Peroxisomal and mitochondrial status of two murine oligodendrocytic cell lines (158N, 158JP): potential models for the study of peroxisomal disorders associated with dysmyelination processes
Abstract
In some neurodegenerative disorders (leukodystrophies) characterized by myelin alterations, the defect of peroxisomal functions on myelin-producing cells (oligodendrocytes) are poorly understood. The development of in vitro models is fundamental to understanding the physiopathogenesis of these diseases. We characterized two immortalized murine oligodendrocyte cell lines: a normal (158N) and a jimpy (158JP) cell line mutated for the proteolipid protein PLP/DM20. Fluorescence microscopy, flow cytometry, and western blotting analysis allow to identify major myelin proteins (PLP colocalizing with mitochondria; myelin basic protein), oligodendrocyte (CNPase and myelin oligodendrocyte glycoprotein), and peroxisomal markers [adrenoleukodystrophy protein, PMP70, acyl-CoA oxidase 1 (ACOX1), l-peroxisomal bifunctional enzyme, and catalase]. Using electron microscopy, peroxisomes were identified in the two cell lines. Gene expression (ATP-binding cassette, Abcd1, Abcd2, Abcd3, and Acox1) involved in peroxisomal transport or β-oxidation of fatty acids was evaluated using quantitative PCR. 4-phenylbutyrate treatment increases expression of ACOX1, l-peroxisomal bifunctional enzyme, PLP, myelin oligodendrocyte glycoprotein, and CNPase, mainly in 158N cells. In both cell lines, 4-phenylbutyrate-induced ACOX1 and catalase activities while only Abcd2 gene was up-regulated in 158JP. Moreover, the higher mitochondrial activity and content observed in 158JP were associated with higher glutathione content and increased basal production of reactive oxygen species revealing different redox statuses. Altogether, 158N and 158JP cells will permit studying the relationships between peroxisomal defects, mitochondrial activity, and oligodendrocyte functions.
Abbreviations used:
-
- 4-PBA
-
- 4-phenylbutyrate
-
- ΔΨm
-
- mitochondrial potential
-
- ABC
-
- ATP-binding cassette
-
- ACOX1
-
- acyl-CoA oxidase 1
-
- ALD
-
- adrenoleukodystrophy
-
- ALDP
-
- adrenoleukodystrophy protein
-
- Ct
-
- cycle threshold
-
- DHE
-
- dihydroethidium
-
- l-PBE
-
- l-peroxisomal bifunctional enzyme
-
- MBP
-
- myelin basic protein
-
- MCB
-
- monochlorobimane
-
- MOG
-
- myelin oligodendrocyte glycoprotein
-
- NAO
-
- nonylacridine orange
-
- PBS
-
- phosphate-buffered saline
-
- PLP
-
- proteolipid protein
-
- PMP70
-
- peroxisomal membrane protein of 70 kDa
-
- P-NALD
-
- pseudo-neonatal adrenoleukodystrophy
-
- ROS
-
- reactive oxygen species
-
- SDS
-
- sodium dodecyl sulfate
-
- TBST
-
- Tris-buffered saline containing Tween 20
-
- VLCFA
-
- very-long-chain fatty acids
-
- X-ALD
-
- X-linked adrenoleukodystrophy
Peroxisomes are single cell membrane organelles ensuring essential cellular functions, particularly in lipid metabolism (Wanders and Waterham 2006a; Schrader and Fahimi 2008). These organelles are involved in the β-oxidation process of long- and very-long-chain fatty acids (LCFA and VLCFA), branched-chain fatty acids, unsaturated fatty acids, and dicarboxylic acids (Nguyen et al. 2008). They participate in the α-oxidation of phytanic acid, the biosynthesis of bile acids, and the degradation of leukotrienes (Schrader and Fahimi 2008). They are also involved in the synthesis of specific fatty acids, such as docosahexaenoic acid (C22:6 n − 3), which are essential for the brain and the retina, as well as the synthesis of plasmalogens, which play essential roles in the growth of neural cells and are important components of myelin, a complex of proteins and lipids (30% proteins and 70% lipids) (Harauz et al. 2004; Hörster et al. 2005). In the CNS, the myelin sheath is formed by membranes that extend from oligodendrocytes that wrap concentrically around nerve fibers, thereby insulating them and facilitating rapid transmission of nerve impulses (Harauz et al. 2004). Consequently, alteration of peroxisomal functions induces lipid modifications that are detrimental for the development and functions of the nervous system.
Besides peroxisome biogenesis disorders (Schrader and Fahimi 2008), several disorders associated with a single defect in the β-oxidation process have been described. They are characterized by either developmental or degenerative pathologies, in particular in the CNS and PNS (Wanders and Waterham 2006a). These pathologies are usually characterized by a dysmyelination of the white matter belonging to the so-called leukodystrophies and are characterized by the accumulation of VLCFA in plasma and tissues because of an impaired β-oxidation in peroxisomes and/or increased elongation (Wanders and Waterham 2006b). X-linked adrenoleukodystrophy (X-ALD, OMIM 300100), the most frequent leukodystrophy, affects either young boys (cerebral childhood ALD, 40% of the cases) leading to a vegetative state or death, or adults (adrenomyeloneuropathy 50% of the cases). X-ALD is caused by mutations in the ABCD1 gene located in Xq28 (Berger and Gartner 2006; Kemp and Wanders 2007) encoding a peroxisomal ATP-binding cassette (ABC) half-transporter called the adrenoleukodystrophy protein (ALDP), which participates in the entry of VLCFA-CoA into the peroxisome. Cerebral childhood ALD is associated with a strong inflammatory reaction and inducible nitric oxide synthase induction in the CNS white matter (Paintlia et al. 2003). Pseudo-neonatal adrenoleukodystrophy (P-NALD; OMIM 264470), characterized by a generalized hypotonia and severe delayed motor development, is caused by mutations in the gene encoding the peroxisomal straight-chain acyl-CoA oxidase 1 (ACOX1) located in 17q25. P-NALD is associated with an enzymatic deficiency of ACOX1, which catalyzes the first and rate-limiting step of straight-chain fatty acid β-oxidation (Poll-The et al. 1988; Jia et al. 2004).
Understanding the mechanism of dysmyelination in these disorders is a major challenge to further developing efficient treatments and/or improving the quality of life of these patients, particularly X-ALD patients. While the mice models deficient in ALDP or ACOX1 do not mimic the human pathologies as they do not develop alterations in the CNS (Forss-Petter et al. 1997), it has been reported that the selective absence of peroxisomes in oligodendrocytes in CNP-Pex5 knockout mice causes progressive demyelination (Kassmann et al. 2007). Similarly, in Nes-Pex5 knockout mice, a clear reduction in myelin fibers is observed (Hulshagen et al. 2008). Consequently, it is important to clarify the relationships between the peroxisomal deficiencies in oligodendrocytes, the VLCFA accumulation, and the dysmyelination process. Currently, several human and murine oligodendrocytic cell lines have been established, and those expressing major myelin proteins could constitute interesting biological models to study these relationships. However, most of these cell lines [human oligodendrial cell lines myelin oligodendrocyte glycoprotein (MOG), MO3.13, and KG-1C (Buntinx et al. 2003); clones JP1.1 and JP1.2, obtained by immortalization of oligodendrocytes from jimpy mice with the temperature-sensitive Simian Virus 40 (SV-40) large T antigen (Bongarzone et al. 1997); the mouse oligodendrocyte cell lines N20.1 and N19 obtained by immortalization of oligodendrocytes from normal mice (Verity et al. 1993)] present oligodendrocytic precursor phenotypes. Interestingly, two murine oligodendrocytic cell lines, 158N and 158JP, obtained by immortalization with the Simian Virus 40 (SV-40) large T antigen of oligodendrocytes from normal and jimpy mice, respectively, show phenotypes of well-differentiated oligodendrocytes (Feutz et al. 1995, 2001; Ghandour et al. 2002). Jimpy mice are characterized by a single mutation (A-to-G) at the splice acceptor site of exon 5 of the proteolipid protein PLP/DM20 gene, producing a deletion of the entire exon 5 and a translation frame shift of the mRNA, which results in a modified C-terminus in the jimpy PLP and of its isoform DM20 (Macklin et al. 1987). The 158N and 158JP cells express the main markers of well-differentiated oligodendrocytes: carbonic anhydrase II, galactocerebroside, and the major myelin proteins, PLP and the myelin basic protein (MBP) known to account for 50% and 30% of myelin proteins, respectively (Harauz et al. 2004; Taylor et al. 2004). So, these cell lines could be suitable in vitro models to study the side effects resulting from an intracellular accumulation of VLCFA on the synthesis of major myelin proteins.
To define whether these cells can be useful to study the relationships between VLCFA accumulation, peroxisomal activity, and the expression of the major myelin proteins, we characterized their peroxisomal equipment by transmission electronic microscopy and analyzed the expression of oligodendrocytic and peroxisomal markers at the transcriptional and/or translational levels by various methods (fluorescence microscopy, flow cytometry, western blotting, and real-time PCR). We were interested in myelin proteins including PLP and MBP, in markers of differentiated oligodendrocytes (CNPase and MOG), as well as in several peroxisomal markers [ALDP (ABCD1), adrenoleukodystrophy related protein (ALDRP) (ABCD2) and peroxisomal membrane protein of 70 kDa (PMP70) (ABCD3), l-peroxisomal bifunctional enzyme (l-PBE), ACOX1, and catalase]. Catalase and ACOX1 activities were also quantified by spectrophotometry and fluorimetry, respectively. Moreover, we tested the ability of 158N and 158JP cells to respond to 4-phenylbutyrate (4-PBA) known to induce peroxisome proliferation and peroxisomal genes (Gondcaille et al. 2005) and to be efficient in reducing VLCFA accumulation in the brain of Abcd1-null mice (Kemp et al. 1998). In addition, as the mitochondrial and redox status can be modified in the case of ABCD1 deficiency and/or VLCFA accumulation (Fourcade et al. 2008; Hein et al. 2008), mitochondrial activity and content as well as redox status were measured by flow cytometry. Finally, as abnormal PLP expression leading to demyelination can modulate mitochondrial activity (Bongarzone et al. 2001), the interaction of PLP with mitochondria was investigated by laser scanning confocal microscopy.
Materials and methods
Reagents
Antibodies raised against MBP (ab53294), PLP (ab28486), and catalase (ab16771) were purchased from Abcam(Abcam, Paris, France); PMP70 antibodies (71-8300) from Invitrogen (Cergy-Pontoise, France); MOG antibodies (MAB2439) from R&D Systems (Minneapolis, MN, USA); CNPase antibodies (C5922) from Sigma-Aldrich (St Louis, MO, USA). Antibodies against ACOX1 and l-PBE have been described elsewhere (Huin et al. 2002) and were produced in the laboratory by Prof. Cherkaoui-Malki. Antibodies raised against ALDP (serum 1664) were a generous gift from Prof. Aubourg (INSERM, Paris, France) (Fouquet et al. 1997). The following dyes were used: Hoechst 33342 and H2-DCFDA (Sigma-Aldrich); dihydroethidium (DHE), nonylacridine orange (NAO), Mitotracker Red, and 3,3′-dihexyloxacarbocyanine iodide [DiOC6(3)] (Molecular Probes, Eugene, OR, USA/Invitrogen); monochlorobimane (MCB) (Biochemica, St. Louis, MO, USA). The Amplex Red catalase assay kit was from Invitrogen. The 4-PBA used was from Sigma-Aldrich.
Cells, cell cultures, and cell treatments
Murine oligodendrocytic cells 158N and 158JP (Feutz et al. 2001) were seeded at 5000–10 000 cells/cm2 either in 75-cm2 culture flasks or in Petri dishes (100 mm in diameter) in Dulbecco’s modified Eagle’s medium supplemented with 5% (v/v) heat inactivated fetal bovine serum (PAN™ Biotech GmbH, Aidenbach, Germany). Cells were incubated at 37°C in a wet atmosphere containing 5% CO2. The conditions of treatment with 4-PBA were the following. After plating cells in culture flasks for 24 h, cells were treated for 72 h with 2.5 mM of 4-PBA.
Transmission electron microscopy of peroxisomes and mitochondria
Transmission electron microscopy was used to visualize peroxisomes and mitochondria in 158N and 158JP cells cultured in the absence or in the presence of 4-PBA (2.5 mM, 72 h). Hepatic sections of 9- to 10-week-old C57 Black/6 males were used as positive controls for peroxisomal analysis. For peroxisomal localization, cells and tissue sections were prepared as follows (Schrader et al. 1994). The samples were fixed for 1 h at 4°C in 2.5% (w/v) glutaraldehyde diluted in cacodylate buffer (0.1 M, pH 7.4), washed in cacodylate buffer (0.1 M, pH 7.4), incubated in the dark for 1 h at 21°C in Tris–HCl (0.05 M, pH 9.0) containing diaminobenzidine (2.5 mg/mL) and H2O2 (10 μL/mL of a 3% solution), washed in cacodylate buffer (0.1 M, pH 7.4) for 5 min at 21°C, post-fixed in 1% (w/v) osmium tetroxide diluted in cacodylate sodium (0.1 M, pH 7.4) for 1 h at 21°C in the dark, and rinsed in cacodylate buffer (0.1 M, pH 7.4). The preparations were then dehydrated in graded ethanol solutions and embedded in Epon. Ultra-thin sections were cut with an ultramicrotome, contrasted with uranyl acetate and lead citrate, and examined under an H7500 electron microscope (Hitachi, Tokyo, Japan).
Immunofluorescence staining procedures, and antigen analysis by conventional fluorescence microscopy, laser scanning confocal microscopy, and flow cytometry
Immunofluorescence staining was performed on cells seeded at 10 000 cells/cm2 on 12-mm glass coverslips. After 3 days of culture, cells were fixed with 2%p-formaldehyde for 5–15 min at 21°C or with 2.5% glutaraldehyde for 1 h at 4°C, washed with phosphate-buffered saline (PBS), pre-incubated with FACS permeabilizing solution (BD-Biosciences, San Jose, CA, USA) for 5 min at 21°C, and incubated with blocking buffer (PBS, 0.05% saponine; Sigma-Aldrich), 10% goat or bovine serum (PAN™ Biotech GmbH) for 20 min at 21°C. After washing in PBS, cells were incubated for 1 h at 21°C with the following primary antibodies (mouse monoclonal antibodies raised against CNPase, catalase, used at 1/100; rabbit polyclonal antibodies directed against MBP, PLP, and PMP70 (ABCD3); ACOX1 and l-PBE, and ALDP (ABCD1) used at 1/60, 1/200, 1/200, 1/100, 1/100, and 1/100, respectively; a rat monoclonal antibody recognizing MOG used at 1/100) diluted in blocking buffer, washed in PBS, and then incubated for 30 min either with a 488-Alexa (or a 594-Alexa) goat anti-rabbit, anti-mouse, or anti-rat used at 1/300. Nuclei were counter-stained with Hoechst 33342 used at 2 μg/mL. After washing with PBS, slides were mounted, observations were made with an Axioskop Zeiss microscope, and digitalized images were obtained with an Axiocam Zeiss camera(Zeiss, Jena, Germany). To investigate the colocalization between mitochondria and PLP, cells were incubated with Mitotracker Red (100 nM, 15 min, 37°C), which stains mitochondria before the immunostaining procedure. Digital images acquisitions were collected with an SP2 AOBS confocal laser microscope (Leica, Wetzlar, Germany) equipped for epifluorescence microscopy. Alexa 488, Mitotracker Red, and Hoechst 33342 were excited with an argon ion laser, a Helium–Neon laser, and a blue diode, respectively. The objective magnification was 40× with a 1.25 numerical aperture Plan Apochromatic oil immersion objective for high resolution (Lizard et al. 1994). Optical sections were obtained at 0.2 μm along the optical axis, and each plane consist of 1024 × 1024 pixels. Laser and diode powers and detection gains were set up such that signals from single-stained controls would not appear in adjacent channels. The focal plane of maximal PLP expression within the cells was selected to maximize the probability of detection of colocalization with mitochondria (Santos et al. 2000). For colocalization of PLP and mitochondria, the ImageJ software was used (ImageJ, NIH, Besthesda, MA, USA).
For flow cytometric analyses, cells were collected by trypsinization (0.25% trypsin/EDTA solution) (Sigma-Aldrich), washed and mixed in PBS, and fixed in freshly prepared 2% (w/v) p-formaldehyde diluted in PBS, pH 7.4, for 10 min at 21°C. Furthermore, the cells were treated with the FACS permeabilizing solution 2 (BD-Biosciences) for 10 min. After washing in PBS, cells were incubated for 20 min with blocking buffer (PBS, 0.05% saponine, 10% goat serum), washed in PBS, and incubated for 1 h at 21°C with the appropriate primary antibody (mouse monoclonal antibodies raised against CNPase, catalase, used at 1/100; rabbit polyclonal antibodies directed against MBP, PLP, PMP70, ACOX1, l-PBE, and ALDP used at 1/60, 1/200, 1/200, 1/100, 1/100, and 1/100, respectively; a rat monoclonal antibody recognizing MOG used at 1/100) diluted in blocking buffer. Then, cells were washed twice with PBS and incubated for 1 h at 21°C either with a 488-Alexa goat anti-rabbit, -mouse, or -rat antibodies used at 1/300, 1/300 and 1/200, respectively. For the different secondary antibodies used, conjugated controls were performed. Cells were washed and mixed in PBS, and immediately analyzed by flow cytometry on a GALAXY flow cytometer (Partec, Münster, Germany). The green fluorescence of 488-Alexa was collected with a 520/10-nm band pass filter. The fluorescent signals were measured on a logarithmic scale. For each sample, 10 000 cells were acquired (dead cells and debris were excluded from the analysis by gating on living cells with the size/structure density plots), and the data were analyzed with FlowMax (Partec) and FlowJo softwares (FlowJo Inc., Ashland, OR, USA).
Protein extraction and western blot analysis
Cells obtained after 3–4 days of culture were trypsinized (0.25% trypsin/EDTA solution), washed with PBS, and lysed in a radioimmunoprecipitation assay (RIPA) buffer [Tris–HCl 0.05 M, pH 8, NaCl 0.15 M, sodium dodecyl sulfate (SDS) 0.1%, Na desoxycholate 0.5%, Nonidet®P-40 (Sigma-Aldrich) 1%, NaF 50 mM, and EDTA 2 mM] in the presence of a complete protease inhibitor cocktail (Roche Diagnostics Inc., Basel, Switzerland) for 20 min on ice. Cell homogenates were cleared by 15 min centrifugation at 20 000 g. The supernatant was collected and used for gel electrophoresis associated with the immunoblot assay. The protein concentration of the samples was determined using the Bio-Rad DC protein assay kit (Ivry-sur-Seine, France), and bovine serum albumin was used as standard. Forty micrograms of total protein extract were diluted in loading buffer 1x (Tris–HCl, 125 mM, pH 6.8, 16% glycerol, 8%β-mercaptoethanol, 4% SDS, and 0.003% bromophenol blue). Proteins were further separated by electrophoresis on a 10% polyacrylamide SDS-containing gel and transferred onto a polyvinylidene difluoride membrane (Bio-Rad). After blocking non-specific sites for 2 h with 5% skim milk and 1% bovine serum albumin in 1x Tris-buffered saline containing Tween 20 (TBST; 10 mM Tris–HCl, 0.1% Tween 20, and 150 mM NaCl, pH 8), the membranes were incubated overnight at 4°C with various primary antibodies diluted in TBST, raised either against mice ALDP (1/300), ACOX1 (1/500), or l-PBE (1/200). After washing the membrane with TBST, it was incubated with horseradish peroxidase-conjugated anti-rabbit IgG (1/10 000) for 1 h at 21°C. The membranes were then washed with TBST and revealed using an enhanced chemiluminescence detection kit (Amersham, Louisville, CO, USA) and autoradiography.
Enzymatic activities: acyl-CoA oxidase 1 and catalase
To perform enzymatic activity, the protein extract was prepared on 8–30 × 106 cells. Cells were mixed in 7.5 mL PBS containing a mixture of protease inhibitors (Roche Diagnostics Inc.), and three successive freezing and thawing cycles were performed. The samples were sonicated, centrifuged (50 000 g, 30 min), and the supernatant was collected. ACOX1 activity was assayed by a fluorimetric assay as described by Oaxaca-Castillo et al. The reaction mixture (200 μL) contained Tris buffer (50 mM, pH 8.3), homovanillic acid (0.75 mM), horseradish peroxidase (20 μg/mL), and acyl-CoA substrate (palmitoyl-CoA at 50 μM final concentration). The reactions were started by the addition of 5–20 μL of enzymatic solution. Catalase activity was quantified with the Amplex Red Catalase Assay Kit (Invitrogen) which uses the highly fluorescent oxidation product, resorufin. The absorbance of resorufin-formed solution was measured at 570 nm using a spectrophotometer (Serlabo Technologies, Entraigues sur la Sorgue, France). One unit of the enzyme is defined as 1 μmol of H2O2 consumed per minute and the specific activity is reported as units per milligram of protein.
Quantitative RT-PCR
Cells were harvested with 0.25% trypsin/EDTA and washed with PBS. Total RNA from oligodendrocytes (158N or 158JP) was extracted using the RNeasy Mini kit (Qiagen, Valencia, CA, USA) following the manufacturer’s instructions. cDNA was generated by reverse transcription using QuantiTect Rev. Transcription Kit (Qiagen) according to the manufacturer’s protocol and analyzed by quantitative PCR using the SYBR Green real-time PCR technology, and an iCycler iQ Real-Time Detection System (Bio-Rad). The primer sequences (Abcd1: forward, 5′-ACATCCCTATCATCACACCCACTG-3′ and reverse, 5′-GAGAACTCTTGCCACAGCCATTG-3′; Abcd2: forward, 5′-GTTCAAAGAGAAGGAGGATGGGATG-3′ and reverse, 5′-TGCTCACGGCACTGGTACATTC-3′; Abcd3: forward, 5′-GCTGGGCGTGAAATGACTAGATTG-3′ and reverse, 5′-CCTTCTCCTGTTGTGACACCATTG-3′; Acox1: forward, 5′-GCCCAACTGTGACTTCCATT-3′ and reverse, 5′-GGCATGTAACCCGTAGCACT-3′; and β-actin: forward, 5′-AACACCCCAGCCATGTACG-3′ and reverse 5′-ATGTCACGCACGATTTCCC-3′) were chosen using the Beacon Designer Software (Bio-Rad). PCR reactions were carried out in duplicate in a final volume of 25 μL containing 12.5 μL of MESA Green qPCR Mastermix (Eurogentec, Uppsala, Sweden), 5 μL of cDNA and forward and reverse primers at 200 nM for Abcd1, 100 nM for Abcd2, or 300 nM for the other genes (Abcd3, Acox1, and β-actin) studied. The PCR enzyme (Taq DNA polymerase) was heat-activated at 95°C for 10 min, and the DNA was amplified for 40 cycles at 95°C for 15 s, 60°C for 30 s, and 72°C for 30 s, followed by a melting curve analysis to control the absence of non-specific products. For each transcript, the amplification efficiency was determined by the slope of the standard curve generated from twofold serial dilutions of cDNA. Gene expression was quantified using cycle threshold (Ct) values and normalized by the β-actin reference gene. The quantitative expression of Abcd1, Abcd2, Abcd3, and Acox1 was determined according to 2−ΔCt with ΔCt = (Ct of the gene studied) − (Ct of the β-Actin gene), or as fold induction of the control.
Flow cytometric characterization of mitochondrial and redox status
The mitochondrial potential (ΔΨm) was measured with DiOC6(3) used at 40 nM (Miguet et al. 2001). The DiOC6(3)-related green fluorescence was analyzed by flow cytometry and collected through a 520/10-nm band pass filter. The mitochondrial mass was studied with NAO (Ratinaud et al. 1988). To this end, cells mixed in PBS at 5 × 105 cells/mL were incubated for 30 min at 37°C with NAO used at 5 nM. After washing and mixing in PBS, cells were analyzed by flow cytometry, and the green fluorescence of NAO was collected through a 520/10-nm band pass filter. Both 2′,7′-dichlorodihydrofluorescein diacetate (H2-DCFDA) and DHE were used to characterize the production of reactive oxygen species (ROS) and of superoxide anions (O2−), respectively (Bass et al. 1983; Rothe and Valet 1990). To measure the production of ROS, cells (106 cells/mL of culture medium) were incubated with H2-DCFDA used at 6 μM final for 10 min at 37°C, and the green fluorescence of 2′,7′-dichlorofluorescein resulting from the oxidation of H2-DCFDA was analyzed by flow cytometry and collected through a 520/10-nm band pass filter. DHE, a non-fluorescent compound rapidly oxidized in ethidium under the action of O2− (Rothe and Valet 1990), was prepared at 10 mM in dimethylsulfoxide, and used at 2 μM on cell samples (106 cells/mL of culture medium). After 15 min of incubation at 37°C, cells were analyzed by flow cytometry. The red fluorescence of ethidium was collected through a 590/10-nm band pass filter.
The redox status was evaluated by the level of intracellular reduced GSH after staining with MCB (Lizard et al. 1998). MCB, prepared at 4 mM, was added at 100–200 μM in cell suspensions (106 cells/mL in PBS). After 15 min of incubation at 37°C, cells were washed and mixed in PBS, and the blue fluorescence of MCB was collected with a 420-nm long pass filter. The fluorescent signals were measured on a logarithmic scale on a GALAXY flow cytometer (Partec) equipped with an argon laser, and with a mercury xenon lamp (to excite MCB). For each sample, 10 000 cells were acquired. The data were analyzed with FlowMax (Partec) and FlowJo softwares (FlowJo Inc.).
Statistical analysis
Statistical analyses were performed on at least three independent experiments using SigmaStat 2.03 software (Systat Software Inc., Chicago, IL, USA) with the Mann–Whitney test, and data were considered statistically different at p < 0.05.
Results
Analysis of oligodendrocytic and myelin markers using fluorescence microscopy and flow cytometry in 158N and 158JP cells
The expression of markers of differentiated oligodendrocytes (CNPase and MOG), and of major myelin proteins (PLP and MBP) was determined by fluorescence microscopy and flow cytometry. High levels of CNPase (Fig. 1a–c) and MOG (Fig. 1d–f) expression were observed. In agreement with previous investigations (Feutz et al. 2001; Ghandour et al. 2002), high levels of MBP (Fig. 1g–i) and PLP (Fig. 1j–l) expression were found. The expressions of CNPase, MOG, MBP, and PLP were either slightly or substantially higher in 158N than in 158JP cells.
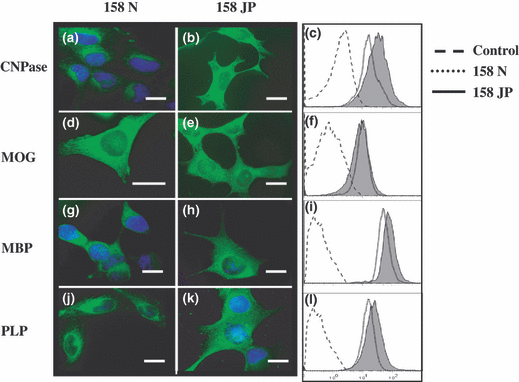
Expression analysis of oligodendrocytic and myelin markers in murine oligodendrocytes 158N and 158JP. Fluorescence microscopy and flow cytometry analysis were accomplished on subconfluent murine oligodendrocytes 158N and JP to determine the expression of oligodendrocyte differentiation markers such as 2′,3′ cyclic nucleotide 3′-phosphodiesterase (CNPase; a–c), myelin oligodendrocyte glycoprotein (MOG; d–f), major myelin proteins (proteolipid protein; PLP; g–i), and myelin basic protein (MBP; j–l). Data shown are representative of three to six independent experiments. Scale bar, 10 μm. Control corresponds to conjugated control.
Peroxisomal and mitochondrial content
Transmission electron microscopy was used to visualize peroxisomes in murine oligodendrocyte 158N and 158JP cells. Tissue sections from mice liver were used as positive controls (Fig. 2a and b). In 158N and 158JP cells, peroxisomes were also detected at the cytoplasmic level (Fig. 2c–f), but they were less numerous than in murine hepatocytes and had a heterogeneous aspect in the diaminobenzidine reaction deposit. We also investigated the morphological aspects of mitochondria in 158N and 158JP cells. Figure 2c–f shows clear morphological differences, in size and shape, between the mitochondria from 158N and 158JP cells. Indeed, the mitochondria from 158JP cells (Fig. 2f) were generally larger and more rod-shaped than the mitochondria of 158N cells (Fig. 2d). Thus, the consequences of PLP mutation and myelin synthesis disruption in the oligodendrocyte cell line 158JP might have a morphological impact on mitochondria shape.
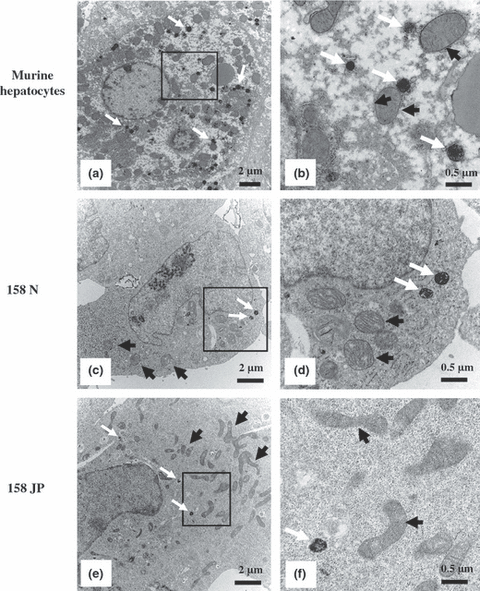
Transmission electron microscopy analysis of peroxisomes and mitochondria in murine oligodendrocytes 158N and 158JP. Livers of 9- to 10-week-old C57 Black/6 males were used as positive controls for peroxisome cytochemical analysis. (a) Numerous peroxisomes (white arrows) are observed in a hepatocyte. (b) These peroxisomes were clearly observed at high magnification in the presence of diaminobenzidine and H2O2. In 158N (c and d) and 158JP (d and e) cells, compared with murine hepatocytes, only a few peroxisomes were observed. Most often, the number of mitochondria (dark arrows) seems lower in 158N (c) than in 158JP (e). Data shown are representative of three independent experiments.
Analysis of peroxisomal markers by fluorescence microscopy, flow cytometry, and western blotting
The expression of the peroxisomal ABC transporters ALDP and PMP70 encoded by the Abcd1 and Abcd3 genes, respectively, and of the peroxisomal enzymes (catalase, ACOX1, and l-PBE) was determined by fluorescence microscopy, flow cytometry, and western blotting on 158N and 158JP cells. On these two cell lines, as shown by fluorescence microscopy and flow cytometry analyses, a high level of ALDP (Fig. 3a–c) and PMP70 (Fig. 3d–f) expression were observed. Substantial expression of catalase (Fig. 3g–i), ACOX1 (Fig. 3j–l), and l-PBE (Fig. 3m–o) were also detected. The expression of these peroxisomal markers was always higher in 158JP than in 158N cells. The expression of ALDP, ACOX1, and l-PBE was confirmed by western blot analysis, which revealed the characteristic bands of these molecules (Fig. 3p). Thus, a major band at 75 kDa for ALDP, and an expected band at 78 kDa for l-PBE were identified (Fig. 3p) (Suzuki et al. 1994; Fouquet et al. 1997). The native 72-kDa ACOX1 protein is known to be cleaved inside the peroxisome mainly into a 50-kDa polypeptide protein (Oaxaca-Castillo et al. 2007). Using a polyclonal antibody, we detected the 72 and 50 kDa bands in 158N and 158JP cells, and these bands were not detected in the deficient ACOX1 fibroblasts (ACOX1−/−) used as negative control (Fig. 3p).
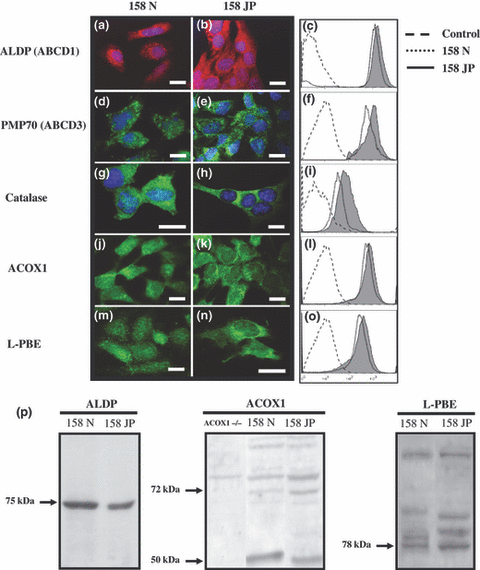
Expression analysis of the peroxisome transporters and peroxisomal enzymes in murine oligodendrocytes 158N and 158JP. Conventional fluorescence microscopy and flow cytometry analyses were performed on subconfluent murine oligodendrocytes 158N and JP for peroxisomal transporters (ALDP (ABCD1; a–c), and PMP70 (ABCD3; d–f) and for peroxisomal enzymes (catalase; g–i), acyl-CoA oxidase1 (ACOX1; j–l), and peroxisomal bifunctional enzyme (l-PBE; m–o). ALDP, ACOX1, and l-PBE expression were also characterized using western blotting (p). Data shown are representative of three to six independent experiments. Scale bar, 10 μm. Control corresponds to conjugated control. To investigate ACOX1 expression by western blotting, we used homogenate from human fibroblasts deficient in ACOX1 (ACOX1−/−).
Analysis of peroxisomal markers by RT-qPCR
The relative expression level of the Abcd1, Abcd2, Abcd3, and ACOX1 genes was determined in 158N and 158JP murine oligodendrocytes by RT-qPCR and evaluated according to 2−ΔCt calculated comparatively to β-actin (Fig. 4). Similar expression levels of Abcd1 and Acox1 were observed in 158N and 158JP cells, while the expression levels of Abcd2 and Abcd3 were higher in 158JP than in 158N cells. Moreover, Abcd2 was very weakly expressed in 158N cells, whereas its expression level in 158JP cells was in the range observed for Abcd1 and Acox1.
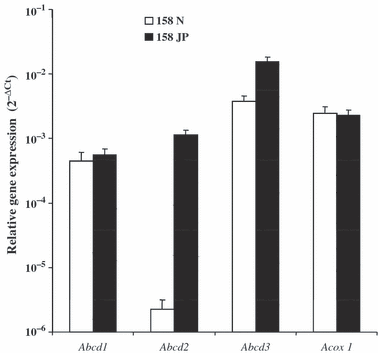
Oligodendrocyte expression levels of genes encoding peroxisome transporters (Abcd1, Abcd2, and Abcd3) or peroxisomal enzyme (Acox1) in murine oligodendrocytes 158N and 158JP, respectively. The mRNA levels were measured using real-time RT-qPCR and normalized to β-actin. Data presented are the mean ± SD of two or three experiments (carried out in duplicate) and are expressed as 2−ΔCt.
4-Phenylbutyrate responses of 158N and 158JP cells
4-Phenylbutyrate has been previously shown to induce peroxisomal genes and peroxisomal proliferation especially in rat hepatocytes (Gondcaille et al. 2005). It was therefore interesting to evaluate the response of 158N and 158JP cells to 4-PBA. Under treatment with 4-PBA (2.5 mM, 72 h), cell growth was strongly inhibited, and significant morphological changes were observed in both cell types (Fig. 5a–d). In 158N cells, the expression of the peroxisomal proteins ACOX1 and l-PBE was enhanced (Fig. 5e), as well as the expression of the myelin protein PLP (Fig. 5f) and the oligodendrocyte markers, MOG and CNPase (Fig. 5g). On 158JP cells, only a weak stimulation of the expression of CNPase was observed (Fig. 5e–g). As 4-PBA was shown to induce the transcription of Abcd2 but not Acox1 in glial cells (Gondcaille et al. 2005), the expression levels of these genes was evaluated by RT-qPCR in both 4-PBA-treated or untreated 158N and 158JP cells. Data from Fig. S1 show that, in 158N cells, the mRNA levels of both Abcd2 and Acox1 was unchanged after 4-PBA treatment, while in 158JP cells 4-PBA induces slightly the mRNA level of Acox1 and strongly the mRNA level of Abcd2.
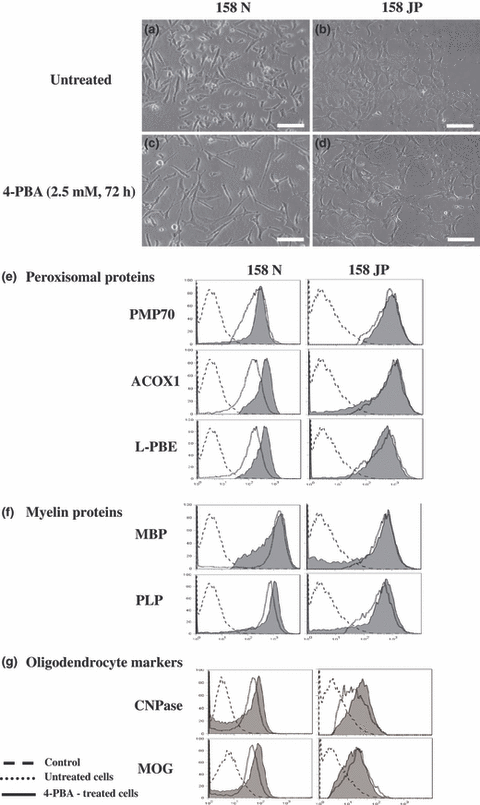
Effect of 4-phenylbutyrate on the expression of peroxisomal proteins, and oligodendrocyte and myelin markers. (a–d) Phase-contrast microscopy showing untreated and 4-PBA (2.5 mM, 72 h)-treated 158N and 158JP cells. Scale bar, 30 μm. Flow cytometric analysis of the expression of peroxisomal proteins (PMP70, ACOX1, and l-PBE; e), major proteins of myelin (PLP and MBP; f), and oligodendrocyte markers (CNPase and MOG; g) on untreated and 4-PBA (2.5 mM, 72 h)-treated 158N and 158JP cells. Data shown are representative of two to three independent experiments. Control corresponds to conjugated control.
In addition, specific activities of peroxisomal enzymes were also compared in untreated and in 4-PBA-treated 158N and 158JP cells. Whereas the catalase activity was similar in 158N and 158JP cells (Fig. 6a), ACOX1 activity was higher in 158JP than in 158N cells (Fig. 6b). In H4IIC3 rat hepatoma cells, used as positive controls, the catalase activity was approximately three times higher than in 158N and JP cells (data not shown). Thus, in both cell types, the 4-PBA treatments resulted in a significant increase in the activity of both enzymes (Fig. 6a and b). Observations made by transmission electron microscopy did not show a peroxisomal proliferation under treatment with 4-PBA in either 158N or 158JP, whereas an increase in the size of peroxisomes was found in 158N cells (data not shown).
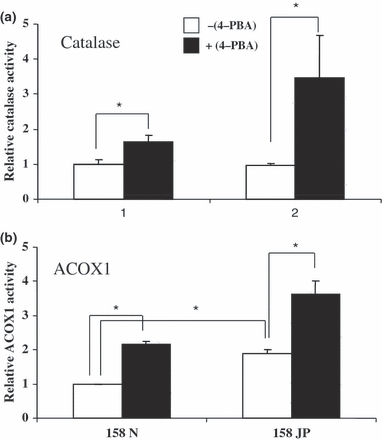
Effects of 4-phenylbutyrate on catalase and ACOX1 activities in murine oligodendrocytes 158N and 158JP. The activities of the peroxisomal enzymes (catalase, a and ACOX1, b) were determined on subconfluent murine oligodendrocytes 158N and JP cultured in the absence or in the presence of 4-PBA used at 2.5 mM for 72 h. The enzymatic activities are expressed in fold induction comparatively to those of 158N cells used as reference. In these cells, catalase specific activity was 21.56 ± 2.99 μmol/min/mg of proteins and ACOX1 specific activity was 6.00 ± 0.28 nmol/min/mg of proteins. Data shown are representative of two independent experiments carried out in triplicate. Results are presented as mean ± SD; *p < 0.05.
Redox and mitochondrial status of 158N and 158JP cells
The oxidation of fatty acids requires both peroxisomal and mitochondrial activities, and the metabolic activities of these organelles are tightly connected to ensure the degradation of fatty acids contributing to produce acetyl-CoA. Therefore, the mitochondrial and redox status of murine oligodendrocytes of 158N and 158JP cells was investigated by flow cytometry with various dyes. The spontaneous production of ROS and of superoxide anions (O2−) measured by flow cytometry after staining with H2-DCFDA and DHE, respectively, was significantly greater in 158JP than in 158N, as well as the intracellular level of reduced GSH quantified with MCB (Table 1). The ΔΨm, and the mitochondrial mass determined by flow cytometry after staining with DiOC6(3) and NAO, respectively, were also significantly higher in 158JP than in 158N cells. These data show substantial differences between the redox and the mitochondrial status of 158N and 158JP cells.
| Assays | 158N | 158JP |
|---|---|---|
| ROS (H2-DCFDA) | 113.59 ± 25.09 | 170.20 ± 9.50* |
| O2− (DHE) | 2.83 ± 0.60 | 14.07 ± 0.92*** |
| GSH (MCB) | 13.80 ± 0.51 | 33.87 ± 2.01*** |
| ΔΨm [DiOC6(3)] | 47.05 ± 10.50 | 122.60 ± 14.67** |
| Mitochondrial mass (NAO) | 34.44 ± 2.80 | 88.15 ± 9.57*** |
- MCB, monochlorobimane; NAO, nonylacridine orange; ROS, reactive oxygen species; ΔΨm, mitochondrial potential. Data are mean ± SD of three independent experiments. Statistic significance of the differences between 158N and 158JP cells; *p < 0.05, **p < 0.005, and ***p < 0.001. Data are expressed as ΔMFI (mean fluorescence intensity)
- ΔMFI = MFI of stained cells − MFI of unstained cells.
Confocal laser microscopic analysis of the interaction of PLP with mitochondria
As 158JP cells express a mutated PLP form (Feutz et al. 2001) and as this protein could modulate the ΔΨm (Bongarzone et al. 2001) and contribute to the induction of a mitochondria-dependent form of cell death (Cerghet et al. 2001), we hypothesized that PLP could play a role in the differences in mitochondrial status observed between 158N and 158JP. Thus, by confocal laser scanning microscopy, after mitochondrial staining with Mitotracker Red and PLP identification by indirect immunofluorescence with Alexa 488, we studied the colocalization of PLP with mitochondria (Fig. 7). The number of colocalization sites of PLP with mitochondria were significantly (p < 0.05) lower in 158N cells (2783 ± 4) (Fig. 7a-d), than in 158JP cells (9581 ± 23) (Fig. 7e-h), supporting the hypothesis that PLP, in addition to its role in myelination, might contribute to other cellular functions.
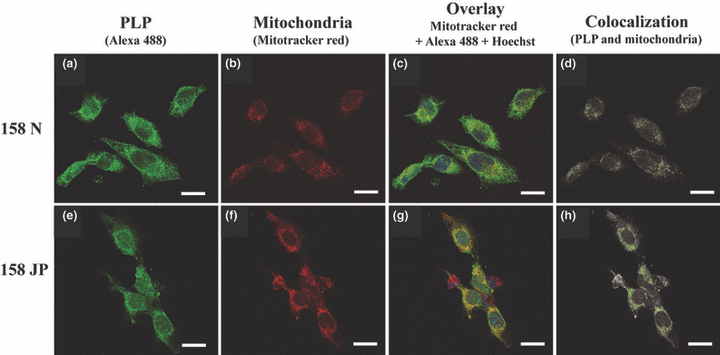
Analysis of the colocalization of PLP and mitochondria by confocal laser scanning microscopy. PLP was revealed with Alexa 488 and mitochondria were stained with Mitotracker Red. The nuclei were counter-stained with Hoechst 33342. Overlay (c and g) was performed on optical sections showing the highest PLP-associated fluorescence (a and e) to maximize the probability of detection of PLP with mitochondria (b and f). The green dots correspond to colocalization sites (d and h). Interestingly, the number of green pixels are strongly higher in 158JP cells (9581 ± 23 green pixels/cell; h) than in 158N cells (2783 ± 4 green pixels/cell; d). The localization of PLP at the mitochondrial level was investigated with the colocalization finder plug-in of ImageJ software. For each slide observed, three microscopical fields were examined (four to six cells per field). Data shown are specific of three independent experiments. Scale bar, 10 μm.
Discussion
The CNS comprises different cell types: neurons, microglial cells, and glial cells consisting of astrocytes and oligodendrocytes. In the CNS, oligodendrocytes synthesize myelin made up of lipid (up to 70% dry weight) and two major proteins: the MBP and the PLP (Baumann and Pham-Dinh 2001; Taylor et al. 2004). The myelin sheath of the CNS is formed by membranes that extend from oligodendrocytes and wrap concentrically around nerve fibers, thereby insulating them and facilitating rapid transmission of nerve impulses (Harauz et al. 2004). Myelin is a dynamic functionally active membrane, and its disruption because of intrinsic or environmental factors (Hörster et al. 2005; Eichler and van Haren 2007) can result in serious neurological disorders including central and peripheral neuropathies (Zhou and Griffin 2003), inflammatory demyelinating diseases such as multiple sclerosis (Steinman 2008), and leukodystrophies such as Pelizaeus–Merzbacher disease, X-ALD, and P-NALD (Koeppen and Robitaille 2002). Given that oligodendrocytes damage is observed in these different pathologies and that it has been demonstrated that oligodendrocytes play a crucial role in the progression of X-ALD (Kassmann et al. 2007), it is important to develop cellular models as better knowledge of oligodendrocyte biology is crucial for the understanding of demyelination and remyelination mechanisms. To this end, murine or rat primary cultures of oligodendrocytes, as well as organotypic cultures can be used. However, these culture methods are difficult to standardize, and it may be advantageous to work on established oligodendrocyte cell lines. Consequently, we did an extensive characterization of the two murine oligodendrocyte cell lines 158N and 158JP isolated from the brain of normal and jimpy mice, respectively.
In agreement with previous investigations (Feutz et al. 2001; Ghandour et al. 2002), both cell lines strongly express the two major proteins of myelin, PLP and MBP, which account for 50% and 30% of whole myelin proteins, respectively (Taylor et al. 2004). In addition, these cells strongly express CNPase and MOG. CNPase is a myelin-associated enzyme localized almost exclusively in the cells elaborating myelin (oligodendrocytes and Schwann cells), and it is involved in the growth of myelin membrane during early oligodendrocyte membrane biogenesis (Sprinkle 1989). Whereas MOG only accounts for 0.1% of oligodendrocytic proteins, it is considered that this protein, which belongs to the immunoglobulin superfamily and plays important roles in the immune response associated with multiple sclerosis (Clements et al. 2003), could also contribute to the stability of myelin structure (Hörster et al. 2005). Thus, as 158N and 158JP cells express major proteins involved in the elaboration, the structure, and the immunoreactivity of myelin, they could be useful to study the effects of intrinsic and extrinsic factors on oligodendrocytic differentiation and study the role of the cellular metabolism in various pathologies associated with important myelin disorders such as the peroxisomal diseases X-ALD and P-NALD.
Several hypotheses have been postulated to explain the physiopathogenesis of the peroxisomal disorders, one of them presenting the toxicity of VLCFA for the myelin-producing cells as central. So, it was important to characterize the peroxisomal equipment of the 158N and 158JP cells. Using transmission electron microscopy, some peroxisomes were detected in both cell lines, but they were less numerous than in mice hepatocytes. Interestingly, by using flow cytometry, western blotting, and/or RT-qPCR, we evidenced that 158N and 158JP express at a similar level the following peroxisomal markers: ALDP and PMP70 corresponding to the peroxisomal ABC transporters ABCD1 and ABCD3, respectively, as well as the peroxisomal enzymes catalase, ACOX1, and l-PBE.
Concerning X-ALD, several pharmacological compounds have been tested in vitro or in vivo to induce peroxisomal β-oxidation. One of them, 4-PBA, has been shown to induce the expression of Abcd2 in mixed primary culture of murine oligodendrocytes and astrocytes (Gondcaille et al. 2005). It was therefore interesting to analyze the effect of 4-PBA on the 158N and 158JP cells. 4-PBA treatments resulted in morphological changes concerning the size and shapes of peroxisomes were only observed in 158N cells. Similarly, enhanced protein expression of ACOX1, l-PBE, PLP, MOG, and CNPase was also only observed in these oligodendrocytes isolated from normal mice, whereas CNPase was only slightly increased in 158JP cells. Although ACOX1 is induced by 4-PBA at both protein and enzymatic activity levels, no 4-PBA effect has been detected for its mRNA. Thus, the ACOX1 expression in treated 158N cells could involve the existence of a post-translational up-regulation mechanism, which has been previously reported for other peroxisome proliferators (Hashimoto 1987). Furthermore, it was also reported that 158JP cells have a defective cAMP metabolic pathway (Feutz et al. 2001). These observations, together with previous results showing that 158JP were unable to respond to basic fibroblast growth factor (Feutz et al. 1995) and dibutyryl cAMP (Feutz et al. 2001), suggest that 4-PBA-induced ACOX1, l-PBE, PLP, and MOG could occur in 158N through a cAMP-dependent pathway. On the other hand, as CNPase expression and catalase activity are enhanced by 4-PBA in both cell lines, our data support that these events could be independent of the cAMP pathway.
Surprisingly, in the absence of any treatment, adrenoleukodystrophy related protein (ALDRP) (ABCD2) gene was found poorly expressed in 158N cells when compared with 158JP cells, which harbor mutation in the PLP gene (Macklin et al. 1987). The increase of the basal expression of Abcd2 gene in 158JP cells raises the question about the relationship between dysmyelination in JP mice oligodendrocytes and Abcd2 gene regulation. Interestingly, PLP gene expression in oligodendrocytes was shown to be regulated via derepression mechanism involving several negative regulatory elements. Accordingly, such repression/derepression mechanisms could explain the low level of Abcd2 mRNA in the myelinating 158N oligodendrocytes when compared with the strong induction of Abcd2 mRNA level in 4-PBA-treated 158JP cells. Therefore, the use of both 158N and 158JP cell lines, instead of primary culture of murine oligodendrocytes, will be greatly helpful to depict the regulation of Abcd2 gene expression and the involved transcription factors, and to understand the substrate specificity of different peroxisomal ABC transporters using gene silencing and/or gene transfection methods.
Peroxisomes and mitochondria are ubiquitous and tightly connected organelles, which have an indispensable role in the cellular metabolism of higher eukaryotes (Schumann and Subramani 2008), and peroxisomal alterations can potentially influence mitochondrial functions (Fourcade et al. 2008; Hein et al. 2008). Consequently, given that peroxisomal and mitochondrial dysfunctions are often associated with neurological and developmental defects and that peroxisomes and mitochondria have been suggested to contribute to pathological conditions associated with oxidative stress (Schrader and Fahimi 2006), the mitochondrial and the redox status were simultaneously studied in 158N and 158JP cells. Interestingly, significant differences were observed between 158N and 158JP cells: the spontaneous production of ROS and O2− was higher in 158JP than in 158N as well as the GSH level; ΔΨm and the whole mitochondrial mass were also higher in 158JP than in 158N. Although the primary causes of these differences are difficult to establish, it is tempting to speculate that the highest expression of mutated PLP observed in 158JP might be involved. This hypothesis is supported by the strong colocalization of PLP and mitochondria observed mainly in 158JP. Therefore, as previously suggested, in addition to its important role in myelin structure and functions, PLP might have additional roles (Campagnoni and Skoff 2001). Indeed, potential roles of PLP in the induction of cell death have been suggested (Bongarzone et al. 2001), and it has been clearly established that increased levels of PLP favor the death of oligodendrocytes and neurons (Boucher et al. 2002; Skoff et al. 2004). Therefore, based on the data obtained in the present investigation and on those of the literature, we suggest that PLP could modulate the mitochondrial and the redox status, which subsequently could contribute to trigger cell death.
Our study shows that 158N and 158JP cells express the main proteins of mature oligodendrocytes (CNPase, MOG, and the two major proteins of myelin, PLP and MBP), contain major peroxisomal proteins and/or mRNAs [ALDP (ABCD1), PMP70 (ABCD3), catalase, ACOX1, and l-PBE], and have different mitochondrial content and activities, and redox status. Interestingly, under treatment with 4-PBA, the expression of some of these proteins is enhanced, especially in 158N cells while Abcd2 gene expression is only modulated in 158JP. Therefore, our data support that 158N and 158JP cells can provide powerful models to explore the relationships between peroxisome, mitochondrial metabolism, and myelin protein expression, and to dissect the complexity of the molecular mechanisms involved in some neurodegenerative diseases, especially X-ALD, P-NALD, and multiple sclerosis.
Acknowledgements
This work was supported by grants from the INSERM, CNRS, the council of Burgundy, the European Leukodystrophy Association (ELA), and the University Hospital of Dijon (CHU de Dijon).




