Hepatitis C virus-human immunodeficiency virus coinfection
Abstract
As a result of shared modes of transmission, chronic hepatitis C infection is common in HIV-infected patients, particularly among those who have used injection drugs as well as men who have sex with men (MSMs). In the era of effective antiretroviral therapy, HCV infection has emerged as a major cause of morbidity and mortality worldwide. Over the last decade, treatment with peginterferon (PEG-IFN) plus ribavirin (RBV) has been recommended for coinfected patients who are at the greatest risk for liver disease; however, the effectiveness of HCV treatment in this population has been disappointing. Challenges to the use of HCV NS3/4A protease inhibitors, telaprevir and boceprevir, patients with HIV/HCV coinfection include the potential for interactions between different drugs, addition of drug toxicities, and the need for therapy with PEG-IFN. Despite these challenges, limited data indicate that telaprevir and boceprevir given in combination with PEG-IFN/RBV increase the rate of viral suppression in coinfected patients with manageable toxicity and drug-drug interaction profile. Accordingly, these agents may be recommended for HCV treatment in carefully selected HIV-infected persons.
Abbreviations
-
- ART
-
- antiretroviral therapy
-
- DAA
-
- direct acting antivirals
-
- HCV
-
- hepatitis C virus
-
- HIV
-
- human immunodeficiency virus
-
- IFN
-
- interferon
-
- PEG-IFN
-
- pegylated interferon
-
- RBV
-
- ribavirin
-
- RR
-
- relative risk
-
- SVR
-
- sustained virological response
Hepatitis C virus (HCV) infection has emerged as a major cause of morbidity and mortality worldwide 1-3. In addition to the high prevalence of chronic HCV, particularly among HIV-infected injection drug users (IDUs), the rate of incident infections with HCV is increasing among HIV-infected men who have sex with men (MSM), so it is important to screen this population 4. Although sexual transmission of HCV is an important focus for prevention, the outcomes of HCV infection include progressive liver disease, end-stage liver disease (ESLD), hepatocellular carcinoma (HCC) and death among patients coinfected with HIV and HCV 5, 6. As a consequence, liver disease is the second leading, and in some cases preventable, cause of death in this population 1. Liver transplantation is an accepted, although limited, option at select medical centres 7.
Over the last decade, treatment with peginterferon (PEG-IFN) plus ribavirin (RBV) has been recommended for coinfected patients who are at the greatest risk for liver disease; however, the effectiveness of HCV treatment in this population has been disappointing. The reasons for limited effectiveness include low rates of treatment initiation, high prevalence of relative and absolute contraindications to the drugs, and, among those treated, low rates of sustained virological response (SVR) – especially individuals coinfected with HCV genotype 1 and those who are African–American 8-10. As such, direct acting anti-virals (DAA) for HCV are urgently needed and could overcome host factors such as immunodeficiency that impair the efficacy of many drugs. Challenges to the use of DAA in patients with HIV/HCV coinfection include the potential for interactions between different drugs, addition of drug toxicities, and the need for therapy with IFN. Despite these challenges, limited data indicate that the HCV protease inhibitors telaprevir and boceprevir, given in combination with PEG-IFN/RBV, increase the rate of viral suppression, with manageable toxicity and drug-drug interactions. These agents are therefore recommended for treatment of HCV in carefully selected HIV-infected persons.
Epidemiology
Owing to shared modes of transmission, 20–30% of HIV-infected patients in the United States are coinfected with HCV 11, 12. HCV is approximately 10 times more infectious than HIV through percutaneous blood exposures 13, 14. As such, injection drug use remains the primary mode of acquisition of HCV infection in many settings. Transmission of HIV and HCV through contaminated blood products is now rare because of effective screening. In the developing world, iatrogenic HCV transmission caused by reuse of injection devices is a large source of incident infection 15. Sexual transmission of HCV is less efficient than sexual transmission of HIV. However, among HIV-infected MSM without percutaneous risk factors, multiple outbreaks of acute HCV infection indicate that sexual transmission occurs in the context of high-risk sexual practices such as unprotected receptive anal intercourse; non-injection recreational drug use is also frequently reported 4, 16, 17. Interestingly, the increased detection of sexual transmission of HCV corresponds to the documented increase in high risk sexual behaviours in MSM who have suppressed HIV replication from ART 18.
Natural history
As early as 1993, Eyster and colleagues reported that HCV RNA levels were higher in people with haemophilia who became HIV infected than in those who remained HIV negative, and liver failure occurred exclusively in coinfected patients 19. The impact of HIV on HCV disease in the era before effective HIV therapy was summarized in a meta-analysis of multiple studies that assessed the correlation between HIV coinfection and the progression of HCV-related liver disease. HIV coinfection was associated with a ~ 6-fold increased relative risk of ESLD and a ~ 2-fold increased relative risk of cirrhosis compared to HCV monoinfection 20. Thus, in the absence of effective antiretroviral therapy (ART), HCV disease is clearly worsened by coinfection with HIV.
Since the availability of effective ART, the impact of HIV on the progression of HCV has been less clear. Although some studies are inconsistent, the treatment of HIV disease has generally been associated with a decreased risk of liver disease progression, particularly with the use of antiretroviral agents with a minimal risk of hepatotoxicity. For example, Qurishi and co-workers reported a lower risk of mortality from liver disease in persons who lived long enough to receive effective ART 21. Brau and colleagues estimated the progression of liver fibrosis in 274 HIV-infected and 382 HIV-uninfected patients 22. Among persons with effectively controlled HIV infection, (defined as an HIV RNA levels < 400 c/ml and/or CD4 cell count > 500/mm3), the progression of fibrosis was similar in persons with and without HIV infection. In contrast, those with inadequately treated HIV disease had accelerated liver disease progression compared to those without HIV coinfection. More recently, two studies failed to detect evidence of significant fibrosis progression in HCV coinfected who underwent paired liver biopsies 23, 24. The first study was a prospective study designed to assess the effect of long-term IFN therapy on the progression of HCV. In that trial, Sherman et al. 23 observed no or minimal fibrosis progression in control patients, most of whom were taking ART. In a second study, Sterling and colleagues observed a similar progression of fibrosis in HCV-infected patients with and without HIV disease 24. Taken together, these and other studies have led to the expert consensus that ART is associated with delayed liver disease progression and is beneficial for most HIV/HCV coinfected patients.
However, despite the positive impact of ART, HIV/HCV coinfected patients remain at greater risk of the progression of liver disease than those with HCV monoinfection. Thein and co-workers conducted a meta-analysis involving 27 studies on the natural history of HCV including 7666 individuals (HIV/HCV coinfection, n = 2636; HCV monoinfection, n = 4970). The overall relative risk (RR) of cirrhosis among coinfected patients relative to monoinfected patients was 2.11 (95% CI 1.51–2.96) 25. This increased risk of cirrhosis in patients with coinfection relative to those with monoinfection was similar in person taking ART (RR 1.72, 95% CI 1.06–2.80) and those not taking ART (RR 2.49, p5% CI 1.81–3.42). Furthermore, in meta-regression analysis, Thein et al. did not detect a significant association detected between ART and risk of cirrhosis. This observation underscores the need for effective HCV treatment in this population.
HCV treatment
Peginterferon plus ribavirin
The standard treatment of HCV in HIV-infected patients is PEG-IFN/RBV. Two formulations of PEG-IFN are available, which are both given once weekly by subcutaneous injection: PEG-IFN alfa 2a (Pegasys®, Hoffman La-Roche Inc, Nutley, NJ, USA; Roche Pharmaceuticals) or PEG-IFN alfa 2b (PegIntron™; Merck Pharmaceuticals, Peglntron, NJ, USA) 26, 27. The optimal dose of oral ribavirin (RBV) varies by genotype. For patients with HCV genotype 2 or 3 infection, RBV 800 mg orally daily in two divided doses is recommended 28. In contrast, higher weight-based dosing may be more effective that fixed dosing in coinfected patients, although one randomized controlled trial failed to detect a higher SVR rate in patients treated with higher dose RBV compared to those treated with 800 mg/day 29, 30. Despite this negative study, most experts recommend weight-based RBV dosing for HCV genotype 1 patients coinfected with HIV. In a meta-analysis of seven randomized trials in HIV-infected patients treated with PEG-IFN/RBV, SVR rates were significantly higher among patients with HCV genotypes 2 and 3 compared to those with genotypes 1 and 4 infection (57% vs 26%) (Table 1) 29-36.
| Parameter | APRICOT 29 | ACTG A5071 32 | RIBAVIC 33 | PRESCO 35 | Laguno 34 | PARADIGM 30 | Laguno 36 | ||
|---|---|---|---|---|---|---|---|---|---|
| Subjects, n | 868 | 133 | 412 | 389 | 95 | 410 | 96 | 86 | |
| Country | Multiple | USA | France | Spain | Spain | Multiple | Spain | Spain | |
| Regimen | |||||||||
| pegIFN | alfa-2aa | alfa-2a | alfa-2b | alfa-2a | alfa-2a | alfa-2a | alfa-2a | alfa-2b-2b | |
| [Ribavirin], mg/day | 800 | 600 → 800 →1000 | 800 | 1000–1220 | 600–1200 | 800 vs 1000–1200 | 800–1200 | 800–1200† | |
| Baseline characteristics | |||||||||
| White, % | 79 | 33 | 93 | 100 | 100 | 63-64 | – | – | |
| Mean CD4+ count, cells/μl | 530 | 474 | 482 | 546 | 570 | 489-519 | 602.3 | 592.5 | |
| Undetectable HIV-1 RNA, % | 60 | 60 | 67 | 72 | 70 | – | 72.9 | 74.1 | |
| Receiving ART, % | 84 | 86 | 83 | 74 | 88 | 88-89 | 83 | ||
| Bridging fibrosis or cirrhosis, % | 16 | 11 | 39 | 27 | 33 | 11–12 | 28.9 | 29.1 | |
| Genotype 1, % | 61 | 77 | 59 | 49 | 55 | 100 | 50.5 | 45.4 | |
| HCV RNA level >800000IU/ml, % | 72 | 83 | – | 66 | 47 | 79–81 | 53.7 | 57.8 | |
| SVR by genotype, % | |||||||||
| 1 | 29 | 14 | – | – | – | 19–22 | – | – | |
| 1 or 4 | – | – | 17 | 35 | 38 | – | 32 | 28 | |
| 2 or 3 | 62 | 73 | 44 | 72.4 | 53 | – | 71 | 62 | |
- a Peginterferon alfa-2a or peginterferon alfa-2b
Peginterferon plus ribavirin plus HCV NS3/4A protease inhibitors
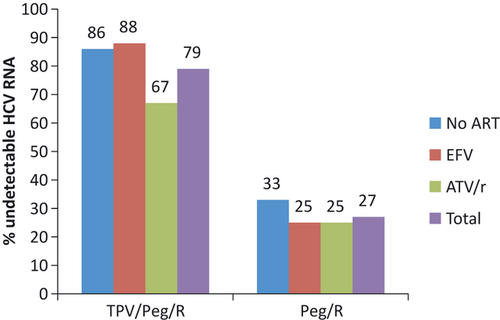
At this time, the HCV NS3/4A protease inhibitors are not approved for the treatment of HCV infection in persons with HIV coinfection. However, preliminary interim data have been reported at scientific meetings from small, ongoing phase 2a studies of telaprevir and boceprevir in combination with PEG-IFN compared to PEG-IFN/RBV plus placebo 37, 38. In the first study, 59 HIV/HCV coinfected patients with HCV genotype 1 were randomized to receive telaprevir plus PEG-IFN/RBV (12 weeks triple therapy followed by 36 weeks of PEG-IFN/RBV) or placebo plus PEG-IFN/RBV for 48 weeks 37. Patients were required to be on no ART (n = 13) or one of two ART regimens tenofovir/emtricitabine or lamivudine with efavirenz (n = 24) or ritonavir-boosted atazanavir (n = 22). Attributable to drug interactions with efavirenz, patients on this regimen were given a higher dose of telaprevir 1125 mg every 8 h compared to the standard 750 mg every 8 h. At the end of treatment week 4, 70% of telaprevir-treated patients had undetectable HCV RNA compared to 5% of PEG-IFN/RBV patients. A similar response pattern was observed at treatment week 12 (Fig. 1). The adverse effect profile was similar to that observed in HCV mono-infected patients with increase gastrointestinal symptoms and rash being reported at a higher frequency in telaprevir patients. Discontinuations caused by adverse events occurred in three telaprevir-treated patients compared to none in the placebo group. There was no adverse impact on control of HIV disease noted and clinically significant drug interactions were not reported. At this time, there are no data on SVR in this population with telaprevir in combination with PEG-IFN/RBV. In the second study, 99 HIV/HCV coinfected patients with HCV genotype 1 were randomized at a 2:1 ratio to take 44 weeks boceprevir or placebo in combination with PEG-IFN/RBV following a 4 week ‘lead-in’ phase of PEG-IFN/RBV 38. Enrolled patients received ritonavir-boosted HIV protease inhibitors (atazanavir, darunavir, and lopinavir) or raltegravir in combination with other antiretroviral drugs including tenofovir or abacavir plus lamivudine or emtricitabine. At weeks 8, 12, and 24 38%, 56% and 70% of patients achieved an undetectable HCV RNA respectively. This response was significantly higher than that observed in the placebo group (Fig. 2). Adverse effects were common in both study groups but were consistent with the profile observed in HCV monoinfected patients. Discontinuation of therapy owing to adverse events was reported in 9% of placebo and 14% of boceprevir-treated patients. At this time, there are no data on SVR in this population with boceprevir in combination with PEG-IFN/RBV. Although both studies are preliminary, these studies support the use of these novel HCV NS3/4A protease inhibitors plus PEG-IFN/RBV in selected patients with HCV genotype 1. However, furthermore studies are needed to address open questions regarding safety, efficacy and interactions with other drugs including antiretroviral agents.
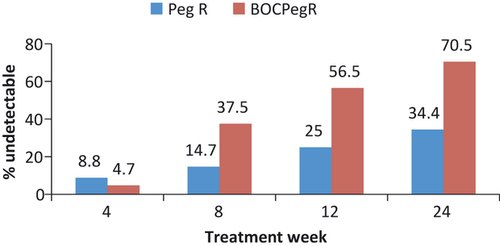
Drug interactions with antiretroviral therapy
In the current era, most HIV-infected patients are receiving combination antiretroviral therapy. Both telaprevir and boceprevir also interact with CYP3A as inhibitors and substrates, raising the potential for interactions with drugs that are metabolized through this pathway. Boceprevir is metabolized primarily by aldo-ketoreductase as well as is a strong inhibitor of and partially metabolized by CYP3A4. Similarly, telaprevir is an inhibitor and substrate of CYP3A4. These effects on CYP3A suggest that drugs that are metabolized by this enzyme may have increased concentrations, and drugs that induce this enzyme may lower telaprevir concentrations.
There are limited studies that assess interactions the HCV protease inhibitors with antiretroviral agents 39. In healthy volunteer studies, when co-administered, boceprevir increased the concentrations of the efavirenz (an inducer of CYP3A), and the concentration of boceprevir was reduced. Similar interactions were observed with the HIV protease inhibitor, ritonavir, an inhibitor of CYP3A4. In the clinical trial, patients on efavirenz and other non-nucleoside reverse transcriptase inhibitors were not enrolled. Thus, based on current data, HIV/HCV coinfected patients treated with boceprevir should receive combination ART that includes ritonavir-boosted HIV protease inhibitors and/or integrase inhibitors such as raltegravir.
Studies have been presented with telaprevir and antiretroviral agents 39. In studies of healthy volunteers, telaprevir was combined with ritonavir-boosted HIV protease inhibitors including atazanavir, darunavir, fosamprenavir and lopinavir. Telaprevir led to significant reductions in the concentrations of darunavir (AUC decreased by 40%) and fosamprenavir (AUC decreased by 47%), but there was less impact with lopinavir (AUC unchanged) and atazanavir (AUC decreased by 17%). Conversely, the HIV protease inhibitors also led to significant reductions in telaprevir concentrations (AUC decreased by 20–54%) with the smallest impact observed with atazanavir. Based on these studies, atazanavir boosted with ritonavir was permitted in the phase 2 study of telaprevir in HIV-infected patients; other HIV protease inhibitors were not allowed. With respect to the NNRTI, efavirenz, co-administration with this agent led to a 20% reduction in the AUC of telaprevir. This effect was offset by the administration of a higher dose of telaprevir, 1125 mg every 8 h. More recently, similar studies demonstrated no significant interactions with the HIV integrase inhibitor, raltegravir and telaprevir.
Conclusions
Approximately ~ 30% of HIV-infected persons in the United States and other parts of the world are coinfected with HCV. In addition to these prevalent cases, sexual acquisition of acute HCV infection has been reported among HIV-infected MSMs. In the era of effective HIV therapy, chronic HCV infection is a leading cause of liver disease and mortality in HIV-infected patients. Whereas treatment of HIV with ART appears to slow the progression of liver disease, coinfected patients remain at greater risk for HCV disease progression than patients with HCV monoinfection. Accordingly, effective HCV treatment is a priority in this population. However, there are multiple challenges to the use of the novel HCV NS3/4A protease inhibitors, telaprevir and boceprevir in HIV/HCV coinfected patients including the potential for drug interactions as well as the relatively poor response and tolerability of PEG-IFN. Nonetheless, limited interim data for these agents in combination with PEG-IFN/RBV support the use of these therapies for the treatment of HCV in carefully selected HIV-infected persons.
Conflict of interest
MS has received grants from : Roche,Vertex, Merck, Abbott, Pharmasset, BMS, BIPI, Tibotec, Merck; Consulting fee/honorarium from: Roche;, Vertex, Merck, Abbott, Pharmasset, BMS, BIPI, Tibotec, Merck.




