Rho family GTPase inhibition reveals opposing effects of mitogen-activated protein kinase kinase/extracellular signal-regulated kinase and Janus kinase/signal transducer and activator of transcription signaling cascades on neuronal survival
Abstract
Rho family GTPases promote the survival of certain neuronal populations. However, pro-survival and pro-death signaling pathways regulated downstream of Rho GTPases are largely unknown. Cerebellar granule neurons (CGNs) exposed to Clostridium difficile toxin B (ToxB), a monoglucosyltransferase that specifically inhibits Rho GTPases, die by a mitochondrial apoptotic cascade. Using a high-throughput immunoblotting screen (BD Powerblot), we found that ToxB markedly reduced the expression of Rac1 and c-Raf, upstream components of a Rac-dependent mitogen-activated protein (MAP) kinase pathway. Moreover, ToxB rapidly suppressed a p21-activated kinase/MAP kinase kinase (MEK)/extracellular signal-regulated kinase (ERK)1/2 signaling cascade that normally promotes degradation of the Bcl-2 homology-3 (BH3)-only protein Bim, a key initiator of mitochondrial apoptosis. In contrast to c-Raf down-regulation, ToxB enhanced expression of the transcription factor, signal transducer and activator of transcription-1 (STAT1). Both STAT1 up-regulation and apoptosis induced by ToxB were prevented by a pan-inhibitor of Janus kinases (JAKs), indicating that JAK/STAT signaling was pro-apoptotic in CGNs. Most significantly, direct inhibition of MEK was sufficient to trigger JAK-dependent STAT1 expression, suggesting that cross-talk between MEK/ERK and JAK/STAT pathways plays a key role in regulating neuronal survival. Finally, ERK dephosphorylation and STAT1 up-regulation induced by ToxB were mimicked by a dominant-negative (N17) mutant of Rac1. These data suggest that the MEK/ERK cascade functions downstream of Rac GTPase to actively repress pro-apoptotic JAK/STAT signaling in healthy CGNs.
Abbreviations used
-
- ActD
-
- actinomycin D
-
- BSA
-
- bovine serum albumin
-
- Casp3
-
- caspase 3
-
- CGN
-
- cerebellar granule neuron
-
- con
-
- vehicle control
-
- DAPI
-
- 4,6-diamidino-2-phenylindole
-
- ERK
-
- extracellular signal-regulated kinase
-
- GFP
-
- green fluorescent protein
-
- JAK
-
- Janus kinase
-
- JAK Inh
-
- JAK inhibitor I
-
- IB
-
- immunoblot
-
- LTox
-
- Clostridium sordellii lethal toxin
-
- MAP
-
- mitogen-activated protein
-
- MEK
-
- MAP kinase kinase
-
- NS
-
- non-specific protein
-
- PAK
-
- p21-activated kinase
-
- PBS
-
- phosphate-buffered saline
-
- PBS-T
-
- PBS containing 0.1% Tween 20
-
- PD
-
- PD98059
-
- p-ERK
-
- phosphorylated ERK
-
- p-MEK
-
- phosphorylated MEK
-
- p-PAK
-
- phosphorylated PAK
-
- PVDF
-
- polyvinylidene difluoride
-
- SDS–PAGE
-
- sodium dodecyl sulfate–polyacrylamide gel electrophoresis
-
- SOCS
-
- suppressor of cytokine signaling
-
- STAT
-
- signal transducer and activator of transcription
-
- ToxB
-
- Clostridium difficile toxin B
-
- Veh
-
- vehicle
Rho family GTPases are members of the Ras superfamily of monomeric GTPases. The three most studied Rho proteins, RhoA, Rac1 and Cdc42, are best known for regulating actin cytoskeletal dynamics (Ridley and Hall 1992; Ridley et al. 1992; Nobes and Hall 1995). Over the past decade, Rho family GTPases have been implicated in a diverse array of cell functions (Etienne-Manneville and Hall 2002). In the CNS, Rho family GTPases regulate multiple signaling pathways that influence neuronal development (Govek et al. 2005). For example, Rho family GTPases modulate neuronal growth cone remodeling (Kozma et al. 1997), synaptic neurotransmitter release (Humeau et al. 2002), dendritic spine morphogenesis and synapse formation (Scott et al. 2003; Tolias et al. 2005; Zhang et al. 2005), and axonal guidance (Yuan et al. 2003; Ng and Luo 2004; Jin et al. 2005). The critical roles that Rho family GTPases play in neuronal physiology are further evidenced by the fact that several Rho GTPase-interacting proteins, oligophrenin1, p21-activated kinase (PAK)3 and alphaPix, are mutated in mental retardation (van Galen and Ramakers 2005).
In addition to their effects on neuronal physiology, Rho family GTPases are also key regulators of neuronal survival. For example, several studies have correlated a loss of plasma membrane-associated Rho family GTPases with a significant increase in neuronal apoptosis (Tanaka et al. 2000; Garcia-Roman et al. 2001; Meske et al. 2003). More recently, a genome-scale, oligonucleotide microarray revealed that the down-regulation of Rho family GTPases is statistically associated with an increase in neuronal apoptosis (Desagher et al. 2005). Consistent with a critical role for Rho family GTPases in neuronal survival, truncation and subsequent loss of function of the Rac1 guanine nucleotide exchange factor, alsinLF, leads to motor neuron apoptosis and juvenile-onset amyotrophic lateral sclerosis (Topp et al. 2004; Kanekura et al. 2005).
In agreement with the above studies, we have previously shown that Rho family GTPase function, particularly Rac activity, is essential for the survival of cultured cerebellar granule neurons (CGNs) (Linseman et al. 2001). Either incubation with large clostridial cytotoxins exhibiting overlapping specificities for inhibiting Rac or overexpression of a dominant-negative Rac1 mutant induces significant apoptosis of CGNs via activation of a mitochondrial death cascade (Linseman et al. 2001; Le et al. 2005). In the present study, we used Clostridium difficile toxin B (ToxB), a specific inhibitor of Rho, Rac and Cdc42 (Just et al. 1995), as a tool to identify novel Rho family GTPase-regulated signaling pathways involved in neuronal survival and apoptosis. ToxB induced down-regulation of several components of a Rac-dependent mitogen-activated protein (MAP) kinase pathway and up-regulation of the transcription factor signal transducer and activator of transcription-1 (STAT1). Further analysis revealed that Rac GTPase inhibition induces CGN apoptosis by inactivating a pro-survival PAK/MAP kinase kinase (MEK)/extracellular signal-regulated kinase (ERK) cascade that ultimately results in the de-repression of a pro-apoptotic Janus kinase (JAK)/STAT pathway.
Materials and methods
Reagents
ToxB and Clostridium sordellii lethal toxin (LTox) were prepared using a method published previously (von Eichel-Streiber et al. 1987). The high-throughput immunoblotting screen was done by BD Pharmingen (Palo Alto, CA, USA) and monoclonal antibodies used for subsequent western blotting of Rac1, c-Raf, ERK1/2, Rab4 and STAT1 were obtained from BD Biosciences (San Diego, CA, USA). Polyclonal antibodies against phospho (p)-PAK1/2/3 (Ser144/141/139), p-MEK1 (Ser298), the dually phosphorylated forms of ERK1 and 2 (p-ERK1 and p-ERK2), and a monoclonal antibody against actin were from Cell Signaling (Beverly, MA, USA). Horseradish peroxidase-linked secondary antibodies and reagents for enhanced chemiluminescence detection were from Amersham Biosciences (Piscataway, NJ, USA). Polyclonal antibody to active caspase 3 was from Promega (Madison, WI, USA). 4,6-Diamidino-2-phenylindole (DAPI), Hoechst dye 33258 and monoclonal antibody against β-tubulin were from Sigma (St Louis, MO, USA). Rabbit polyclonal antibody against Bim was from Stressgen (San Diego, CA, USA). Cy3- and FITC-conjugated secondary antibodies for immunofluorescence were from Jackson Immunoresearch Laboratories (West Grove, PA, USA). PD98059 (PD), U0126 and the small molecule JAK inhibitor I [2-(1,1-dimethylethyl)9-fluoro-3,6-dihydro-7H-benz[h]-imidaz[4,5-f]isoquinolin-7-one; JAK Inh] were from Calbiochem (San Diego, CA, USA).
CGN culture
Rat CGNs were isolated from 7-day-old Sprague–Dawley rat pups of both sexes (15–19 g) as described previously (Linseman et al. 2001). Neurons were plated on 35-mm diameter plastic dishes coated with poly-l-lysine at a density of 2.0 × 106 cells/mL in basal modified Eagle's medium containing 10% fetal bovine serum, 25 mm KCl, 2 mm l-glutamine, and penicillin (100 units/mL)/streptomycin (100 µg/mL) (Life Technologies, Grand Island, NY, USA). Cytosine arabinoside (10 µm) was added to the culture medium 24 h after plating to limit the growth of non-neuronal cells. Using this protocol, the cultures were approximately 95% pure for granule neurons. In general, experiments were performed after 6–7 days in culture.
Cell lysis and immunoblotting
After treatment as described in the results section, cells were kept on ice while the culture medium was aspirated and the cells were washed once with 2 mL ice-cold phosphate-buffered saline (PBS) (pH 7.4). The cells were then incubated for 10 min in lysis buffer (200 µL per 35-mm well) containing 20 mm HEPES (pH 7.4), 1% Triton X-100, 50 mm NaCl, 1 mm EGTA, 5 mmβ-glycerophosphate, 30 mm sodium pyrophosphate, 100 µm sodium orthovanadate, 10 µg/mL leupeptin and 10 µg/mL aprotinin. The cells were then harvested into the buffer by scraping and cell debris was removed by centrifugation at 6000 g for 2 min. A protein assay kit (Pierce Chemical Co., Rockford, IL, USA) was used to determine the protein concentration of the samples. Approximately 80 µg supernatant protein were diluted with sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS–PAGE) sample buffer, boiled for 5 min and electrophoresed through polyacrylamide gels. Proteins were then transferred to polyvinylidene difluoride (PVDF) membranes (Amersham Biosciences) and processed for immunoblot analysis.
Non-specific binding sites were blocked in PBS (pH 7.4) containing 0.1% Tween 20 (PBS-T) and 1% bovine serum albumin (BSA) for 1 h at room temperature (25°C). The membranes were then incubated for 1 h with primary antibodies diluted in blocking solution. Excess primary antibody was removed by washing the membranes five times with PBS-T over 30 min. Appropriate horseradish peroxidase-conjugated secondary antibodies diluted in PBS-T were then incubated with the membranes for 1 h. Excess secondary antibody was removed with five washes of PBS-T over 30 min. Immunoreactive proteins were detected by enhanced chemiluminescence. In general, western blots shown are representative of the results obtained from three independent experiments. Densitometry was used to quantitate changes in protein expression.
BD Pharmingen Powerblot™
CGNs were incubated with either ToxB (40 ng/mL, final concentration) or vehicle (2 µg/mL BSA in PBS, final concentration) for 24 h and then lysed according to the manufacturer's instructions. Lysates from three different CGN cultures were pooled and sent to BD Clontech for high-throughput immunoblotting against a panel of 1009 purified monoclonal antibodies (BD PowerBlot™). Raw data in the form of image files of the actual blots and densitometric measurements of the immunoreactive proteins were obtained from the manufacturer. The blots shown for Rac1, c-Raf and STAT1 are representative of 2 × 2 comparisons of duplicate control and ToxB lysates.
Quantitation of apoptosis
After treatment, Hoechst dye (8 µg/mL, final concentration) was added directly into the wells for 30 min to stain CGN nuclei. The cells were subsequently washed with 1 mL PBS and then incubated in 4% paraformaldehyde in PBS for 30 min at room temperature. The cells were washed twice more with 1 mL PBS before quantitation. Cells were scored as apoptotic when chromatin condensation or fragmentation was seen. About 300 cells were counted in a minimum of three 60 × fields of a 35-mm dish, and final counts represent data obtained from at least three independent experiments performed in duplicate.
Immunocytochemistry
CGNs were plated at a density of 4.5 × 105 cells per coverslip on glass coverslips coated with polyethyleneimine. After treatment, the cells were fixed in 4% paraformaldehyde, washed once in PBS, and then permeabilized and blocked in 0.2% Triton X-100 and 5% BSA in PBS (pH 7.4). Primary antibodies were diluted in 2% BSA and 0.2% Triton X-100 in PBS. Cells were incubated in the primary antibody for approximately 16 h at 4°C. They were subsequently washed five times in PBS over 30 min and then incubated for 1 h at room temperature with Cy3- or FITC-conjugated secondary antibodies and DAPI diluted in 2% BSA and 0.2% Triton X-100 in PBS. The cells were washed five more times over 30 min with PBS before mounting the coverslips on slides with anti-quench composed of 0.1%p-phenylenediamine in 75% glycerol in PBS. Fluorescent images were captured using a 63 × oil immersion objective on a Zeiss (Thornwood, NY, USA) Axioplan 2 microscope equipped with a Cooke (Romulus, MI, USA) Sensicam deep-cooled charge-coupled device (CCD) camera and a Slidebook software analysis program for digital deconvolution (Intelligent Imaging Innovations Inc., Denver, CO, USA).
Adenovirus preparation and infection
Adenoviral dominant-negative Rho family GTPases were constructed as described previously (Allen et al. 2002). Briefly, Rac1 was cloned from a mouse cDNA library. A dominant-negative (T17N) mutant of Rac1 was generated by PCR-based site-directed mutagenesis. Adenoviral green fluorescent protein (GFP) and GFP/N17Rac1were generated using the AdEasy system (Qbiogene, Inc., Carlsbad, CA, USA). The adenoviruses produced express either GFP alone or in combination with the dominant-negative Rac1 mutant. CGN cultures were infected on day 4 in vitro with adenoviruses at a final titer of 50 infectious particles per cell. Using these conditions, the adenoviruses infected approximately 15–20% of CGNs in the cultures (infection efficiency based on GFP fluorescence detection). At 96 h after infection, cell lysates of infected cells were prepared for immunoblot analysis. Owing to the mechanisms involved in infecting primary neurons, CGNs require at least 48 h for infection to be effective (based on visualization of GFP fluorescence). Therefore, infecting them for 96 h is essentially equivalent to exposing the cells for approximately 48 h to the dominant-negative mutant.
Data analysis
Results represent the mean ± SEM for the number (n) of independent experiments performed. Statistical differences between the means of unpaired sets of data were evaluated by one-way anova, followed by a post hoc Tukey's test. A p-value of < 0.05 was considered statistically significant.
Results
ToxB induces down-regulation of Rac1 and c-Raf in CGNs
ToxB covalently modifies Rho family GTPases by monoglucosylation of a highly conserved threonine residue in the switch 1 region of these G proteins (Just et al. 1995). Addition of a glucose molecule at this site prevents Rho GTPases from interacting with their effectors causing the interruption of downstream signaling pathways (Fig. 1a). ToxB specifically modifies and inhibits Rho, Rac, Cdc42, RhoG and TC10 without affecting other small GTPases such as Ras, Rab or Arf (Just et al. 1995; Just and Gerhard 2004). We have shown previously that incubation of CGNs with ng/mL concentrations of ToxB induces significant apoptosis through a mitochondrial death cascade (Linseman et al. 2001; Le et al. 2005). Consistent with this, CGNs incubated for 24 h with ToxB (40 ng/mL) exhibited a marked increase in fragmented and/or condensed nuclei and significant activation of caspase 3, compared with vehicle-treated CGNs (Fig. 1b). In the present study, we used ToxB to investigate signaling pathways regulated by Rho family GTPases that influence neuronal survival.
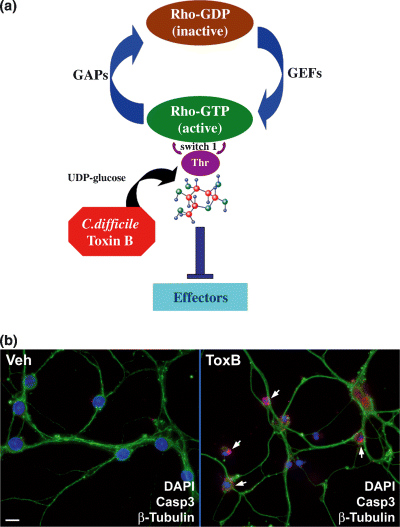
Rho family GTPase-inhibitory ToxB induces CGN apoptosis. (a) Mechanism of action of ToxB; ToxB monoglucosylates a critical threonine residue in the switch 1 region of Rho family GTPases, preventing their interaction with downstream effectors. ToxB specifically modifies and inhibits Rho, Rac, Cdc42, RhoG and TC10 without affecting other small GTPases. GAP, GTPase-activating protein; GEF, guanine nucleotide exchange factor. (b) CGNs were incubated for 24 h in complete medium (containing serum and 25 mm KCl) with either vehicle (Veh; 2 µg/mL BSA in PBS) or ToxB (40 ng/mL). Cells were then fixed with 4% paraformaldehyde, blocked and permeabilized in 5% BSA and 0.2% Triton X-100 in PBS. The cells were incubated with a polyclonal antibody against active caspase 3 (Casp3; shown in red), a monoclonal antibody to β-tubulin (shown in green) and nuclei were stained with DAPI (shown in blue). Cells incubated with vehicle displayed healthy nuclei, an intact microtubule network and essentially no caspase 3 activation, whereas ToxB-treated CGNs exhibited condensed and/or fragmented nuclei, a disrupted tubulin cytoskeleton and extensive immunoreactivity for active caspase 3 (indicated by the arrows). The images shown are representative of those captured in three separate experiments. Scale bar 10 µm.
To identify proteins that change significantly in expression in response to inhibition of Rho family GTPase function, we initially used a proteomics screening approach. CGNs were incubated with either vehicle or ToxB for 24 h and cell lysates were combined from three separate CGN preparations. Neuronal lysates were then analyzed using the BD PowerBlot™ (BD Pharmingen), a high-throughput immunoblotting screen consisting of more than 1000 monoclonal antibodies to a diverse array of proteins (Malakhov et al. 2003; Li et al. 2004; Kim and Lotan 2005). Independent confirmatory western blots were then performed with new CGN preparations using the same monoclonal antibodies as used in the initial PowerBlot™ screen.
As we recently reported, Rac1 GTPase was markedly decreased in expression in CGNs incubated with ToxB for 24 h (Fig. 2a; upper blots, arrows), whereas Rho and Cdc42 expression did not change under these conditions (see Le et al. 2005). In a similar manner, expression of c-Raf, an upstream serine–threonine kinase in the MAP kinase signaling pathway, was also significantly reduced in CGNs exposed to ToxB (Fig. 2a; lower blots, arrows). Confirmatory western blots verified that the ToxB-induced loss of Rac1 and c-Raf expression observed in the PowerBlot™ screen could be reproduced in a distinct CGN preparation (Fig. 2b). In contrast to these dramatic changes in the expression of Rac1 and c-Raf, the MAP kinases ERK1 and ERK2 showed only minimal decreases in immunoreactivity following ToxB, and the small GTPase Rab4 showed no significant change in expression (Fig. 2b).
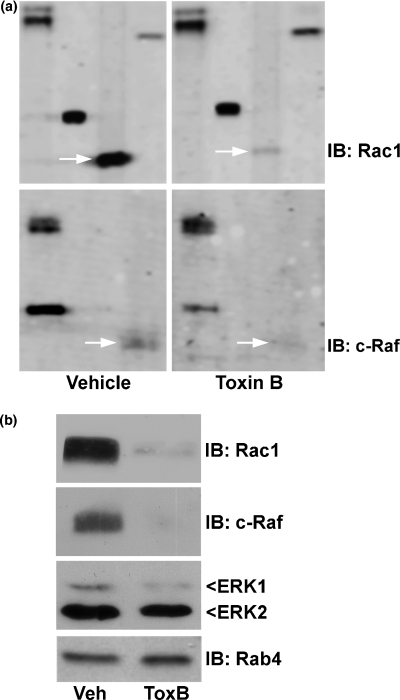
ToxB induces the down-regulation of Rac1 and c-Raf in CGNs. (a) CGNs were incubated for 24 h in complete medium (containing serum and 25 mm KCl) with either vehicle (2 µg/mL BSA in PBS) or ToxB (40 ng/mL). Lysates were prepared according to the manufacturer's directions for the PowerBlot™ screen. TIFF files of the blots comparing duplicate control and ToxB lysates were obtained from BD Pharmingen. The immunoblots (IB) shown represent portions of the membranes probed with antibodies against Rac1 (upper blot) and c-Raf (lower blot), which are indicated by the arrows. The additional bands represent other proteins on the PowerBlot™ membranes and are shown to indicate the overall quality of loading. (b) Independent western blots in a new CGN preparation confirmed the ToxB-induced decreases in expression of Rac1 and c-Raf. The blots were then stripped and re-probed with monoclonal antibodies against ERK1/2 and Rab4. In contrast to the marked decreases in Rac1 and c-Raf, ERK1/2 immunoreactivity was only slightly decreased in CGNs treated with ToxB, whereas the expression of Rab4 was unchanged.
Inhibition of Rho family GTPases inactivates a PAK/MEK/ERK1/2 signaling pathway that normally targets pro-apoptotic Bim for degradation
Time course experiments demonstrated that Rac1 immunoreactivity decreased rapidly following exposure to ToxB, with losses of greater than 50 and 75% after 2 and 4 h of Rho family GTPase inhibition respectively (Fig. 3a). Rac and Cdc42 GTPases can each stimulate ERK1/2 signaling via the interaction of their common effector, PAK, with Raf, MEK or ERK kinases (Li et al. 2001; Eblen et al. 2002; Sundberg-Smith et al. 2005). Consistent with Rac/Cdc42 activity regulating a PAK/MEK/ERK1/2 signaling pathway in CGNs, incubation with ToxB rapidly decreased the phosphorylation of PAK, MEK1 and ERK1/2 (Fig. 3b). Interestingly, significant reductions in p-PAK, p-MEK and p-ERK were detected within 1 h of ToxB addition, preceding any detectable loss of Rac1 immunoreactivity (see Fig. 3a). These data suggest that the ToxB-induced glucosylation of Rac/Cdc42 occurs within 1 h and is sufficient to cause a rapid inactivation (dephosphorylation) of the downstream effector PAK. In contrast, a significant loss of Rac1 immunoreactivity was not observed until a slightly later time point, indicating that the degradation of Rac1 is not required for the acute inhibition of downstream PAK signaling.

The PAK/MEK/ERK1/2 pathway is suppressed and Bim is increased in CGNs subjected to ToxB. (a) CGNs were treated for various periods of time up to 24 h with ToxB (40 ng/mL) in complete medium (containing serum and 25 mm KCl). The cells were then lysed and proteins were resolved by SDS–PAGE and transferred to PVDF membranes. The blot was probed with a monoclonal antibody against Rac1. ToxB induced a significant decrease in Rac1 immunoreactivity within 2 h, which persisted for 24 h. The change in Rac1 expression relative to the control treatment was measured quantitatively by densitometry and is shown above the blot. (b) CGNs were incubated with ToxB (40 ng/mL) for the times indicated (0–8 h). Cell lysates were resolved by SDS–PAGE and proteins were transferred to PVDF membranes. The blots were probed with the following polyclonal antibodies: p-PAK1/2/3 (Ser144/141/139), p-MEK1 (Ser298) and p-ERK1/2 (tyrosine–threonine dually phosphorylated). Incubation with ToxB induced a rapid decrease in the phosphorylated (active) forms of PAK, MEK1 and ERK1/2. NS, a non-specific protein detected by the p-MEK1 antibody and shown as a loading control. Densitometric analysis of the changes in p-PAK expression relative to the control condition is shown above the blot. (c) CGNs were incubated with PD (20 µm) for the times indicated (0–24 h). Cell lysates were resolved by SDS–PAGE on a polyacrylamide gel, and proteins were transferred to PVDF membranes and immunoblotted for p-ERK1/2. NS, a non-specific protein detected by the p-ERK1/2 antibody and shown as a loading control. Densitometric analysis was performed to determine the change in p-ERK2 expression with respect to the control condition and is shown above the blot. (d) CGNs were incubated for 24 h with either vehicle (Veh; 2 µg/mL BSA in PBS), ToxB (40 ng/mL) or PD (20 µm). Incubation of CGNs with either ToxB or the MEK inhibitor (PD) resulted in an increased level of Bim protein. Quantification of the change in Bim expression compared with the control condition is shown above the blot.
One recently described pro-survival action of ERK1/2 is phosphorylation of the BH3-only protein, Bim, which targets this pro-apoptotic Bcl-2 family member for degradation via the proteasome (Ley et al. 2003; Luciano et al. 2003). To determine whether ERK1/2 has this pro-survival effect in CGNs, we evaluated the effect of PD, an inhibitor of the upstream kinase MEK, on Bim expression. As expected, incubation of CGNs with PD significantly reduced ERK1/2 activation as shown by decreased phosphorylation of ERK1/2 over an extended period of at least 24 h (Fig. 3c). Moreover, sustained inactivation of ERK1/2 for 24 h with PD resulted in an increase in Bim protein comparable to that observed after 24 h of ToxB exposure (Fig. 3d). These data suggest that ToxB suppresses a Rac/Cdc42-dependent PAK/MEK/ERK1/2 pathway, resulting in decreased proteasomal degradation of Bim. The consequent increase in Bim expression ultimately contributes to CGN death by initiating a mitochondrial apoptotic cascade.
Clostridial toxins with overlapping specificities for inhibiting Rac elicit a JAK-dependent induction of STAT1 and JAK-dependent CGN apoptosis
In contrast to the dramatic reductions in the expression of Rac1 and c-Raf (see Figs 2a and b), inhibition of Rho family GTPases with ToxB induced a marked increase in expression of the transcription factor STAT1 (Fig. 4a). The induction of STAT1 protein following ToxB exposure was largely blocked by co-incubation with actinomycin D (ActD), an inhibitor of gene transcription (Fig. 4b). Moreover, a small molecule pan-JAK inhibitor, JAK Inh (Fig. 4c), also prevented the induction of STAT1 elicited by ToxB (Fig. 4b).
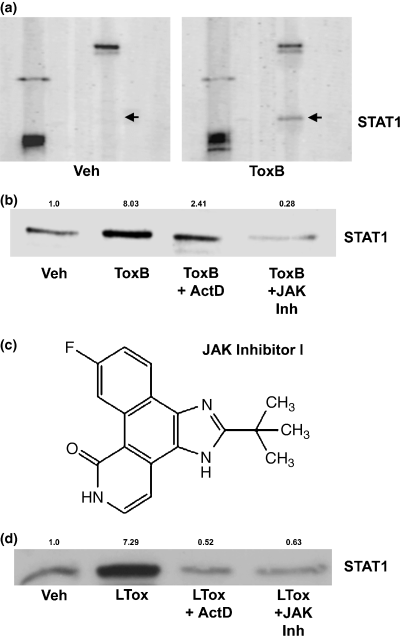
STAT1 expression is up-regulated in CGNs treated with Rac-inhibitory clostridial toxins in a transcriptional- and JAK-dependent manner. (a) A portion of the membrane from the PowerBlot™ probed for STAT1. Cells were incubated for 24 h with either vehicle (Veh; 2 µg/mL BSA in PBS) or ToxB (40 ng/mL) in complete medium (containing serum and 25 mm KCl). CGNs were lysed and processed for the BD PowerBlot™. A monoclonal antibody was used to detect expression of STAT1 (indicated by the arrows). Other proteins blotted with distinct antibodies are shown to give an indication of the overall equivalency of protein loading. (b) CGNs were incubated for 24 h with either vehicle (2 µg/mL BSA in PBS), ToxB (40 ng/mL), ToxB + ActD (4 µm), or ToxB + JAK Inh (1 µm). STAT1 expression was up-regulated with ToxB, and this effect was blocked by either ActD or JAK Inh. The quantitative change in STAT1 expression relative to the control condition is shown above the blot. (c) Molecular structure of JAK Inh. (d) CGNs were incubated for 24 h with either vehicle, LTox (1 µg/mL), LTox + ActD, or LTox + JAK Inh. The induction of STAT1 observed after incubation with LTox was blocked by co-incubation with either ActD or JAK Inh. Quantification of the change in STAT1 expression compared with the control condition is shown above the blot.
To further establish which Rho family GTPase(s) is involved in regulating STAT1 expression in CGNs, we examined the potential of LTox to induce STAT1. LTox is a monoglucosyltransferase that shows overlapping specificity with ToxB for inhibiting Rac, whereas it inhibits Cdc42 to a much lesser extent and does not inhibit Rho, RhoG or TC10 (Genth et al. 1996; Popoff et al. 1996; Just and Gerhard 2004). Consistent with STAT1 expression being up-regulated in CGNs principally as a result of Rac inhibition, incubation with LTox elicited an increase in STAT1 protein similar to that induced by ToxB (Fig. 4d). STAT1 induction by LTox was similarly blocked by co-incubation with either ActD or JAK Inh. Collectively, these results suggest that inhibition of Rac GTPase leads to stimulation of a JAK/STAT pathway that, in turn, transcriptionally up-regulates STAT1.
STAT1 has recently been shown to play a pro-apoptotic role in the neuronal death induced during ischemic brain injury (Takagi et al. 2002). To determine whether a JAK/STAT pathway exerts a similar pro-apoptotic effect in neurons in which Rho family GTPases (and particularly Rac) are inhibited, we assessed the neuroprotective effects of JAK Inh in ToxB-treated cells. CGNs incubated with ToxB alone demonstrated a marked increase in nuclear condensation and fragmentation relative to vehicle-treated CGNs, and this effect was significantly attenuated by JAK Inh (Fig. 5a). Quantification of apoptosis in CGNs co-incubated with ToxB and JAK Inh revealed significant neuroprotection by the pan-JAK inhibitor at submicromolar concentrations (Fig. 5b). These data indicate that inhibition of Rho family GTPases, and Rac in particular, elicits the activation of a JAK/STAT pathway that plays a significant pro-apoptotic role in CGNs.
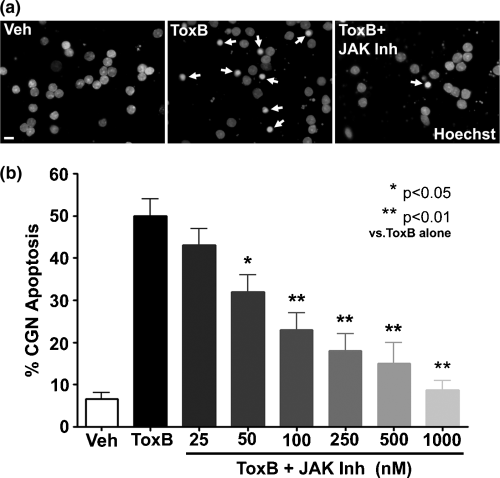
Blockade of JAK activity protects CGNs from apoptosis induced by inhibition of Rho family GTPases. (a) Nuclear staining of CGNs incubated with either vehicle (Veh; 2 µg/mL BSA in PBS), ToxB (40 ng/mL), or ToxB + JAK Inh (1 µm) for 24 h in complete medium (containing serum and 25 mm KCl). Following incubation, cells were fixed with paraformaldehyde and nuclei were stained with Hoechst. CGNs incubated with ToxB exhibited many condensed and/or fragmented nuclei (indicated by arrows). CGNs co-incubated with ToxB and JAK Inh were significantly protected from apoptosis and their nuclei were morphologically similar to those of controls. Scale bar 10 µm. (b) Quantitation of apoptosis in CGNs incubated with ToxB and various concentrations of JAK Inh for 24 h. JAK Inh significantly protected CGNs from ToxB-induced apoptosis in a dose-dependent manner. Values are mean ± SEM of four independent experiments, each performed in duplicate. *p < 0.05, **p < 0.01 versus ToxB alone (one-way anova with a post-hoc Tukey's test).
The MEK/ERK cascade signals downstream of Rac to repress a pro-apoptotic JAK/STAT pathway in CGNs
The data presented so far indicate that both suppression of the MEK/ERK pathway and induction of a JAK/STAT pathway contribute to CGN apoptosis elicited by inhibition of Rho family GTPases. Next, we investigated whether there is any cross-talk between these two signaling pathways. Several previous studies have shown that ERK activity can negatively regulate JAK/STAT signaling in non-neuronal cells (Jain et al. 1998; Sengupta et al. 1998; Krasilnikov et al. 2003). To determine whether ERK1/2 similarly regulates JAK/STAT signaling in CGNs, we examined the effects of two pharmacological inhibitors of MEK (PD and U0126) on STAT1 expression. Incubation of CGNs for 24 h with PD induced a marked increase in STAT1 protein that was completely blocked by co-incubation with JAK Inh (Fig. 6a). In a similar manner, U0126 significantly reduced ERK1/2 phosphorylation while concomitantly increasing STAT1 expression (Fig. 6b). STAT1 induction elicited by U0126 was completely blocked by JAK Inh; however, the JAK inhibitor had no effect on the loss of p-ERK1/2. These results demonstrate that endogenous MEK/ERK signaling actively represses a JAK-dependent induction of STAT1 in healthy CGNs.
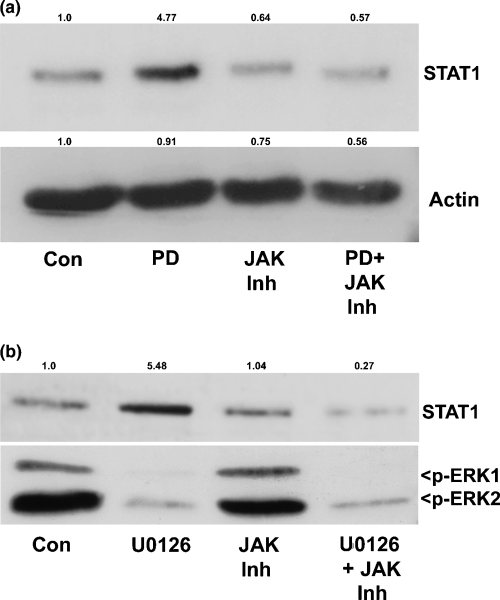
Inhibition of the MEK/ERK pathway induces STAT1 expression. (a) CGNs were incubated with either control vehicle (Con; 0.2% dimethylsulfoxide), PD (20 µm), JAK Inh (1 µm), or PD + JAK Inh in complete medium (containing serum and 25 mm KCl). After 24 h, cells were lysed, and proteins were resolved by SDS–PAGE on polyacrylamide gels, transferred to PVDF membranes and immunoblotted with a monoclonal antibody against STAT1. The blot was also cut and probed with a polyclonal antibody to actin to demonstrate equal loading. The MEK/ERK pathway inhibitor PD caused a marked induction of STAT1 in CGNs that was completely blocked by JAK Inh. Densitometric analysis was performed and quantitative changes in STAT1 and actin expression with respect to the control condition are shown above each blot. (b) CGNs were incubated with either control vehicle, U0126 (10 µm), JAK Inh or U0126 + JAK Inh in complete medium for 24 h. Cells were then lysed and resolved by SDS–PAGE. Proteins were transferred to PVDF membranes and probed with antibodies against p-ERK1/2 and STAT1. The MEK inhibitor U0126 significantly up-regulated STAT1, and the effect was blocked by JAK Inh. Quantification of the change in STAT1 expression relative to control levels is shown above the blot.
The preceding results show that inhibition of Rho family GTPase function with ToxB results in sequential changes in two signaling pathways that regulate CGN survival. First, inhibition of Rac (and/or Cdc42) function leads to inactivation of a PAK/MEK/ERK1/2 cascade and a consequential decrease in the ERK-dependent degradation of pro-apoptotic Bim. Second, loss of MEK/ERK signaling ultimately results in the de-repression of JAK/STAT activity and induction of pro-apoptotic STAT1. Interestingly, the MEK inhibitors PD and U0126 did not overtly induce CGN apoptosis (data not shown), indicating that the induction of Bim and STAT1 is necessary, but not sufficient, to induce cell death; furthermore, this finding suggests that changes in other pathways downstream of Rho GTPase inhibition are required to initiate apoptosis.
As mentioned previously, we have recently shown that the pro-apoptotic effects of ToxB and LTox in CGNs are largely due to inhibition of Rac GTPase because expression of dominant-negative N17Rac1 is entirely sufficient to mimic the neuronal death elicited by these toxins; furthermore, expression of dominant-negative N17Cdc42 or N19RhoA does not induce CGN apoptosis (Le et al. 2005). To determine whether the above signaling pathways are altered in a similar manner by N17Rac1 and ToxB, we evaluated the effects of adenoviral N17Rac1 on ERK1/2 activity and STAT1 expression. Compared with findings in CGNs infected with adenoviral GFP alone (a negative control), co-expression of GFP and N17Rac1 led to a pronounced inactivation (dephosphorylation) of ERK1/2 and concurrent induction of STAT1 (Fig. 7a). Thus, both the MEK/ERK and JAK/STAT signaling pathways are regulated downstream of Rac activity in CGNs (Fig. 7b).
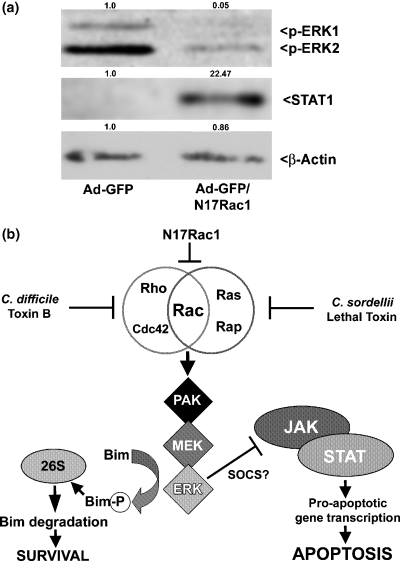
Opposing effects of MEK/ERK and JAK/STAT signaling pathways on neuronal survival acting downstream of Rac. (a) CGNs were infected with either adenoviral green fluorescent protein (Ad-GFP) alone or in combination with N17Rac1 for 96 h. The cells were then lysed, resolved by SDS–PAGE on polyacrylamide gels, transferred to PVDF membranes, and probed with antibodies to p-ERK1/2, STAT1 and actin. N17Rac1 significantly decreased p-ERK1/2 and induced STAT1. Densitometric analysis of protein expression changes for p-ERK2, STAT1 and actin relative to the Ad-GFP control is shown above each blot. (b) The overlapping specificities of ToxB, LTox and N17Rac1 for inhibiting Rac indicate that this small GTPase plays a key role in promoting CGN survival. The PAK/MEK/ERK1/2 pathway, activated downstream of Rac, promotes CGN survival by two different pathways. First, ERK phosphorylates the pro-apoptotic BH3-only protein, Bim, which targets Bim for degradation by the proteasome. A second pathway involves the MEK/ERK-dependent negative regulation of pro-apoptotic JAK/STAT signaling. The latter may involve ERK-dependent up-regulation of SOCS proteins, which act as endogenous repressors of JAK/STAT.
Discussion
Numerous recent studies have demonstrated a key pro-survival role for Rho family GTPases in diverse neuronal cell types (Tanaka et al. 2000; Garcia-Roman et al. 2001; Meske et al. 2003; Topp et al. 2004; Kanekura et al. 2005). Consistent with these findings, we have shown that the activity of Rho family GTPases, and particularly Rac, acting downstream of integrins and growth factors, is essential for the survival of CGNs (Linseman et al. 2001; Le et al. 2005). Despite their critical involvement in neuronal survival, relatively little is known about the molecular mechanisms by which Rho family GTPases exert their neuroprotective effects. In the present study, we used the large clostridial cytotoxin ToxB as a tool with which to selectively inhibit the activity of Rho, Rac and Cdc42 in CGNs, with the aim of identifying novel proteins that influence neuronal survival downstream of Rho family GTPases. Based on preliminary data obtained from a high-throughput immunoblotting screen, we revealed two signaling cascades regulated downstream of Rho family GTPases that have opposing effects on neuronal survival.
First, inhibition of Rho family GTPases with ToxB resulted in the down-regulation of Rac1 and c-Raf, two components of a Rac-dependent MAP kinase pathway. We and others (Zhang et al. 2003; Le et al. 2005) have shown previously that Rac1 is a putative caspase substrate and that its down-regulation in ToxB-treated CGNs is partially blocked by co-incubation with pan-caspase inhibitors but not proteasome inhibitors (Le et al. 2005). In the present study, Rac1 immunoreactivity decreased markedly after only 2–4 h of ToxB exposure, and this loss of Rac1 protein persisted for 24 h. Consistent with Rac1 regulating a MAP kinase pathway in CGNs, ToxB was further shown rapidly to suppress a PAK/MEK/ERK1/2 signal transduction cascade. We observed significant reductions in levels of p-PAK, p-MEK and p-ERK before any detectable loss of Rac1 immunoreactivity (i.e. within 1 h of ToxB addition), suggesting that the glucosylation of Rac1 occurs very rapidly in CGNs after exposure to ToxB. Furthermore, this rapid glucosylation of Rac1 is sufficient to inhibit activity of the downstream effector PAK, whereas the subsequent degradation of Rac1 is not essential for this acute inhibition of PAK signaling.
Recently, ERK1/2 have been shown to phosphorylate the BH3-only protein, Bim, and target it for degradation via the proteasome (Ley et al. 2003; Luciano et al. 2003). Consistent with a similar anti-apoptotic function for ERK1/2 in CGNs, inhibition of MEK/ERK signaling at the level of either Rac/Cdc42 (ToxB) or MEK (PD) resulted in a marked increase in the expression of Bim. These data suggest that a Rac/Cdc42-dependent MEK/ERK pathway is active in healthy CGNs and promotes neuronal survival, in part, by targeting pro-apoptotic Bim for degradation. This finding is consistent with previous results showing that the MEK/ERK pathway promotes neuronal survival by regulating the expression of additional Bcl-2 family members. For example, previous research has demonstrated that the ability of the neuropeptide pituitary adenylate cyclase-activating polypeptide to protect CGNs against either potassium deprivation or ceramide toxicity is dependent on ERK1/2 activity and, more specifically, ERK1/2-mediated up-regulation of Bcl-2 (Villalba et al. 1997; Falluel-Morel et al. 2004). Thus, the MEK/ERK cascade can significantly affect the expression of multiple components of the mitochondrial apoptotic machinery in neurons.
Second, incubation of CGNs with either ToxB or LTox induced a marked increase in the transcription factor STAT1. STAT1 is a putative tumor suppressor protein that is pro-apoptotic in non-neuronal cells (Calo et al. 2003; Thomas et al. 2004; Townsend et al. 2004), as well as in ischemia-induced neuronal death (Takagi et al. 2002). The induction of STAT1 elicited by Rac-inhibitory clostridial toxins was prevented by co-incubation with either ActD or JAK Inh, indicating that the up-regulation of STAT1 was transcriptionally mediated and JAK-dependent. Consistent with a JAK/STAT pathway signaling apoptosis downstream of Rac GTPase inhibition, inclusion of JAK Inh protected CGNs from ToxB-induced death. To our knowledge, these data are the first to show a neuroprotective effect of a small molecule pan-JAK inhibitor in any paradigm of neuronal injury. Interestingly, JAK Inh did not prevent the increase in Bim expression elicited by either ToxB or PD (data not shown), indicating that Bim is not a transcriptional target of STAT1 in CGNs. However, STAT1 has been shown to cooperate with p53 to regulate the expression of other BH3-only proteins (e.g. Noxa) as well as the multidomain, pro-apoptotic protein Bax in mouse embryonic fibroblasts (Townsend et al. 2004). Thus, the JAK-dependent up-regulation of STAT1 observed following inhibition of Rac GTPase may contribute to the mitochondrial apoptosis of CGNs by modulating the expression of pro-apoptotic Bcl-2 family proteins other than Bim.
One intriguing result of the present study is that suppression of the MEK/ERK pathway and stimulation of the JAK/STAT pathway are not autonomous events occurring after Rho family GTPase inhibition, but that there is significant cross-talk between these two signaling cascades in CGNs. In agreement with previous observations in non-neuronal cells showing that ERK activity can suppress JAK/STAT signaling (Jain et al. 1998; Sengupta et al. 1998; Krasilnikov et al. 2003), direct inhibition of the MEK/ERK pathway with either PD or U0126 induced the expression of STAT1 in CGNs, an effect that was blocked by JAK Inh. These data identify a novel pro-survival function of the MEK/ERK cascade in neurons; MEK/ERK acts downstream of Rho family GTPases to actively repress a pro-apoptotic JAK/STAT pathway in healthy CGNs. The mechanism underlying this repression is currently unclear, but it may involve suppressor of cytokine signaling (SOCS) proteins, endogenous inhibitors of JAK/STAT signaling (Cooney 2002; Wormald and Hilton 2004), because the ERK pathway has been shown previously to regulate the expression of SOCS proteins (Terstegen et al. 2000).
Finally, in agreement with our previous work showing that Rac GTPase plays a key pro-survival role in CGNs (Linseman et al. 2001; Le et al. 2005), adenoviral expression of N17Rac1, a dominant-negative mutant of Rac, resulted in decreased activation (dephosphorylation) of ERK1/2 and increased expression of STAT1. Thus, N17Rac1, a more selective inhibitor of Rac function, mimicked the effects of ToxB on the MEK/ERK and JAK/STAT signaling pathways. Collectively, the above data reveal opposing effects on neuronal survival of two signaling cascades acting downstream of Rho family GTPases (principally Rac) in CGNs.
In conclusion, we have identified two contrasting signaling cascades, MEK/ERK and JAK/STAT, that are regulated downstream of Rho family GTPases (principally Rac) in CGNs. The MEK/ERK pathway is pro-survival and targets Bim for degradation, whereas the JAK/STAT pathway is pro-apoptotic. Inhibition of Rac function inactivates MEK/ERK and stimulates JAK/STAT, ultimately triggering JAK-dependent CGN apoptosis. Most significantly, we have elucidated a novel mechanism of cross-talk in neuronal survival wherein the MEK/ERK pathway acts as an endogenous repressor of pro-apoptotic JAK/STAT signaling in healthy neurons.
Acknowledgements
This work was supported by a Department of Veterans Affairs Merit Review Entry Program Award (to DAL). AKZ was supported by a Neuroscience Program Training Grant (T32 HD041697-03) from the National Institutes of Health. The authors thank Dr Hiroshi Udo and Dr Eric Kandel for preparation of the adenoviral GFP and N17Rac1.




