Human immunodeficiency virus-1 B and non-B subtypes with the same drug resistance pattern respond similarly to antiretroviral therapy
Abstract
Clin Microbiol Infect 2012; 18: E66–E70
We analysed the 12-week virological response to protease inhibitor (PI) or non-nucleoside reverse transcriptase inhibitor (NNRTI) therapy in 1108 patients carrying B or non-B human immunodeficiency virus (HIV)-1 subtypes with matched resistance mutation patterns. Response rates were not significantly different for non-B and B subtypes stratified for treatment status (51.5% vs. 41.5% in naïve patients; 46.7% vs. 38.7% in experienced patients) or regimens (46.9% vs. 39.7% with PI; 56.7% vs. 40% with NNRTI). No difference in response was detected in patients harbouring B and non-B subtypes with any resistance profile. Further studies are advisable to fully test this approach on larger datasets.
Introduction
The majority of data on highly active antiretroviral therapy (HAART) efficacy and resistance to antiretroviral drugs have been obtained from clinical trials involving mainly patients harbouring human immunodeficiency virus (HIV)-1 subtype B virus. However, non-B subtypes are highly prevalent worldwide, and non-B subtypes are expanding in areas previously homogeneous for subtype B [1–5].
Previous studies did not show significant variations in virological and immunological responses to HAART between subtype B and all non-B subtypes grouped together [6–10]. Few studies have addressed treatment response with specific subtypes [11–14].
Moreover, even though most of the resistance-associated mutations characterized in subtype B viruses are also found in treatment-failing patients harbouring non-B subtypes [15,16], some differences have been documented between different subtypes [17,18].
Our aim was to explore a novel approach to compare the impact of specific patterns of mutations on virological response to treatment in patients harbouring different HIV-1 subtypes.
Methods
Details of the patients included in this analysis were obtained from the Antiretroviral Resistance Cohort Analysis database. Patient cases were selected on the basis of availability of baseline HIV-1 genotype obtained at a maximum of 12 weeks before starting or changing regimen and availability of a 12-week (range: 8–16 weeks) follow-up HIV-1 RNA determination. Response to therapy was defined as viral load suppression below 50 HIV-1 RNA copies/mL at week 12. Subtyping was based on a partial HIV-1 pol sequence, as previously reported [3].
HIV-1 genotype was coded as a vector derived from the following conditions: occurrence of any thymidine analogue mutation (K65R, L74I/V, Q151M, 69ins, and M184I/V), any major non-nucleoside reverse transcriptase inhibitor (NNRTI) mutation (K103N/S, Y181C/I/V, Y188C/H/L, and G190A/E/S/T), and number (0, 1–3, or >3) of major protease inhibitor (PI) mutations (D30N, I47A/V, G48V, I50L/V, I54L/M, L76V, V82A/F/L/S/T, N88D/S, I84V, and L90M). Response to therapy was evaluated in patients carrying either non-B or B subtypes after their assignment to a final vector combining resistance pattern and treatment type.
Distributions of individuals were compared with the use of the chi-squared test or Fisher exact test (categorical parameters) and standard non-parametric methods (non-categorical parameters). The crude and Mantel–Haenszel-adjusted ORs of response to therapy with 95% CIs were calculated in univariate and multivariate analyses.
Results
The study included 1108 HIV-1-positive individuals referred to 65 clinical centres of 13 Italian regions in the period 1997–2009. Of these patients, 126 (11.4%) harboured non-B subtypes, represented by CRF02_AG (20.6%), F1 (19.8%), C (15.9%), A (7.9%), G (7.1%), unique recombinant forms (6.3%), and others (22.3%), as shown in Fig. 1.
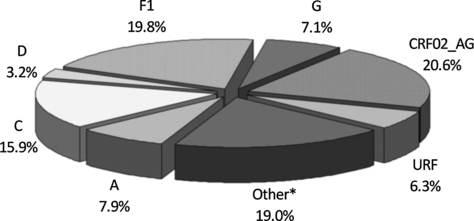
Distribution of non-B subtypes detected in 126 patients of the study population.*Other non-B clades were CRF01_AE (n = 5), CRF12_BF (n = 4), CRF06_cpx (n = 4), CRF15_01B (n = 3), CRF10_CD (n = 2), CRF09_cpx (n = 2), CRF13_cpx (n = 1), CRF20_BG (n = 1), CRF28_BF (n = 1), CRF31_BC (n = 1).
The prevalence of non-B subtypes was higher among drug-naïve than among drug-experienced patients (20.7% vs. 7.6%, respectively; p <0.0001). The demographic, immunological and virological data of the patients stratified between naïve and experienced individuals are shown in Table 1A. No difference in the proportion of drug resistance was observed between non-B and B subtypes when they were grouped according to naïve or previously experienced individuals (Table 1B). Notably, no difference in transmitted drug resistance between subjects carrying non-B and B subtypes was observed.
| Overall population | Naïve patients | Experienced patients | |||||
|---|---|---|---|---|---|---|---|
| B | Non-B | p | B | Non-B | p | ||
| (A) | |||||||
| Ethnic group, % (n) | |||||||
| Europeans | 77.3 (856) | 81.4 (206) | 56.1 (37) | <0.0001a | 79.4 (579) | 65.7 (34) | <0.0001a |
| Africans | 3.0 (33) | 0.8 (2) | 21.2 (14) | 0.1 (1) | 26.7(16) | ||
| Latin Americans | 2.4 (27) | 4.0 (10) | 1.5 (1) | 1.9 (14) | 3.3 (2) | ||
| Asians | 0.5 (6) | 1.2 (3) | 1.5 (1) | 0.3 (2) | 0 | ||
| NA | 16.8 (186) | 12.6 (32) | 0.2 (13) | 18.2 (123) | 13.3 (8) | ||
| Risk factor, % (n) | |||||||
| Heterosexual sex | 48 (532) | 58.9 (149) | 78.8 (52) | 0.013a | 38.4 (280) | 85.0 (51) | <0.0001a |
| Men having sex with men | 20.4 (226) | 29.3 (74) | 12.1 (8) | 19.3 (141) | 5.0 (3) | ||
| Intravenous drug use | 28 (310) | 9.1 (23) | 6.1 (4) | 38.5 (281) | 3.3 (2) | ||
| Otherb | 3.6 (40) | 2.8 (7) | 3.3 (2) | 3.7 (27) | 6.7 (4) | ||
| Gender, % (n) | |||||||
| Males | 68.8 (758) | 73.9 (184) | 60.6 (40) | 0.046a | 69.74 (507) | 45.0 (27) | <0.0001a |
| Age (years), median (IQR) | 40 (36–45) | 41 (34–47) | 35 (32–41) | 0.001c | 41 (37–45) | 36.5 (32–46) | 0.001c |
| HIV-1 RNA (log copies/mL), median (IQR) | 4.6 (3.9–5.2) | 5.1 (4.7–5.5) | 4.9 (4.2–5.4) | 0.049c | 4.3 (3.7–5) | 4.3 (3.9–4.7) | NSc |
| CD4 count (cells/μL), median (IQR) | 237 (118–380) | 198 (64–323) | 182 (64–286) | NSc | 262 (150–411) | 209 (101–317) | 0.029c |
| (B) | |||||||
| Any resistance, % (n) | 57.0 (631) | 9.9 (25) | 6.1 (4) | NSa | 76.9 (561) | 68.3 (41) | NSa |
| NRTI resistance, % (n) | 52.0 (576) | 7.5 (19) | 6.1 (4) | NSa | 70.6 (515) | 63.3 (38) | NSa |
| NNRTI resistance, % (n) | 28.7 (318) | 3. 2 (8) | 4.6 (3) | NSa | 39.4 (287) | 33.3 (20) | NSa |
| PI resistance, % (n) | 24.3 (269) | 2.8 (7) | 0 | NSa | 33.3 (243) | 31.7 (19) | NSa |
| Number of previous drug regimens, median (IQR) | 3 (0–7) | – | – | – | 5 (3–8) | 3 (1–7) | 0.001c |
| Total patients, % (n) | 100 (1108) | 79.3 (253) | 20.7 (66) | – | 92.4 (729) | 7.6 (60) | – |
- HIV, human immunodeficiency virus; NA, not available; NNRTI, non-nucleoside reverse transcriptase inhibitor; NRTI, nucleoside/nucleotide reverse transcriptase inhibitor; NS, not significant; IQR, interquartile range; PI, protease inhibitor.
- aChi squared test.
- bOther: professional risk, transfusions, vertical transmission.
- cWilcoxon test (ns: not significant).
Complete previous treatment history was available for 424 patients. All of these subjects were NRTI-experienced, and 53 and 109 patients had previously received NNRTI-containing or PI-containing therapy, respectively; 232 received both NNRTIs and PIs. Overall, there were 883 subjects beginning a PI-based HAART regimen, of whom 31.8% had previously received PI treatment. Among these individuals, 54.5% (n = 153), 36.3% (n = 102) and 9.2% (n = 26) harboured a virus population carrying 0, 1–3 and >3 major protease mutations before starting or changing therapy. An antiretroviral regimen containing an NNRTI was started in 225 individuals, seven of whom had a transmitted NNRTI mutation.
As shown in Fig. 2, the proportion of subjects achieving virological suppression was higher among individuals carrying non-B subtypes than among those carrying subtype B (49.2% vs. 39.4%, p 0.035). This association was not linked to treatment status and type, and the statistical significance was lost in the subgroup analyses considering groups of drug-naïve (51.5% vs. 41.5%) or pretreated individuals (46.7% vs. 38.7%) and PI-based regimens (46.9% vs. 39.7%) or NNRTI-based regimens (56.7% vs. 40%).
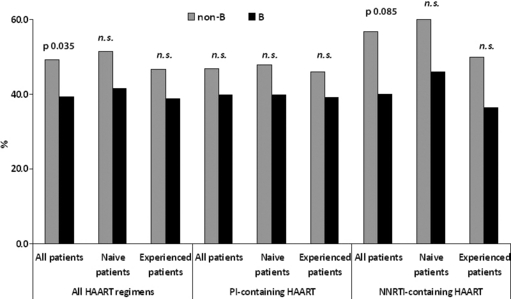
Proportions of subjects achieving virological suppression at the median week 12. Proportions of virological suppression are shown among the overall population and naïve or experienced subjects, starting an antiretroviral regimen including a protease inhibitor (PI) or a non-nucleoside reverse transcriptase inhibitor (NNRTI).
Predictors of virological suppression in the univariate analysis included subtype (for non-B subtypes: OR 1.49, 95% CI 1.02–2.16; p 0.035), viral load at baseline (per 1 log higher: OR 0.55, 95% CI 0.47–0.65; p <0.0001), time of virological follow-up (per 1 week higher: OR 1.01, 95% CI 1.00–1.11; p 0.051), number of previous regimens (per one regimen higher: OR 0.95, 95% CI 0.92–0.97; p <0.0001), and calendar year (per 1 year later: OR 1.21, 95% CI 1.14–1.28; p <0.0001). By contrast, ethnicity, mode of transmission, gender and age, NNRTI-containing regimen or PI-containing regimen did not influence the outcome of therapy. In the multivariate model, baseline viral load (per 1 log higher: OR 0.41, 95% CI 0.34–0.50; p <0.0001), number of previous regimens (per one regimen higher: OR 0.92, 95% CI 0.89–0.95; p <0.0001) and calendar year (per 1 year later: OR 1.27, 95% CI 1.19–1.37; p <0.0001) remained significant predictors of virological success. The procedure used to code HIV-1 genotype generated 35 and 12 vectors for PI-treated and NNRTI-treated patients, respectively. Table 2 shows the patterns of mutations identified and the proportions of patients infected with B or non-B subtypes who achieved virological success. Among patients on PI-based or NNRTI-based regimens, the rate of virological response was not different between subtype B and non-B subtypes for any of the evaluated resistance patterns.
| Vectors | Mutation pattern | Subtype | Response to HAART | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Any TAM | K65R | L74V | Q151M | M184V | PI mutationsa | Non-B, % (n) | B, % (n) | Non-B, % (n) | B, % (n) | |
| 1 | 0 | 0 | 0 | 0 | 0 | 0 | 15.4 (59) | 84.6 (324) | 40.7 (24) | 36.7 (119) |
| 2 | 0 | 0 | 0 | 0 | 1 | 0 | 13.2 (9) | 86.8 (59) | 44.4 (4) | 57.6 (34) |
| 3 | 1 | 0 | 0 | 0 | 0 | 0 | 4.4 (4) | 95.6 (86) | 75.0 (3) | 41.9 (36) |
| 4 | 1 | 0 | 0 | 0 | 1 | 0 | 5.2 (4) | 94.8 (73) | 75.0 (3) | 42.5 (31) |
| 5 | 1 | 0 | 0 | 0 | 0 | 1 | 10.9 (7) | 89.1 (57) | 28.6 (2) | 26.3 (15) |
| 6 | 1 | 0 | 0 | 0 | 1 | 1 | 4.1 (3) | 95.9 (71) | 33.3 (1) | 42.3 (30) |
| 7 | 0 | 0 | 0 | 0 | 0 | 1 | 12.5 (2) | 87.5 (14) | 100 (2) | 42.9 (6) |
| 8 | 0 | 0 | 0 | 0 | 1 | 1 | 7.7 (1) | 92.3 (12) | 0 (0) | 33.3 (4) |
| 9 | 0 | 0 | 1 | 0 | 0 | 0 | 50 (2) | 50 (2) | 100 (2) | 50 (1) |
| 10 | 0 | 1 | 0 | 0 | 0 | 0 | 20 (1) | 80 (4) | 100 (1) | 100 (4) |
| 11 | 1 | 0 | 1 | 0 | 1 | 0 | 10 (1) | 90 (9) | 100 (1) | 66.7 (6) |
| 12 | 1 | 0 | 1 | 0 | 1 | 1 | 20 (1) | 80 (4) | 100 (1) | 50 (2) |
| 13 | 1 | 0 | 1 | 0 | 1 | 2 | 20 (1) | 80 (4) | 100 (1) | 100 (4) |
| Any TAM | K65R | L74V | Q151M | M184V | NNRTI mutationsb | |||||
| 14 | 0 | 0 | 0 | 0 | 0 | 0 | 16.3 (24) | 83.7 (123) | 62.5 (15) | 50.4 (62) |
| 15 | 0 | 0 | 0 | 0 | 0 | 1 | 50 (1) | 50 (1) | 100 (1) | 100 (1) |
| 16 | 0 | 0 | 0 | 0 | 1 | 0 | 12.5 (2) | 87.5 (14) | 0 (0) | 57.1 (8) |
| 17 | 1 | 0 | 0 | 0 | 1 | 0 | 6.9 (2) | 93.1 (27) | 50 (1) | 18.5 (5) |
- HAART, highly active antiretroviral therapy; NNRTI, non-nucleoside reverse transcriptase inhibitor; PI, protease inhibitor; TAM, thymidine analogue mutation.
- a0, no mutations; 1, 1–3 mutations; 2, >3 mutations.
- b0, no mutations; 1, any mutations.
Patients harbouring a wild-type HIV-1 genotype (vector 1) and beginning PI-based HAART were the only group allowing comparisons between subtype B and some of the individual non-B subtypes. No significant difference was observed in this group when subtypes F1 and C were compared with subtype B (response rates of 23.1% and 44.4% vs. 36.7%, respectively). By contrast, a better response in patients carrying CRF02_AG (n = 12) than in those with subtype B (75.0% vs. 36.7%, p 0.012) was observed.
Discussion
In the overall population, we observed a better response to HAART with non-B subtypes, independently of treatment status at baseline and use of NNRTI-based or PI-based therapy. However, no difference was found according to subtype after stratification for treatment status. Response to treatment has been previously analysed with subtype B viruses as compared with non-B subtypes considered as a single cumulative group [6–10]. This approach may mask differences among specific subtypes in disease progression and drug susceptibility. Indeed, few studies have investigated treatment response with specific subtypes [11,12]. Some reports indicated subtype-independent effects of HAART, whereas other studies suggested that subtype D, as compared with subtypes C and A, negatively impacts on disease progression and response to treatment [13,14].
Our data indicate that the efficacy of NNRTI-containing or PI-containing HAART is similar with B and non-B subtypes when the same resistance pattern is detected. Response to therapy with specific non-B subtypes was difficult to analyse, owing to the limited number of cases. However, subtypes F1 and C appear to respond similarly to subtype B, supporting previous results and extending them to subtype F1 [13]. However, viral suppression was achieved in a higher proportion of HIV genotype-matched patients harbouring CRF02_AG. This result is compatible with a possible influence of subtype-specific polymorphisms on the treatment outcome. Although it has been previously suggested that certain polymorphisms can negatively impact on the outcome of patients with non-B subtypes [19,20], our findings indicate that HIV-1 pol minor mutations and polymorphisms do not significantly reduce the response to HAART. These data are in agreement with a recent paper indicating that minor changes not belonging to known resistance mutations are not predictors of development of resistance in non-B subtypes [16].
Owing to its retrospective nature, our study has several limitations, particularly the lack of any information about adherence to treatment. However, a number of factors possibly affecting adherence, such as ethnicity, age, gender, and risk factors, were considered as possible confounders in our regression model. None of these covariates had a detectable impact on the virological outcome of patients stratified according to treatment status in the multivariate analysis.
A limited proportion of subjects in our study achieved viral suppression on therapy. This result may be partly explained by: (i) the high proportion (more than 30%) of patients with HIV-1 viral load above 105 copies/mL; and (ii) the relatively short follow-up, which was deliberately chosen to focus on the impact of genotype on virological response.
This study provides a new methodological approach for the evaluation of HAART efficacy in non-B subtypes as compared with subtype B based on coding and matching for baseline genotype. It cannot be ruled out that specific combinations of mutations and specific polymorphisms exert different effects on drug susceptibility with a particular subtype, and larger datasets are required to definitively determine this.
Acknowledgements
This work was partially supported by: Programmi di Ricerca Scientifica di Rilevante Interesse Nazionale, grant 2005064042 to C. Balotta), Programma Nazionale AIDS 2006 (grant 30G.44 to C. Balotta), Ministero della Salute, Ricerca Corrente (grant 80207 to S. Paolucci), Programma Nazionale AIDS 2009 (grant 40H81 to M. Zazzi), and the European Community’s Seventh Framework Programme (FP7/2007–2013) under the project ‘Collaborative HIV and Anti-HIV Drug Resistance Network (CHAIN)’ (grant agreement number 223131).
Transparency Declaration
The authors have no conflicting interests regarding this work. A part of the results has been previously presented at the ‘International HIV and Hepatitis virus drug resistance workshop and curative strategies’, Dubrovnik, June 8–12 2010.




