Transforming growth factor β1 and ethanol affect transcription and translation of genes and proteins for cell adhesion molecules in B104 neuroblastoma cells
Abstract
Transforming growth factor (TGF) β1 and ethanol retard the migration of young, post-mitotic neurons to the developing cerebral cortex. The coordination of this migration depends upon cell adhesion proteins (CAPs). We examined the effects of TGFβ1 and ethanol on genes related to both TGF and CAPs. Rat B104 neuroblastoma cells were treated with TGFβ1 (0 or 10 ng/mL) and ethanol (0 or 400 mg/dL) for 6–48 h. Total RNA was purified from each sample and analyzed using the Rat U34A GeneChip (Affymetrix). Candidate genes were those up- or down-regulated by either TGFβ1 or ethanol. Twenty transcripts of CAPs were identified as being expressed by B104 cells and as being affected by treatment with TGFβ1 or ethanol. The expression was verified for five representative genes (neural cell adhesion molecule, L1, and integrins α1, α7, and β1) using assays with real-time reverse transcriptase–polymerase chain reactions. Each of these genes showed time-dependent changes. The changes were reflected in increases in protein expression that appeared within 24 or 48 h. Thus, the effects of TGFβ1 and ethanol on CAPs parallel changes described in vivo and likely underlie changes associated with ethanol-induced alterations in neuronal migration.
Abbreviations used
-
- CAPs
-
- cell adhesion proteins
-
- MAPK
-
- mitogen-activated protein kinase
-
- nCAM
-
- neural cell adhesion molecule
-
- qRT–PCR
-
- quantitative reverse transcriptase polymerase chain reaction
-
- RT
-
- reverse transcriptase
-
- TGF
-
- transforming growth factor
-
- TPBS
-
- phosphate-buffered saline with Tween-20
Neuronal migration and neurite outgrowth are two processes that are critical to brain development. Migration is the active movement of post-mitotic neurons from the zones where cells proliferate to the anlage where they differentiate (Hu 2006). Neurite outgrowth is the identification and elongation of both dendrites and axons (Mintz et al. 2006).
Both neuronal migration and neurite outgrowth are regulated by transforming growth factor (TGF) β1. TGFβ1 promotes migration by moving neural precursors out of the proliferative population (Luo and Miller 1999a; Miller and Luo 2002; Siegenthaler and Miller 2005) and by increasing the rate of migration (Siegenthaler and Miller 2004). TGFβ ligands and their receptors are strategically expressed by elements key to the migratory processes, principally radial glia (Flanders et al. 1991; Pelton et al. 1991; Krieglstein et al. 2002; Miller 2003). TGFβ1 also promotes dendritic growth (Ishihara et al. 1994; Abe et al. 1996; Ho et al. 2000). TGFβ regulation of these ontogenetic processes apparently is mediated by cell adhesion proteins (CAPs) such as neural cell adhesion molecule (nCAM), L1, and isoforms of integrin (Siegenthaler and Miller 2004).
Prenatal exposure to ethanol affects early neuronal development. Ethanol (i) delays the onset, (ii) retards the rate, and (iii) disrupts the cessation of neuronal migration (e.g. Miller 1986, 1993; Kotkoskie and Norton 1988; Komatsu et al. 2001; Sun et al. 2002; Mooney et al. 2004; Siegenthaler and Miller 2004, 2006). Likewise, dendritic and axonal growth are altered by ethanol (Lindsley 2006). Ethanol can stunt (e.g. Hammer and Scheibel 1981; Schapiro et al. 1984; Fabregues et al. 1985; Yanni and Lindsley 2000) or enhance growth (Miller 1987; Miller et al. 1990, 1999; Messing et al. 1991; Roivainen et al. 1993, 1995; Clamp and Lindsley 1998). Indeed, ethanol affects CAP (specifically L1) mediated cell adhesion (Ramanathan et al. 1996; Wilkemeyer et al. 1999; Bearer 2001) and neurite outgrowth (Bearer et al. 1999; El Bitar et al. 1999; Bearer 2001; Watanabe et al. 2004).
Ethanol profoundly affects TGFβ1 systems and TGFβ1-regulated activities. In vivo expression of endogenous TGF ligands and receptors is altered in rats prenatally exposed to ethanol (Miller 2003). This includes radial glia used as guides for neuronal migration. It is noteworthy that radial glia elaborate CAPs (e.g. Sheppard et al. 1991; Seki and Arai 1999; Ortino et al. 2003). In slice cultures, ethanol blocks exogenous TGFβ1-enhanced neuronal migration (Siegenthaler and Miller 2004). This blockage is paralleled by ethanol-induced increases in the expression of CAPs. The present study tests the hypothesis that ethanol interferes with TGFβ1-regulated transcription of CAPs.
Materials and methods
Subjects
B104 rat neuroblastoma cells (ATCC; Bottenstein and Sato 1979) were examined because they behave like neuronal progenitors (cf. Luo and Miller 1999a; Jacobs and Miller 2001; Miller and Luo 2002). These cells respond to both TGFβ1 and ethanol in manners parallel to those of primary cultured cortical neurons.
B104 cells were plated on poly d-lysine-coated plastic wells. They were grown in a serum-free medium: Eagle's Minimal Essential Medium supplemented with 1.0 mm glutamine, 33 mm glucose, 180 mm gentamycin (Fisher, Houston, TX, USA), and 10% fetal calf serum. This medium was changed every 2–3 days, and cultures were split when they reached confluency (about every 4 days). After splitting, the B104 neuroblastoma cells were raised in fresh fetal calf serum-supplemented medium for 24 h and then switched to a medium devoid of serum (Eagle's Minimal Essential Medium with 5.0 mg/L insulin, 5.0 mg/L transferrin, and 5.0 µg/L selenium; Collaborative Biomedical, Bedford, MA, USA).
Cultures were maintained in the serum-free condition for at least 4 h, and then the cells were treated with TGFβ1 (0 or 10 ng/mL) and ethanol (0 or 400 mg/dL). These concentrations were used because 10 ng/mL of TGFβ1 maximally stimulates neuronal migration (Siegenthaler and Miller 2004) and 400 mg/dL of ethanol induces changes in cell migration (cf. Miller 1993; Siegenthaler and Miller 2004) and proliferation (cf. Miller and Nowakowski 1991; Luo and Miller 1997) in vitro that replicate the in vivo situation wherein blood ethanol concentrations are ∼150 mg/dL. In the present study, B104 cells were treated with a substance(s) for 3–48 h. Ethanol is a volatile substance. Therefore, it was necessary to stabilize the ethanol in the medium. This was accomplished using closed chambers (e.g. Adickes et al. 1988; Luo and Miller 1997, 1999a).
Microarray methods
The microarray experiments used the Rat U34A GeneChip (Affymetrix, Santa Clara, CA, USA) to screen for expression changes in the B104 cells. To obtain RNA for expression analysis, total RNA was extracted from cell preparations from three samples per treatment group at each of three time points (6, 12, and 18 h) using the RNeasy kit (Qiagen, Valencia, CA, USA). The quality and quantity of the purified RNA was assessed using ultraviolet spectrophotometry and comparison of 28S:18S ratios with the Bioanalyzer RNA NanoChip (Agilent, Palo Alto, CA, USA). One sample per treatment group per time point was labeled and processed according to standard procedures, washed and stained on the Fluidics Station (Affymetrix) according to the EukGE-WS2 protocol, and scanned using the Agilent G2500A Gene Array Scanner.
After scanning the GeneChips, pivot tables containing the Microarray Suite 5-calculated values were imported into GeneSpring 7.0 (Agilent). In GeneSpring, array data were normalized by adjusting the median intensity of each array to a value of 1.0, and then normalizing expression of each gene in the treated sample to that of the matching control sample for each time point.
The present study focused on transcripts related to cell adhesion processes. The initial list of possible genes screened included > 300 different probe sets (approximately 200 different genes) for CAPs. Transcripts pursued were those (i) that were expressed in at least two of the 12 samples and (ii) that exhibited a change of > 50% with treatment or time.
Verification method
To validate changes in the expression of candidate transcripts, three independent replicate RNA samples were generated for each treatment at each time point (6, 18, and 48 h). These samples were tested with a real-time quantitative reverse transcriptase (RT) polymerase chain reaction (qRT–PCR). For the RT reaction, 250 ng total RNA (25 µL in water) from each RNA preparation was incubated with 250 pmoles of an oligo (dT)24 primer at 70°C for 10 min. Then, 3.5 µL 10 × PCR Gold buffer (Applied Biosystems, Foster City, CA, USA), 15 µL of 25 mm MgCl2 (Applied Biosystems), 2.0 µL of 25 mm dNTP, 0.50 µL RNase inhibitor (40 U/mL; Ambion, Austin, TX, USA), and 0.63 µL SuperScript II reverse transcriptase (200 U/mL; Invitrogen, Carlsbad, CA, USA) were added. The reaction was incubated at 25°C for 10 min, 48°C for 30 min, 95°C for 5 min. Subsequently, samples were diluted five-fold with nuclease-free water.
For quantification of transcript differences, 1.0 µL of the RT reaction from each of the samples was evaluated in triplicate PCR reactions for each gene of interest on 96-well plates. Each 25 µL PCR reaction contained 1 × TaqMan Universal PCR Master Mix (Applied Biosystems), 0.20 µm of each custom designed forward and reverse primer, 0.25 µL of SYBRGreen dye (Invitrogen) and 1.0 µL of diluted RT. These reactions were cycled according to recommended conditions.
Amplification in the absence of template failed to produce any signal that would have occurred due to primer dimerization and extension. End point melt-curve analysis confirmed the presence of single amplicons in each reaction well.
Quantitative immunoblotting studies
Samples were processed by a rigorous two-stage normalization approach (Mooney and Miller 2000). Accordingly, three independent samples were harvested from each treatment group at six time points (3, 6, 12, 18, 24, and 48 h). Solubilized cells were centrifuged, the proteinaceous supernatant was collected, and aliquots of the supernatant containing equal quantities of total protein were loaded onto the lanes of a sodium dodecyl sulfate polyacrylamide gel. Each aliquot contained 30 µg of protein. At least one sample from each condition was placed on a single blot to facilitate direct comparisons of the treatment. In addition, an internal standard (pooled homogenate of whole brains from 12-day-old rats) was applied to two lanes and a set of rainbow molecular weight standards was added to another lane.
Proteins were separated by electrophoresis, transferred to nitrocellulose filters, and immunoblots for each protein of interest were produced. Filters were washed with 5.0% non-fat dry milk in 0.010 m phosphate-buffered saline (pH 7.4) and 0.10% Tween-20 (TPBS) at room temperature for (20°C) 1 h to block non-specific immunoreactivity. Subsequently, the filters were incubated with a primary antibody for 1.5 h at room temperature. Four primary antibodies were used: anti-nCAM (diluted 1: 2000 in TPBS; Sigma, St. Louis, MO, USA), anti-integrin α1 (diluted 1 : 500 in TPBS; Santa Cruz Biotech., Santa Cruz, CA, USA), anti-integrin α7 (diluted 1 : 500 in TPBS; generously provided by Stephen J. Kaufman, University of Illinois, Urbana, IL, USA), and anti-integrin β1 (diluted 1 : 1000 in TPBS; BD Biosciences, San Jose, CA, USA). The filters were incubated with a secondary antibody conjugated to horseradish peroxidase (diluted 1 : 2000 in TPBS; Amersham, Arlington Heights, IL, USA) for 1 h. The immune complexes were detected with the enhanced chemiluminescence method (Amersham). The relative amount of each protein imaged on the films was quantified using a Kodak Image Station.
To control for variation in loading, expression in each lane was normalized against the amount of β-actin or β-tubulin expression depending on the molecular weight of the antibody of interest. Blots generated for proteins with molecular weights below 50 kDa were normalized to β-actin. Blots for proteins of molecular weights higher than 50 kDa were normalized to β-tubulin. Neither TGFβ1 nor ethanol affected the expression of β-actin or β-tubulin. The blots were stripped of immunolabeling and re-probed with the β-actin (diluted 1 : 4000 in TPBS; Sigma) or β-tubulin (diluted 1 : 1000 in TPBS; Santa Cruz) antibodies. Each experiment was repeated three times for each primary antibody.
Controls for non-specific binding included preparations in which the primary or secondary antibody was omitted. In a control for specific binding, blots were processed with primary antibody preabsorbed with an excess of antigen. No immunostaining was detected in these controls.
Statistical analyses
Analyses of the data from the qRT–PCR studies were performed using repeated measures anova comparing the differences in the number of cycles to threshold (ΔCt) between the gene of interest and a reference gene (RNA polymerase II). Group differences were calculated by determining the mean difference in the ΔCt values per sample group (the ΔΔCt), and a fold change (FC) calculated according to the formula FC = 2−ΔΔCt The means (and standard errors of the means) were calculated based on three samples per timepoint for each of the four treatment groups.
Data from the immunoblots were compared by normalizing against the mean values determined for the internal standards (Mooney and Miller 2000). This approach allowed for comparisons of data from multiple blots. Secondarily, data were normalized against actin or tubulin to equate for loading variation. Data were compared with two-way analyses of variance for treatment and time. Significant differences were further pursued by post hoc t-tests. Comparisons of the effectiveness of TGFβ1 and ethanol were made with a Pearson's correlation test.
Results
Transcript identification
RNA was harvested from B104 neuroblastoma cells that were treated with TGFβ1 (0 or 10 ng/mL) and ethanol (0 or 400 mg/dL). Two distinct bands of RNA (18S and 28S) were isolated from each sample (Fig. 1). DNA contamination and RNA breakdown products were virtually nonexistent.
RNA isolation. Samples of RNA were harvested from B104 cells treated with transforming growth factor (TGF) β1 (0 or 10 ng/mL) and ethanol (Et: 0 or 400 mg/dL) and analyzed using ultraviolet spectrophotometry. On the left is a ladder of standard RNAs of known sizes. CT, control.
A cluster analysis of gene expression was performed to identify transcripts for CAPs that were uniquely expressed in a treatment- or time-dependent manner. Sixty-seven transcripts were affected by TGFβ1 and/or ethanol. Further screening showed that 20 of these genes were unique (some were identified by multiple probe sets) and were meaningfully expressed by B104 cells (Fig. 2).

Microarray analyses of B104 neuroblastoma cells. The amounts of mRNA for cell adhesion proteins (CAPs) were determined in samples taken from cells treated with transforming growth factor β1 (TGFβ1) and/or ethanol (Et) for 6, 12, or 18 h. Twenty genes (from 22 probe sets; two were from the same gene) were identified as being affected by TGFβ1 or ethanol. Quantitative data were normalized to expression detected in untreated controls (Ct) at each time point. Darker boxes represent situations in which transcript expression was increased and lighter boxes for reduced transcript expression. Note that the scale goes to 2.0-fold and is occasionally saturated, as in the case of osteopontin, where TGFβ1-induced increases were up to 2.6-fold. The transcripts listed in bold are those that were further examined in the quantitative real-time RT–PCR and immunoblotting assays.
Each gene was differentially affected in a time-dependent manner. For example, TGFβ1 maximally stimulated integrin β1 and tyrosine kinase receptor (Ptk-3) within 6 h and the expression of these transcripts later fell, even below control amounts. This contrasted with (i) TGFβ1-mediated stimulation of osteopontin, which consistently increased between 6 and 18 h, and (ii) TGFβ1-mediated transient depression of solute carrier family 8 and vCAM at 6 h, which was followed by an increase at 18 h. Ethanol also had variable effects on the identified genes; often these effects paralleled those of TGFβ1. In cultures treated with TGFβ1 and ethanol, the effect tended to be additive (e.g. solute carrier family 8 and integrin α1 at 18 h) or similar to the effect of TGFβ1 or ethanol alone (e.g. osteopontin).
Verification with real-time polymerase chain reaction
Five of the genes identified by the microarray analyses (for nCAM, L1, and integrin subunits α1, α7, and β1) were selected for verification. These genes were selected (i) because they exhibited different patterns of TGFβ1- and ethanol-induced changes and (ii) because previous studies showed that TGFβ1 and ethanol affected the expression of the translated protein (Luo and Miller 1999a; Miller and Luo 2002; Siegenthaler and Miller 2004).
qRT–PCR assays were used to confirm the array data. Sample amplification curves for the analysis of integrin α7 are shown in Fig. 3. The series of sigmoidal functions described the effects of TGFβ1 and ethanol on the number of cycles needed to generate detectable amounts of transcript. In the example shown, the number of cycles needed to generate threshold amounts of integrin α7 mRNA was greater for TGFβ1- and ethanol plus TGFβ1-treated samples. This contrasts with the lack of a treatment-induced change in the expression of control transcript for RNA polymerase II.
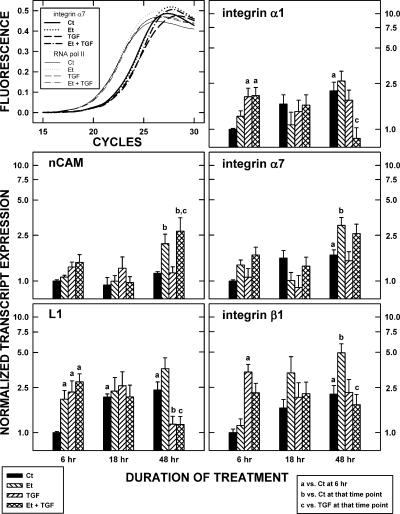
Verification of transcript expression. Top left: a representative set of growth curves illustrates the increase in the amount of integrin α7 transcript (measured with SYBRgreen fluorescence) with increasing numbers of polymerase chain reaction (PCR) cycles (coarse lines). The signal was corrected for background fluorescence. Transforming growth factor (TGF) β1 and ethanol (Et) caused a shift in the growth curves, indicating that more cycles were needed to detect a certain amount of a transcript. The implication was that TGFβ1 and ethanol reduced the amount of mRNA in the samples. No changes were evident in the control (Ct) mRNA for RNA polymerase II (fine lines). Histograms: the effect of TGFβ1 and ethanol on the expression of five cell adhesion protein (CAP) transcripts was determined using real-time PCR. Data were standardized against the amount of transcript expressed in the controls at time 6 h. Data represent the means of three samples and T-bars are the standard errors of the means. Statistically significant differences (p < 0.05) relative to the control samples at 6 h, to the control within the time point, or to the cells treated with TGFβ1 within the time point, are noted by ‘a’, ‘b’, and ‘c’, respectively.
Each of the five transcripts was detected by qRT–PCR for untreated and treated cells. The expression of mRNAs for CAPs often was stable over time for the controls. There were, however, significant (p < 0.05) increases in the controls at the 18 h time point in the expression of L1 and at the 48 h time point for L1 and integrins α1 and β1 (i.e. compared to the controls at 6 h). The implication is that the B104 cells were secreting something that eventually caused an autocrine/paracrine-mediated up-regulation in these mRNAs.
Time- and/or treatment-induced changes in mRNA expression were common. For example, integrin β1 expression was up-regulated by TGFβ1 within the first 6 h and that elevated expression was maintained for at least the next 42 h. In general, the responses are complex, largely because of the dynamism of the changes in the untreated controls (see above). At 48 h, additive effects of TGFβ1 and ethanol can be discerned (nCAM) and counteracting effects of TGFβ1 and ethanol were apparent (L1, integrin α1, and integrin β1).
Protein expression
The effects of TGFβ1 (0 or 10 ng/mL) and ethanol (0 or 400 mg/dL) on the expression of nCAM and integrins α1, α7, and β1 was determined using a quantitative immunoblotting method (Fig. 4). [L1 was not included in this analysis because of difficulties in obtaining reliable, quantifiable immunoblots.] In general, protein expression was constant in the control preparations, i.e. there was no change in expression over time. This was particularly notable for nCAM and integrin β1. There was, however, a transient fall over the 9 hours of treatment between 3 and 12 h in the amount of integrin α1. Thereafter, no statistically significant changes were detected.
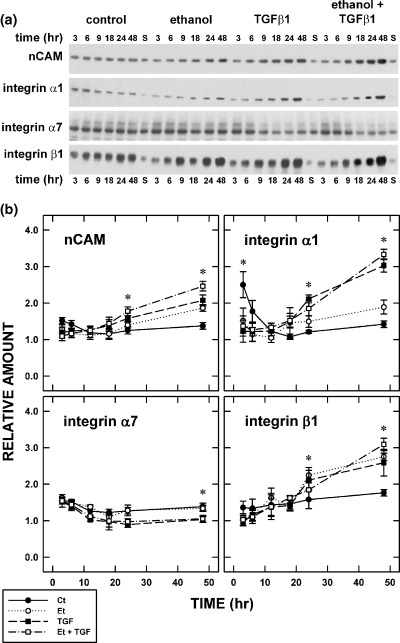
Expression of cell adhesion proteins. (a) Immunoblots depict the temporal change in expression of four representative cell adhesion proteins (CAPs): neural cell adhesion molecule (nCAM) and integrin α1, α7, and β1. Samples were collected at six times (3, 6, 9, 18, 24, and 48 h) after the introduction of transforming growth factor β1 (TGFβ1) and/or ethanol. (b) Quantitative immunblot analyses were used to assess the effects of TGFβ1 and/or ethanol (Et). Each data point is the mean of three samples and the error bars signify standard errors of the means. Statistically significant differences (p < 0.05) are identified by an asterisk.
Treatment with TGFβ1 or ethanol induced a temporal increase in the expression of nCAM, integrin α1, and integrin β1 (Fig. 4). Some increases reached statistical significance (p < 0.05) at 24 h; the effects of TGFβ1 on nCAM and integrins α1 and β1 and the combined effects of TGFβ1 and ethanol on nCAM and integrin α1. Differences were best appreciated following treatment for 48 h. All four exemplary CAPs were significantly (p < 0.05) affected by TGFβ1; however, only nCAM and integrins α1 and β1 were affected by ethanol. Combined TGFβ1 and ethanol treatment consistently affected CAP expression; generally this was an increase in expression except for integrin α7.
Integration of changes in cell adhesion protein transcript and protein expression
At 48 h, it was evident that expression of most CAP protein was significantly (p < 0.05) increased by TGFβ1 or ethanol treatment. This is rather surprising considering that the changes in CAP transcript expression varied in time and direction. In general, the effects of ethanol on CAP transcript and protein expression echoed the effects of TGFβ1. RNAs and proteins for various CAPs (e.g. nCAM, and integrins α1, α7, and β1) are affected by ethanol. The effects of ethanol on RNAs proceeded on different timelines. In some cases, the effects of ethanol were quite rapid; e.g. transcript expression rose within 6 h for integrin β1. The associated increase in integrin β1 protein expression, however, only became evident by 24 h. The notable exception was integrin α7; neither the transcript nor protein of which was affected by treatment with TGFβ1 or ethanol.
Across the five transcripts that were examined with real-time PCR, the correlation between TGFβ1-treated and ethanol-treated samples (when the samples were normalized against the untreated samples at each time point) was particularly high at 6 h (r = 0.846) and 18 h (r = 0.768). The correlation was weak at 48 h (r = − 0.081). On the other hand, following normalization of the data against the 6 h timepoint with treatment group, then the correlation between the TGFβ1-treated and ethanol-treated samples was strong (r = 0.504).
Discussion
Methodological considerations
The present study examined the effects of TGFβ1 and ethanol on CAP transcription and translation. Before discussing the data, however, it is important to address methodological issues. B104 cells have been used as a model of developing neurons. They permit controlled analyses of cells that behave like neuronal progenitors; they are renewable and give rise to neuron-like cells (Bottenstein and Sato 1979; Luo and Miller 1997; Miller and Luo 2002). Possibly most important is that they are ideal for studies of growth factors because they do not require serum or any exogenous mitogens to sustain them. The proliferative response of B104 cells (Luo and Miller 1997) and the substances that they elaborate (e.g. nCAM) (Luo and Miller 1999a; Miller and Luo 2002; present study) mimic the activities of developing neurons both in situ (Siegenthaler and Miller 2004, 2005) and in vivo (Miller and Nowakowski 1991).
Microarray screening approaches have been criticized for the difficulty in identifying thresholds of significance and for generating false positives. In this report, we chose to specifically examine cell adhesion protein transcripts, rather than focus on the genes with the most robust or most significant changes in expression. Thus, our analysis was susceptible to producing more false positives than an approach based on these types of thresholds. Nonetheless, we independently validated a sampling of the array findings using RNA from additional experimental preparations, and observed a high degree of validity and reproducibility of the original findings. Based on the present empirical studies, we estimate that the analysis of single functionally defined gene groups with microarrays generated a small percentage of false positive results (type I errors), but had reasonable power to detect true positive changes (and avoid type II errors). That said, the method is not so reliable that verification of the results can be omitted, but given sufficiently stringent criterion, data from the microarray method can be used to guide molecular inquiries.
Transforming growth factor β1-affected transcription
TGFβ1 regulates the expression of transcripts of various CAPs. These effects vary among CAPs. The expression of some transcripts is increased and for others it is depressed. Moreover, the effects are time-dependent. Some genes respond relatively early to the TGFβ1 and others are not recruited until 18 h post-treatment. The changes in gene expression are largely mirrored by later changes in the expression of the associated protein. The implication is that TGFβ1 initiates receptor-mediated pathways that ultimately lead to the translation of CAPs. Though studies of neural cells show that CAP expression is regulated by TGFβ1 (Luo and Miller 1999a; Miller and Luo 2002; Siegenthaler and Miller 2004), other studies show that TGFβ1 promotes CAP transcript and protein expression in non-neural tissues (e.g. Li et al. 2001; Chakraborty et al. 2002; Wright et al. 2003).
CAPs such as integrins and cell adhesion molecules (CAMs) are important in neuronal migration and neurite outgrowth (e.g. Dulabon et al. 2000; Bruses and Rutishauser 2001; Kamiguchi 2003; Bamdad et al. 2004; Schmid et al. 2004). As mentioned above, these processes are regulated by TGFβ1 (Ishihara et al. 1994; Abe et al. 1996; Ho et al. 2000). CAP expression in organotypic cultures of the cerebral cortex shows that the effects of TGFβ1 are concentration-dependent (Siegenthaler and Miller 2004). Increasing amounts of TGFβ1 promote increasing expression of CAPs. Nevertheless, the bioeffect of TGFβ1 on neuronal migration is bimodal; at low concentrations, TGFβ1 is facilitatory, whereas at higher concentrations, it retards neuronal migration. Presumably this is because a microenvironment that is too rich in CAPs is not conducive to migration, possibly due to over-adhesive neuron–glia interaction.
Effects of ethanol
Ethanol has broad, time-dependent effects on the expression of CAPs. One CAP that has received a great deal of attention as a target of ethanol is L1 (e.g. Ramanathan et al. 1996; Bearer et al. 1999; Wilkemeyer et al. 1999, 2003; Bearer 2001). For example, in NG108-15 cells, ethanol affects L1 expression and L1-mediated cell-cell adhesion. These properties (i) may be cell line-specific (Wilkemeyer and Charness 1998) and (ii) may not relate to ethanol-induced defects in neuronal migration (Vallejo et al. 1997; Sakata-Haga et al. 2004). On the other hand, ethanol affects the expression of other CAPs, including nCAM and integrins, in other situations, e.g. B104 cells (Luo and Miller 1999a), dissociated cortical neurons (Miller and Luo 2002), cerebral cortical cells in organotypic cultures (Siegenthaler and Miller 2004), and whole animals (Miñana et al. 2000; Ozer et al. 2000). Thus, the effects of ethanol on CAPs expressed in the developing neural cells are complex and cannot be distilled to a specific transcript/protein.
Effects of ethanol on transforming growth factor β1-mediated changes in cell adhesion protein transcript and protein expression
The combined effects of TGFβ1 and ethanol can be additive or antagonistic. One example is the effects of TGFβ1 and ethanol on nCAM expression. In B104 neuroblastoma cells, the effects are additive (Luo and Miller 1999a; present study). Yet in astrocytes and C6 astrocytoma cells, the inhibitory effects of TGFβ1 and ethanol cancel one another. Though these data can be interpreted as TGFβ1 altering ethanol toxicity, because TGFβ1 (and not ethanol) is naturally expressed in the developing nervous system (Flanders et al. 1991; Pelton et al. 1991; Krieglstein et al. 2002; Miller 2003), it is more parsimonious to conclude that ethanol interferes with the effects of TGFβ1.
On review of the data on the effects of TGFβ1 and ethanol, patterns in the changes of CAP transcript and protein emerge.
- (i)
Changes in transcript expression do not reliably lead to changes in protein expression. For example, although transcript expression for integrin α7 is increased by combined TGFβ1 and ethanol treatment, TGFβ1 and ethanol slightly, but significantly (p < 0.05), depress the expression of integrin α7 protein.
- (ii)
The time line for changes in protein expression does not follow in lock-step with changes in transcript expression. Often it takes 24 h for a detectable change in protein expression to emerge, yet a change in transcript expression may be apparent within 6 or 18 h of treatment.
Despite the similar patterns in TGFβ1- and ethanol-induced responses, there are many changes that do not fit into patterns. For example, the timing and direction of the effect of TGFβ1, ethanol, or TGFβ1 and ethanol vary widely among the CAP transcripts. The implications from the varied effects of TGFβ1 and ethanol are (i) that neither is a simple trigger of the transcription/translation of CAPs and (ii) that some other factor(s) is involved. Such paradoxical effects are also evident in the effect of TGFβ1 and ethanol on mitogen-activated protein kinase (MAPK). MAPK is stimulated by mitogenic growth factors, e.g. basic fibroblast growth factor, epidermal growth factor, and platelet-derived growth factor (e.g. Luo and Miller 1999b). Interestingly, even though TGFβ1 and ethanol are anti-proliferative factors, both TGFβ1 and ethanol stimulate MAPK phosphorylation in proliferating B104 cells (Luo and Miller 1999a). With combined treatment, activation of MAPK is exacerbated, as is expression of CAPs such as nCAM. The answer to this and related riddles may lie from understanding (i) non-consensual systems that are activated by TGFβ1 and ethanol, i.e. the SMADs and protein kinase C, respectively, and (ii) TGFβ1- and ethanol-induced changes in transcript and protein turnover/stability.
Acknowledgements
We thank Terri Novak and Wendi Burnett for their help in processing samples in the RNA and protein analyses, respectively. This research has been supported by the National Institute on Alcohol Abuse and Alcoholism (AA06916, AA07568 and AA15413) and the Department of Veterans Affairs.




