Modifications in DARPP-32 phosphorylation pattern after repeated palatable food consumption undergo rapid habituation in the nucleus accumbens shell of non-food-deprived rats
Abstract
In non-food-deprived rats a palatable meal induces a transient increase in dopamine output in the prefrontal cortex and nucleus accumbens shell and core; habituation to this response develops with a second palatable meal, selectively in the shell, unless animals are food-deprived. A palatable meal also induces time-dependent modifications in the dopamine and cAMP-regulated phosphoprotein of Mr 32 000 (DARPP-32) phosphorylation pattern that are prevented when SCH 23390, a selective dopamine D1 receptor antagonist, is administered shortly after the meal. This study investigated whether dopaminergic habituation in the shell had a counterpart in DARPP-32 phosphorylation changes. In non-food-deprived rats, two consecutive palatable meals were followed by similar sequences of modifications in DARPP-32 phosphorylation levels in the prefrontal cortex and nucleus accumbens core, while changes after the second meal were blunted in the shell. In food-deprived rats two consecutive meals also induced similar phosphorylation changes in the shell. Finally, SCH 23390 administered shortly after the first palatable meal in non-food-deprived rats inhibited DARPP-32 phosphorylation changes in response to the first meal, and prevented the habituation to a second meal in terms of dopaminergic response and DARPP-32 phosphorylation changes. Thus, dopamine D1 receptor stimulation plays a role in the development of habituation.
Abbreviations used:
-
- AMPA
-
- α-amino-3-hydroxy-5-methylisoxazole-4-propionate
-
- DARPP-32
-
- dopamine and cAMP-regulated phosphoprotein of Mr 32 kDa
-
- mPFC
-
- medial prefrontal cortex
-
- NAc
-
- nucleus accumbens
-
- NAcC
-
- NAc core portion
-
- NAcS
-
- NAc shell portion
-
- PKA
-
- cAMP-dependent protein kinase
-
- VS
-
- vanilla sugar
Transient increases in extraneuronal dopamine levels in the nucleus accumbens (NAc) signal the emotional salience of a perceived stimulus, independently of its actual positive (rewarding) or negative (aversive) motivational valence (Kalivas and Duffy 1995; Horvitz 2000). The consumption of a palatable food induces in rats an increase in dopamine output in discrete limbic areas, such as the medial prefrontal cortex (mPFC) and NAc in its shell (NAcS) and core (NAcC) portions (Bassareo and Di Chiara 1997; Bassareo et al. 2002). When rats are food-deprived, a second palatable meal produces a dopaminergic response of the same intensity of the first in the three areas (Bassareo and Di Chiara 1999), whereas rapid habituation to a second palatable meal selectively develops in the NAcS of non-food-deprived rats (Bassareo and Di Chiara 1997; Bassareo et al. 2002). Thus, in non-food-deprived rats the NAcS dopaminergic response to a palatable food prevalently signals the novelty component of its emotional value, while in food-deprived animals it mainly reflects the drive state of hunger.
The dopaminergic response to a first palatable meal is associated with consistent modifications in the phosphorylation pattern of some cAMP-dependent protein kinase (PKA) substrates (Rauggi et al. 2005). In particular, an increase in the phosphorylation levels of Thr34 dopamine and cAMP-regulated phosphoprotein of Mr 32 000 (DARPP-32), GluR1 and NR1 subunits of α-amino-3-hydroxy-5-methylisoxazole-4-propionate (AMPA) and NMDA receptors, respectively, paralleled by a decrease in phospho-Thr75 DARPP-32 levels, is observed 30 min after meal in the mPFC, where phosphorylation levels are back to basal values within 1 h. Similar initial phosphorylation changes are observed in the whole NAc that are then followed by opposite modifications 2–3 h after the meal (Rauggi et al. 2005). This sequence of early and delayed phosphorylation changes in the mPFC and NAc is prevented when SCH 23390, a selective dopamine D1 receptor antagonist, is administered 5 min after palatable food consumption (Rauggi et al. 2005).
The aim of this study was to verify whether rapid habituation of the dopaminergic response to repeated palatable food consumption in the NAcS of non-food-deprived rats had a counterpart in the modifications in dopaminergic signaling, in particular in the DARPP-32 phosphorylation pattern. Thus, we investigated whether the phosphorylation changes observed in the NAcS after two consecutive palatable meals were different from those that occur in the mPFC and NAcC, where no rapid habituation occurs. Moreover, to further clarify the relationship between the phenomenon of habituation in dopamine output and the modifications in the DARPP-32 phosphorylation pattern, the phosphorylation changes induced in the NAcS, NAcC and mPFC by a palatable meal in food-deprived and non-food-deprived rats were compared. Finally, we studied whether administration of SCH 23390 or eticlopride, a selective dopamine D2 receptor antagonist, soon after the first palatable meal prevented the development of habituation in the NAcS. The results obtained indicated that the dopamine D1 receptor signaling cascade plays a role in the development of rapid habituation in the shell.
Materials and methods
Animals
Experiments were carried out on male Sprague–Dawley rats (Charles River, Calco, Italy) that were allowed at least 1 week of habituation to the animal colony and that weighed 200–225 g when the experimental procedures began. Animals were housed five per cage, were kept in an environment maintained at a constant temperature and humidity with free access to food and water, and they were moved to a different cage or apparatus only for the time required for behavioral manipulation. A 12 h reverse light/dark cycle (7:00 am lights off, 7:00 pm lights on) was used. Experiments were carried out from 9:00 am to 5:00 pm under a red light and controlled noise conditions in a testing room separated from and adjacent to the main animal room, under the same conditions of temperature and humidity. The procedures used were in accordance with the European legislation on the use and care of laboratory animals (EEC Council Directive 86/609) and were approved by the University of Siena Ethics Committee. All efforts were made to minimize the number of animals used and their suffering.
Immunoblotting
Rats were killed, their heads were briefly immersed (3–5 s) in liquid nitrogen, and the brains were rapidly removed and cut into 1 mm slices using an ice-cold metal brain matrix (ASI Instruments, Inc., Warren, MI, USA). The mPFC and NAc were quickly dissected out from the slices that had been identified using the Atlas of Rat Brain corresponding to plates 7–9 and 10–12, respectively (Paxinos and Watson 1998). The mPFC (corresponding to the cingulate area, the pre-limbic cortex, and part of the infralimbic area) was dissected out from slice 2, approximately 3.7–2.7 mm from the bregma. The NAc was identified in slice 3, approximately 2.7–1.7 mm from the bregma. The shell and core regions were bilaterally dissected out using a 16 gauge dissection needle and the anterior commissura was the point of reference.
Tissues were flash-frozen in liquid nitrogen and stored at −80°C until assayed. Frozen tissue samples were prepared by solubilization in boiling 1% sodium dodecyl sulfate and 50 mM NaF. Small aliquots of the homogenate were used for protein determination by a modified Lowry protein assay method (DC protein assay, Bio-Rad Laboratories, Hercules, CA, USA). Proteins (30 μg) were separated by electrophoresis on precast 10% Bis–Tris polyacrylamide gels (XT-Criterion, Bio-Rad Laboratories), with XT-MES (Bio-Rad Laboratories) as the running buffer for DARPP-32, and then transferred to nitrocellulose membranes. Immunoblotting was carried out with phosphorylation-state-specific antibodies against phospho-Thr34 DARPP-32 and phospho-Thr75 DARPP-32 (Cell Signaling Technology, Beverly, MA, USA), or antibodies that are not phosphorylation-state-specific against total DARPP-32 (Cell Signaling Technology). Membranes were then incubated with peroxidase-conjugated affinity-purified secondary antibodies (DARPP-32: anti-rabbit, Pierce Biotechnology Inc., Rockford, IL, USA). Antibody binding was detected using a chemiluminescence detection system (Pierce Biotechnology Inc.) and quantified with the Versa Doc 1000 Imaging System (Bio-Rad Laboratories). Samples containing the same amount of total proteins from rats in each experimental group were run on the same immunoblots and then analyzed together. In the regions studied, the total amount of DARPP-32 was unmodified in the different experimental groups compared to the respective control group (data not shown). To control for equal loading, blots were reprobed with the respective non-phosphorylation-state-specific antibody; when a greater than 10% difference in the levels of total DARPP-32 was detected, protein concentrations were determined again and a new immunoblotting experiment was performed. Thus, although levels of phosphorylated proteins were not normalized to the respective total protein levels, only the data obtained with equal protein loading were utilized. For each experiment, values obtained from experimental groups were calculated as the percentage of their respective control values.
Microdialysis procedure
Anaesthetized rats (pentobarbital 50 mg/kg, scopolamine 0.4 mg/kg, i.p.) were placed in a stereotaxic instrument and a concentric vertical probe was lowered into the NAcS (AP +1.7 mm, L ±1.2 mm from bregma, V −8.0 mm from skull surface) (Paxinos and Watson 1998). Microdialysis probes were made from semipermeable dialysis tubing (AN 69, Hospal, Bologna, Italy). The length of the permeable portion of the membrane was 2.0 mm. Probes were implanted in the left or right NAcS, balanced within the groups. After surgery, rats were housed individually in microdialysis boxes (20 × 30 × 30 cm) with a grid floor and an open top. Twenty-four h of recovery and habituation to the chamber were allowed before the beginning of microdialysis. During this period and up to the end of the experiment, water and standard food pellets were always available. On the day of the experiment, Ringer’s solution (147 mM NaCl, 2.2 mM CaCl2, 4 mM KCl) was infused through the probe at a flow rate of 1 μL/min. After a 2 h equilibration period, dialysate samples were collected every 15 min. At least four samples were obtained for the estimation of dopamine basal levels. Samples were immediately analyzed by reverse-phase HPLC with electrochemical detection (ESA Coulochem II with a 5014 A analytical cell, ESA Inc., Chelmsford, MA, USA). The potential of the first electrode was set at +175 mV, and that of the second, the recording electrode, at −175 mV. The mobile phase consisted of an aqueous solution containing: 33 mM NaH2PO4, 0.1 mM Na2EDTA, 1 mM sodium dodecyl sulfate, 20% methanol (vol/vol) and 15% acetonitrile (vol/vol), pH 5.7. A flow-rate of 1.0 mL/min was used. Data were acquired by PC using EZChrom 6.6 software (Scientific Software Inc., San Ramon, CA, USA) and quantified based on peak area by comparison with a standard curve run before and after each experiment. Rats were killed at the end of the experiment to verify probe placement. Microdialysis data were utilized only when the correct location of the probes had been microscopically confirmed on cresyl violet-stained brain sections.
Vanilla sugar pellet preparation
Vanilla sugar (VS) pellets were made daily: standard food pellets (Harlan Italy, S. Pietro al Natisone, Italy) were crushed by mortar and pestle and the fragments were dampened with water and rolled in powdered vanilla sugar (Zucchero Vanigliato, Cannamela S.p.A., S. Lazzaro di Savena, Italy) to obtain regular pellets weighing approximately 150 mg.
Repeated exposure to VS pellets
During the week preceding the exposure to VS, rats were habituated to handling, to the test room and to being housed individually in small cages (20 × 30 × 30 cm) or in microdialysis chambers for 10 min. On the day of the experiment, rats were moved to the test room, individually placed in the cage and after 5 min a small tray was introduced into the cage. Rats in the VS-exposed groups were given five VS pellets, while an empty tray was presented to the control (time 0) groups. All the rats in the VS-exposed groups ate all five VS pellets within 1–3 min. Six hours after the first pellet consumption, groups of rats were given five VS pellets. Rats were killed within 5 min after empty tray presentation (time 0) or 30 min, 1, 2, 6 h after the first VS pellet consumption and 30 min and 2 h after the second VS meal. For microdialysis experiments, sample collection continued after the first and second VS consumptions until dopamine levels returned to baseline values.
Experimental protocols
Experiment 1: Modifications in the phosphorylation levels of DARPP-32 in the NAcS, NAcC and mPFC after repeated VS pellet consumption in non-food-deprived and food-deprived rats
This experiment was carried out in order to evaluate whether the habituation in dopamine output selectively observed in the NAcS in response to the consumption of a second VS meal (Fig. S1) had a counterpart in the VS-induced modifications in the DARPP-32 phosphorylation pattern. Moreover, to further study this possible correlation, the response to repeated VS consumption was examined in food-deprived rats that do not show habituation in dopamine output after a second VS meal (Bassareo and Di Chiara 1999; Fig. S1). Rats were divided into two groups: one group was not food-deprived, while the second was food-deprived for about 16 h before the first VS pellet exposure. Rats in each group were then divided into subgroups: the first subgroups were not exposed to VS pellets (time 0 groups); the other subgroups were exposed to VS pellets once or twice and then killed at different intervals (30 min, 1, 2 or 6 h after the first VS consumption or 30 min or 2 h after the second VS meal).
Experiment 2: Effects of acute administration of the dopamine D1 receptor antagonist SCH 23390 on the modifications of DARPP-32 phosphorylation levels after repeated VS pellet consumption in non-food-deprived rats
SCH 23390, a dopamine D1 receptor antagonist, administered immediately after a VS meal has been shown to prevent the occurrence of both the early and late modifications in the DARPP-32 phosphorylation pattern (Rauggi et al. 2005). This experiment was carried out in order to establish whether SCH 23390 administration would also interfere with rapid habituation. Rats were divided into groups: one group was not exposed to VS pellets (time 0 group); the other groups were exposed to VS pellets once or twice; 5 min after the first VS meal, half of the animals received saline (1 mL/kg) and the other half received SCH 23390 (0.03 mg/kg) i.p. Rats were killed at different intervals after VS pellet consumption (30 min or 2 h after the first VS meal, or 30 min or 2 h after the second). The dose of SCH 23390 was chosen on the basis of previous studies (Gambarana et al. 1995, 1999; Fenu et al. 2001; Rauggi et al. 2005).
Experiment 3: Effects of acute administration of the dopamine D2 receptor antagonist eticlopride on the modifications in DARPP-32 phosphorylation levels after repeated VS pellet consumption in non-food-deprived rats
This experiment was aimed at examining whether dopamine D2 receptor transmission was involved in the modifications of the DARPP-32 phosphorylation pattern after palatable food consumption and/or in the development of rapid habituation. However, as the administration of a selective dopamine D2 receptor antagonist increases extraneuronal dopamine levels in striatal areas (Imperato and Di Chiara 1988; Westerink and de Vries 1989; Rahaman and McBride 2000), the effects of eticlopride administration (0.05 mg/kg, i.p.) on the phosphorylation pattern of DARPP-32 were evaluated in preliminary experiments (Appendix S1). Then, eticlopride (0.05 mg/kg, i.p.) was administered to rats 5 min after the first VS consumption. Rats were divided into groups: one group was not exposed to VS pellets (time 0 group); the other groups were exposed to VS pellets once or twice; 5 min after the first VS meal, half of the animals received saline (1 mL/kg, i.p.) and the other half received eticlopride. Rats were killed at different intervals after VS pellet consumption (30 min or 2 h after the first VS meal, or 30 min or 2 h after the second VS meal). The dose of eticlopride was chosen on the basis of previously published results that demonstrated the lack of relevant modifications of spontaneous behavior after its administration (Scheggi et al. 2000).
Experiment 4: Effects of acute administration of the dopamine D1 receptor antagonist SCH 23390 on the dopaminergic output in the NAcS in response to repeated palatable food consumption in non-food-deprived rats
This experiment was carried out in order to study the role of dopamine D1 receptors in the development of rapid habituation in the NAcS of non-food-deprived rats. Rats were implanted with microdialysis probes and 24 h later they underwent microdialysis. Five min after the first VS pellet consumption rats were injected i.p. with saline (1 mL/kg) or with SCH 23390 (0.03 mg/kg). Six h after the first VS meal, five VS pellets were introduced in the cage and samples were collected.
Drugs
SCH 23390 and eticlopride were dissolved in deionized/distilled water and injected in a volume of 1 mL/kg rat body weight. Control rats received the corresponding dose of saline. Pentobarbital was dissolved in a mixture of 12% ethanol, 38% propylene glycol, 50% deionized/distilled water (vol/vol) and was injected in a volume of 4 mL/kg rat body weight. Scopolamine was dissolved in deionized/distilled water. Pentobarbital was purchased from Sigma Chemical Co. (St. Louis, MO, USA). All other drugs and chemicals were purchased from commercial sources.
Statistical analysis
Statistical analyses were performed on commercially available software (Prism 4.0a, GraphPad Software Inc., San Diego, CA, USA). Western blot data were analyzed using anova and post-hoc analysis was performed by Bonferroni’s test, when p < 0.05, unless otherwise specified. Microdialysis data were analyzed using repeated measures anova (ranova) with dopamine concentration as the between-subject variable and time as the within-subject variable; post-hoc analysis was performed by Bonferroni’s test, when p < 0.05.
Results
Experiment 1: Modifications in the phosphorylation levels of DARPP-32 in the NAcS, NAcC and mPFC after repeated VS pellet consumption in non-food-deprived and food-deprived rats
In the NAcS, analysis of phospho-Thr75 and phospho-Thr34 DARPP-32 levels at time 0 in non-food-deprived and food-deprived rats demonstrated increased phospho-Thr34 DARPP-32 levels in the food-deprived group (phospho-Thr75 DARPP-32 levels, 103.5 ± 5.8%; phospho-Thr34 DARPP-32 levels, 133.9 ± 10.9%, p < 0.01, t-test). In non-food-deprived rats, decreases in phospho-Thr75 DARPP-32 levels and increases in phospho-Thr34 DARPP-32 levels were observed 30 min after the first VS pellet consumption; 1 h after the VS meal the levels of the two phosphorylated forms of DARPP-32 were back to time 0 values, while at 2 h phospho-Thr75 DARPP-32 levels increased and phospho-Thr34 DARPP-32 levels decreased (Fig. 1a and b). As previously shown (Rauggi et al. 2005), 6 h after the palatable meal, the DARPP-32 phosphorylation pattern was back to basal levels and at this time a second VS meal was presented. All rats rapidly ate the five VS pellets. However, phospho-Thr75 and -Thr34 DARPP-32 levels, measured 30 min and 2 h after the second VS consumption, were not significantly different from those at time 0 (Fig. 1a and b). In food-deprived rats phospho-Thr75 DARPP-32 levels were similarly decreased 30 min after the first and second VS meal, whereas they did not increase 2 h after the first and the second VS meal; phospho-Thr34 DARPP-32 levels showed a similar increase 30 min after the first and second VS pellet consumptions and they did not decrease at 2 h (Fig. 1c and d). The phosphorylation levels of the GluR1 and NR1 subunits of AMPA and NMDA receptors were also determined: at time 0 they were similar in food-deprived compared to non-food-deprived rats (phospho-GluR1, 112.7 ± 4.0%; phospho-NR1, 104.6 ± 2.7%); after the first and second VS meal, phospho-GluR1 and phospho-NR1 levels were modified consistently with the early (30 min) changes in phospho-Thr34 DARPP-32 levels (Table S1).
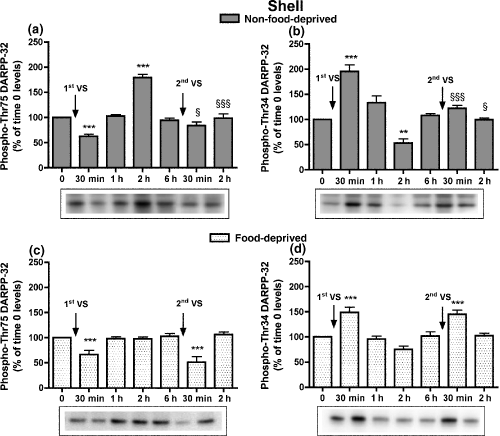
Time-course of the modifications in phospho-Thr75 (a) and phospho-Thr34 DARPP-32 levels (b) in the NAcS of non-food-deprived and food-deprived rats (c, d) after the first and second VS pellet consumptions. Cumulative data (mean ± SEM of percentage modification in phospho-DARPP-32 compared to levels in time 0 group) are shown in graphical format. Representative immunoblots are presented in the lower panels. Rats were killed at different times after the consumption of the first and second VS meals. Rats were exposed for the second time to VS pellets 6 h after the first VS meal; the control group (time 0) was not exposed to VS pellets (n = 6–8 in each group). anova: non-food-deprived rats, phospho-Thr75 DARPP-32, F6,45 = 36.97, p < 0.001; phospho-Thr34 DARPP-32, F6,46 = 26.39, p < 0.001; food-deprived rats, phospho-Thr75 DARPP-32, F6,54 = 10.30, p < 0.001; phospho-Thr34 DARPP-32, F6,55 = 14.57, p < 0.001. Bonferroni’s test: **p < 0.01, ***p < 0.001 versus time 0 group; §p < 0.05, §§§p < 0.001, versus the group killed at the corresponding time after the first VS meal.
In the NAcC, phospho-Thr75 and phospho-Thr34 DARPP-32 levels at time 0 were similar in food-deprived compared to non-food-deprived rats (phospho-Thr75 DARPP-32 levels, 106.6 ± 5.8%; phospho-Thr34 DARPP-32 levels, 102.0 ± 6.4%). In non-food-deprived rats phospho-Thr75 DARPP-32 levels decreased at 30 min and increased at 2 h by the same extent after the first and the second VS meal; consistently, phospho-Thr34 DARPP-32 levels increased 30 min and decreased 2 h after the first and second VS pellet consumptions (Fig. 2a and b). In food-deprived rats after the first and second VS meals, phospho-Thr75 DARPP-32 levels were decreased at 30 min, but were unmodified at 2 h, and phospho-Thr34 DARPP-32 levels were increased at 30 min, but unmodified at 2 h (Fig. 2c and d). In non-food-deprived and food-deprived rats, the modifications in phosphorylation levels of Thr75 and Thr34 DARPP-32 after the first and second VS consumptions were not statistically different. Phospho-GluR1 and -NR1 levels at time 0 were similar in food-deprived versus non-food-deprived rats (103.4 ± 3.1%, and 109.0 ± 7.3%, respectively), and after the first and second VS meals they were modified consistently with the early modifications in phospho-Thr34 DARPP-32 levels (Table S1).
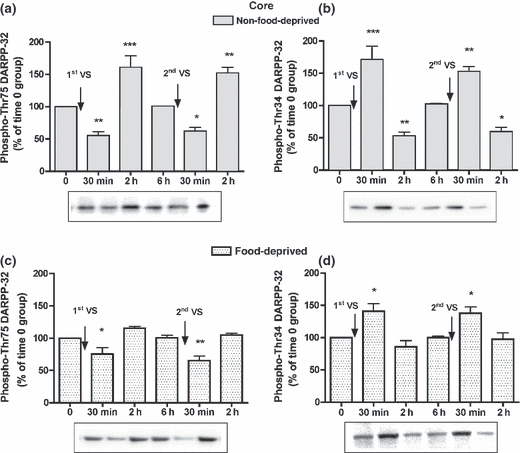
Time-course of the modifications in phospho-Thr75 (a) and phospho-Thr34 DARPP-32 levels (b) in the NAcC of non-food-deprived and food-deprived rats (c, d) after the first and second VS pellet consumptions. Cumulative data (mean ± SEM of percentage modification in phospho-DARPP-32 compared to levels in time 0 group) are shown in graphical format. Representative immunoblots are presented in the lower panels. The control group (time 0) was not exposed to VS pellets (n = 5 in each group). anova: non-food-deprived rats, phospho-Thr75 DARPP-32, F5,30 = 26.05, p < 0.001; phospho-Thr34 DARPP-32, F5,30 = 25.30, p < 0.001; food-deprived rats, phospho-Thr75 DARPP-32, F5,28 = 12.14, p < 0.001; phospho-Thr34 DARPP-32, F5,28 = 8.05, p < 0.001. Bonferroni’s test: *p < 0.05, **p < 0.01, ***p < 0.001 versus time 0 group.
In the mPFC, phospho-Thr75 and phospho-Thr34 DARPP-32 levels at time 0 were similar in non-food-deprived and food-deprived rats (phospho-Thr75 DARPP-32 levels, 104.6 ± 7.2%; phospho-Thr34 DARPP-32 levels, 94.4 ± 6.2%). After the first and the second VS meals, in non-food-deprived and food-deprived rats phospho-Thr75 DARPP-32 levels decreased and phospho-Thr34 DARPP-32 levels increased at 30 min, while at 2 h they were similar to time 0 levels (Fig. 3). Phospho-GluR1 and phospho-NR1 levels at time 0 were similar in food-deprived and non-food-deprived rats (99.5 ± 14.0%, and 102.0 ± 12.4%, respectively), and after the first and second VS meals they were modified consistently with the early modifications in phospho-Thr34 DARPP-32 levels (Table S1).
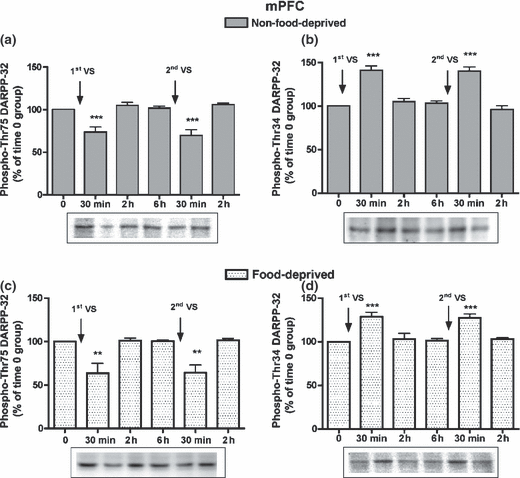
Time-course of the modifications in phospho-Thr75 (a) and phospho-Thr34 DARPP-32 levels (b) in the mPFC of non-food-deprived and food-deprived rats (c, d) after the first and second VS meals. Cumulative data (mean ± SEM of percentage modification in phospho-DARPP-32 compared to levels in time 0 group) are shown in graphical format. Representative immunoblots are presented in the lower panels. The control group (time 0) was not exposed to VS pellets (n = 5–6 in each group). anova: non-food-deprived rats, phospho-Thr75 DARPP-32, F5,34 = 15.23, p < 0.001; phospho-Thr34 DARPP-32, F5,33 = 29.95, p < 0.001; food-deprived rats, phospho-Thr75 DARPP-32, F5,29 = 9.97, p < 0.001; phospho-Thr34 DARPP-32, F5,29 = 11.86, p < 0.001. Bonferroni’s test: **p < 0.01, ***p < 0.001 versus time 0 group.
The results of this experiment showed that in non-food-deprived rats the modifications in DARPP-32, GluR1 and NR1 phosphorylation levels observed in the NAcS after the second VS consumption were significantly reduced compared to those induced by the first VS meal; whereas in the NAcC and mPFC the phosphorylation changes induced by the two consecutive VS meals were similar. Moreover, the changes in phosphorylation levels in the NAcS and NAcC of food-deprived rats were observed only 30 min after VS consumption, while in the mPFC the delayed modifications (2 h) did not develop in non-food-deprived nor in food-deprived rats.
Experiment 2: Effects of acute administration of the dopamine D1 receptor antagonist SCH 23390 on the modifications of DARPP-32 phosphorylation levels after repeated VS pellet consumption in non-food-deprived rats
In the NAcS of saline-treated groups, after the first VS meal phospho-Thr75 DARPP-32 levels decreased and phospho-Thr34 DARPP-32 levels increased at 30 min, while at 2 h phospho-Thr75 DARPP-32 levels increased and phospho-Thr34 DARPP-32 levels decreased (Fig. 4a and b). After the second meal, phospho-Thr75 and phospho-Thr34 DARPP-32 levels were unmodified compared to time 0 values and they were different from levels measured after the first VS meal (Fig. 4a and b). In rats administered SCH 23390, no modifications were observed after the first VS meal, but after the second VS meal phospho-Thr75 DARPP-32 levels decreased at 30 min and phospho-Thr34 DARPP-32 levels increased at 30 min and decreased at 2 h, compared to the levels in the groups killed at time 0 and at corresponding times after the first VS meal (Fig. 4c and d). Phosphorylation levels of GluR1 and NR1 subunits in saline-treated rats were modified as in Experiment 1, and in SCH 23390-treated rats their modifications were consistent with the early changes in phospho-Thr34 DARPP-32 levels (Table S2).
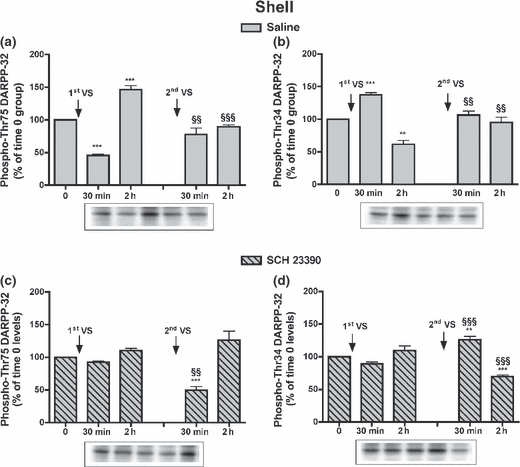
Effect of SCH 23390 administration on phospho-DARPP-32 levels in the NAcS after the first and second VS pellet consumptions: levels of phospho-Thr75 (a) and phospho-Thr34 DARPP-32 (b) in rats receiving saline or SCH23390 (c, d). Cumulative data (mean ± SEM of percentage modification in phospho-DARPP-32 compared to levels in respective time 0 group) are shown in graphical format. Representative immunoblots are presented in the lower panels. Rats received saline (1 mL/kg, i.p.) or SCH 23390 (0.03 mg/kg, i.p.) 5 min after the first VS consumption. After 6 h, rats were exposed for the second time to VS pellets; the control group (time 0) was not exposed to VS pellets (n = 5–7 in each group). anova: saline-treated rats, phospho-Thr75 DARPP-32, F4,26 = 37.68, p < 0.001; phospho-Thr34 DARPP-32, F4,27 = 21.59, p < 0.001; SCH 23390-treated rats, phospho-Thr75 DARPP-32, F4,27 = 16.71, p < 0.001; phospho-Thr34 DARPP-32, F4,27 = 27.60, p < 0.001. Bonferroni’s test: **p < 0.01, ***p < 0.001 versus time 0 group; §§p < 0.01, §§§p < 0.001 versus the group killed at the corresponding time after the first VS meal.
In the NAcC of saline-treated rats the changes in Thr75 and Thr34 DARPP-32 levels were similar to those observed in Experiment 1, although the decreases in phospho-Thr75 (at 30 min) and phospho-Thr34 DARPP-32 levels (at 2 h) in this experiment were not significant (Fig. 5a and b). In SCH 23390-treated groups, phospho-Thr75 DARPP-32 levels were statistically similar to time 0 levels after both VS consumptions, while phospho-Thr34 DARPP-32 levels after the second VS meal increased at 30 min, compared to time 0 levels, and decreased at 2 h, compared to the levels determined 2 h after the first VS meal (Fig. 5c and d). Phospho-GluR1 and -NR1 levels in saline-treated rats were modified as described in Experiment 1, and in SCH 23390-treated rats they were modified consistently with the early modifications in phospho-Thr34 DARPP-32 levels (Table S2).
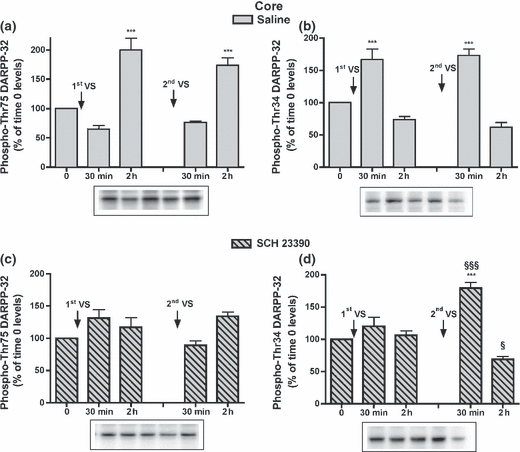
Effect of SCH 23390 administration on phospho-DARPP-32 levels in the NAcC after the first and second VS pellet consumptions: levels of phospho-Thr75 (a) and phospho-Thr34 DARPP-32 (b) in rats receiving saline or SCH 23390 (c, d). Cumulative data (mean ± SEM of percentage modification in phospho-DARPP-32 compared to levels in respective time 0 group) are shown in graphical format. Representative immunoblots are presented in the lower panels (n = 5–7 in each group). anova: saline-treated rats, phospho-Thr75 DARPP-32, F4,25 = 26.85, p < 0.001; phospho-Thr34 DARPP-32, F4,26 = 28.30, p < 0.001; SCH 23390-treated rats, phospho-Thr75 DARPP-32, F4,27 = 4.37, p < 0.01; phospho-Thr34 DARPP-32, F4,27 = 26.13, p < 0.001. Bonferroni’s test: ***p < 0.001 versus the respective control group; §p < 0.05, §§§p < 0.001 versus the group killed at the corresponding time after the first VS meal.
Experiment 3: Effects of acute administration of the dopamine D2 receptor antagonist eticlopride on the modifications of DARPP-32 phosphorylation levels after repeated VS pellet consumption in the NAcS of non-food-deprived rats
Eticlopride administration modified the DARPP-32 phosphorylation pattern in the NAcS and NAcC after 30 min, through a prevalent increase in dopamine D1 receptor stimulation (Experiment S2 in Appendix S1). These results suggested that eticlopride would not represent a confounding factor when administered shortly after VS consumption, as its effect would likely be superimposed over the dopamine D1 receptor stimulation induced by VS meal. Thus, the effects of eticlopride administration on the response of non-food-deprived rats to repeated VS consumption were examined in the NAcS. In the saline-treated groups, phosphorylation changes (anova: phospho-Thr75 DARPP-32, F4,39 = 20.36, p < 0.001; phospho-Thr34 DARPP-32, F4,39 = 15.38, p < 0.001) were similar to those observed in Experiment 1: phospho-Thr75 DARPP-32 levels decreased 30 min and increased 2 h after the first VS meal (57.8 ± 9.1%, p < 0.01 and 160.5 ± 14.2%, p < 0.001, respectively), while they were similar to time 0 levels after the second VS meal (30 min, 94.8 ± 4.1; 2 h, 105.5 ± 5.8); phospho-Thr34 DARPP-32 levels increased 30 min and decreased 2 h after the first VS meal (143.3 ± 12.8, p < 0.001 and 60.4 ± 7.4, p < 0.01, respectively), while they were similar to time 0 levels after the second VS meal (30 min, 98.5 ± 5.1; 2 h, 96.4 ± 6.1). In eticlopride-treated rats, phospho-Thr75 and phospho-Thr34 DARPP-32 levels differed between groups (phospho-Thr75 DARPP-32, F4,39 = 22.54, p < 0.001; phospho-Thr34 DARPP-32, F4,39 = 59.25, p < 0.001). In particular, phospho-Thr75 DARPP-32 levels decreased 30 min and increased 2 h after the first VS meal (56.6 ± 6.9 and 144.9 ± 10.3, respectively, p < 0.001 for both comparisons), while they were similar to time 0 levels after the second VS meal (30 min, 107.5 ± 6.5; 2 h, 105.8 ± 5.2); phospho-Thr34 DARPP-32 levels increased 30 min and decreased 2 h after the first VS meal (194.6 ± 7.2, p < 0.001 and 75.5 ± 9.0, p < 0.05, respectively), and they were similar to time 0 levels after the second VS meal (30 min, 112.3 ± 5.1; 2 h, 103.3 ± 3.9). Thus, the administration of a dopamine D2 receptor antagonist after the first VS consumption did not alter the sequence of modifications in the DARPP-32 phosphorylation pattern; indeed, the VS meal-induced early increase in phospho-Thr34 DARPP-32 levels summed up to that induced by eticlopride (Experiment S2 in Appendix S1). Moreover, eticlopride administration did not interfere with the development of rapid habituation.
Experiment 4: Effects of acute administration of the dopamine D1 receptor antagonist SCH 23390 on the dopaminergic output in the NAcS in response to repeated palatable food consumption in non-food-deprived rats
Dopamine baseline values were assessed in the group assigned to saline administration and in the group assigned to SCH 23390 administration (saline, 7.6 ± 0.7 pg/10 μL; SCH 23390, 8.1 ± 0.9 pg/10 μL); five VS pellets were then placed in the microdialysis cage. Five min after VS consumption rats were administered saline (1 mL/kg) or SCH 23390 (0.03 mg/kg) i.p.; 6 h later a second VS meal was presented. In the saline-treated rats, dopamine levels increased 15, 30, and 45 min after the first VS meal (p < 0.01 for all comparisons) but not after the second (Fig. 6). In the SCH 23390-treated rats, dopamine levels increased 15, 30, and 45 min after the first and the second VS pellet consumption and, in this group, the increases in dopamine levels after the first VS meal were not different from those observed after the second VS meal (Fig. 6). This experiment indicates that the inhibition of dopamine D1 receptors prevented the occurrence of rapid habituation in the dopaminergic response in the NAcS of non-food-deprived rats.
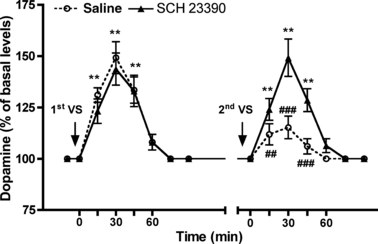
Effect of SCH 23390 administration on NAcS extraneuronal dopamine levels in response to repeated VS pellet consumptions in non-food-deprived rats. Rats naive to VS were implanted with a probe in the NacS. After the assessment of baseline levels, five VS pellets were introduced in the cage; 5 min later saline (1 mL/kg) or SCH 23390 (0.03 mg/kg) were administered i.p. and samples were collected; 6 h later, five VS pellets were introduced in the cage and samples were collected. Values represent the mean ± SEM of percentage of basal dopamine levels (n = 5 in the saline group and 6 in the SCH 23390 group). anova: saline-treated rats, ranova, F12,64 = 20.14, p < 0.001; SCH 23390-treated rats, ranova, F12,77 = 17.04, p < 0.001. Bonferroni’s test: **p < 0.01, versus time 0 levels; ##p < 0.01, ###p < 0.001 versus corresponding time levels after the first VS meal.
Discussion
The present results confirmed that rapid habituation in dopamine output increase in response to a second palatable meal selectively develops in the NAcS of non-food-deprived rats. In the mPFC and NAcC, where no habituation in dopamine output develops (Bassareo and Di Chiara 1997; Bassareo et al. 2002; Gambarana et al. 2003), the phosphorylation changes observed in response to the second VS meal were similar to those observed after the first VS meal. Conversely, in the NAcS the phosphorylation changes induced by the second VS meal were reduced compared to those observed after the first. Thus, in the areas analyzed the modifications in signaling appeared to match the modifications observed in extraneuronal dopamine levels after palatable food consumption, although with a different time scale in the NAc. In fact, a blunted increase in dopamine output in response to a second palatable meal can be already observed 2 h after the first meal in the NAcS (Gambarana et al. 2003); that is, at a time when the levels of the modified phosphorylated species were steadily back to normal values in the mPFC, and the delayed phosphorylation changes in the NAcS and NAcC were reaching their peaks. Thus, in order to avoid confounding overlapping in the neurochemical responses studied, the second VS meal was presented 6 h after the first; that is, 2 h after reaching the signaling basal values in the NAc (Rauggi et al. 2005).
Food-deprived rats did not develop habituation in NAcS dopamine output after a second palatable meal (Fig. S1), in agreement with previous results (Bassareo and Di Chiara 1999). Caloric deprivation and the associated feeling of hunger seem to render the lack of novelty insufficient to reduce the NAcS dopaminergic response to a second VS meal (Bassareo and Di Chiara 1999). In these animals the early modifications in signaling after the first VS meal were similar to those observed in non-food-deprived rats, but the early changes induced by the second VS meal had the same intensity of those observed after the first. Interestingly, no delayed modifications were observed and in the NAcS the time course of VS meal-induced dopaminergic response coincided with that of VS meal-induced phosphorylation changes, as it occurs in the mPFC of non-food-deprived animals.
The NAcS dopaminergic response to palatable food consumption reflects the meal actual emotional value that in non-food-deprived rats has a strong novelty component, whereas in fasted rats it is mainly dependent on food caloric content. This difference is dependent on the internal state of the animal (food-deprived vs. non-food-deprived) that is responsible for the observed different NAcS responses to the same food. Extraneuronal dopamine levels at time 0 and their increase in response to the first VS meal were similar in non-food-deprived and food-deprived rats. However, in food-deprived rats basal phospho-Thr34 DARPP-32 levels were increased, the dopamine D1 receptor-triggered phosphorylation changes were back to basal values at 1 h and the delayed modifications, that are dependent on mGluR5 activity (Rauggi et al. 2005), were absent. Moreover, the emotional value of VS pellets, because of the small caloric content, was unmodified at a second encounter and it prevailed on the lack of novelty, thus preventing the occurrence of habituation. That is, the intensity of food emotional value plays a crucial role in the control of dopamine D1 receptor-dependent signaling events. Finally, in the NAcC of food-deprived rats the phenomenon of habituation in signaling was not observed after two consecutive VS meals and only the early modifications in phosphorylation were present. These data seem to exclude the existence of a functional link between delayed modifications and habituation development, as in the NAcC the lack of habituation was observed in the presence (non-food-deprived rats) or in the absence (food-deprived rats) of late phosphorylation changes.
In order to assess whether a relationship existed between the modifications in dopamine D1 receptor-dependent signaling and the development of habituation in the NAcS of non-food-deprived rats, animals were administered SCH 23390 soon after the first VS meal, a treatment that prevents the occurrence of the entire signaling sequence (Rauggi et al. 2005). These animals, after consuming a second VS meal 6 h later, did not develop habituation in the NAcS dopaminergic response, in terms of dopamine output or signaling modifications. The observed phosphorylation changes mainly consisted in increased Thr34 DARPP-32, GluR1 and NR1 (Table S1) phosphorylation levels that are mediated by the dopamine D1-receptor-adenylyl cyclase-PKA cascade (Greengard 2001). Thus, their presence indicated that 6 h after its administration, the effect of SCH 23390 had completely subsided. The observed VS meal-induced neurochemical modifications after a first VS meal were prevalently dependent on dopamine D1 receptor stimulation, as the administration of eticlopride, a selective dopamine D2 receptor antagonist, 5 min after food ingestion did not influence the sequence of phosphorylation changes. SCH 23390 administration did not interfere with the VS meal-induced increase in dopamine extraneuronal levels in the NAcS; although in this condition the increase in dopamine output could not be associated to signaling modifications. The second VS meal induced an increase in dopamine output similar to that observed after the first VS consumption that was associated with intense signaling modifications.
Dopamine D1 receptors are stimulatory on adenylyl cyclase that, in turn, activates PKA. Thirty min after the VS meal rats showed increased phosphorylation levels of three PKA substrates, the NMDA NR1 subunit at Ser897, the AMPA GluR1 subunit at Ser845, and DARPP-32 at Thr34 in the mPFC, NAcC and NAcS. Phospho-Thr34 DARPP-32 is a potent phosphatase-1 inhibitor; thus, when its levels are increased dopamine D1 receptor-dependent signaling is likely strengthened (Hemmings et al. 1984). Phospho-Thr34 DARPP-32 plays an important role in the dopamine D1 receptor-mediated regulation of other neuronal ion channels and ion pumps such as of N/P-type Ca2+ channels and Na+,K+-ATPase (Fienberg et al. 1998). Moreover, it induces long-term potentiation (Calabresi et al. 2000) and regulation of gene transcription through various signal transduction cascades that modify the phosphorylation of transcription factors (Hyman and Malenka 2001). The fact that rapid habituation is observed within 2 h after the first VS meal (Bassareo and Di Chiara 1997; Gambarana et al. 2003) seems to exclude that long-term changes in synaptic plasticity are involved in this phenomenon. The present data also rule out that the delayed increase in phospho-Thr75 DARPP-32 and the associated late changes may play a role in the development of habituation. Thus, palatable meal-induced dopamine D1 receptor stimulation is involved in triggering the sequence of early and delayed phosphorylation changes (Rauggi et al. 2005), and a strict relationship seems to link the dopamine D1 receptor-dependent signaling and the mechanisms that underpin the development of rapid habituation in the NAcS of non-food-deprived rats. Finally, it is likely that other limbic areas directly connected with the NAcS and involved in encoding the actual emotional value of food, such as the baso-lateral amygdala (Miranda et al. 2003; Phillips et al. 2003), may contribute to the rapid development of habituation.
The presence of rapid habituation in the NAcS and its absence in the mPFC and NAcC are distinct aspects of the same phenomenon, i.e. the switch of a palatable food taste from novelty to familiarity. Further studies on the mechanisms that underpin this switch may help to clarify the implication of increased dopamine output in the limbic areas in response to natural hedonic stimuli. In non-food-deprived rats, the dopaminergic response in the limbic areas to the consumption of a novel palatable food is a crucial indicator of the competence to acquire an appetitive behavior instrumental in earning that food again (Gambarana et al. 2003). In this context, the lack of habituation in the mPFC and NAcC to successive food encounters may play a role in reducing neophobia, and promoting the association between a contingent action and food taste in a goal-directed behavioral procedure (Ghiglieri et al. 1997). Interestingly, after 10 days of training, when rats have acquired the instrumental behavior and the reinforcing value of palatable food is established, no dopaminergic response to VS consumption is present in the mesolimbic areas of trained animals, in terms of dopamine output (Gambarana et al. 2003) and phosphorylation changes (Scheggi, unpublished results). That is, when the reinforcing property of a palatable food has been encoded, in non-food-deprived animals dopamine seems no longer to play a role in signaling its emotional value, as also observed in different learned behaviors (Horvitz et al. 2007).
Acknowledgements
This study was supported by a grant from the Ministero dell’Università e della Ricerca Scientifica (MIUR) and a grant from the University of Siena (PAR). The authors wish to thank Ms. Colleen Pisaneschi for language editing of the manuscript.




