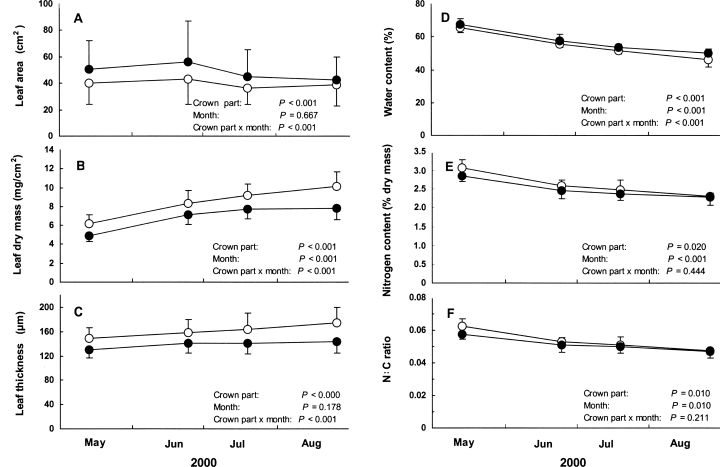
Seasonal changes in (A) leaf area, (B) leaf dry mass per area, (C) leaf thickness, (D) leaf water content, (E) leaf nitrogen content, and (F) leaf N : C ratio in the upper and lower crowns of Quercus acutissima (mean + SD or –SD). See the Materials and Methods section for detailed descriptions of statistical models. (○), Upper crown leaves; (●), lower crown leaves.
Feeding preference, performance and leaf consumption of larvae
When fourth and fifth instar A. yamamai larvae were simultaneously offered upper-crown and lower-crown leaves, they consumed a significantly larger dry weight of upper-crown leaves (Fig. 2). This indicates that A. yamamai larvae prefer upper-crown leaves to lower-crown leaves.
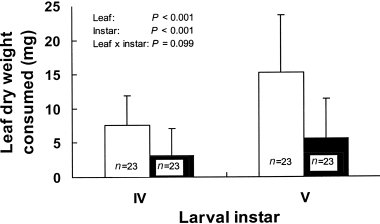
Leaf dry weight consumed by fourth and fifth instar larvae of Antheraea yamamai over 6 h when the larvae were simultaneously offered upper-crown and lower-crown leaves of Quercus acutissima (mean + SE). See the Materials and Methods section for a detailed description of the statistical model. (□), Upper crown leaves; (▪), lower crown leaves.
The percentage mortalities of larvae reared on upper-crown leaves and those on lower-crown leaves were 12.5% (10/80) and 20% (16/80), respectively. No significant difference was found between them (Fisher’s exact probability = 0.284). Those larvae that died during rearing were excluded in the analyses described later here.
Larval fresh weight was somewhat greater in individuals reared on lower-crown leaves than in those reared on upper-crown leaves at each instar for males and females; the difference as a whole was significant for both sexes (Fig. 3). Interactions between leaf and instar were significant, indicating that differences in larval fresh weight between two categories of leaves vary according to instars.
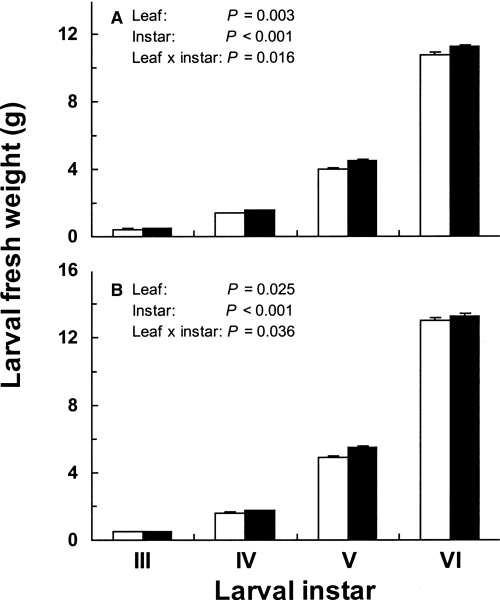
Fresh weight of Antheraea yamamai larvae reared on either upper-crown or lower-crown leaves of Quercus acutissima (mean + SE). Because the data did not meet the sphericity assumption (Mauchly’s test; P < 0.001), Greenhouse-Geisser-adjusted probability was used. See the Materials and Methods section for a detailed description of the statistical model. (A) Male; (□), upper crown leaves (n= 31); (▪), lower crown leaves (n= 26). (B) Female; (□), upper crown leaves (n= 33); (▪), lower crown leaves (n= 44).
In contrast, pupal fresh and dry weights were larger in individuals reared on upper-crown leaves than in those reared on lower-crown leaves; significant differences were found in male dry pupae and in female fresh and dry pupae (Fig. 4A,B). In males, neither development time nor RGR significantly differed between the two categories of leaves. In females, however, both indices were significantly larger on upper-crown leaves (Fig. 4C,D).
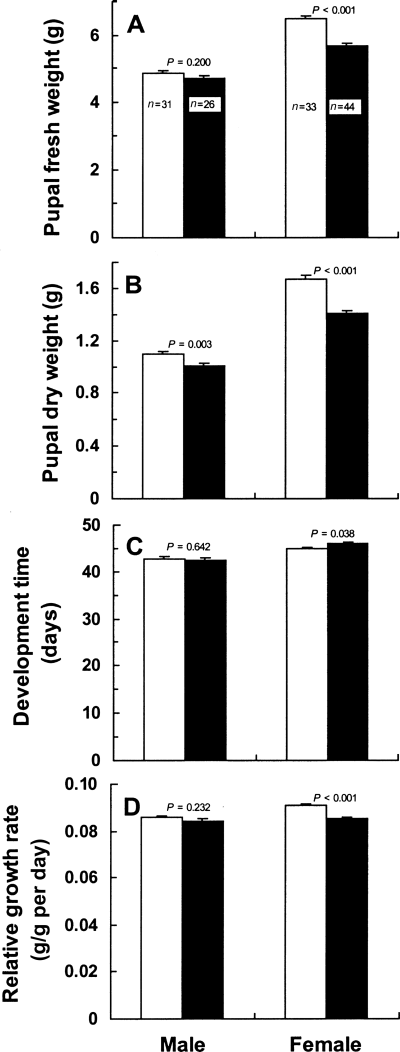
(A) Pupal fresh weight, (B) pupal dry weight, (C) development time from the third instar to the start of spinning for pupation, and (D) relative growth rate of Antheraea yamamai larvae reared on either upper-crown or lower-crown leaves of Quercus acutissima (mean + SE). Data were analyzed by using the t-test. (□), Upper crown leaves; (▪), lower crown leaves.
When sixth instar larvae were fed with either upper-crown leaves or lower-crown leaves, both males and females consumed a significantly larger amount (wet and dry weights) of upper-crown leaves (Fig. 5).
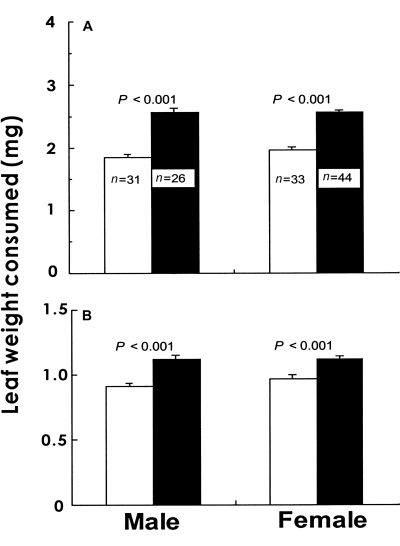
Leaf (A) wet and (B) dry weights consumed by sixth instar larvae of Antheraea yamamai over 6 h when the larvae were fed with either upper-crown or lower-crown leaves of Quercus acutissima (mean + SE). (□), Upper crown leaves; (▪), lower crown leaves.
DISCUSSION
Leaf traits in the upper and lower crowns
In Q. acutissima, upper-crown leaves are smaller and thicker, and have higher nitrogen content than lower-crown leaves (Fig. 1). This is consistent with the theory proposed by Mooney and Ehleringer (1997) that leaves developing under conditions of high light intensity, or sun leaves, tend to have a smaller area, greater thickness, and higher nitrogen content compared with those developing under conditions of low light intensity, or shade leaves. Le Corff and Marquis (1999) and Fortin and Mauffette (2002) obtained similar results for deciduous oaks (Q. alba and Q. velutina) and the sugar maple (A. saccharum), respectively. However, Yamasaki and Kikuzawa (2003) found that sun leaves had lower nitrogen content than shade leaves in a beech (Fagus crenata). Thus, we should be cautious about inferring a generalized positive relationship between light intensity and nitrogen content. Unfortunately, several studies have examined the effects of sun and shade leaves on folivore performance without analyzing the nitrogen content of leaves (Futuyma & Saks 1981; Harrison 1987; Watt 1992; Suomela et al. 1995).
Water content is lower in upper-crown leaves than in lower-crown leaves in Q. acutissima (Fig. 1D). Fortin and Mauffette (2002) also found lower water content in upper-crown leaves of the sugar maple. This is probably attributable to higher transpiration rates in upper-crown leaves in greater light intensity (Grace 1997).
Performance and feeding preference
The most commonly used index for folivore performance is the RGR, expressed as amount of growth attained (dry matter) per unit of body weight (dry matter) per day (Schoonhoven et al. 1998). Pupal weight is positively related to adult size (Stamp & Bowers 1990; Scheirs et al. 2002) and varies with sex (Bauce et al. 1994; Pöykkö & Hyvärinen 2003). Thus, folivore performance should be represented by the RGR for each sex based on larval development time and pupal dry weight. As far as we know, no studies have satisfied these conditions to examine the effect of upper-crown and lower-crown leaves or sun and shade leaves on folivore performance. The RGR values obtained in the present study meet these conditions.
The RGR of female Japanese oak silkmoths is significantly higher on upper-crown leaves than lower-crown leaves, whereas male RGR values do not significantly differ between the two categories of leaves (Fig. 4D). Clearly, the higher RGR of females on upper-crown leaves results from not only a larger dry weight of pupae (Fig. 4C), but also a shorter development time (Fig. 4B). Hence, the effects of upper-crown and lower-crown leaves on folivore performance are likely to vary with the sex.
Upper-crown leaves of Q. acutissima have significantly higher nitrogen content and N/C ratios than lower-crown leaves, as mentioned previously, although the differences are rather small. The higher RGR of females on upper-crown leaves is consistent with the theory that folivore performance increases with nitrogen content and N/C ratio of leaves (Schoonhoven et al. 1998; Awmack & Leather 2002). However, this is not the case with males. The different effects of the nitrogen content and N/C ratio of leaves on female and male RGR values might be partly because females need more protein than males to produce eggs (Nation 2002).
The higher RGR of female larvae on the upper-crown leaves is, however, inconsistent with the theory that folivore performance increases with the water content of leaves (Scriber 1977; Schoonhoven et al. 1998; Henriksson et al. 2003), because upper-crown leaves of Q. acutissima contain lower water content than lower-crown leaves (Fig. 1D). This does not necessarily mean that the larval growth of A. yamamai is independent of leaf water content. The present experiment allowed the larvae to supplement leaf water by taking water from moistened sanitary cotton in rearing boxes. Upper-crown leaves may be more suitable for larval growth than lower-crown leaves only when larvae are free from water deficiency.
The present results raise two points that are relevant when we examine folivore performance. First, the performance based on fresh weight may depend on whether it is measured at larval or pupal stadia. Larvae of the Japanese oak silkmoth on lower-crown leaves have a larger fresh weight at larval stadia (Fig. 2), but they, especially females, attain a smaller fresh weight at pupal stadia (Fig. 4A). This inconsistency seems to be linked to excess water intake from lower-crown leaves, which contain a higher water content than upper-crown leaves. Larvae appear to ingest a greater amount of lower-crown leaves (Fig. 5) to compensate for the lower nitrogen content of the foliage, so they have excess water intake. Larvae defecate rather liquid frass when they are ready to spin cocoons (Gardiner 1982; M. Oishi, unpubl. data, 2001). Larvae fed with lower-crown leaves could excrete excess water in the frass, and they would thus become pupae with a smaller fresh mass.
The second point of note is that performance based on pupal weight may depend on whether it is measured by fresh weight or dry weight. Male pupae of the Japanese oak silkmoth had similar fresh weights when reared on upper-crown and lower-crown leaves, but they had a significantly larger dry weight when reared on the former (Fig. 4A,B). Unlike male pupae, female pupae had larger fresh and dry weights when reared on upper-crown leaves. This suggests that female pupae do not have so much excess water as male pupae when they feed on lower-crown leaves, although we cannot offer any reason for this. In any case, considering that larvae on the lower-crown leaves have an excess of water, conversion formulae for calculating dry weights of larvae and pupae from their fresh weights (Stamp & Bowers 1990; Bauce et al. 1994; Henriksson et al. 2003) should be treated with caution.
Leaf thickness, which is related to toughness, usually reduces folivore feeding and performance (Choong 1996; Schoonhoven et al. 1998). Larvae of A. yamamai, however, exhibited a preference for and better performance on upper-crown leaves (2, 4), despite the fact that these leaves are thicker than lower-crown leaves (Fig. 1C). Because fourth and fifth instar larvae were used in the preference experiment, they may have easily overcome the thickness of the upper-crown leaves. In fact, first and second instar larvae of A. yamamai are unable to eat expanded leaves (Teramoto 2001).
Feeding preference of A. yamamai larvae for upper-crown leaves is in accordance with better performance (larger pupal dry weight for males and higher RGR for females) on the foliage. However, the experiment for feeding preference was carried out when the differences in nitrogen content and N/C ratio between the two categories of leaves were rather small (late July and early August). Hence, there is a possibility that larvae do not select leaves by judging the nutritional value. Larvae might use as feeding stimulants physical or chemical characters that have very little relationship to leaf nutritional value.
ACKNOWLEDGMENTS
We thank M.T. Kimura for his critical reading of an early draft of the manuscript. This study was supported by a Grant-in-Aid from the Ministry of Education, Science, Sports and Culture of Japan (No. 10640615).




