Liver cancer stem cells: implications for a new therapeutic target
Abstract
Hepatocellular carcinoma (HCC) is an aggressive tumour with a poor prognosis. Current therapeutic strategies against this disease target mostly rapidly growing differentiated tumour cells. However, the result is often dismal due to the chemoresistant nature of this tumour type. Recent research efforts on stem cells and cancer biology have shed light on new directions for the eradication of cancer stem cells (CSCs) in HCC. The liver is a distinctive organ with the ability of tissue renewal in response to injury. Based on the hypothesis that cancer development is derived from the hierarchy of the stem cell system, we will briefly discuss the origin of liver stem cells and its relation to HCC development. We will also summarize the current CSC markers in HCC and discuss their relevance to the treatment of this deadly disease.
Liver cancer consists mainly of hepatocellular carcinoma (HCC) and cholangiocarcinoma (CC). HCC is the fifth most common cancer in the world and is especially prevalent in Southeast Asia (1, 2). Over 80% of HCC is complicated with liver cirrhosis and chronic hepatitis (3–6). Chronic viral infections, including the hepatitis B virus (HBV) and hepatitis C virus (HCV), are major risk factors contributing to HCC development (3–9). Apart from hepatotropic viruses, other major risk factors leading to cirrhosis include alcohol abuse and aflatoxin intake (3–6, 10). CCs arise from the biliary epithelium, which is either intra- or extrahepatic. This tumour is less common compared with HCC, but its incidence and mortality have increased drastically over the past two decades. HCV infection is also a risk factor of intrahepatic but not extrahepatic CC (11).
The front-line treatment for HCC is liver transplantation or resection (12–17). However, over 80% of HCC cases are presented at an advanced stage and no longer operable with these surgical treatments. Chemotherapy via either transarterial chemoembolization or systemic treatment is the second-line treatment, and yet the overall response rate is unsatisfactory due to the highly chemoresistant nature of this tumour (18–21). Apart from conventional systemic chemotherapy, targeted therapy potentially provides another treatment modality for advanced stage HCC patients. In recent studies, molecular targets involved in several important pathways contributing to HCC growth, including vascular endothelial growth factor, epidermal growth factor and their downstream signalling cascades, have been examined clinically (22). Yet, in essence, both systemic chemotherapy and targeted therapy are directed against the rapidly growing, differentiated HCC tumour cells (23).
Recent evidence on the presence of cancer stem cells (CSCs) in different solid tumours, including brain (24, 25), colon (26, 27), breast (28, 29), prostate (30), melanoma (31), pancreatic (32) and head and neck cancer (33), has provided a novel therapeutic direction for the treatment of cancers, including liver cancer. The new concept of CSCs is based on the idea that stem cells are present in cancer tissue, like in normal tissues, and are part of the hierarchy of cells. In other words, just as there are normal stem cells in normal tissues, CSCs are found in tumour tissues. Like normal stem cells, CSCs are involved in tissue renewal. Because a number of genes involved in self-renewal turn out to be cancer-associated, efforts are currently being made to elucidate the mechanism of cancer development from the perspective of a stem cell hierarchy. Based on this hypothesis, the source of HCC and CC is beginning to be verified using isolated liver stem/progenitor cells. This review will first discuss the possible sources of the cellular origin of liver stem/progenitor cells and their relation to liver cancer development, following which we will discuss the current CSC markers for liver cancer and, finally, present insights into new therapeutic approaches for more specific targeting and elimination of CSCs.
Liver stem cells: hepatocytes
Fetal liver is one of the major sources of bipotential progenitor cells, as evidenced by their extensive colonization of diseased rat liver (34). Under normal circumstances, individual hepatocytes have a life expectancy of over a year. Therefore, the liver hardly renews itself, with only 0.01% of hepatocytes in active cell cycle at a time (35). In response to parenchymal cell loss, the hepatocytes self-replicate in order to restore the original liver mass. In rodents, the liver can restore its original liver mass in approximately 10 days when two-thirds of the liver is resected by partial hepatectomy. The detailed mechanisms controlling this regenerative process have been studied previously (36, 37). In response to an extracellular stimulus, quiescent hepatocytes exit the G0 phase and enter the cell cycle, under the control of growth factors. Hepatocyte proliferation begins in the periportal region of the liver and spreads to the centrilobular region. Serial transplantation models have shown that hepatocytes have a nearly infinite capacity to proliferate in the diseased livers of animals (38). After partial hepatectomy, hepatocytes expressed several preneoplastic markers, such as the placental form of glutathione-S-transferase and γ-glutamyl transferase (39, 40). Bralet and colleagues showed that in a rat model, a significant proportion (18.3%) of regenerative hepatocytes originated from mature hepatocytes. After diethylnitrosamine (DEN) treatment, 17.7% of HCCs originated from mature hepatocytes, this figure matching precisely the proportion of mature hepatocyte-derived regenerative hepatocytes before DEN treatment. This showed a direct lineage relationship between mature hepatocytes and HCC (40).
Studies have examined the transplantation potential of adult hepatocytes in different animal models. Laconi et al. (41) demonstrated the complete replacement of the recipient liver by donor hepatocytes through the injection of hepatocytes into retrorsine-pretreated F344 rats after partial hepatectomy. On the other hand, if rats were simply administered retrorsine before receiving two-third partial hepatectomy, complete liver regeneration could be accomplished through the activation, expansion and differentiation of small hepatocyte-like progenitor cells (SHPCs). When such cells were established in a short-term culture and then transplanted into syngeneic rats, they gave rise to differentiated hepatocytes. Mature hepatocytes are the source of SHPCs after retrorsine treatment (42). This supports the occurrence of differentiation of mature hepatocytes when the proliferation of hepatocytes is suppressed.
In chronic hepatitis, between 0.3 and 3% of all hepatocytes are killed daily, and approximately 0.5% of the hepatocytes are needed to maintain the parenchymal mass (43). The loss of hepatocytes parallels with the proliferation rate of hepatocytes, as evidenced by the 0.1–3.6% and 1–14% expression of proliferating cell nuclear antigen and Ki67 immunostaining respectively (44, 45). In response to chronic hepatitis infection, self-replication of hepatocytes is triggered, through which the parenchymal mass can be maintained. Falkowski et al. (46) reported an increase in hepatocyte proliferation in hepatitis C-induced histological damage until cirrhosis resulted. The decline in hepatocyte proliferation can be partly due to cell senescence. The number of senescent mature hepatocytes correlates positively to the activation of what are known as oval cells – potential stem cell compartments located within the smallest branches of the intrahepatic biliary tree (47).
Liver stem cells: oval cells
‘Oval cells’, which are small cells with a characteristic ovoid nucleus and a high nucleus to cytoplasm size ratio, appear in the periportal region and then infiltrate along the bile canaliculi (48). Oval cells are bipotential, capable of differentiating into either hepatocytes or cholangiocytes (49–51). Oval cell activation has been extensively studied in the rodent system, where a carcinogen was used to inhibit hepatocyte replication in response to regenerative stimuli such as a partial hepatectomy or carbon tetrachloride administration (52). In addition, the activation of oval cells has been demonstrated in fatty liver disease in ObOb mice or PARP1 (−/−) mice, in which the replication of mature hepatocytes is inhibited by oxidative stress (53). The bipotential characteristic of oval cells has also been shown in several transplantation models. Yasui et al. (54) demonstrated that oval cells from Long–Evans Cinnamon rats could generate a number of functional albumin-secreting hepatocytes in recipient animals. In addition, an oval cell-enriched population could cure murine tyrosinaemia (55).
The existence and importance of human counterparts to the oval cell-like progenitor, often referred to as SHPCs, are now being recognized. In severe hepatocellular necrosis, chronic viral hepatitis, alcoholic and non-alcoholic fatty liver disease, where mature liver cells are unable to regenerate owing to inhibition or replicative exhaustion, activation of the potential stem cell compartment leads to formation of reactive ductules with a high expression of oval cell markers. Activation of these progenitor cells correlates with the level of tissue damage and inflammation (56, 57). The existence of these cells is of great prognostic significance to human liver diseases (57). Under conditions such as alcoholic or non-alcoholic fatty liver disease, the appearance and activity of SHPCs are markers of disease severity (57). In chronic hepatitis, SHPC activation correlates with the degree of inflammation (58). Many markers have been used to identify oval cells, including γ-glutamyl transpeptidase, glutathione-S-transferase, OV6, α-foetoprotein (AFP), neural cell adhesion molecule 1 and chromogranin A. There is also speculation that oval cells are derived from bone marrow precursor cells because they express some of the antigens of haematopoietic cells such as c-kit, flt-3, Thy-1 and CD34. However, because of the localization of oval cells at the transitional zone between periportal hepatocytes and the biliary cells lining the smallest terminal bile ducts, such a speculation is questionable.
Liver stem cells: bone marrow cells
Sell et al. (59) challenged the notion of the canal of Hering as the source of oval cell reactions. They demonstrated that intraportal stem cells participated in the response of the liver to periportal necrosis induced by allyl alcohol, suggesting another alternative source of cells to the canal of Hering. Using a lethally irradiated and bone marrow sex-mismatched transplant rat model, studies have shown with increasing evidence that oval cells/hepatocytes are occasionally derived from bone marrow cells (BMCs) after liver damage (60). Using a similar transplantation approach in mice to trace the fate of BMCs, about 1–2% of hepatocytes in murine liver were found to be possibly derived from the bone marrow in the absence of any liver injury (61), meaning bone marrow contributes to the normal hepatocyte turnover process. Recent reports have demonstrated the therapeutic role of BMCs in repairing tissue of non-haematopoietic lineage, such as skeletal muscle (62). Clinical studies in which patients received gender-mismatched bone marrow or liver transplant, and possessed nontrivial frequencies of donor liver/bone marrow-derived cells, provided further evidence that liver stem cells originate from BMCs (61, 63). Two approaches were used to support the above hypothesis. In the first approach, the livers of female patients who had received a bone marrow transplant from male donors were examined for the presence of cells of donor origin, using a Y-chromosome specific probe to perform in situ hybridization. In the second, Y-positive cells were sought in female livers engrafted into male patients but that were later removed because of recurrent disease. In both sets of patients, Y-chromosome-positive hepatocytes were identified, but the degree of BMC engraftment varied because of different degrees of parenchymal damage (64). Following these studies, further confirmation of the ability of granulocyte colony-stimulating factor-mobilized CD34+ stem cells in peripheral blood to transdifferentiate into hepatocytes was obtained (65). However, there have also been studies showing no real BMC engraftment in allografted liver (66).
A representative study demonstrating the use of BMCs in the treatment of liver disease was conducted by Lagasse et al. (65). In their experiment, they successfully used wild-type BMCs to differentiate into functional hepatocytes expressing the enzyme fumaryl-acetoacetate hydrolase (FAH) in tyrosinaemic (fah−/−) animals. In addition, it was shown that a small number of Sca-1+ (KTLS), c-kit+, Thy-1-low, lineage-negative BMCs was sufficient to generate functional hepatocytes in recipient animals (65), suggesting that a haematopoietic progenitor, rather than a non-haematopoietic component of the bone marrow, is responsible for this transdifferentiation process. However, other groups have discovered that the functional hepatocyte is the result of the fusion between a donor bone marrow-derived macrophage and a fah−/− hepatocyte nucleus (67). Although the exact significance of BMCs to liver disease is far from fully understood, the possibility that, without cell fusion, damaged hepatocytes can alter the lineage commitment of haematopoietic stem cells towards that of hepatocytes cannot be excluded. However, the current data demonstrate only a very low frequency at which haematopoietic cells can generate hepatocytes, such that it is not a significant source under most conditions (68).
Liver stem cells and cancer
Hepatocytes, oval cells and BMCs may all be sources of liver progenitor/stem cells. In clinical pathology, combined HCC–CC exists, revealing the possibility of stem/progenitor involvement in cancer development. There is also a close correlation between the degree of progenitor/stem cell activation and the severity of inflammation and fibrosis in chronic hepatitis. The role of liver stem cells in HCC carcinogenesis has also been illustrated experimentally. Hepatocytes have been found to be directly involved in hepatocarcinogenesis in 2-acetylaminflourene and DEN-treated rats in which hepatocytes were labelled with β-galactosidase (69, 70). The direct involvement of oval cells in HCC carcinogenesis was demonstrated by Dumble et al. (71), where such cells isolated from p53-null mice formed tumours when transplanted into athymic nude mice. The participation of BMCs in hepatocarcinogenesis remains controversial. Bone marrow-derived liver stem cells demonstrated low malignancy in chimeric mice that had genetically modified BMCs (72). Another study indicated BMCs as the origin of poorly differentiated HCCs. Interestingly, poorly differentiated cells (HA22T/VGH and SK-Hep-1) expressed markers of BMCs whereas well-differentiated cells like HepG2, Huh-7 and PLC/PRF/5 did not (73).
How do liver stem cells drive cancer initiation? It has been proposed that an excessive and persistent self-renewal signal is one of the key events in the early stages of carcinogenesis (74). Specifically, using highly purified liver stem/progenitor (c-kit−CD49+CD45−Ter119−) cells, the activation of two major self-renewal regulators Wnt/β-catenin and BMI-1 could drive tumour formation (75). Hepatocarcinogenesis is a multistep process that can last more than 30 years after chronic HBV or HCV infection is first diagnosed. Before cancer development, most patients suffer from concomitant cirrhosis and chronic hepatitis, which subsequently induce liver regeneration via liver stem/progenitor cell activation (2). Deregulation of the self-renewal pathway in normal liver stem/progenitor cells plays a significant role in hepatocarcinogenesis (Fig. 1). According to this new ‘cancer stem cell’ model, a tumour is hierarchically organized into a heterogeneous population of cells, within which resides two different cell populations: CSCs with the ability to divide and expand the CSC pool, and a second, larger population of partially differentiated non-tumorigenic cancer cells derived from CSCs, which form the bulk of the tumour. This model proposes that within the whole tumour bulk, only CSCs possess the distinct survival mechanisms and stem cell properties crucial for the maintenance and propagation of the tumour (23).
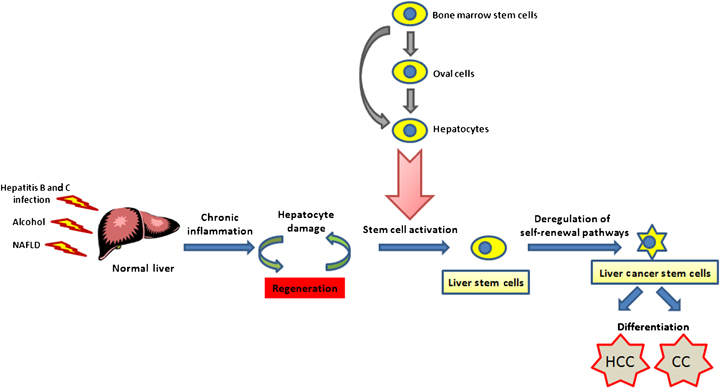
Stem cell model for multistep hepatocarcinogenesis. In response to chronic inflammation by hepatitis B virus, hepatitis C virus infection, alcohol and other non-alcohol fatty liver diseases (NAFLD), normal liver undergoes regeneration owing to hepatic injury. Normal liver stem cells are activated either intrahepatically (oval cells or hepatocytes) or extrahepatically (bone marrow cells). It is believed that liver cancer stem cells originate from normal liver stem/progenitor cells that have undergone genetic alterations with excessive and persistent self-renewal. All three types of cells may be involved in hepatocarcinogenesis. In other words, liver cancer [hepatocellular carcinoma (HCC) and cholangiocarcinoma (CC)] may arise from differentiation of liver cancer stem cells.
Liver cancer stem cell markers
To date, it has been shown that CSCs in HCC can be identified by several cell surface antigens CD133, CD90, CD44, OV6 and the epithelial cell adhesion molecule (EpCAM), or by selecting for the side population (SP) cells in Hoechst dye-staining. Given the phenotypic similarities between CSCs and normal stem cells (which include the ability to self-renew and increased transmembrane efflux capability), it is reasonable to infer that the surface phenotype of CSCs resembles that of normal hepatic stem cells. Hence, Ma et al. (76) used a severe partial hepatectomy model in mice to study the role of stem cells in liver regeneration, so as to gain an insight into the role of stem cells in carcinogenesis. They discovered that Prominin-1, the murine homologue of human CD133, was significantly upregulated in association with the liver regeneration process. In subsequent functional studies using sorted HCC cells, CD133+ cells, and not CD133− cells, displayed properties of CSCs. The CD133+ cells also had significantly greater tumorigenicity in immunodeficient mice, higher colony-forming efficiency and proliferation ability in vitro and could be induced to differentiate into non-hepatocyte-like lineages, indicative of multipotency. The expression of ‘stem-ness’ genes involved in self-renewal pathways was also markedly higher in CD133+ cells, and further studies showed that these cells were more resistant to conventional chemotherapy than their CD133− counterparts (77). Moreover, in HCC cell lines, CD133 was found to be expressed in only a minute proportion of HCC cells, and not in normal hepatocytes (76). Its expression was also detected in human HCC specimens, where increased CD133 expression correlated with inferior prognosis and tumour recurrence (78). However, the prognosis value using a single marker is still controversial (79).
CD90 has emerged as another putative marker for liver CSCs. Among a variety of stem cell-related surface markers, Yang et al. (80) found that the expression of CD90 correlated with tumorigenic potential in a panel of liver cell lines. CD90 is a marker expressed on hepatic stem/progenitor cells during liver development (81), and its expression has been identified in murine breast CSCs (82) and in primarily cultured CD133+ glioblastoma cells (83). In order to exclude lymphocytes from among all the cells marked by CD90+, the authors combined its use with CD45 to isolate non-lymphoid CD90+ cells. They then found these CD90+CD45− cells to be present in all the HCC cell lines and tumour specimens studied. Blood samples collected from the same HCC patients also contained CD90+CD45− cells, indicating the presence of putative CSCs in the circulation (84). The group went on to perform multimarker analysis of CD90+ cells and found that most of these cells also expressed CD44. CD44 is a cell surface receptor for hyaluronic acid and possibly contributes to tumour metastasis. Blockade of CD44 by a neutralizing antibody induced apoptosis of CD90+ cells and prevented local and systemic tumour formation in mice. Together, these results suggest that CD44 could serve as a marker capable of further specifying putative CD90+CD45− liver CSCs (84).
Very recently, using a transcription profiling approach, EpCAM has been identified as a potential early biomarker of HCC (85). This surface molecule is highly expressed in fetal hepatoblasts, hepatic stem cells, bile duct epithelium and also in premalignant hepatic tissues and a subset of HCC, but not in most normal adult hepatocytes (85, 86). Yamashita and colleagues have devised a classification system defined by EpCAM and AFP expression levels that enable prognostic stratification. Furthermore, EpCAM has been shown to be a direct transcriptional target in the Wnt/β-catenin signalling pathway, a pathway that has been suggested to play an important role in governing the self-renewal of cancer cells (87). EpCAM may hence serve as a biomarker for the activation of Wnt/β-catenin signalling. More recently, EpCAM-positive HCC cells were found to be more tumorigenic and invasive when compared with EpCAM-negative cells (88).
As described previously, oval cells are one of the important origins of liver stem cells. Among many markers, OV6 is widely chosen as the oval cell marker of choice. Yang et al. (89) have demonstrated that OV6+ HCC cells possess a greater tumorigenic ability and chemoresistance to standard chemotherapy when compared with OV6− cells. The Wnt/β-catenin pathway plays a pivotal role in the activation and expansion of oval cells in human HCC. Given the increased transmembrane efflux capability in CSCs, Chiba et al. (90) reported that SP cells, which comprised 0.25 and 0.8% of the cell population, were highly proliferative and relatively resistant to chemotherapy in vitro. The increased tumorigenic potential of SP was demonstrated in vivo in a non-obese diabetic/severe combined immunodeficient (SCID) mouse model where 103 liver SP cells consistently led to tumour formation whereas 106 non-SP cells did not. In addition, it was found that SP cells isolated from HCC cell lines may be related to the metastatic potential of HCC (91).
The putative CSC markers are summarized in Table 1. The currently identified markers for HCC CSCs, however, have their limitations. Notably, none of these markers are exclusively expressed by CSCs in HCC tumours, but instead are often expressed by other stem/progenitor cell populations in patients. In targeting cells that express these markers, there is the risk of depleting normal stem/progenitor cells simultaneously. Our challenge is to identify markers that are more specific, or to use several markers in combination, so as to not only be able to differentiate CSCs from their differentiated counterparts, but also, equally importantly, to differentiate CSCs from normal stem cells.
Liver stem/progenitor cells: therapeutic implications
Because the majority of HCC patients are presented at an advanced stage, only about 20% are eligible to receive potentially curative therapy such as liver resection or transplantation (12–17). Systemic chemotherapy for advanced HCC has been used, but the response rate is unsatisfactory owing to chemoresistance by mechanisms such as the over-expression of p-glycoprotein in HCC tumours (18–21). There is still a limited understanding of the pathogenesis underlying this disease. Investigation of the signalling pathways in HCC pathogenesis has led to the development of targeted therapies against HCC, using drugs including sorafenib, erlotinib and bevacizumab (92), which target rapidly growing differentiated tumour cells. Nevertheless, increasing evidence for the existence of liver CSCs suggests the possibility of targeting the undifferentiated or dedifferentiated CSCs, which only constitute a small proportion of a tumour. Notably, liver tumours with progenitor cell characteristics have been found to be particularly aggressive and related to poor patient survival (93). The identification of liver CSC markers and their related pathways has hence become one of the most important goals of present-day cancer research, and working towards this goal would first require an intricate knowledge of the biological characteristics of these cells.
Hepatic regeneration after chronic liver injury involves the recruitment of progenitor cells from the adult liver stem cell compartment. These progenitor cells are c-kit positive and may play a role in HCC carcinogenesis (94, 95). Based on the theory that HCC may arise from the maturation of these progenitor cells, Knight et al. (96) have suggested the possibility of using imatinib as an antitumour agent to target c-kit-positive progenitor cells. Imatinib has been used in two phase II human trials for HCC patients, but the results were unsatisfactory (97, 98). It is possible that, by targeting liver progenitor cells, imatinib and other drugs that perturb similar pathways play a more prominent role in chemoprevention. Apart from c-kit, CD133+ cells have been suggested to be another type of critical tumorigenic progenitors in HCC (76), conferring chemoresistance by preferential activation of the AKT/PKB pathway (77). Aldehyde dehydrogenase expression also discriminates CD133 liver stem cell populations (99). Smith et al. (100) targeted CD133+ cells using a murine antibody to human CD133 (AC133) conjugated to a potent cytotoxic drug, monomethyl auristatin. By doing so, they demonstrated a dramatic reduction in the proliferation rate of Hep3B cells in vitro and delayed tumour growth in a SCID mouse model (100). In a subsequent study, CD90+CD44+ cells were shown to be tumorigenic and formed metastatic lesions in the lungs of immunodeficient mice (80). Moreover, blockage of CD44 in CD90+CD44+ cells by antibody to human CD44 prevented the formation of local and metastatic tumour nodules in a nude mouse model (80). Recently, Yamashita et al. (87) suggested EpCAM+ cells to be tumorigenic progenitor cells, in which the Wnt signalling pathway plays an important role. Suppression of EpCAM by siRNA inhibited Hep3B cell growth.
The self-renewal, growth and survival of human liver stem/progenitor cells involve a diverse network of regulatory mechanisms, including the signalling pathways that have emerged to be attractive therapeutic targets in HCC. Wnt/β-catenin signalling has been proposed to play a crucial role in the self-renewal of liver stem cells (87), and its deregulation has been extensively reported in HCC (85, 101). Several experimental studies have demonstrated decreased proliferation and increased apoptosis by inhibiting the Wnt signalling pathway with siRNA (102). In addition, other self-renewal pathways, such as the Sonic Hedgehog signaling pathway, were found to be frequently deregulated in HCC when compared with their non-tumour counterparts (103). Suppression of the Sonic Hedgehog pathway by siRNA not only decreased HCC cell proliferation but also chemosensitized the cells to 5-fluorouracil and to the induction of cell apoptosis (104).
In contrast to the above pathways, Notch has been found to be downregulated in HCC (105), and its over-expression led to decreased HCC cell proliferation and the induction of apoptosis (106). A recent study has shown that PTEN plays a role in the expansion of the CD133 liver CSC population in liver-specific PTEN-deleted mice, which supports PTEN as a promising target in HCC therapy (107). In addition, transforming growth factor-β (TGF-β) family proteins have also emerged as key players in promoting the growth of stem cells in their undifferentiated state (108). A recent study has found an unexpected functional link between interleukin (IL)-6 and TGF-β signalling in the modulation of HCC and proposed IL-6 to be another important therapeutic target for HCC. The polycomb gene product BMI-1 has been most recently reported to be a novel therapeutic target for the eradication of CSCs in HCC, as it plays a crucial role in the growth of liver cancer cells (109) and its expression correlates significantly with poor HCC patient survival (110).
Survival of stem-like cells in response to chemotherapeutic drugs is thought to be governed by the presence of active transmembrane adenosine triphosphate-binding cassette (ABC) transporter family members, such as MDR1, ABCG2 and ABCC2. It is believed that stem-like cells known as SP cells, which are known for their ability to efflux the DNA-binding dye Hoechst 33342, confer resistance to chemotherapeutic drugs, including cisplatin and doxorubicin, through expressing high levels of such ABC transporters. In SP cells purified from HCC cell lines, inhibition of MDR1 (111), ABCG2 (112) and ABCC2 (113), either by inhibitors or the antisense approach, reverses their chemoresistance. These cells have been shown to harbour other CSC-like properties, and may be related to the metastatic potential and chemoresistance of HCC (91). A novel approach to treat HCC is to induce differentiation of liver CSCs. Such differentiation therapy would force hepatoma cells to differentiate and lose their self-renewal property (114, 115). Dedifferentiation is a key early event in the pathogenesis of HCC and often associated with reduced expression of liver-enriched transcription factors. Yin et al. (116) have demonstrated that hepatocyte nuclear factor-4α, a key liver-enriched transcription factor and central regulator of the differentiated hepatocyte phenotype, promotes the reversion of tumours towards a less invasive phenotype. Overall, Table 2 summarizes the potential therapeutic targets for liver stem/progenitor cells to date.
| Targets | References |
|---|---|
| 1. Liver stem/progenitor cell markers | |
| c-kit | Knight et al. (96) |
| CD133 | Ma et al. (76); Smith et al. (100) |
| CD44 | Yang et al. (84) |
| EpCAM | Yamashita et al. (88) |
| 2. Self-renewal pathways | |
| Transcription factor BMI-1 | Chiba et al. (109) |
| Notch | Qi et al. (106) |
| Wnt/β-catenin | Yang et al. (102) |
| Sonic Hedgehog | Wang et al. (104) |
| 3. Growth | |
| PTEN | Rountree et al. (107) |
| Interleukin-6 | Tang et al. (108) |
| 4. Survival-ABC multidrug efflux transporters | |
| Mdr-1 | Wakamatsu et al. (111) |
| ABCG2 | Hu et al. (112) |
| ABCC2 | Folmer et al. (113) |
| Side population (SP) | Shi et al. (90) |
| 5. Differentiation therapy | |
| Hepatocyte nuclear factor-4α (HNF4α) | Yin et al. (116) |
- ABC, adenosine triphosphate-binding cassette; EpCAM, epithelial cell adhesion molecule.
Conclusions
The role of hepatic progenitor/stem cells has been described. Current anticancer drugs kill differentiated cancer cells and cause a reduction in tumour mass. However, the cancer often recurs after treatment and this can be attributed to the presence of CSCs. Drug discovery in the direction of combating CSCs rather than the bulk population of cancer cells is currently underway. Current research is now looking into a new generation of anticancer drugs that will selectively and specifically destroy various populations of CSCs while leaving the normal stem cell population unharmed, to allow for the complete regeneration of normal tissue. Understanding the interdependent regulatory pathways of these cancer-initiating progenitor/stem cells holds promise for the development of new therapeutic strategies for HCC. Figure 2 lists a selection of inhibitors potentially useful as drugs against liver CSCs, by targeting their surface antigens or the signalling pathways important for the CSC phenotype. It is hoped that in future this new approach will facilitate drug discovery, and equate to improved outcomes for patients with this deadly disease.
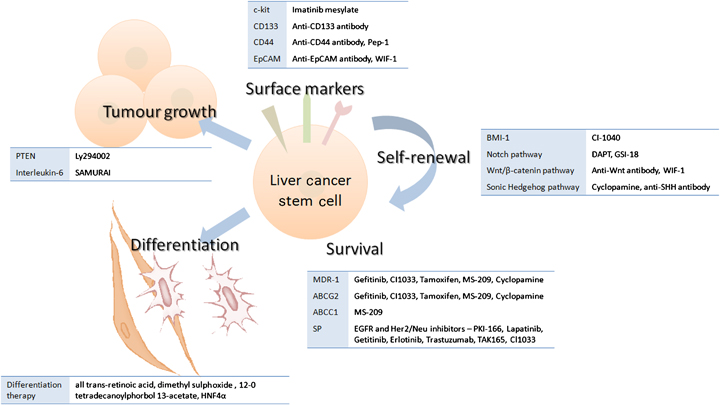
A selection of inhibitors of current liver cancer stem cell markers and signalling pathways involved in self-renewal, growth, survival and differentiation.
Acknowledgements
Financial support: This study was funded by Hong Kong Research Grants Council Collaborative Research Fund (HKU 1/06C).




