Gender-specific features of plasmatic and circulating cell alterations as risk factors in cardiovascular disease
Themed series on ‘Gender - specific issues in cardiovascular therapy’
Abstract
Cardiovascular disease (CVD) is the leading cause of death in the Western countries. Several epidemiological studies have hypothesized a gender disparity in the pathogenesis and progression of CVD. For instance, women develop CVD when they are about 10 years older than men and, typically, after menopause. However, considering that women are often excluded from research studies, sex differences in CVD remains a frontier for discovery. Very important is thus the identification of risk factors allowing us to diagnose or predict cardiovascular events taking into account gender disparities. In this review, we will examine some of the major challenges in the discovery and validation of cardiovascular biomarkers in a gender perspective. In particular, we will consider classical (hypertension, smoking, diabetes, dyslipidemia, physical inactivity) and novel (inflammation markers, markers of endothelial dysfunction, markers of coronary disease) risk factors reporting gender differences. The aim of this review was to provide an overview on current knowledge on sex-associated cardiovascular determinants with the aim to improve CVD diagnostic and prognostic clinical courses and to develop new and gender-biased prevention strategies.
Introduction
Cardiovascular disease (CVD) is the number one cause of death in industrialized countries. Several epidemiological studies, the Framingham in particular [1], have investigated into the evolution of CVD hypothesizing the presence of a gender difference in the pathogenetic and progression determinants detectable in men and women. For instance, women develop CVD when they are about 10 years older than men and typically after the menopause. Thus, premenopausal women have a lower risk of CVD than age-matched men [2]. Indeed, female hormones, especially estrogen, may be implicated in the mechanisms underlying this gender disparity. In fact, they play an important role in protecting the vascular system, i.e., enhancing endothelial function and vasodilation, inhibiting vascular smooth muscle (VSM) cell proliferation, and improving HDL and LDL cholesterol levels [2]. However, identification of risk factors is crucial in determining cardiovascular events in the two sexes [3]. Particularly important is the control of hypertension, lipids, and other factors as central obesity and impaired glucose regulation that, contributing to the metabolic syndrome, atherosclerotic disease, and type 2 diabetes, increase the risk of coronary and cardiovascular mortality. Marked gender differences have been identified in the clinical manifestations of atherosclerosis and in the pattern of symptoms in the two sexes. In men, cholesterol is more important than in women, in whom arterial hypertension, diabetes, and their combination has a greater importance in determining cardiovascular risk. In women, the control of blood pressure and glucose metabolism should be a priority. Angina, the most common manifestation of coronary heart disease (CHD), is frequently uncomplicated in women, whereas in men, it tends to evolve to an acute coronary syndrome. The clinical presentation of acute ischemic syndromes is also different in men and women and, because of the frequent atypical symptoms, women tend to underestimate the importance of them. In this review, in addition to well-established risk factors for CVD, we will examine some of the major challenges in the discovery and validation of cardiovascular biomarkers.
In particular, gender-specific aspects of plasmatic and circulating cells alterations will be considered.
Biomarkers in Cardiovascular Disease
In the cardiovascular literature, the term biomarker has generally referred to molecules circulating in the blood or urine. A recent National Institute of Health working group defines a biomarker as a characteristic that is objectively measured and evaluated as an indicator of normal biologic processes, pathogenic processes or pharmacologic responses to a therapeutic intervention [4]. The Framingham Risk Score, the most commonly used model for estimating risk of CVD, lists eight risk factors considered classical risk factors. Among these factors, some such as hypertension, diabetes, abnormal cholesterol, smoking, physical inactivity, and obesity are considered modifiable; others, such as age, family history, and gender are considered nonmodifiable (Table I). Moreover, an increasing number of novel biomarkers, particularly inflammatory markers and markers of atherosclerotic burden, have been added to the classical risk factors [5] (Table II). These novel biomarkers include molecules circulating in the blood or urine that can be used for both the diagnosis and the prevention of CVD. These molecules can be classified on the basis of their function (e.g., marker of exposition, markers of effects, etc.) or in their biochemical or biologic properties (e.g., proteins metabolites, hormones, cytokines, etc.). A lot of these classical or novel biomarkers have been considered risk factor gender dependent (Table III).
| Modifiable | Nonmodifiable |
|---|---|
| Hypertension | Age |
| Diabetes | Family history |
| Dyslipidemia | Gender |
| Smoking | |
| Physical inactivity | |
| Obesity |
| Inflammation markers | Endothelial dysfunction markers | Coronary disease markers |
|---|---|---|
| CRP | sICAM | NBP |
| sCD40L | sVCAM | |
| IL-18 | E-selectin | |
| MCP-1 | TSP-1 | |
| Fibrinogen | P-selectin | |
| ADMA | ||
| Ox-LDL | ||
| Annexin V | ||
| EMPs |
- ADMA, asymmetric dimethylarginine; CRP, C-reactive protein; EMP, endothelial microparticle.
| Risk factors in men | Risk factors in women |
|---|---|
| Dyslipidemia | Hypertension |
| IL-18 | Diabetes |
| MCP-1 | CRP |
| sCD40 | |
| Fibrinogen | |
| sICAM | |
| ADMA | |
| BNP |
- ADMA, asymmetric dimethylarginine; BNP, brain natriuretic peptide; CRP, C-reactive protein.
Classical Risk Factors
Hypertension
The single most important classical risk factor for CVD is hypertension [6]. In the adult population, hypertension is the most prevalent chronic disorder [7]. It is more common in younger men than women, but this trend is inverted at approximately 60 years of age thereafter hypertension is more common in women. Menopause’s contribution to this phenomenon is complex. Estrogen deficiency after menopause precipitates a number of factors, and these have established the ‘menopausal metabolic syndrome’ as a concept in postmenopausal women [8]. However, studies have indicated that changes in the prevalence of hypertension, and overall cardiovascular risk profiles in postmenopausal women, might be because of aging and not estrogen deficiency. Undoubtedly, there is a strong multicolinearity between the two phenomena. Furthermore, hormone replacement therapy (HRT) may reduce age-induced blood pressure increases, thus decreasing cardiovascular risks. However, recent results have questioned HRT’s role in CVD prevention in postmenopausal women and trials have unequivocally shown that CVD risk in postmenopausal women with hypertension can effectively be reduced by common antihypertensive drugs.
Diabetes
Altered glucose metabolism and diabetes mellitus are important risk factors for the development of CVD. The prevalence of diabetes increases sharply with increasing age and is higher in older women than in older men [9]. High testosterone levels in women increase the likelihood of diabetes, whereas the risk is lowered in men [10]. Also, women with gestational diabetes are more likely to develop diabetes in later life [11]. Moreover, the European Heart Survey of Acute Coronary Events found that women with diabetes are more likely to have ST-segment elevation myocardial infarction than other women presenting with acute coronary symptoms and has a high incidence of hospital mortality [12]. Although the EUROASPIRE study based on data from 4437 patients with CHD shows that the prevalence of known diabetes, newly diagnosed diabetes, or impaired fasting glucose is similar in men (46%) and women (47%), [13], the relative risk of death from CHD and nonfatal myocardial infarction attributable to diabetes is greater in women. A recent meta-analysis of 22 studies found that the relative risk for fatal CHD associated with diabetes is 50% higher in women [14]. Thus, in women diabetes, together with hypertension, are the two most important cardiovascular risk factors especially when they occur in association [15].
Dyslipidemia
Serum cholesterol is a significant risk factor for myocardial infarction for both men and women and increases with age [16]. In fact, several studies have shown that in men total and LDL cholesterol are higher until 5th decade of life and that the decrease of testosterone levels occurring with age is associated with a more unfavorable lipid profile. Similarly, in women, the menopause is associated with an increase in plasma triglycerides, total and LDL cholesterol, lipoprotein A and a decline of HDL levels [17,18]. In the PROCAM study [19], it has been demonstrated that hypertriglyceridemia: (i) is much more common among men (18.6%) than women (4.2%), (ii) increases with age in women, and (iii) remains nearly constant at about 20% in men aged 35 years or more. Moreover, the data obtained by a longitudinal analysis of 4474 male PROCAM participants (aged 40–64 years) with a follow-up of 4 years suggest that hypertriglyceridemia is an additional risk factor for CHD, when excessive triglycerides coincide with a high ratio of plasma cholesterol and with low HDL-cholesterol values [19].
Smoking
Cigarette smoking is a major modifiable risk factor for CVD, including coronary artery disease (CAD), stroke, peripheral vascular disease and thrombosis [20].
The increased risk of thrombogenesis associated with smoking appears to be affected through increased platelet aggregation and degenerative changes in the vascular endothelium [21,22]. The magnitude of risk increases with an increase in smoking intensity and results greater for women than for men. The increased risk in the women has been associated with an increased platelet aggregation and degenerative changes in the vascular endothelium [23,24].
Physical inactivity
A sedentary lifestyle is considered by various national and international organizations to be one of the most important modifiable risk factors for cardiovascular morbidity and mortality. Well-designed clinical investigations, supported by basic animal studies, have demonstrated that the beneficial effects of exercise are related to direct and indirect protective mechanisms. These benefits may result from an improvement in cardiovascular risk factors, enhanced fibrinolysis, improved endothelial function, decreased sympathetic tone and other as-yet-undetermined factors [25]. However, gender disparity has not yet been considered by literature on this argument.
Novel Risk Factors
Inflammation markers
Over the past few years, it has become increasingly clear that inflammation is intimately related to the pathogenesis of atherosclerosis and its complications [26,27]. C-reactive protein (CRP), soluble CD40 ligand (sCD40L), IL-18, monocyte chemotactic protein-1 (MCP-1), and fibrinogen are inflammatory markers that result in endothelial activation. CRP is considered as an independent predictive factor of cardiovascular events and a possible mediator of atherogenesis. Accumulating evidence suggests that high CRP levels (greater than or equal to 3.0 mg/L) in the plasma are one of the most powerful predictors of atherosclerosis and vascular death [28] and have been associated with risk of acute myocardial infarction, angina, and stroke [29,30]. The mechanistic basis of the predictive value of CRP may be its ability to incite endothelial dysfunction. In this vein, recent studies demonstrate that CRP potently downregulates endothelial NO synthase (eNOS) transcription in endothelial cells (ECs) and destabilizes eNOS mRNA, with resultant decreases in both basal and stimulated NO release [31]. Preliminary observations also suggest that CRP upregulates nuclear factor kB (NF-kB) signaling in ECs while attenuates endothelial progenitor cell survival and differentiation. Recently, CRP has been demonstrated to potently upregulate angiotensin-type 1 receptor (AT1-R) in vascular SMCs in vivo and in vitro, augmenting VSM proliferation, migration, reactive oxygen species (ROS) production, and restenosis. Thus, CRP is not only an inflammatory marker of atherosclerosis/coronary events but is also a mediator of the disease because it contributes to the substrate underlying lesion formation, plaque rupture, and coronary thrombosis via interacting with, and altering, the EC phenotype. Whether CRP values differ between men and women or increase with age has still not been defined, and the literature data are contradictory. In fact, some studies affirm that CRP levels increase with age and that the values are higher in women but not in men [32], by contrast, a study on the Japanese population indicates that the CRP values are higher in men compared to women [33]. Moreover, a significant association of high CRP levels with abnormal glucose metabolism has been found in women than men [34].
CD40L is a protein of the tumor necrosis factor family involved in the pathogenesis of atherosclerosis via its inflammatory and prothrombotic properties [35]. Soluble form of CD40L is released in the peripheral blood from ECs, macrophages, or activated T lymphocytes, and platelets. Attention has recently been given to the clinical validity of soluble CD40L (sCD40L), which derives prevalently from platelet activation [35].
Notably, sCD40L highly correlates with instable plaque and may be an important predictor of plaque instability and eventually plaque complication [36]. Observational and perspective studies supported this view by showing that sCD40L is a predictor of cardiovascular events including myocardial infarction and stroke [37,38]. Elevated plasma concentrations of this protein have been reported in individuals with unstable angina and in patients with moderate hypercholesterolemia, as well as in individuals with type 1 or 2 diabetes [39–41]. Moreover, an association between high levels of sCD40L and CRP with microalbuminuria has been found in hypertensive premenopausal women and not in men and postmenopausal women [42].
IL-18, a member of the IL-1 cytokine family, is highly expressed in atherosclerotic plaques, when compared with normal arteries, and is localized mainly in plaque macrophages [43]. High levels of IL-18 mRNA have been found in unstable plaques, and serum levels of IL-18 have been determined to be greater in patients who have suffered a myocardial infarction or who experience unstable angina [44]. Animal models support the proatherogenic role of IL-18 and the beneficial effect of its inhibition on plaque progression [45]. It also promotes adhesion molecule expression on the endothelium and promotes plaque instability by enhancing matrix metalloproteinases secretion [46]. In a well-characterized cohort of young postmyocardial infarction patients, the IL-18 concentration has been found higher in men (in both patients and controls) [47].
Monocyte chemo-attractant protein-1 (MCP-1) is a chemokine responsible for the recruitment of monocytes to sites of inflammation that appears to play a critical role in the promotion of plaque instability [48]. Cardiovascular cells, including ECs, VSMCs (vascular SMCs) and cardiac myocytes, can produce MCP-1 in response to a variety of stimuli, and its expression has been identified in advanced murine and human atheroma, which, by triggering and sustaining leukocyte accumulation, may in turn promote chronic inflammation [49]. Different levels of MCP-1 have been found in men than women. In particular, it has been shown that the subjects with high estrogen status have significantly lower plasma MCP-1 levels than subjects with low estrogen status [50].
Fibrinogen, a glycoprotein mainly synthesized in hepatic cells and in megakaryocytes, can bind to GpIIB/IIIa surface proteins creating bridges between platelets. In addition, being involved in the coagulation cascade, fibrinogen stimulates smooth muscle cell migration, promotes platelet aggregation, and increases blood viscosity [51]. Fibrinogen is associated with atherosclerosis and thrombosis and is considered an independent risk factor for CHD, myocardial infarction, and stroke. Moreover, it has been reported that (i) fibrinogen levels of women are generally higher than those of men of the same age; and (ii) in women plasma fibrinogen rises with menopause [52].
Soluble molecules as markers of endothelial dysfunction
An altered endothelial function has been found in patients with established CVD as well as in those with increased cardiovascular risk [53]. Moreover, an important concept has been well established and refined in the course of years: a key role of cell-to-cell adhesion and aggregation as a general mechanism involved in the promotion of vascular occlusion. The rationale for this concept is based on the observation that an increase of cell–cell aggregation, including an increased ability to adhere to the ECs, can be detected in circulating cells in the atherosclerotic disease [54]. In few words, both homotypic and heterotypic cell–cell interactions play a key role in the onset or progression of plaque formation. Several cell types have been implicated. Some are well-known actors in this scenario (platelets), whereas others are new. For instance, erythrocyte aggregation and increased adhesiveness have been shown to occur during unfavorable conditions, e.g., in terms of atherosclerotic biorheology, and have been associated with increased concentrations of fibrinogen during hypertension, diabetes, and inflammation [55,56]. Moreover, it has been hypothesized that if erythrocyte undergoes changes in its redox state, it becomes a source of reactive oxygen species and, consequently, increases its aggregability and adhesiveness to the endothelium and to other blood cells (for example platelets) thus, contributing to vascular damage [57].
On the basis of this scenario, it has been hypothesized that some soluble forms of adhesion molecules play an important role in the pathogenesis of EC injury and progressive formation of atherosclerotic lesions [58]. Among these molecules, soluble intercellular adhesion molecule-1 (sICAM-1), soluble vascular cell adhesion molecule-1 (sVCAM-1), E-selectin, thrombospondin-1 (TSP-1), and P-selectin have been demonstrated to promote the adherence of monocytes and lymphocytes to ECs contributing to atheroma formation [46]. sICAM-1 and sVCAM-1, released in the blood from ECs, have been considered as inflammatory markers associated with the prediction of death, particularly CVD-related death. Moreover, the sICAM-1 has been associated with increased risk of CVD-associated death in women but not in men [59]. TSP-1 and P-selectin are proteins released in abundance from activated platelets and accumulated in sites of vascular injury. TSP-1 induces the expression of VCAM-1 and ICAM-1 on endothelium resulting in a significant increase of monocyte attachment [60]. P-selectin (CD62P) mediates the rolling of blood cells on the surface of the endothelium and initiates the attachment of leukocytes circulating in the blood to platelets, ECs, and other leukocytes at sites of tissue injury and inflammation. The levels of soluble P-selectin have been found higher in the hypertensive patients, and significantly higher in the hypertensive patients with diabetes [61]. In literature, there are still few studies on the role of P-selectin in CVD in relation to gender disparity. Moreover, in patients with metabolic syndrome, higher levels of P-selectin have been detected in plasma from men with respect to women [62].
Besides adhesion molecules, molecules as asymmetric dimethylarginine (ADMA), oxidized low-density lipoprotein (ox-LDL), Annexin V, and circulating endothelial microparticles (EMPs) have been considered markers of endothelial damage. ADMA, an endogenous competitive nitric oxide (NO)-syntase inhibitor, is constantly produced in the course of normal protein turnover in many tissues, including vascular ECs, and is derived from the hydrolysis of methylated proteins. Increased plasma levels of ADMA induce dysfunction of the endothelium, which becomes clinically evident by impaired endothelium-dependent vasodilation, hyperaggregability of platelets, and enhanced monocyte adhesion [63,64]. In a study on hypercholesterolemic patients, it has been demonstrated that ADMA alters the adhesive behavior of circulating mononuclear cells predisposing these individuals to atherosclerosis [65]. Moreover, sex-dependent differences have been found in ADMA plasmatic concentration in different age groups. In fact, it has been demonstrated that in women at the onset of menopause, the plasma levels of ADMA increase significantly with respect to age-matched men [66].
Recently, oxidized low-density lipoprotein (ox-LDL), a marker of oxidative stress, has been proposed as marker of endothelial dysfunction and atherogenesis. Oxidized LDL may be formed by oxidative processes during migration of the LDL particles in the vessel wall [67]. The plasma levels of ox-LDL are related to the presence of angiographically detected complex and thrombotic lesion morphology in patients with unstable angina [68]. Ox-LDL induces atherosclerosis by stimulating monocyte infiltration and smooth muscle cell migration and proliferation. It acts through the lectin-like receptor for ox-LDL (LOX-1), a membrane glycoprotein expressed in ECs, macrophages, VSM cells, and platelets [69]. LOX-1 binding to ox-LDL enhances nitric oxide (NO) catabolism as a result of superoxide generation, and decreases NO release via attenuated eNOS activity. LOX-1-mediated NF-kB activation by ox-LDL is crucial for increasing the expressions of the following adhesion molecules: E- and P-selectins, intracellular adhesion molecule-1 (ICAM-1), vascular cell adhesion molecule-1 (VCAM-1), and monocyte chemotactic protein-1 (MCP-1), which brings proinflammatory changes to the vessel wall [70]. Moreover, increasing levels of ox-LDL seem to be preferentially associated with loss of systolic function in men (with less impairment of diastolic function). By contrast, in women, systolic function remains better preserved but with decreasing diastolic function [71].
In agreement with an accumulation of ox-LDL and elevated inflammatory state, high amounts of Annexin V has been found in atherosclerotic plaques. Annexin V, a calcium-dependent protein, is widely distributed in human tissues and released upon injury [72]. The expression of Annexin V has been found abnormally increased in the myocardium of hypertensive patients with left ventricular hypertrophy exhibiting increased cardiomyocyte apoptosis. Moreover, Annexin V is known to bind to negatively charged phospholipids exposed by activated and/or apoptotic cells as well as ox-LDL [73]. Considering that atherosclerotic plaques contain high amount of ox-LDL and high amounts of Annexin V, the ox-LDL/Annexin V ratio has been considered a better marker of the severity of coronary stenosis than ox-LDL alone [73].
Endothelial microparticles are small membrane-shed vesicles generated from EC surfaces in response to cellular activation or injury/apoptosis and can potentially reflect endothelial dysfunction [74]. Although the mechanisms leading to their in vivo formation remain obscure, the release of EMPs from cultured cells can be caused in vitro by a number of cytokines and apoptotic stimuli. Circulating EMPs have procoagulant properties that rely on the exposure of phosphatidylserine (PS) and on the possible presence of tissue factor, the main initiator of blood coagulation. Recent studies indicate that EMPs are able to decrease nitric oxide–dependent vasodilation, increase arterial stiffness, promote inflammation, and initiate thrombosis at their phosphatidylserine-rich membrane, which highly co-expresses tissue factor [75]. Regarding to plasmatic Annexin V and EMPs, no gender-associated differences have been proved so far. Molecules involved in endothelial dysfunction have been reported in 1.
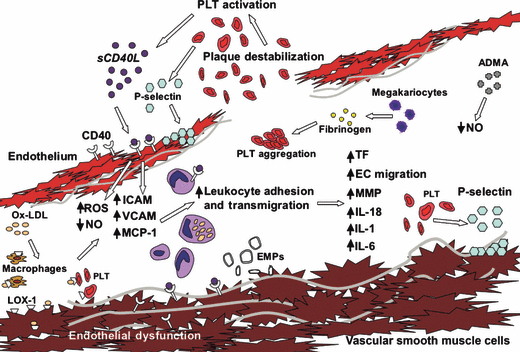
Molecules involved in endothelial dysfunction. When sCD40L engages CD40 on endothelial cells (ECs), signaling results in production of ROS, which antagonize NO synthesis, and promote endothelial dysfunction. This signaling also results in upregulation of cellular adhesion molecules and secretion of chemokines that promote leukocyte recruitment. Both macrophages and T cells express CD40 and CD40L and can further activate both ECs and SMCs, leading to production of endothelial microparticles and inhibiting EC migration. Plaque thrombogenicity is further enhanced via CD40/CD40L–mediated tissue factor (TF) expression, which in turn activates platelets (PLT). The activated PLT generate more sCD40L, reinforcing the inflammatory reaction. When ox-LDL binds LOX-1, which is expressed on PLT, macrophages and ECs, a secretion of chemokines occurs. An increased asymmetric dimethylarginine secretion causes endothelial NO synthase inhibition reducing NO levels. This drawing has already been published by Szmitko et al. [45] and modified by the authors.
Markers of acute coronary syndrome and coronary disease
Recently, brain natriuretic peptide (BNP) has been validated for diagnosis and prognosis of acute coronary syndrome and coronary disease [76]. BNP, a member of the natriuretic peptide family, is released from cardiac myocytes in response to increased stretch resulting from high filling pressure, high arterial pressure or cardiac dilatation [77,78]. Physiological actions of BNP reduce the adverse stimulus of stretch by causing both arterial and veno-dilatation and reduction in blood volume through natriuresis, and suppression of secretion of renin and aldosterone [79]. Because synthesis of BNP requires activated gene transcription, concentrations of BNP may be stable in the blood for about 3 days. Therefore, the long half-life of BNP, the duration of the signal in the blood and established cause and effect relationship between stimulus and release of BNP make it an ideal candidate as a biomarker of heart failure. Moreover, it has been shown that plasma concentrations of BNP increases with age (45–85 years) and both median and 95th percentiles were significantly higher in women compared to men [80]. In the Framingham Heart Study (911 subjects, mean age 55 years with 62% women), plasma concentrations of BNP have been found to vary by age and sex [81]. Moreover, it has been reported that plasmatic BNP level influences left ventricular remodeling, a potent predictor of cardiovascular mortality more prominent in asymptomatic men [82].
Conclusions
Data from epidemiologic studies indicated that current biomarkers can provide a modest utility for predicting risks in ambulatory individuals, highlighting the need to identify additional biomarkers from different biologic pathways. A recommendation from the WHO also underlined the importance of gender disparity in this respect. During the past years, several new putative cardiovascular biomarkers have thus been identified or proposed to the attention of the clinicians. These biomarkers could help to identify individuals of both sexes at risk for CVD, could be used in primary prevention in the two sexes but, in addition, they could also help in cardiovascular complications because of drug treatments. For example, cardiovascular toxicity associated with cancer chemotherapy represents one of the most important challenges in the clinical management of oncological patients and the search for more accurate risk assessment markers is mandatory and critical for the improvement of novel therapeutic strategies. Hence, in this context, the most important goal of future studies should be to better analyze gender disparity and to point out possible gender-associated biomarkers providing differential diagnostic and therapeutic courses for men and women.




