Possibilities for therapeutic interventions in disrupting Chlamydophila pneumoniae involvement in atherosclerosis
Themed series on ‘Sudden Cardiac Death – Cardiovascular Therapy’ Meeting June 18–19, 2009 Copenhagen, Denmark
Abstract
Strong sero-epidemiologic, pathologic, and experimental evidence suggests that Chlamydophila pneumoniae (Cpn) infection may play a causative role in the development of atherosclerosis. Cpn is an obligate intracellular gram-negative bacterium that is responsible for 10% of cases of community-acquired pneumonia. In addition to its presence in the respiratory tract, live Cpn has been found within atherosclerotic plaques. Experimental findings have established Cpn’s ability to infect vascular cells and elicit important atherogenic responses. Furthermore, Cpn infection can promote atherosclerotic development in different animal models. To date however, large-scale antibiotic clinical trials have not been effective in preventing major cardiovascular events. It is becoming apparent that Cpn undergoes a persistent state of infection, which is refractory to current chlamydial antibiotics. New treatment strategies that are effective toward acute and persistent forms of Cpn infection are needed in order to effectively eradicate the bacterium within the vascular wall. Possible therapeutics targets include Cpn-specific proteins and machinery directly involved in their survival, replication and maintenance. Alternatively, selectively targeting host cell pathways and machinery required for Cpn’s actions in vascular cells also represent potential treatment strategies for atherosclerosis.
Introduction
Atherosclerosis is the leading cause of morbidity and mortality in North America. It is characterized as a chronic inflammatory disease with contributions from both the innate and adaptive arms of the immune system [1]. The involvement of an active host immune response in disease progression has prompted interest in the possible role of infectious agents as risk factors for coronary artery disease. Chlamydophila pneumoniae (Cpn), a unique obligate intracellular gram-negative bacterium, has emerged as the most plausible pathogen to contribute to atherogenesis. The current review will discuss: (i) the involvement of Cpn in atherogenesis, (ii) the success of treatment strategies for Cpn as they relate to cardiovascular disease, and (iii) novel therapeutic targets for persistent Cpn infection.
Cpn and atherosclerosis
Chlamydophila pneumoniae is primarily known as a respiratory pathogen responsible for acute and chronic conditions such sinusitis, pneumonia and bronchitis. Cpn infection is highly prevalent with an estimated 50% of the population by the age of 20 and 70–80% of older adults displaying serologic evidence of exposure [2]. Saikku et al. [3] first established the link between Cpn infection and cardiovascular disease by demonstrating that high antibody titers against Cpn were associated with the incidence of chronic stable coronary artery disease or acute myocardial infarction. Since then numerous cross-sectional and prospective sero-epidemiologic studies have provided varied findings [4]. Despite these results, pathologic evidence has been strongly supportive of a role for Cpn in heart disease. Cpn has been detected in atherosclerotic carotid and coronary arteries but not in healthy tissues by electron microscopy, immunofluorescence and polymerase chain reaction (PCR) methods [5–7]. Cpn strains retrieved from these plaques are still viable by evidence of their ability to be cultured [8,9]. Furthermore, Cpn specific T cells have also been detected in these atherosclerotic plaques demonstrating activation of the host immune response to Cpn replication [10].
The mechanism whereby Cpn infection leads to atherosclerotic development has received considerable research attention. Multiple laboratories have established that Cpn has the ability to infect and actively replicate in cells relevant to atherosclerotic injury (monocyte/macrophages, endothelial cells and vascular smooth muscle cells [11–13]). Infection of these cell types stimulates cellular responses that support the involvement of Cpn in all stages of atherogenesis (1). The migration of Cpn from the lungs to the vessel wall is proposed to be through the infection of alveolar-derived monocytes which play a role as a vector for the bacteria [14]. Once at the vessel wall, Cpn can be released from the monocytes and subsequently re-infects the cells in the vicinity. The transfer process between peripheral mononuclear cells and endothelial cells is disturbed by shear stress and thus favors atherogenic prone areas [15]. This may explain why Cpn is not found in healthy tissues and provides potential evidence for the involvement of Cpn in the initiation of atherogenesis. To further support this hypothesis, Cpn-infected endothelial cells display an upregulation of adhesion molecules like vascular cell adhesion molecule (VCAM)-1, intracellular cell adhesion molecule (ICAM)-1 and monocyte chemotactic protein (MCP) -1 [16–20]. These changes resulted in increases in monocyte adhesion and transendothelial migration [16,18]. Chlamydial heat shock protein 60 (cHSP60), which is secreted during Cpn infection, can additionally decrease endothelial nitric oxide synthase expression and activity [21]. Foam cell formation is also induced by infection of macrophages [22–24]. Cpn continues to promote the inflammatory environment by stimulating production of pro-atherogenic cytokines and reactive oxygen species in macrophages, endothelial cells, and vascular smooth muscle cells [21,22,25–30]. Cpn and Cpn-derived components can directly and indirectly stimulate smooth muscle cell proliferation through endothelial cell infection, a key step in plaque formation [31–34]. Cpn may also be involved in atherothrombosis as it has been shown to induce the production of degradative enzymes such as matrix metalloproteases (MMPs) and pro-coagulant factors such as tissue factor (TF) and plasminogen-activating inhibitor (PAI-1) in vascular cells [26,35–37].
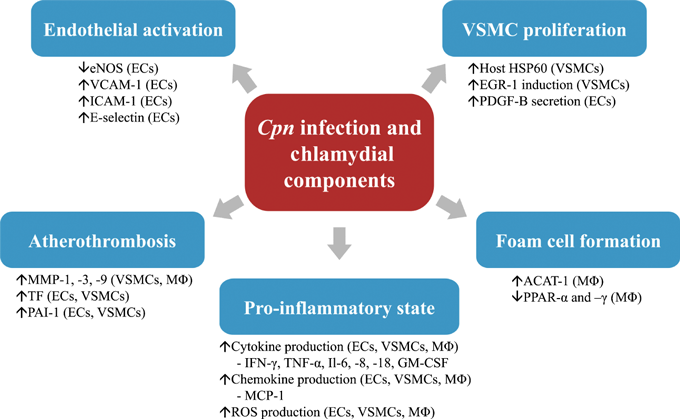
Pathophysiologic mechanisms of Cpn infection in atherosclerosis. Cpn and its components contribute to key steps of atherosclerotic development by eliciting specific cellular responses in endothelial cells (ECs), vascular smooth muscle cells (VSMCs) and Macrophages (MØ). ACAT-1, acyl-coenzyme A: cholesterol acyltransferase 1; EGR-1, early growth response gene 1; eNOS, endothelial nitric oxide synthase; GM-CSF, granulocyte–macrophage colony-stimulating factor; HSP60, heat shock protein 60; ICAM-1, intracellular cell adhesion molecule 1; IFN-γ, interferon gamma; Il-(6,8 and 18), interleukin (6,8 and 18); MCP-1, monocyte chemotactic protein 1; MMP- (1,3 and 9), matrix metalloprotease (1,3 and 9); PAI-1, plasminogen-activating inhibitor 1; PDGF-B, platelet-derived growth factor subunit B; PPAR-(α and –γ), peroxisome proliferator-activated receptor (alpha and gamma); ROS, reactive oxygen species; TF, tissue factor; TNF-α, tumor necrosis factor alpha; VCAM-1, vascular cell adhesion molecule 1.
Numerous animal studies have also produced supportive data for a cause-and-effect role of Cpn in atherosclerosis (Table I). Rabbits fed with a normal diet or an atherogenic diet and then infected with Cpn display significant accelerated lesion development [38–40]. Studies in mice also demonstrate the ability of Cpn infection to accelerate the process; however, this is dependent upon the initiation of the lesion through diet prior to infection [41–44]. Regular mice fed with an atherogenic diet following infection did not exhibit any changes [45]. Furthermore, these changes in both animal models appear to be specific to Cpn, as Chlamydia trachomatis and Mycoplasma pneumoniae infection in mice and rabbits, respectively, were not able to induce these changes [39,41,46]. Further studies have also shown that Cpn infection leads to endothelial dysfunction and neotintima formation [47–49]. Importantly, we have recently demonstrated using a novel ex vivo porcine coronary artery model that Cpn can induce arterial thickening in the absence of a host immune response (2) [50]. These findings again support a causative role for Cpn in the development of atherosclerosis.
| References | Animal Model | Diet | Cpn strain | Inoculation(s) | Effect |
|---|---|---|---|---|---|
| [47] | Apo E−/− Mouse | Regular | IOL-207 | 3 | Endothelial dysfunction |
| [106] | Apo E−/− Mouse | Regular | IOL-207 | 3 | Endothelial dysfunction |
| [107] | Apo E−/− Mouse | Regular | AR-39 | 3 | Accelerates atherosclerosis |
| [108] | Apo E−/− Mouse | Regular | ? | 2 | Accelerates atherosclerosis |
| [109] | Apo E−/− Mouse | Regular | K6 | 2 | No effect |
| [110] | Apo E−/− Mouse | Atherogenic | K7 | 3/4 | No effect |
| [111] | Apo E/LDLr−/− Mouse | Regular | TWAR 2043 | 6 | ↓ Fibrous cap area |
| [44] | LDLr−/− Mouse | Atherogenic | AR-39 | 12 | Accelerates atherosclerosis |
| [41] | LDLr−/− Mouse | Atherogenic | AR-39 | 9 | Accelerates atherosclerosis |
| [41] | LDLr−/− Mouse | Regular | AR-39 | 9 | No effect |
| [112] | BALB/C Mouse | Regular | TWAR | 3 | Inflammatory changes |
| [42] | C57BL/6J Mouse | Regular | AR-39 | 3 | Inflammatory changes |
| [43] | C57BL/6J Mouse | Atherogenic | AR-39 | 3 | Accelerates atherosclerosis |
| [45] | C57BL/6J Mouse | Atherogenic (post-inoculation) | AR-39 | 3 | No effect |
| [38] | NZW Rabbit | Normal | VR1310 | 1 | Lesion development |
| [39] | NZW Rabbit | Normal | VR1310/AR-39 | 1/3 | Lesion development |
| [40] | NZW Rabbit | Atherogenic | VR1310 | 3 | ↑Intimal thickening |
| [113] | Swine | Atherogenic | AR-39 | 1 | Endothelial dysfunction |
| [48] | Swine | Atherogenic | IOL-207 | 1 | Endothelial dysfunction |
| [49] | Swine | Not identified | AR-39 | 3 | Neointima formation |
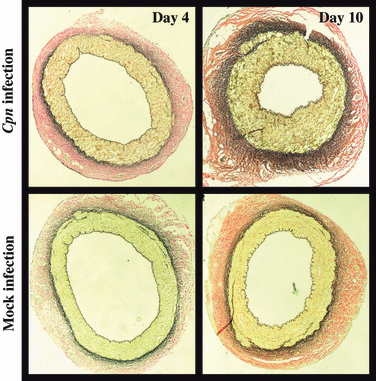
Cpn-induced arterial thickening in isolated coronary arteries. Representative elastic staining of coronary cross-sections from infected (Live Cpn, top panels) and mock infected (Heat-inactivated Cpn, bottom panels) at days 4 and 10 postinfection.
Cpn treatment in cardiovascular disease
Establishing a causative role for Cpn in atherogenesis opens a window of opportunity for the use of antibiotics as a treatment modality for cardiovascular disease. Both respiratory and vascular Cpn strains have displayed in vitro susceptibility to antibiotics like tetracycline, fluoroquinolones and macrolides [51,52]. The newer generation macrolides, azithromycin, clarithromycin and roxithromycin have been the drugs of preference for use in cardiovascular interventions [4]. Azithromycin can be effectively taken up by atherosclerotic plaques [53] and significantly inhibited the Cpn-induced atherosclerotic development in rabbits [40,54]. Retrospective studies in humans examining the incidence of acute myocardial infarction in patients who have been treated with antibiotics in the preceding 3–5 years have delivered opposing conclusions [55,56].
Several randomized antibiotic trials have also been completed (Table II). Gupta et al. performed the first small-scale antibiotic trial on patients with cardiovascular disease [57]. They administered either azithromycin or a placebo to patients after they had suffered a myocardial infarction. The placebo group demonstrated a 4-fold increase in cardiovascular events compared to the azithromycin group and this effect correlated positively with Cpn Immunoglobulin G (IgG) titers [57]. This positive result justified pursuing additional trials. The Azithromycin in Coronary Artery Disease: Elimination of Myocardial Infection with Chlamydia (ACADEMIC) study examined the effects of antibiotic treatment in patients with stable coronary artery disease. Despite extending the duration of the treatment from 3 or 6 days up to 3 months (compared with the previous study), no changes in cardiovascular events were observed. Three subsequent high-powered large-scale trials followed including the Weekly Intervention With Zithromax Against Atherosclerotic-Related Disorders (WIZARD), Clarithromycin for patients with stable coronary heart disease (CLARICOR) and Azithromycin and Coronary Events Study (ACES). The WIZARD study demonstrated a 30% reduction in the incidence of death and myocardial infarction at 6 weeks but these effects could not be sustained throughout the follow-up period, perhaps because of an insufficiently long-treatment period [58]. This question was answered by the ACES study in which a year-long treatment period did not result in any reduction in cardiovascular events [59]. The CLARICOR study confirmed these negative results [60].
| References | Year | Trial | Patient population (n) | Antibiotic(s) | Treatment protocol | Follow-up (months) | Benefit |
|---|---|---|---|---|---|---|---|
| [57] | 1997 | Stable CAD (60) | Azithromycin | 500 mg/day (3 days) or 500 mg/day (2 × 3 days, 3 months apart) | 18 | Yes | |
| [61] | 1999 | ROXIS | ACS (202) | Roxithromycin | 300 mg/day (30 days) | 1 & 6 | Yes |
| [114] | 2000 | ACADEMIC | Stable CAD (302) | Azithromycin | 500 mg/day (3 days) + 500 mg/week (3 months) | 24 | No |
| [62] | 2002 | CLARIFY | ACS (148) | Clarithromycin | 500 mg/day (85 days) | 36 | Yes |
| [63] | 2002 | STAMINA | ACS (325) | Azithromycin or amoxicillin | Azi : 500 mg/day, Amo : 2000 mg/day (1 week) | 12 | Yes |
| [64] | 2003 | ANTI-BIO | ACS (872) | Roxithromycin | 300 mg/day (6 weeks) | 12 | No |
| [64] | 2003 | AZACS | ACS (1439) | Azythromycin | 500 mg (day 1) + 250 mg (days 2-5) | 6 | No |
| [58] | 2003 | WIZARD | Stable CAD (7747) | Azithromycin | 600 mg/day (3 days, week 1) + 600 mg/week (11 weeks) | 30 | No |
| [66] | 2005 | PROVE-IT | ACS (4162) | Gatifloxacin | 400 mg/day (2 weeks) + 400 mg/day (10 days/month, duration of study) | 24 | No |
| [59] | 2005 | ACES | Stable CAD (4012) | Azithromycin | 600 mg/week (1 year) | 46.8 | No |
| [60] | 2006 | CLARICOR | Stable CAD (4373) | Clarithromycin | 500 mg/day (2 weeks) | 36 | No |
- ACS, Acute coronary syndrome; CAD, Coronary artery disease.
A second series of clinical trials performed during this same period investigated the effect of antibiotics on patients with acute coronary syndromes. Initial pilot studies, Roxithromycin in Non-Q Wave Coronary Syndromes (ROXIS), Clarithromycin in Acute Coronary Syndrome Patients in Finland (CLARIFY) and South Thames Trial Of Antibiotics in Myocardial Infarction And Unstable Angina Pectoris (STAMINA) all noted significant reductions in cardiovascular events with macrolide treatment [61–63]. However, just as in the case with patients with stable coronary artery disease, larger scale trials Antibiotic Therapy in Acute Myocardial Infarction (ANTIBIO), Azythromycin in Acute Coronary Syndrome (AZACS), Pravastatin Or Atorvastatin Evaluation and Infection Therapy (PROVE-IT) all concluded that antibiotic treatments were not effective in preventing acute cardiovascular events [64–66].
Taken as a whole, it is possible to conclude from all of these studies that antibiotic treatment has no beneficial effect on cardiovascular outcomes in these disease populations. However, these antibiotics trials do not provide definitive information regarding the role of Cpn in the development of atherosclerosis. It is still not clear whether macrolides, the primary antibiotics used in the trials, are effective in fully eradicating Cpn infection within a vascular environment. Under unfavorable conditions such as nutrient deprivation, heat shock, inflammatory responses, antibiotic treatment, Chlamydia species have the ability to transfer to a nonreplicating metabolically active form known as persistent bodies [67]. This persistent form of infection has been shown to be refractive to azithromycin treatment [68]. Prolonged treatment with azithromycin, clarithromycin and levofloxacin in a continuous infection model was able to reduce but not eliminate Cpn [69]. Furthermore, the high percentage of Cpn detection within the atherosclerotic plaque paired with the difficulty of isolating viable Cpn provides potential evidence for Chlamydial persistence within the diseased vessel wall [4]. Despite the capacity for macrolides to accumulate to effective, relevant concentrations within atherosclerotic plaques, it remains ineffective against the persistent form of Cpn infection. To date, there is no reliable serologic marker for persistent Cpn infection [2]. As such, it is impossible to determine whether these patients did suffer from persistent infection. If they did, this could explain the lack of positive results.
It is also important to note that none of these trials actually addressed the potential causative nature of Cpn infection in atherosclerosis. Patients enrolled in the trials all displayed severe atherosclerotic plaque development and had already suffered an initial major cardiovascular event. This particular target group does not provide any insight into the ability of Cpn to initiate atherogenesis. Even if total eradication of Cpn could be achieved, the damage already inflicted may be too severe from which to recover. In the rabbit models where azithromycin treatment was able to inhibit Cpn-induced atherosclerosis, the antibiotic treatment was given shortly after Cpn inoculation [40,54]. However, this protective role was lost if the treatment regimen was given 6 weeks past inoculation [54]. This suggests that there may be a critical window of time for the initiation of Chlamydial specific antibiotics. However, this becomes very difficult to examine in humans. Initial Cpn infections of the respiratory tract are often asymptomatic and thus are rarely treated unless severe. In addition, continuous long-term antibiotic treatment may induce toxicity and antibiotic resistance.
A preventative chlamydial vaccine approach could, in theory, represent an ideal treatment therapy. However, no effective chlamydial vaccine for humans is currently available and development remains at the experimental stage. The unique nature of the bacterium poses a number of challenges for chlamydial vaccine design. First, protective immunity to Cpn correlates with a cell-mediated immune response, in particular production of CD8+ T cells [70,71]. Additionally, mucosal antibodies also appear to play a role in the protective response [72,73]. This highlights the importance of stimulating a specific adaptive immune response. Second, genetic manipulation of Cpn cannot be performed, which rules out the use of a live attenuated vaccine model. Third, natural infection with the bacterium only confers partial immune protection and re-infection is a common occurrence. Thus, it is imperative that the immune response elicited be strong enough to eliminate infection and be long lasting. As a result, the selection of the immunogen, the formulation of the vaccine, choice of adjuvant, and immunization schedule are all important considerations for an effective chlamydial vaccine. Deoxyribonucleic acid (DNA) vaccines have demonstrated the most promise in animal models. Work using this approach in mice demonstrated that plasmids encoding key chlamydial proteins such as major outer membrane protein (MOMP), outer membrane protein-2 (omp2), cHSP60, and Adenosine diphosphate/Adenosine triphosphate (ADP/ATP) translocase were able to significantly reduce the bacterial load within the lungs [74–77]. A multi-epitope approach directed to CD8+ T-cells has also provided partial protection [78]. A novel Cpn immunogen, LcrE, a structural component of the type 3 secretion (T3S) system has recently been discovered [79]. The DNA vaccine encoding this protein was able to induce CD4+ and CD8+ cell activation, type 1 cytokine secretion and neutralizing antibodies. More importantly, unlike the previous designs, this vaccine was effective in eliminating the infection upon challenge with Cpn. One important limitation in translating this approach to clinical application is that DNA vaccines have not been successful in humans [80]. Although these findings are encouraging, there remains considerable work before a vaccine is ready for human use.
Alternative therapeutic targeting
The ineffectiveness of current antibiotics in effectively treating Cpn infections in atherosclerosis has prompted the need to explore alternative treatment strategies and therapeutic targets. The obligate intracellular nature of Cpn has allowed Cpn to develop effective mechanisms that permit its replication and survival within a host. However, this also affords opportunities to develop specific strategies and novel potential therapeutic targets for the treatment of atherosclerosis (Table III).
| Cpn targets |
| Transporters (ATP/ADP translocase, Na+ transporting proteins) |
| Metabolism machinery (glycolysis enzymes, pentose phosphate enzymes) |
| Secretion apparatus (T3S, T2S) |
| Effector proteins (Cpn 0585, Tarp, CPAF, Ser/Thr protein kinases, cHSP60) |
| Host targets |
| Pro-apoptotic signaling pathways |
| Cell signaling pathways (ERK 1/2, NFkB) |
| Pattern recognition receptors (TLRs, NODs) |
Chlamydial species are characterized by a biphasic life cycle that lasts between 48 and 72 h [81] and involves two morphologically distinct forms of the bacteria, the elementary body (EB) and the reticulate body (RB) [82]. EBs, the metabolically inactive and infective forms, will specifically bind cell-surface receptors and mediate their uptake by rearrangement of the actin cytoskeleton [83]. Upon endocytosis, EBs will differentiate into metabolically active RBs (2 h) within the newly formed chlamydial inclusion. RBs replicate asynchronously via binary fission (12–24 h) before re-differentiation back into EBs (24–48 h). EBs exit the cell through cytolysis or extrusion (48–78 h) [82]. Chlamydial gene expression during this process is temporal and RNA transcripts are organized as early, mid- and late-cycle [84]. These transcripts encode proteins that are directly involved with chlamydial replication processes, which include RNA, DNA and protein synthesis, nutrient translocation, metabolism, and cytokinesis. In addition to this replication machinery, chlamydiae also express genes encoding effector proteins that can modulate host cell functions [85]. Persistent infection may display altered expression patterns for certain genes [86,87], and this may pose additional challenges.
As an intracellular organism, Cpn depends upon host cell energy stores and metabolites to effectively replicate. Chlamydia imports ATP from the host via constitutively expressed ATP/ADP translocases, and glucose is metabolized via glycolysis and pentose phosphate pathway enzymes expressed in RBs [88,89]. Additionally, the Chlamydia genome carries sequences encoding multiple transporters involved in or dependent upon Na+ gradients. Dibrov et al. [90] have suggested that this transport machinery might be important during the late stage replication when conventional cytoplasmic energy stores are depleted. Specific inhibition of these chlamydial transporters and metabolism machinery might prove to be effective in preventing Cpn replication.
Effector proteins are produced by chlamydial RBs and they interact with host machinery involved in invasion, vesicle trafficking, immune/inflammatory response, and cell survival pathways. The inability to perform genetic manipulation in Chlamydiae has presented issues in the identification and characterization of these proteins. However, the combinatory use of comparative genomics, DNA arrays and genome scale expression has allowed for the identification, localization, and function of multiple chlamydial effector proteins. Secretion of these factors is thought to be mediated primarily through the use of a T3S apparatus [91], although type II secretion (T2S) has also been employed [92]. Different chlamydial species including Cpn carry a complete set of genes for the T3S complex and genes encoding T3S system components have been shown to be actively expressed during replication and even persistent infection [93]. The importance of these molecular secretory systems to the action of effector proteins, make them prime inhibitory targets for treatment of both acute and persistent infection. Secreted proteins sharing a large bi-lobal hydrophobic motif are directed to integrate into the inclusion membrane. These proteins are referred to as Inc proteins and appear to be involved in selective vesicular trafficking in chlamydiae. One member of the Inc family, Cpn 0585, has been shown to selectively recruit and interact with multiple host Rab GTPases [94]. Rab GTPases are members of the Ras-like small GTPase family, which is involved in the generation, transport, docking, and fusion of vesicles. This Inc protein was shown to be upregulated during persistent infection [81]. Interestingly, ectopic expression of Cpn 0585 in Cpn-infected cells has been shown recently to disrupt chlamydial replication [94]. As such, competitive inhibition of this Inc protein through the development of Cpn 0585 analogs could prove to be beneficial.
While some effector molecules are destined for the inclusion membranes, others, which include translocated actin recruiting phosphoprotein (Tarp) and chlamydial protease-like activity factor (CPAF), are released into the host cytoplasm. Upon attachment of Cpn to the host cell membrane, Tarp is translocated into the cytoplasm to mediate nucleation of actin filaments which enables chlamydial invasion [95]. Strategies aimed to inhibit the action of Tarp, such as production of anti-Tarp antibodies, represent realistic possibilities to prevent the spread of Cpn infection. The CPAF produced and secreted in the host cytoplasm during Cpn infection cleaves the eukaryotic transcription factor RFX5 responsible for expression of major histocompatibility complex (MHC) [92]. Inhibition of this action would improve host adaptive immunity toward Cpn and could be explored as a strategy to improve chlamydial vaccine efficacy. Other CPAF cleavage targets identified to date in Cpn-infected hosts include various cytoskeleton proteins [96] and hypoxia inducible transcription factor (HIF-1) [97]. Additionally, CPAF expressed during Chlamydia trachomatis infection was shown to cleave pro-apoptotic BH3-only proteins [98]. Cpn infection has also been shown to inhibit apoptosis during both acute and persistent infection [99–101]; however, alternative effector proteins may be involved. Induction of premature apoptosis in Chlamydia trachomatis infected cells can disrupt chlamydial development and form morphologically normal but noninfectious elementary bodies [102]. A treatment combining the inhibition of anti-apoptotic effectors with direct activation of alternative pro-apoptotic pathways could represent a legitimate Cpn eradication strategy. Furthermore, secreted effector proteins may mediate Cpn-induced cell signaling pathways that are involved in proliferation (e.g. ERK1/2) and pro-inflammatory cytokine production (NFkB) during atherosclerosis. This could occur through direct phosphorylation/dephosphorylation of the particular pathway, as Ser/Thr protein kinases and phosphatases are expressed during the Cpn replication cycle [81]. Chlamydial effector proteins may also activate these pathways via binding of extracellular and intracellular pattern recognition receptors (PRRs) that recognize pathogen-associated molecular patterns (PAMPs). Toll-like receptors (TLRs) and cell surface PRRs have been shown to play an important role in the development of atherosclerosis. cHSP60, a stress protein produced and released during both acute and chronic infection, has been shown to stimulate smooth muscle cell proliferation and inflammatory responses through TLR activation [31,103,104]. Chlamydial virulence factors have also been regarded as potential stimulators of nucleotide-binding oligomerization domain (NOD) proteins, intracellular PRRs, which are involved in Cpn-induced endothelial cell activation leading to inflammatory responses [105]. This effector protein–mediated activation of cell signaling pathways could be targeted as a viable treatment for atherosclerosis by directly (binding effector protein) or indirectly(competitive inhibition of effector protein target, inhibition of signaling pathways) inhibiting the action of these effector proteins.
Conclusion
Significant pathologic and experimental data support a role for Cpn in atherosclerotic plaque development. The development of effective therapies for the persistent form of Cpn infection is needed in order to prevent the contribution of Cpn to the disease process. Targeting specific proteins involved in insuring the survival of Cpn during chronic infection may prove to be beneficial. A better understanding of the persistent infection process within the atherosclerotic lesions would be very helpful in achieving these goals.
Sources of support
This work was supported by a grant from the Canadian Institutes for Health Research and with indirect research support from the St. Boniface Hospital and Research Foundation. Justin Deniset received a Studentship from the Manitoba Health Research Council.




