Fournier's gangrene in a cat
Abstract
Objective – To describe the clinical course of a cat diagnosed with Fournier's gangrene.
Case Summary – A 2-year-old castrated male cat was presented to an emergency hospital for evaluation of acute onset of lethargy, mucoid anal discharge, and fever. During hospitalization, with provision of supportive care, an area of necrotizing fasciitis around the prepuce and anus developed and surgical debridement was performed. Severe sepsis developed secondary to the necrotizing fasciitis and the cat was eventually euthanized.
New Information Provided – The purpose of this report is to document the first case of Fournier's gangrene in a cat that presented for mucoid anal discharge, lethargy, and mild ataxia, and to alert emergency clinicians to this disease process. Early detection of the disease with prompt, aggressive supportive care and surgical debridement is necessary for successful treatment.
Introduction
Necrotizing fasciitis is a fulminant, rapidly progressive, potentially life-threatening, bacterial infection of the fascial and subcutaneous tissue.1 In human medicine, Fournier's gangrene is defined as a necrotizing soft tissue infection of the genital and anorectal regions characterized by tissue necrosis (cellulitis, fasciitis, and myositis), rapid progression, lack of frank suppuration, and severe systemic toxicity.2 To the authors' knowledge, this is the first report of Fournier's gangrene in veterinary medicine and demonstrates the importance of early recognition and aggressive therapy.
Case Summary
A 2-year-old castrated male domestic short hair cat, weighing 6.1 kg was presented to an emergency service for evaluation of acute onset of lethargy, mild ataxia, and mucoid anal discharge. The cat had no previous medical problems, was retrovirus (FeLV and FIV) negative, and lived indoors only. He had received his first vaccinations at the time of castration but had received no additional vaccinations. The owners reported that the cat had a good appetite and energy level before the day of presentation. Within the previous week, a stray kitten had been introduced to the household but no other environmental changes had been made. The owners reported that although the new kitten had not yet received veterinary care, he appeared to be healthy at home.
On presentation, the cat was extremely lethargic, had an elevated temperature of 40.4°C (104.8°F), was mildly hypotensive (88–96 mm Hg systolic blood pressure measured by an ultrasonic Doppler flow monitora), was estimated to be 8% dehydrated, and had a painful swelling (3/10 pain score3) around the rectum that was edematous in appearance. Further physical exam findings included tachycardia at 220/min with strong and synchronous pulses, a soft nonpainful abdomen, and a normal direct fundic examination. There was no evidence of cutaneous lesions at the initial examination. A venous blood gas performed at admission showed a metabolic acidosis with respiratory compensation (pH 7.287, reference interval, 7.265–7.424; HCO3 14.6 mmol/L, reference interval, 18–23.2 mmol/L; base excess, −8.7 mmol/L; PCO2 31.5 mm Hg, reference interval, 33.2–47.3 mm Hg) and mild hyperglycemia (9.2 mmol/L [165 mg/dL]; reference interval, 4.0–7.3 mmol/L [72–132 mg/dL]). The PCV and total plasma protein (PCV/TPP) were 50% (reference interval 37–54%4) and 79 g/L (7.9 g/dL) (reference interval 60–75 g/L [6.0–7.5 g/dL]5) respectively. Initial treatment consisted of an IV crystalloid fluid bolus (16.5 mL/kg, lactated Ringer's solutionb), after which the cat seemed more alert and had a systolic blood pressure of 136 mm Hg. IV fluid treatment was started with lactated Ringer's solution (85 mL/kg/d) with added potassiumc (16 mEq KCl/L) and B vitaminsd (2 mL/L) to meet the cat's ongoing fluid needs and replace the fluid deficit (420 mL calculated deficit based on a 6-kg cat at 8% dehydration) over 24 hours. In addition, buprenorphinee (0.01 mg/kg, IV, q 8 h) and metronidazolef (7.5 mg/kg, IV, q 12 h) were initiated. Three-view body radiographs were taken and were within normal limits. Because of initial financial constraints, separate thoracic and abdominal radiographs could not be taken. A full abdominal ultrasonographic examination was not completed due to the late night presentation but an emergency ultrasonographic screening examination revealed no obvious masses or free fluid. A diagnostic peritoneal lavage was performed by instilling sterile saline (10 mL/kg) into the abdomen via a 22-Ga needle. Cytologic examination revealed a mild amount of protein and mucinous material but no cells or bacteria. Differential diagnoses for the fever and lethargy at that time included gastrointestinal perforation, pancreatitis, hepatitis, cholangiohepatitis, viral infection (feline infectious peritonitis [FIP], FeLV, FIV, herpes virus, calici virus), toxoplasmosis, occult bacterial infection, immune-mediated disease, and neoplasia. A CBC, general chemistry profile, urinalysis, urine culture, and thyroxine level were submitted for analysis.
On the following morning and first full day of hospitalization, the cat's body temperature increased to 41.7°C (107°F), and he remained lethargic. A recheck PCV/TPP showed a decrease to 38%/56 g/L (5.6 g/dL) that was thought to be due to hemodilution, red cell destruction, or as a result of cytokine-induced decrease in RBC production. The patient remained normotensive throughout the day. The results of the serum biochemistry profile obtained at admission revealed an elevated aspartate transferase and alanine transferase, and the CBC count revealed a normal leukocyte count with toxic changes graded subjectively as 1+ in the segmented neutrophils (see Tables 2 and 3). The pathologist's review of the blood smear revealed no evidence of red cell destruction thus making the differential of immune-mediated red cell destruction less likely. The thyroxine level was low at 16.7 nmol/L (1.3 μg/dL) (reference interval, 27–56.6 nmol/L [2.1–4.4 μg/dL]) and was suspected to be due to euthyroid sick syndrome. The urinalysis, collected at admision, revealed a urine specific gravity of 1.077, 2+ proteinuria, 2+ urobilinogen, 1+ bilirubinuria, and 2+ blood. An abdominal ultrasonographic examination, the following morning, showed a hypoechoic liver, mild mesenteric lymphadenopathy, and a small amount of abdominal effusion likely secondary to the diagnostic peritoneal lavage. Treatment with IV fluids, and buprenorphine, were continued and vitamin K1g (5 mg, PO, q 24 h) was added to protect against a possible liver-related coagulopathy. Treatment with metronidazole was discontinued due to a lack of persistent anal discharge or diarrhea and treatment with clindamycinh (12.5 mg/kg, IV, q 12 h) and enrofloxacini (5 mg/kg, IV, q 12 h) was initiated to provide broader antibacterial coverage. At this time additional diagnostic testing for FeLV, FIV, and Toxoplasma gondii, and feline pancreatic lipase immunoreactivity was declined by the owner.
| Day 1 | Day 3 | Day 6 | Day 13 | Reference interval | |
|---|---|---|---|---|---|
| Blood glucose (mmol/L) | 10.9 | 12.8 | 9.9 | 9.3 | 3.9–7.0 |
| Blood glucose (mg/dL) | 197 | 230 | 179 | 167 | 70–126 |
| Aspartate aminotransferase (U/L) | 90 | 176 | 46 | 7 | 17–43 |
| Alkaline aminotransferase (U/L) | 152 | 142 | 81 | 25 | 32–38 |
| Total bilirubin (μmol/L) | 6.8 | 65.0 | 6.8 | 1.71 | 1.71–8.6 |
| Total bilirubin (mg/dL) | 0.4 | 3.8 | 0.4 | 0.1 | 0.1–0.5 |
| Total protein (g/L) | 78 | 50 | 45 | 50 | 61–83 |
| Total protein (g/dL) | 7.8 | 5.0 | 4.5 | 5.0 | 6.1–8.3 |
| Albumin (g/L) | 45 | 22 | 20 | 23 | 31–41 |
| Albumin (g/dL) | 4.5 | 2.2 | 2.0 | 2.3 | 3.1–4.1 |
| Day 1 | Day 3 | Day 6 | Day 13 | Referenceinterval | |
|---|---|---|---|---|---|
| WBC count (× 109/L) | 5.2* | 19.4* with bands | 7.0 | 0.2 | 4.4–18.2 |
| Hematocrit (%) | 49.8 | 24 | 17.6 | 18.5 | 30.6–48.5 |
| Platelet count (× 109/L) | 56 | 132 | 58 | 6 | 200–900 |
- * The presence of 1+ toxic change.
Throughout the second through fifth days of hospitalization, the patient remained lethargic and febrile at 39.2–40.0°C (102.6–104.0°F). On physical examination the cat appeared nauseated, was painful on abdominal palpation, and was weak and barely ambulatory. Petechiation on the ventral abdomen around the area of peritoneal lavage was present and on the fourth day areas of petechiation were seen around the rectum. He remained normotensive during this time with a systolic blood pressure of 90–130 mm Hg. During this time a repeat CBC, serum biochemistry profile, and daily PCV/TPP revealed an increase in liver enzymes including the total bilirubin, hypoalbuminemia (see Table 2), an increase in WBC with the presence of band neutrophils (see Table 3), and a progressive anemia (see Table 1). The prothrombin time (PT) was within reference interval and the activated partial thromboplastin time (aPTT) was mildly prolonged at 23.2 seconds (reference interval 10.8–16.7 s). The patient's blood type was A, and the owners consented to infectious disease testing (FeLV, FIV, T. gondii, FIP PCR) and a feline pancreatic lipase immunoreactivity assay on the third day of hospitalization. Possible causes for the changes in liver enzymes considered at this time included primary hepatocellular disease, anemic hypoxia, sepsis-induced cholestasis, or extrahepatic bile duct obstruction. The progressive hypoalbuminemia was suspected to be secondary to a decreased production, because albumin is a negative acute-phase protein, or hepatic failure, although loss through endothelial damage and subsequent capillary leak (third spacing), a protein-losing enteropathy, or protein-losing nephropathy could not be ruled out.
| Day ofhospitalization | PCV(%)/total plasmaprotein (g/L) [g/dL] |
|---|---|
| Admission | 50/79 [7.9] |
| 1 | 38/56 [5.6] |
| 2 | 33/63 [6.3] |
| 3 | 24/56 [5.6] |
| 4 | 25/59 [5.9] |
| 5 | 22/58 [5.8] |
| 6 | 19/53 [5.3]* |
| 7 | 20/55 [5.5]* |
| 8 | 26/54 [5.4] |
| 9 | 22/49 [4.9] |
| 10 | 20/53 [5.3] |
| 11 | 22/59 [5.9] |
| 12 | 23/59 [5.9] |
| 13 | 19/58 [5.8] |
- * Pretransfusion values on days when packed RBC transfusions were given.
In order to further investigate possible liver disease, a repeat abdominal ultrasonographic examination was performed and revealed a hypoechoic liver with distended gallbladder and a tortuous cystic duct. A fine needle aspirate of the liver was obtained. This test was chosen over a liver biopsy because of the short turnaround time for cytology (approx 24 h) and the minimal invasiveness of the test. The distended gallbladder was attributed to anorexia, and no investigations were performed as a result. The cytologic examination of the liver aspirate showed possible neutrophilic-lymphoplasmacytic inflammation with no evidence of neoplastic cells.
Crystalloid IV fluids were continued and hetastarchj was started at 10 mL/kg/d. A nasoesophageal (NE) feeding tube was placed and feedings with a liquid enteral dietk were started (30 kcal, q 6 h). Treatment with enrofloxacin, clindamycin, and vitamin K1 were continued; dolasetronl (0.3 mg/kg, IV, q 24 h) was started to control the nausea, and ursodiolm (90 mg, PO, q 24 h) and S-adenosylmethioninen (90 mg, PO, q 24 h) were added for additional hepatic support.
During the sixth and seventh day of hospitalization, the patient remained febrile at 39.4–40.6°C (103–105°F) and became tachycardic (250/min) with a gallop rhythm. The cat was more painful around the tail and perineum (5/10 pain scale3) and the areas of bruising around either side of the anus developed into large well-demarcated regions of swelling, erythema, purpura, and suspected necrosis extending down the caudal thighs (see Figure 1). A moderate amount of exudate was visible at the edges of the ulcerated tissue. A recheck CBC and chemistry profile showed improvement in the liver values (see Table 2) and a decreased hematocrit (see Table 3). The cat's anemia was progressive (see Table 1). Previously submitted diagnostic testing that became available during this time included FeLV/FIV (negative) and the urine culture (negative for bacterial growth). The cat was given 1 U of type A packed RBCs over 4 hours, a ketamineo/fentanylp infusion (2.5 μg/kg/min and 2.5 μg/kg/h, respectively) was started, and the buprenorphine was discontinued. The NE tube feedings were well tolerated. Because the cat's fever had not yet resolved, and he was not showing clinical improvement, the antibiotics were changed to metronidazole (7.5 mg/kg, IV, q 12 h) and ticarcillin with clavulanate potassiumq (50 mg/kg, IV, q 8 h). Because of continued anemia and a systolic blood pressure of 85 mm Hg, a second unit of packed RBCs was given over 4 hours after which the cat was normotensive. A dermatologic consultation was performed and differential diagnoses included a cellulitis or vasculitis due to infection and other causes such as an autoimmune disorder. Biopsies of the affected area were recommended for histopathology and tissue culture.
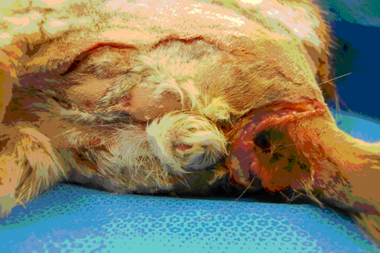
Patient in right lateral recumbancy. An approximately 9 cm area of swelling and skin discoloration (possible necrosis) can be seen around the anus and prepuce. Note the clear line of demarcation with purulent discharge.
Because of the delicate anatomical area involved and the potential need for skin flap procedures, a surgical procedure was scheduled after a weekend when a board-certified veterinary surgeon with special expertize in soft tissue reconstruction would be available (on the 9th day of hospitalization). General anesthesia was induced with ketamine (5.3 μg/kg, IV) and midazolamr (0.26 mg/kg, IV) and maintained with isofluranes in 100% oxygen and a fentanyl infusion (2.5 μg/kg/h). The cat was not given an epidural as part of his anesthetic protocol because he was thrombocytopenic, had a local skin infection, and was presumed to be septic/bacteremic. Surgical debridement of the necrotic perianal area was performed and the area was left open without bandaging, with the plan to return to surgery in 2–3 days to attempt closure. Wedge biopsy samples were obtained aseptically and submitted for histopathology and aerobic and anaerobic bacterial cultures. An esophageal feeding tube was placed and the NE tube was removed. The patient remained stable under anesthesia throughout the procedure. Additional pending diagnostic test results were finalized on the same day and included FIP mRNA PCR and T. gondii (both seronegative).
Supportive care was continued over the following 3 days of hospitalization. On the 13th day of hospitalization, physical examination abnormalities included elevated temperature at 39.9°C (103.8°F), tachycardia (220/min) with a gallop rhythm, pale mucous membranes, and bilateral serosanguinous nasal discharge. The cat was normotensive at 96–100 mm Hg (systolic) and his PCV/TPP again dropped to 19%/58 g/L (5.8 g/dL). The area of necrosis around the perineal area and scrotum had mild serous discharge and appeared more erythematous. The cause of the serosanguinous nasal discharge was suspected to be due to a progressive thrombocytopenia. A CBC and biochemical profile revealed a severe leukopenia and thrombocytopenia with minimal changes in the biochemical profile (see Tables 2 and 3) and a normal PT/aPTT. These findings were suspected to be the result of a chronic widespread cytokine release with leukocyte consumption and a decreased bone marrow response. Because the cat was worsening clinically, and the prognosis was poor, the owners elected euthanasia.
The results of the anaerobic culture were negative for growth and the aerobic culture produced a moderate growth of Enterococcus faecium, Staphylococcus epidermidis (β-lactamase positive), and Escherichia coli. The S. epidermidis was resistant to amoxicillin/clavulanic acid, ampicillin, cefazolin, ciprofloxacin, clindamycin, oxacillin, and trimethoprim sulfa but was sensitive to gentamycin, tetracycline, and vancomycin. Although susceptibility to ticarcillin and clavulanate potassium was not tested, Staphylococcus species that are resistant to oxacillin must be considered resistant to ticarcillin/clavulanic acid.6 Based on the results of the culture and susceptibility and information provided about ticarcillin and clavulanate potassium, the antimicrobials used in this case did not adequately treat the Staphylococcus species present. Histopathology of the biopsy samples revealed severe acute necrosuppurative panniculitis with multifocal intralesional fibrinoid vascular necrosis consistent with Fournier's gangrene (see Figure 2).
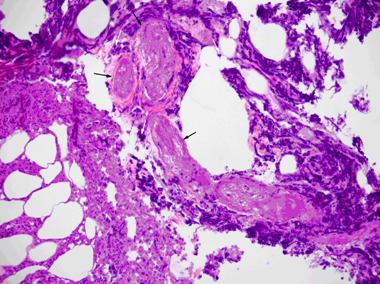
Photomicrograph of a biopsy sample obtained from the effected perianal skin and subcutis: the subcutaneous tissue exhibits necrosuppurative panniculitis with infiltration of degenerative neutrophils (white arrows) and several necrotic blood vessels with hyalinized, hypereosinophilic walls characteristic of fibrionoid vascular necrosis (black arrows). H&E stain × 20.
Discussion
Necrotizing fasciitis is a rare infection of the deep layers of the skin and subcutaneous tissues that is often accompanied by septic shock.7 In human medicine, a specific type of necrotizing fasciitis of the genital, perianal, and perineal regions of the body is known as Fournier's gangrene.2,8 To the authors' knowledge, there have been no reports of Fournier's gangrene in the veterinary literature.
Although uncommon, Fournier's gangrene is an important disease process that has a mortality rate of 20–50% in humans.9 Fournier's gangrene was originally described in 1883 and was characterized by a sudden onset of necrotizing fasciitis in healthy young men, rapid progression of lesions to gangrene and the absence of a definite cause (idiopathic).8 These 3 characteristics were seen in the case reported here. More recently, a concurrent problem has been found in approximately 95% of human cases with the source of infection being anorectal abscesses, genitourinary infections, or traumatic injures.10 Most often, the patient is found to have a predisposing problem. In one study, the 5 most common predisposing factors included diabetes mellitus, hypertension, chronic liver disease, liver cirrhosis, and chronic renal insufficiency.8 In another study, diabetes mellitus was seen in 10–60% of human cases of Fournier's gangrene10 and in a study that included all types of necrotizing fasciitis, 91% of humans had some associated immunodeficiency.11 The case reported here did not have any evidence of a predisposing condition.
Fournier's gangrene and other forms of necrotizing fasciitis can be difficult to diagnose and the diagnosis is usually made primarily based on clinical signs. General signs include a nonspecific history, fever, lethargy, erythema, edema, and pain of the affected site.1 As seen in this case, the hallmark clinical sign of necrotizing fasciitis is extreme pain that is disproportionate to the appearance of the affected area.1,10 In cases of necrotizing fasciitis in veterinary medicine, results of routine blood tests are usually nonspecific, reflecting severe systemic inflammation, vasculitis, and sepsis or organ dysfunction.1 In human medicine, advanced imaging such as ultrasonography, computed tomography, or magnetic resonance imaging is used to detect small pockets of fluid or gas, or both.10 A definitive diagnosis of necrotizing fasciitis or Fournier's gangrene is made at surgery with the finding of gangrenous tissue or purulent material and an increased ease of separation of fascia from other tissues by blunt dissection.1,10
Fournier's gangrene is considered a polymicrobial infection with aerobic and anaerobic bacteria present, although anaerobic organisms are less commonly isolated.8 A majority of cases are caused by normal flora of the lower gastrointestinal tract including E. coli, Staphylococcus spp., Streptococcus spp., and Enterobacteraceae.10 The culture of normal flora or organisms of low virulence adds to the difficulty in making a diagnosis as the organisms may not be considered pathogenic. In cases of Fournier's gangrene it is thought that the polymicrobial nature of the infection in combination with local trauma or underlying disease processes allows for the development of synergistic and virulent behavior.12 This virulent behavior results in massive release of cytokines and thrombus formation in the small vessels. This is known as obliterative endarteritis and is thought to be a key pathophysiologic event.8 Culture of E. faecium, S. epidermidis, and E. coli in this case is similar to culture results obtained in human cases of Fournier's gangrene.8,10,12 We suspect that it was the synergistic effect of these pathogens, massive cytokine release, and microthrombi formation that led to the clinical deterioration seen in this case. A second contributing factor in this case included the antimicrobial resistance of S. epidermidis. Although S. epidermidis is often considered a nonpathogenic commensal organism of the skin, one case report in human medicine concluded that advanced age and local tissue factors can predispose a patient to the development of necrotizing fasciitis with this organism alone.13 Histopathologic examination of biopsy samples obtained from cases of Fournier's gangrene have characteristic findings of necrosis of the superficial and deep fascial planes, fibrinoid coagulation of the nutrient arterioles, and polymorphic nuclear cell infiltration, as were described in this case.10
The diagnosis of necrotizing fasciitis or Fournier's gangrene is often not readily apparent at the time of initial examination. Treatment with IV fluids and broad-spectrum antimicrobials with coverage against Staphylococcus spp., Streptococcus spp., Enterobacteriaceae, and anaerobes should be promptly initiated pending the results of any diagnostic testing.10 In the case presented here, metronidazole was the first antimicrobial used for treatment of diarrhea due to its enteric anaerobic coverage. Once hospitalized, the cat's fever persisted and it did not develop further diarrhea; thus, the antimicrobial treatment was changed to clindamycin and enrofloxacin to provide better coverage against potential β-hemolytic Streptococcus spp., T. gondii, and gram-negative species. Once the cat was determined to be seronegative for T. gondii, clindamycin was discontinued and ticarcillin with clavulanate potassium and metronidazole were initiated to provide additional coverage against Enterococcus spp. and anaerobes. Although the patient was being treated with broad-spectrum antimicrobial coverage, results of the tissue culture available after euthanasia revealed that a resistant S. epidermidis, sensitive only to gentamicin, tetracycline, and vancomycin, was present.
The most important component of treatment in cases of Fournier's gangrene is aggressive surgical excision of all necrotic tissue to remove the bacterial nidus,8 and to prevent further spread along facial planes. One study in humans demonstrated that a 24-hour delay in debridement increased the mortality rate by 11.5% and a 6-day delay was associated with a mortality rate of 76%.10 Complications of delayed surgical intervention include respiratory failure, renal failure, septic shock, pneumonia, hepatic failure, disseminated intravascular coagulation, and upper gastrointestinal bleeding.8 Multiorgan system failure secondary to gram-negative sepsis is the most common cause of death in these cases.10 Unfortunately in this case, despite multiple examinations each day and frequent nursing care, bruising of the affected area was only noted on the 4th day of hospitalization, but abscess formation was not recognized until the 7th day of hospitalization and surgical debridement of the area was not performed until the 9th day of hospitalization. In retrospect, several aspects of case management could have been carried out differently to have allowed for more efficient use of diagnostic testing and treatment. Earlier sampling of the affected area when initial edema was noted either via punch biopsies, fine-needle aspirates, or tissue cultures would have provided information regarding antimicrobial sensitivity in a more timely fashion. This would have allowed for more appropriate antimicrobial use and may have prevented the patient's decline. Also, the use of enrofloxacin every 12 hours, although published as acceptable,14 may not have allowed for its maximum effectiveness. Because enrofloxacin is a concentration-dependent drug, it is the concentration of the medication and not the duration that is important in determining its efficacy. Thus, using enrofloxacin every 24 hours might have allowed for a higher concentration and more effective use of this medication. Overall, earlier recognition of Fournier's gangrene as the underlying disease process and more aggressive diagnostic testing and earlier surgical debridement may have prevented the patient's rapid decline and sepsis.
More recently, studies investigating the use of hyperbaric oxygen as a treatment modality has been investigated.10,11,15,16 Although results regarding the use of this therapy are mixed, hyperbaric oxygen theoretically increases tissue oxygen tension, leukocyte activation, oxygen free radical reduction, capillary angiogenesis, fibroblast proliferation, and vasoconstriction, and decreases anaerobe multiplication.10
To the authors' knowledge this is the first case of Fournier's gangrene reported in the veterinary literature. This case illustrates the classic findings of Fournier's gangrene as reported in the human literature and demonstrates the importance of early recognition, and aggressive supportive care with prompt surgical debridement. Emergency clinicians should consider this disease process in cases presenting with fever, pain disproportionate to the apparent degree of injury, and nonspecific history. Close and repeated evaluation of the perianal, perineal, and scrotal area is important and prompt surgical intervention is necessary to prevent sepsis, organ dysfunction, and death.
Acknowledgements
The authors would like to thank Patty Ewing, DVM, MS, DACVP and Melanie Buote, DVM, DACVP, anatomic pathologist for providing the image used for Figure 2, and for their repeated analysis of clinical pathology abnormalities throughout the case.
Footnotes
aUltrasonic Doppler Flow Detector, Model 811-B, Parks Medical Electronics Inc, Aloha, OR.
bLactated Ringer's solution, Hospira Inc, Lake Forest, IL.
cPotassium Chloride, Hospira Inc.
dVitamin B complex, Butler Animal Health Supply, Dublin, OH.
eBuprenex, Hospira Inc.
fFlagyl, FLIVA Inc, Pomona, NY.
gPhytonadione, Aton Pharma Inc, Lawrenceville, NY.
hAntirobe, Hospira Inc.
iBaytril, Bayer Health Care LLC, Animal Health Division, Shawnee Mission, KS.
jEspan, B. Braun Medical Inc, Irvine, CA.
kClinicare, Abbott Laboratories, Columbus, OH.
lAnzemet, Sanofi-Aventis U.S. LLC, Bridgewater, NJ.
mActigal, Watson Laboratories Inc, Corona, CA.
nSAMe, Nutramax Laboratories, Edgewood, MD.
oKetamine Hydrochloride Injection, Fort Dodge Animal Health, Fort Dodge, IA.
pFentanyl Citrate, Hospira Inc.
qTimentin, GlaxoSmithKline, Research Triangle Park, NC.
rVersed, Hospira Inc.
sIsoflurane, Webster Veterinary Supply Inc, Sterling, MA.




