Histiocytic sarcomas in flat-coated retrievers: a summary of 37 cases (November 1998–March 2005)
Abstract
Thirty-seven cases of histiocytic-like sarcomas (HLSs) in flat-coated retriever dogs were evaluated retrospectively. This tumour accounted for 36% of the malignant tumours seen in this breed during the study period. The median age at presentation was 8.2 years. Thirty-four dogs presented with a swelling or mass in a muscle group or surrounding a joint. The remaining three presented for rib (1), cutaneous (1) or primary splenic origin (1). A high rate of metastasis to local lymph nodes (45%), thorax (20%) and abdominal organs (20% confirmed) was seen. Overall metastastic rate by the time of death was 70%. The median survival for all dogs was 123 days. The most significant prognostic indicator was presence of distant metastasis at the time of diagnosis with median survival of 68 or 200 days, with or without metastasis, respectively. Chemotherapy and radiation therapy significantly improved survival. Dogs given chemotherapy survived a median of 185 versus 34 days for dogs that were not (P = 0.0008). Dogs treated with radiation survived a median of 182 versus 60 days for those that were not (P = 0.0282). Dogs receiving only palliative therapy survived a median of 17 versus 167 days in dogs receiving any kind of radiation, chemotherapy, surgery or combinations. A set protocol of radiation and CCNU (RTCCNU) induced minimal toxicity and provided a median survival of 208 versus 68 days for all other dogs. While this tumour carries a poor long-term prognosis in flat-coated retrievers, it is reasonable to treat these dogs for palliation of signs and extension of life.
Introduction
Flat-coated retrievers remain a relatively small population of dogs throughout the Americas and Europe. For several years, there has been a suspicion of increased neoplasia in the breed. A survey of neoplasms in flat-coated retrievers in Great Britain showed a high incidence of malignant tumours in submitted biopsy specimens, in particular a large number of undifferentiated sarcomas.1 In this survey, tumours that were all of a similar type, but all listed as unclassifiable sarcomas, accounted for roughly 35% of the malignant tumours sampled. Many of the tumours were found deep in the skeletal muscle or surrounding joints. Although the classification was not always clear, the description of the tumours was always similar with either spindle cells, a mixed population of round and elongated cells, mainly round cells or multinucleated giant cells predominating. The authors commented that the pathology seemed almost specific for the breed. A small number of the undifferentiated tumours were further examined and classified as malignant fibrous histiocytomas (MFH) but with a much more aggressive character than is usually associated with this tumour.2 Metastasis was common in this group of dogs, and widespread metastasis was present at the time of diagnosis in a small number of dogs.
A tumour with the same description as reported by Morris was found in flat-coated retrievers presenting to the Section of Diagnostic Imaging and Radio-Oncology University of Zürich and also Washington State University Veterinary Teaching Hospital (WSUVTH). The tumour was referred to as histiocytic sarcoma (HS) by pathologists in these clinics. The disease seemed to occur with a much higher incidence in this breed and in almost a breed-specific manner. Reports of HSs in the veterinary literature have previously concentrated primarily on pathology and have lacked information on survival, treatment options and appropriate staging procedures. Although HS has been seen increasingly in other breeds, the flat-coated retriever accounted for such a high percentage of cases (48% of HS cases seen at Zurich over the past 4.5 years), and they were selected to examine as a separate group. The purpose of this retrospective study was to examine the clinical records of flat-coated retrievers presenting with HS to describe the clinical presentation of this disease and to evaluate the impact of therapy on clinical outcome.
Materials and methods
Flat-coated retrievers presenting with malignant tumours were identified using the clinical data bank of SDIRO, University of Zurich (June 1995–9 February 2005), and the Veterinary Pathology data bank, University of Zürich (November 1998–31 December 2002). All cases found presented after November 1998. One additional case was added from the clinical data bank at WSUVTH presenting 19 April 2004. End point for evaluation of cases was 31 March 2005. From cases identified, patients were selected which had been diagnosed histologically with HS. Prior to 2001, this terminology had not been used by all pathology groups, so biopsy samples of flat-coated retrievers presenting with a variety of tumours, including polymorphic cell sarcoma, malignant fibrous histiocytoma and undifferentiated sarcoma, were reviewed. Only dogs for which information on treatment and date of death was available were included. Information obtained included date of presentation, age at presentation, sex, history and clinical signs, results of staging procedures (abdominal ultrasound, thoracic radiographs, radiographs of the primary lesion, CBC and blood chemistries), results of therapy and date of death. For the six cases that presented to external veterinary practices, veterinarians were called for information, and copies of the records were sent for examination.
Each biopsy was reviewed by one of two pathologists and in some cases by both. The histologic classification of the tumours was based on morphologic features evaluated on HE-stained sections. Cases with biopsies compatible with HS were included based on this updated diagnosis. Immunohistochemistry (IHC) for CD18 protein was performed on 20 of the biopsy samples using archived formalin-fixed, paraffin-embedded 3 um sections. The tissues were pretreated for 5 min at 37 ° C with 0.1% protease [Type XXVII (Sigma, Buchs, Switzerland) diluted in 0.05 M Tris buffer, pH 7.6]. After 30-min incubation with the primary and monoclonal antibody [CA16.3C10 for the CD18-antigen (Dr P. F. Moore, University of California at Davis) working dilution of 1:5], the reaction product was visualized using the labelled streptavidine-biotin method (DAKO ChemMate Detection Kit, K 5003, DAKO Diagnostics AG, Zug, Switzerland). One biopsy sample was stained for lysozyme to aid in diagnosis, and one biopsy was sent as fresh tissue to University of California at Davis (laboratory of Peter Moore) for immunophenotyping.
Time to treatment failure (TTF), time to treatment failure of radiation therapy (TTF RT) and survival were estimated using Kaplan–Meier survival plots. Dogs were censored only for continued life as all deaths were either directly attributable to tumour, or tumour could not be excluded as a cause. The starting point for both survival and TTF was the date of presentation; TTF RT was calculated from the end of treatment. Only dogs that had received radiation, chemotherapy or surgery were included in calculations of TTF, and only dogs receiving radiation were included in calculations of TTF RT. Prognostic variables examined included age, sex, type of therapy used, stage and hematocrit at presentation. Twenty-seven dogs had adequate information available for staging using the WHO criteria for soft tissue sarcomas.3 Calculations of significance of stage at time of diagnosis (WHO stage, primary tumour stage, lymph node metastasis and distant metastasis) utilized only these dogs plus three dogs with identified distant metastasis. Dogs with tumour appearing in a lymph node were assigned to the category of lymph node positive at the outset. Multiple skin masses were placed in the distant metastasis group. Response to any treatment was only evaluated if tumour was present when therapy was given. Treatment failure was defined as the return or progression of tumour after the first therapy used. (i.e. If one therapy was first used and another was tried at relapse, TTF was the time to failure of the first therapy only.) Failure did not have to be local. Univariate analyses for prognostic variables were evaluated using Log Rank (Mantel–Cox) or Breslow–Gehan–Wilcoxon test for significance. Multivariate analyses were performed using Cox regression. All calculations were done using a commercially available software program (Stat View, SAS Institute Inc., SAS Campus Drive, Cary, NC 27513, USA). A P value <0.05 was considered significant in all calculations.
Results
Thirty-seven cases of pure bred flat-coated retrievers with HLSs were reviewed (Table 1). Mean and median age at presentation was 8.2 years (range 5–12 years). There were 16 intact males, 5 castrated males, 6 intact females and 10 spayed females. In 25 dogs, the first sign noticed was limping, and pain was a problem at the time of diagnosis. One additional dog developed a painful swelling in the thigh muscles after being diagnosed with the tumour in multiple skin sites. Pain was often more severe than the presence of the mass alone could account for, and the area of the tumour often was painful to palpation. In one dog, the tumour had ruptured and contained a necrotic centre; and in several dogs, aspiration cytology yielded material thought to represent abscessation or necrosis. Of the 29 dogs where histories were complete and detailed, 15 dogs (52%) had signs for over 2 months before a diagnosis was made. Thirty-four dogs presented with a swelling or mass in a muscle group or surrounding a joint. The majority of the tumours were located in the forelimb with 10 near the elbow and 12 in the upper arm, shoulder or axillary area. Eleven tumours were located in the hind leg localized to the proximal hind leg (n = 3), stifle (n = 6) or tarsus (n = 2). The remaining tumours were located one each in the spleen, temporal mandibular joint, rib and multiple cutaneous sites. Tumours were associated with joints in 23 patients, but whether the mass originated in the joint or invaded from surrounding structures could not always be determined.
| Dog | Age (years) | Sex | Tumour size | Location | Abdominal ultrasound | Thoracic radiographs | Metastasis found at presentation* | Stage | Survival (days)† | TTF (days) | Therapies applied |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 5 | F | T3 | Stifle | Yes | No | Incomplete staging | 17 | None | ||
| 2 | 11 | M | T3 | Stifle | No | No | Incomplete staging | 467 | 467 | Sx, Cx | |
| 3 | 7 | FS | T4 | Elbow | No | No | Incomplete staging | 30 | 30 | Sx | |
| 4 | 6 | M | T4 | Stifle | No | No | Incomplete staging | 34 | 34 | Sx | |
| 5 | 7 | M | T3 | Upper arm (medial to humerus) | No | No | Incomplete staging | 85 | Sx | ||
| 6 | 8 | MC | T3 | Elbow | No | No | Incomplete staging | 235 | 235 | Sx | |
| 7 | 11 | M | T4 | Shoulder musculature | Yes | Yes | Splenic masses | 3 | 68 | 68 | None |
| 8 | 11 | F | T4 | Spleen | Yes | Yes | Pancreas and stomach | 4 | 100 | 100 | Sx, Cx |
| 9 | 10 | F | T4 | Thigh musculature | Yes | Yes | Internal illiac nodes, lung mass | 4 | 34 | 34 | Sx, Cx |
| 10 | 8 | FS | T3 | Elbow | Yes | Yes | Renal masses* | 3 | 197 | 63 | Sx, Cx, RT |
| 11 | 10 | M | T4 | Elbow | Yes | Yes | Splenic masses | 3 | 38 | 38 | RT |
| 12 | 9 | FS | T4 | Humerus and surrounding muscle | No | Yes | Incomplete staging, axillary mass was lymph node? | 23 | 23 | None | |
| 13 | 9 | FS | T3 | Axilla | Yes | Yes | Liver nodules,* splenic nodules cytologically reactive, axillary mass was LN? | 3 | 63 | 63 | Cx, RT |
| 14 | 9 | FS | T3 | Tarsus | Yes | Yes | None found | 3 | 436 | 436 | Sx, Cx, RT |
| 15 | 5 | F | T3 | Stifle | Yes | Yes | Abdominal nodes | 3 | 561 | 534 | Sx, Cx, RT |
| 16 | 9 | M | T4 | Tarsus | Yes | Yes | Splenic nodules* | 3 | 596 | 553 | Sx, RT |
| 17 | 8 | M | T3 | Axilla | Yes | Yes | Lung or mediastinal mass | 4 | 154 | 154 | Cx |
| 18 | 8 | M | T3 | Shoulder joint | Yes | Yes | Splenic nodules* | 3 | 182 | 182 | Cx, RT |
| 19 | 7 | M | T4 | Elbow | No | Yes | Incomplete staging | 200 | 200 | Cx, RT | |
| 20 | 12 | FS | T4 | Lateral to humerus | Yes | Yes | None found | 3 | 123 | 123 | Cx, RT |
| 21 | 8 | M | T3 | Elbow | Yes | No | Incomplete staging | 1 | None | ||
| 22 | 10 | M | T4 | Axillary mass | Yes | Yes | Liver nodules,* scapular lymph node | 3 | 48 | 48 | Cx |
| 23 | 7 | M | T4 | Dermal nodules | Yes | Yes | Lung mass, internal iliac lymph node enlarged, spleen and renal mass* | 4 | 28 | 28 | Sx, Cx, RT |
| 24 | 8 | FS | T3 | Stifle | Yes | Yes | None found | 3 | 29 | 29 | RT |
| 25 | 9 | FS | T3 | Shoulder musculature | Yes | Yes | Axillary node? | 3 | 362 | 343 | Sx, Cx, RT |
| 26 | 7 | MC | T3 | Elbow | Yes | Yes | None found | 3 | 259 | 176 | Cx, RT |
| 27 | 9 | FS | T4 | Subcutaneous tissue in flank | No | Yes | Multiple skin nodules, multiple peripheral nodes | 4 | 60 | 60 | None |
| 28 | 7 | M | T3 | Elbow | Yes | Yes | None found | 3 | 137 | 137 | RT |
| 29 | 8 | M | T3 | Shoulder musculature | No | No | Incomplete staging | 14 | None | ||
| 30 | 10 | MC | T3 | Elbow | Yes | Yes | Lung nodules believed to be fibrosarcoma in origin* | 4 | 117 | 117 | Cx, RT |
| 31 | 11 | FS | T4 | Rib | Yes | Yes | Sternal* and scapular nodes | 3 | 62 | 59 | Sx, Cx, RT |
| 32 | 9 | M | T3 | Shoulder musculature | Yes | Yes | Scapular node | 3 | 167 | 57 | Cx, RT |
| 33 | 8 | MC | T4 | Pelvis | Yes | Yes | Liver and splenic masses | 4 | 185 | 185 | Cx |
| 34 | 7 | MC | T4 | Shoulder joint | Yes | Yes | (Lung-broncho-interstial pattern), skin mass on prepuce, scapular node | 4 | (239) | Cx | |
| 35 | 5 | M | T4 | TM joint | Yes | Yes | Masses in liver and spleen, mandibular node | 4 | (230) | Cx, RT | |
| 36 | 7 | F | T4 | Stifle | Yes | Yes | Internal iliac node | 3 | (52) | Cx | |
| 37 | 5 | FS | T3 | Elbow | Yes | Yes | Axillary node | 3 | 208 | 208 | Sx, Cx, RT |
- Cx, chemotherapy; F, female intact; FS, female spayed; M, male intact; MC, male castrate; p.sx, post-surgery; RT, radiation therapy Sx, surgery; TTF, time to treatment failure.
- * Organs were suspicious from ultrasound but not sampled or cytology was non-diagnostic.
- † Bolded values in parenthesis indicate the animal was still alive, 31 March 2005.
Histologically, tumours were composed of densely arranged, pleomorphic, round to spindle-shaped cells with abundant eosinophilic cytoplasm and occasional cytoplasmic vacuolation. Nuclei were pleomorphic, ovoid with coarse chromatin and several pleomorphic nucleoli. Anisokaryosis and anisonucleoliosis were prominent features (Fig. 2). Multinucleated giant cells were common. Phagocytosis by tumour cells was occasionally seen. The antibody for CD18 failed in our lab to stain tumour or positive controls on formalin-fixed tissues. The one biopsy stained for lysozyme was positive. Tumour submitted as fresh tissue to University of California at Davis (laboratory of Peter F. Moore) for immunophenotyping also failed to show typical staining for histiocytic origin (likely due to small sample size and poor tissue handling), although it was read morphologically as HS. Because histiocytic origin could not be conclusively established, we refer to the tumour as HLS for the remainder of this report.
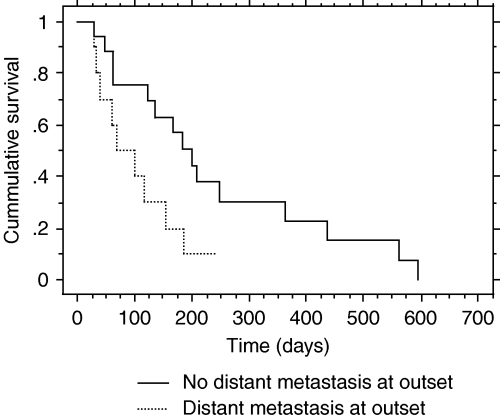
Kaplan–Meier cumulative hazard plot of survival with and without distant metastasis at the outset. Median survival with no metastasis at outset is 200 days. Median survival with distant metastasis at outset is 68 days. P = 0.0325.
Twenty-nine dogs had thoracic radiographs at presentation, and six (21%) had changes consistent with metastasis, five with lung nodules and two with sternal lymph node enlargement. One dog with evidence of thoracic metastasis also was diagnosed simultaneously with a fibrosarcoma. Bone lysis at the tumour site was seen in 29% (9/31) of dogs evaluated with radiographs. Twenty-six dogs had abdominal ultrasound, and 13 had suggestive lesions in the spleen, liver and/or abdominal lymph nodes.4 In five cases, abdominal metastasis was confirmed with cytology or biopsy, but in the remaining cases either aspiration was not attempted or the diagnosis on cytology was unclear. Splenic lesions were seen in nine, with five cytologically confirmed HLS; hepatic lesions were seen in four, with one cytologically confirmed HLS; renal lesions were seen in one case and enlarged abdominal lymph nodes in three cases, but none were aspirated. Local lymph nodes were evaluated in 31 dogs via palpation and aspiration when possible. Ten dogs had metastasis to the local lymph node at first presentation, and four dogs had tumours in the axillary location that were difficult to distinguish from lymph nodes. Of the 17 dogs with no identifiable lymph node metastasis at presentation, three developed lymph node metastasis by the time of disease progression or relapse. In all, 55% (17/31) of the dogs developed metastasis to the local lymph node. Of the 29 dogs with complete blood counts available on the first day, anaemia (hematocrit ≤42%) was found in 14 (48%).
Therapy varied widely in the patient population and was often determined by stage of disease. Six dogs received only palliative therapy consisting of pain medication or corticosteroids. Radiation therapy (defined as equal to or more than 24 Gy) was used in 18 patients. All treatments were administered with a betatron or a linear accelerator. Sixteen received a coarse fractionated protocol; three of these received 3 × 8 Gy on scheduled day 0, 7 and 21, and 13 received 5 × 6 Gy given over 10–14 days. Two dogs received more traditional protocols to of 12 × 4 Gy and 14 × 3.5 Gy. Treatments were planned to incorporate the tumour mass and 3 cm margins within the 90% isodose line. Eight dogs had the draining lymph node also irradiated. Twenty-three patients received at least one dose of doxorubicin or CCNU. At least three doses of chemotherapy with either CCNU (60 mg/m2 every 3–4 weeks) or doxorubicin (30 mg/m2 every 3 weeks) were received by 16 dogs; one dog received both drugs. Fourteen dogs were treated with both radiation and chemotherapy. Chemotherapy was given 3 weeks after finishing radiation in 10 dogs (all CCNU) and simultaneously with or prior to radiation in four dogs (one doxorubicin, three CCNU). Only one patient underwent surgery with a curative attempt (amputation). Fourteen dogs were considered to have had a debulking surgery (defined as removal of at least 50% of the tumour), but only five out of these could have been considered tumour stage 0 (microscopic disease only) after surgery. Twenty-nine patients were known to have received prednisone and/or nonsteroidal anti-inflammatories. Eleven dogs received only one form of therapy (five chemotherapy, three surgery, three radiation therapy). Nineteen dogs received combinations of therapies. Nine dogs received a set protocol of RTCCNU consisting of radiation with 5 × 6 Gy, 3 weeks pause and then CCNU at 60 m2 for four doses given at 3-week intervals.
The survival for all dogs in this case series was 123 days median (±30 days) and 158 days mean (±29 days). A summary of the significant univariate analyses is presented in Table 2, with P values. Age, sex, hematocrit, bone lysis at the tumour site, primary tumour stage, WHO disease stage and presence of lymph node metastasis did not significantly affect survival or treatment outcomes. Significant pretreatment predictors of survival were distant metastasis or any metastasis present. Dogs without distant metastasis survived a median of 200 days while dogs with distant metastasis survived a median of 68 days, with P = 0.03 (Fig. 2). Dogs with metastasis of any kind survived a median of 117 days while dogs with no observable metastasis at presentation survived a median of 200 days. Dogs with distant metastasis at the outset also failed radiation earlier (22 versus 162 days).
| Yes | No | |||||
|---|---|---|---|---|---|---|
| Variable | Number evaluated | Median survival in days | Mean survival in days | Median survival in days | Mean survival in days | P value (test) |
| Predictors of survival | ||||||
| Distant metastasis | 27 | 68 ± 32 | 97 ± 19 | 200 ± 26 | 243 ± 47 | 0.0325 (LMC) |
| n = 10 | n = 17 | |||||
| Any metastasis at start | 29 | 117 ± 24 | 141 ± 28 | 200 ± 53 | 292 ± 65 | 0.0353 (LMC) |
| n = 10 | n = 19 | |||||
| Radiation therapy | 37 | 182 ± 24 | 221 ± 42 | 60 ± 19 | 123 ± 42 | 0.0282 (BGW) |
| n = 19 | n = 18 | |||||
| Chemotherapy | 37 | 185 ± 14 | 218 ± 35 | 34 ± 7 | 98 ± 42 | 0.0008 (BGW) |
| n = 23 | n = 14 | |||||
| ≥3 doses chemotherapy | 37 | 200 ± 17 | 254 ± 41 | 48 ± 25 | 110 ± 33 | 0.0004 (BGW) |
| n = 16 | n = 21 | |||||
| ≥3 doses CCNU | 19 | 200 ± 15 | 260 ± 48 | 117 ± 39 | 97 ± 24 | 0.0008 (BGW) |
| n = 12 | n = 7 | |||||
| Any therapy | 37 | 167 ± 38 | 201 ± 32 | 17 ± 6 | 30 ± 11 | <0.0001 |
| n = 31 | n = 6 | |||||
| RTCCNU | 37 | 208 ± 31 | 264 ± 55 | 68 ± 29 | 144 ± 32 | 0.0159 (BGW) |
| n = 9 | n = 28 | |||||
| Predictors of time to treatment failure ≥3 doses CCNU | 19 | 182 ± 21 | 238 ± 52 | 117 ± 39 | 97 ± 24 | 0.0117 (LMC) |
| n = 12 | n = 7 | |||||
| Predictors of time to failure of radiation therapy | 18 | 22 ± 36 | 45 ± 28 | 162 ± 43 | 206 ± 50 | 0.0362 (LMC) |
| n = 3 | n = 15 | |||||
| Distant metastasis at start | 18 | 22 ± 36 | 45 ± 28 | 162 ± 43 | 206 ± 50 | 0.0362 (LMC) |
| n = 3 | n = 15 | |||||
| ≥3 doses CCNU | 13 | 163 ± 2 | 208 ± 58 | 85 ± 36 | 66 ± 27 | 0.0492 (LMC) |
| n = 10 | n = 3 | |||||
- BGW, Breslow–Gehan–Wilcoxon; LMC, log rank (Mantel–Cox).
When therapies were evaluated independently, use of radiation therapy and chemotherapy were strong predictors of increased survival. Dogs that did not receive radiation therapy had a median survival of 60 versus 182 days in dogs that received radiation therapy (P = 0.0282). Nineteen dogs could be evaluated for response to radiation. Response was generally rapid and was judged at the third week post therapy. In 13 dogs, the response was complete (CR); in five, there was a partial response (PR) and in one, there was progressive disease (PD). Differences between survivals with curative or coarse fractionated radiation protocols were not detected, although only two dogs received curative protocols. Radiation of the draining lymph node was not a significant predictor of survival, TTF or TTF RT. Side-effects of radiation were hair loss or grade 1 skin toxicity in 14 dogs and grade 2 (due to moist desquamation) in five dogs.5
Dogs receiving chemotherapy of any kind had a median survival of 185 days while in dogs that received no chemotherapy drugs the median survival was 34 days (P = 0.0008). A minimum of three doses of a given chemotherapy drug brought greater increased survival (200 days median versus 48 days), and the use, specifically, of a minimum of three doses of CCNU increased survival in dogs receiving CCNU (200 days median versus 117 days). Fifteen of the 23 dogs that received chemotherapy could be evaluated for response to therapy. In 12 dogs, CCNU was given and a CR was seen in five, PR in three, stable disease in two and PD in two. Doxorubicin combined with vincristine and cyclophosphamide was given to one dog and a PR was seen. A difference between CCNU and doxorubicin was not able to be calculated due to the small number of dogs receiving doxorubicin.
Surgery examined alone did not significantly increase survival. Dogs receiving any therapy (radiation, chemotherapy, surgery or combinations) lived significantly longer than dogs receiving only palliative therapy (167 versus 17 days, with P < 0.0001). Use of RTCCNU gave a median survival of 208 versus 68 days in all other cases (Fig. 3).
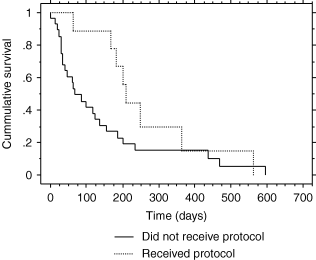
Kaplan–Meier cumulative hazard plot of survival with RTCCNU protocol (CCNU and palliative radiation) or without protocol. Median survival with protocol is 208 days. Median survival of all other dogs is 68 days. P = 0.0159.
Overall, time to failure of any treatment attempted was 137 days median. WHO stage was not a significant predictor of TTF. The only factor correlating positively with TTF was giving at least three doses of CCNU. Use of prednisone had a negative correlation with TTF. Median TTF RT in those dogs that received radiation was 120 days. Distant metastasis at the start of therapy and use of prednisone negatively affected the length of response to radiation therapy. Use of at least three doses of CCNU predicted a better duration of response to radiation (163 days median versus 85 days). Multivariate analysis could not separate responses from radiation therapy or chemotherapy because of the number of dogs receiving multiple therapies. Multivariate analysis did, however, identify use of 3 doses of CCNU, or 3 doses of chemotherapy in general, as the strongest predictors of survival.
Twenty-nine dogs were euthanized due to tumour. One was euthanized for fibrosarcoma, and the histiocytic tumour was felt to be in remission. Generally, tumour progression at recurrence was rapid in onset, included signs compatible with metastasis, and dogs were euthanized shortly after signs returned. Two dogs had complete necropsies. In one dog with only an elbow mass at presentation, tumour was found in liver, kidney and the axillary lymph node. In the second dog with tumour originally located in the muscles over the humerus, metastases to the scapular and axillary lymph nodes were found with no involvement of internal organs. Four dogs were euthanized for signs which may have been tumour related (one with seizures, one with vomiting and diarrhoea and two with acute dyspnea) and one died suddenly with cause of death not determined.
Discussion
Our results confirm as in previous studies that the flat-coated retriever breed has a high incidence of HLSs. In the Swiss population, 36 cases were found at a single institution within a 6.5-years period. The actual number of flat-coated retrievers presenting for other problems was not available, but the population of dogs in this breed in Switzerland is low (estimated to be only around 1000 during any year), and fewer cases of these tumours were seen in dogs of other breeds. HLS accounted for roughly 36% (30/83) of malignant tumours in this breed presenting to SDIRO, University of Zürich. This equals the 35% of all malignant tumours in the breed found by Morris et al.1 The tumour described here is similar to that previously diagnosed as MFH2 or HS diagnosed in other breeds with IHC.6 In domestic animals, MFH was at one time considered a tumour that was locally aggressive and rarely metastatic, but this idea has been changing most likely because the term MFH in the past was used to describe a diverse group of sarcomas, some of which were true tumours of histiocytic origin.7 Several reports of MFH or HS in the soft tissues or spleens of pure bred and mixed breed dogs have been published in the veterinary literature.8–19 In reports from the United States, however, the flat-coated retriever appears only once and in very small numbers.12
Presentation of dogs in this study was similar to other reports. Affected dogs tended to be older with mean and median age of 8.2 years, which is compatible with previous reports.1,2,18 There were 21 males and 16 females which, although not statistically significant, is comparable to the human literature, which reports a male predisposition.20–22 The tumour arising within muscle or joints was very typical in this group and has been reported also by Morris et al.1 along with the predominance in the front limbs (22 of the 37 dogs). The pain and inflammation associated with this tumour made it easy to confuse clinically with a bacterial process or mast cell tumour and often led to fever, inappetence, lethargy, weight loss, poor quality of life with a quick decline and rapid euthanasia. This clinical course has not been previously reported as a characteristic of the tumour in flat-coated retrievers but was noted in 10 dogs with MFH giant cell variant.18 In contrast, the fact that some dogs had signs for long periods (52% had signs of lameness for >2 months prior to presentation) before the actual tumour was diagnosed would seem to indicate an indolent course before the tumour actually becomes apparent.
The tumour caries a very poor prognosis as shown by the overall median survival of 123 and 158 days mean. This also has not been previously described for the breed but was reported by Waters et al.18 for MFH giant cell variant and is often a conclusion when HSs are discussed in other papers.9,14,15 A survival of only 17 days for dogs that palliative therapy with pain medication or corticosteroids also highlights the aggressive nature of this tumour. The number presenting with lymph node metastasis at the outset was as high as 45% (including dogs with tumours in the area of lymph nodes). With the addition of the three dogs which developed metastasis later, 55% of all the dogs eventually developed lymph node metastasis. Of dogs screened adequately, 19% (5/26) had confirmed abdominal metastasis and 21% (6/29) had thoracic metastasis. Of 31 dogs monitored for metastasis, 22 had confirmed metastasis before death for an overall metastasis rate of at least 70%. This rate is high for a sarcoma but is comparable with the 90% metastatic rate reported for dogs with the giant cell variant of MFH,18 the 91% rate reported for HSs of the joint9 and the widespread metastasis seen in two dogs with MFH.13 Our slightly lower metastastic rate may reflect success of therapy but more likely reflects a lack of complete necropsies at death and true identification of all metastasis.
Staging provided prognostic indicators but also highlighted problems with staging systems used for this tumour in general. Using the WHO staging system for soft tissue sarcomas in animals, the primary tumour stage and WHO disease stage gave differences in survival but they were not statistically significant. What was significant was the presence of distant metastasis or the presence of any metastasis. Human sarcoma staging systems place any lymph node metastasis into stage IV disease,23 whereas the animal system allows lymph node metastasis in the stage III category. At least for this sarcoma, it would seem wise to place any metastasis into the stage IV category. Size of the tumour (>5 cm) is often a significant predictor of survival in humans.22,24–30 Since all tumours in the dogs of this study were >5 cm this could not be evaluated. Distal lesions are often thought to carry a better prognosis in human patients.27 The two patients with distal lesions in our study were long-term survivors (436 and 596 days), but with only two cases no conclusion can be drawn. It would seem that HLS in the flat-coated retriever is more likely to be treatable if found early in the course of the disease and in a distal location.
Therapy did prolong survival, but which therapy was best and how long survival was prolonged is difficult to assess. Dogs that received more aggressive therapy tended to be dogs with less widespread disease from the outset, and this admittedly biased any results found with therapy even with multivariate analysis. Dogs with metastasis to lymph nodes or more distant sites tended to be given only chemotherapy. Also, the protocol for treating these dogs changed over time based on our clinical impression as to what seemed to be working, and we were able to convince more owners to treat even severely symptomatic dogs. Combinations of therapies were recommended as more cases were treated. Surgery did not significantly affect survival, but whether more dogs could have been cured with aggressive surgery and radiation remains to be seen. It is doubtful because of the strong tendency seen for metastasis even when local control was achieved. Radiation and chemotherapy individually seemed to be associated with prolonged survival (survival with radiation of 182 days median and with three doses of chemotherapy 200 days median), but in actuality, the two could not be separated because most dogs got some combination of both. Failure of radiation did not appear to be affected by dose which is in contrast to the human data.20 Dogs receiving a set protocol of RTCCNU survived a median of 208 days.
The behaviour of these tumours with frequent rapid metastasis to lymph nodes and spleen is not typical for a soft tissue sarcoma. The extreme pain and inflammation associated with this tumour and response to minimal radiation schemes are also not typical of a sarcoma. The actual cell of origin of this tumour is not known, and this is the reason for confusions in the nomenclature. Most commonly in the veterinary and human fields, it is felt that MFH arises from an undifferentiated mesechymal cell or fibroblast.2,13,27 Some veterinary pathologists examining these tumours, however, have shown many to be of a primary histiocytic origin or true dendritic cell origin.2,12 Recently, even tumours previously diagnosed as synovial cell sarcomas have been found to represent true histiocytic tumours.9 Kerlin and Hendrick12 proposed a hybrid diagnosis of malignant histiocytosis and MFH because of similarities between the tumours and hypothesized that an undifferentiated precursor could differentiate towards either tumour type. Affolter and Moore6 also documented tumours of histiocytic origin in both localized and disseminated forms. We selected the CD18 antibody for IHC analysis to help support a diagnosis of our tumours as histiocytic in origin. CD18 is not a histiocytic marker as it is actually a panleukocytic protein, which histiocytes happen to express in high levels. A panel of antibodies is actually needed to accurately determine the origin of these tumours but nearly all require fresh tissues to function.8 The CD18 antibody is often able to stain formalin-fixed tissues and is therefore used when archived tissue is all that is available. Unfortunately with our formalin-fixed tissues the antibody did not work. The one sample submitted as fresh tissue for IHC analysis also did not stain characteristically but was read morphologically as HS. Interestingly, flat-coated retrievers have a high incidence of both histiocytomas as juveniles1 and HLS or MFH as adults. Histiocytomas have been shown to be of Langerhan's cell origin.31,32
Histiocytic tumours in people are also poorly understood and defined. A true HS might be comparable to the disease known as Langerhans histiocytosis in the human, although canine HS have only rarely been shown to be of Langerhan's cell origin. MFH in humans has higher metastatic rates as compared with other sarcomas. The levels vary among different studies and metastasis at outset is often not reported, but they are generally in the 30–40% range.20,26,28,33,34 Additionally, the most common site of metastasis is the lung, not the regional lymph node. Chemotherapy is believed to play a role in the treatment of MFH in humans8 as well as radiation and surgery.20
Additional work needs to be done to identify the origin of this tumour in the flat-coated retriever and all affected dogs and apply a uniform name for the tumour type. Better controlled therapeutic trials based on the tumour type and what is known so far about response to various therapies could then be initiated. The extreme over-representation within the flat-coated retriever breed must indicate a genetic tendency, and this is another area for investigation. Our current recommendation for flat-coated retrievers with this tumour type is to stage them carefully with thoracic radiographs, abdominal ultrasound with aspiration of suspicious lesions and lymph node examination. Surgery is recommended for localized lesions but amputation may be of little benefit because of the high potential for rapid metastasis and the fact that good local control can be achieved with radiation therapy, particularly combined with chemotherapy.