The effect of injecting drug use history on disease progression and death among HIV-positive individuals initiating combination antiretroviral therapy: collaborative cohort analysis
*See Appendix S1 for all members of study groups.
Abstract
Background
We examined whether determinants of disease progression and causes of death differ between injecting drug users (IDUs) and non-IDUs who initiate combination antiretroviral therapy (cART).
Methods
The ART Cohort Collaboration combines data from participating cohort studies on cART-naïve adults from cART initiation. We used Cox models to estimate hazard ratios for death and AIDS among IDUs and non-IDUs. The cumulative incidence of specific causes of death was calculated and compared using methods that allow for competing risks.
Results
Data on 6269 IDUs and 37 774 non-IDUs were analysed. Compared with non-IDUs, a lower proportion of IDUs initiated cART with a CD4 cell count <200 cells/μL or had a prior diagnosis of AIDS. Mortality rates were higher in IDUs than in non-IDUs (2.08 vs. 1.04 per 100 person-years, respectively; P<0.001). Lower baseline CD4 cell count, higher baseline HIV viral load, clinical AIDS at baseline, and later year of cART initiation were associated with disease progression in both groups. However, the inverse association of baseline CD4 cell count with AIDS and death appeared stronger in non-IDUs than in IDUs. The risk of death from each specific cause was higher in IDUs than non-IDUs, with particularly marked increases in risk for liver-related deaths, and those from violence and non-AIDS infection.
Conclusion
While liver-related deaths and deaths from direct effects of substance abuse appear to explain much of the excess mortality in IDUs, they are at increased risk for many other causes of death, which may relate to suboptimal management of HIV disease in these individuals.
Introduction
Injecting drug use (IDU) is one of the most frequent routes of HIV transmission in many industrialized countries [1] and is responsible for up to one-third of HIV transmission globally, outside of sub-Saharan Africa [2]. Since the introduction of combination antiretroviral therapy (cART) in 1996, mortality rates related to HIV infection have significantly decreased [3–9]. Rates of morbidity and mortality subsequent to initiation of cART are higher in HIV-positive IDUs than in other HIV-positive persons [10–13], although some studies found only limited evidence for this effect [6,14,15].
Several factors may contribute to the relatively poor response to treatment observed in HIV-positive patients who have a history of IDU. They have been shown to have decreased access to HIV care and treatment [16,17], more comorbid conditions associated with drug use and addiction [such as hepatitis C virus (HCV) coinfection], poorer adherence to treatment [18], and more adverse drug interactions [19,20]. They are also more likely to come from particular ethnic or racial groups that have historically been disadvantaged with respect to health outcomes [21]. In some studies, immunological or virological responses to cART appeared to be lower in HIV-positive IDUs than in other patients [11,22]. However, it is important to distinguish between those who are and are not actively injecting drugs, as the former will have additional risks from overdose, accidents and violence.
Given the high prevalence of IDU among HIV-positive individuals receiving cART, it is important to understand what factors affect disease progression and death in this group: for example, in order to design programmes to reduce disparities in health outcomes between IDUs and non-IDUs receiving cART. We examined determinants of disease progression and death among IDUs and non-IDUs initiating cART in participants in a large multinational collaboration of HIV treatment programmes, and compared causes of death in IDU and non-IDU populations.
Methods
Patient and cohort eligibility
The Antiretroviral Therapy Cohort Collaboration (ART-CC) is a multinational collaboration of HIV cohort studies. The collaboration has been described in detail elsewhere [12,23,24]. In brief, it was established in 2001, updated in 2004, 2006 and 2008, and includes cohort studies from Canada, Europe and the USA. Cohort studies were eligible to join if they had enrolled at least 100 HIV-1-positive antiretroviral-naïve patients aged 16 years and over who initiated potent cART with at least three antiretrovirals, including nucleoside reverse transcriptase inhibitors (NRTIs), protease inhibitors (PIs), and nonnucleoside reverse transcriptase inhibitors (NNRTIs), with a duration of follow-up of at least 1 year. Fourteen cohort studies provided information on causes of death and were included in analyses presented in this paper.
All studies that joined the collaboration have been approved by their local ethics committees or institutional review boards, use standardized methods of data collection, and schedule follow-up visits at least once every 6 months.
Data collection
Patient selection and data extraction were performed at the data centres of the participating cohort studies. Anonymized data from each cohort on a predefined set of demographic, laboratory and clinical variables were pooled and analysed centrally. Data managers checked for duplicated records, and ensured that patients included in more than one cohort had only one record in the combined data set.
Primary outcome
The primary endpoint in this study was HIV disease progression, defined as (1) a new AIDS-defining disease [based on the clinical part of the 1993 US Centers for Disease Control and Prevention (CDC) revision of the AIDS case definition] or (2) death from any cause. We utilized an intent-to-continue-treatment approach, and therefore ignored changes to treatment regimen, including treatment interruptions and terminations. We measured time from the initiation of cART to the date on which the endpoints occurred. Patients who remained alive were censored at their last visit plus 50% of the average time between visits for that cohort. For example, if a cohort had, on average, 6 months between follow-up visits, patients who did not die would be censored at last visit plus 3 months. This allocates follow-up time in an unbiased way to those who did not die, as the average time from last follow-up to death in those who died is approximately 50% of the interval between scheduled visits.
Secondary outcomes
The secondary outcomes in this study were causes of death. All deaths with International Classification of Diseases (ICD) version 9 or ICD10 or free text coding were reviewed by a computer program and also by a clinician and an epidemiologist and then reviewed in committee when discordant. Cause of death was determined utilizing a standardized protocol developed by the Copenhagen HIV Programme for coding causes of death in HIV-positive individuals [25]. Two cohorts participating in ART-CC [Italian Cohort of Antiretroviral-Naïve Patients (ICONA) and the Veterans Aging Cohort Study (VACS)] did not provide causes of death and were omitted from analyses. The two cohorts from Germany did not provide cause of death prior to 2002 for patients in Frankfurt and prior to 2003 in Cologne and Bonn clinics. Patients enrolled in these cohorts prior to these years were excluded.
Prognostic variables
Prognostic variables measured at baseline (initiation of cART) included gender, age (four categories, from 16 to ≥50 years), CD4 cell count (six categories, from <25 to >350 cells/μL), plasma HIV RNA (three categories, from 3 to ≥5 log10 HIV-1 RNA copies/mL), CDC HIV disease stage at baseline, first prescribed cART regimen (PI-based, NNRTI-based, three NRTIs, or other) and year of first therapy (three categories, from 1996 to 2006).
Statistical analyses
Baseline variables in patients infected via IDU and non-IDU were compared using χ2 tests for categorical variables or the Wilcoxon rank sum test for continuous variables. Hazard ratios for progression to AIDS and death were estimated separately in IDUs and non-IDUs using Cox proportional hazards models, and were compared using Wald tests for interaction (assuming log-linear interactions for variables with more than two categories). We compared rates of death in IDUs and non-IDUs and estimated rate ratios stratified by CD4 count (<200 vs. ≥200 cells/μL) and time since starting cART (0–6 months, 6–12 months and 1–5 years) and tested for homogeneity across these strata [26]. Causes of death in IDUs and non-IDUs were compared using Fisher's exact test or χ2 analysis; and using Cox models adjusted for sex, age, prior AIDS diagnosis, baseline CD4 cell count, baseline HIV-1 RNA and year in which cART was started, and stratified by cohort. In models for specific causes of death, patients who died from other causes were censored at the date of death.
We estimated and graphed cause-specific cumulative incidence of deaths classified as AIDS-related, liver-related, violent (including suicide and overdose) and other (including unknown). The cumulative incidence function is similar to the Kaplan–Meier (KM) estimate, but accounts for censoring resulting from competing causes of death: the KM estimate is the cumulative risk of death from that cause conditional on having not died of another cause. Estimated cumulative incidence functions were stacked to illustrate the contribution of each specific cause to total cumulative mortality [27].
Results
A total of 44 043 HIV-positive men and women were eligible for analyses. The majority of study participants were male (32 032; 72%), initiated PI-based regimens (26 345; 59%) and had CDC HIV stage A or B disease at baseline (33 868; 77%). At baseline, the median age was 37 years [interquartile range (IQR) 31, 44 years], the median CD4 count was 215 cells/μL (IQR 90, 345 cells/μL) and the median HIV-1 RNA was 4.94 log10 copies/mL (IQR 4.41, 5.40 log10 copies/mL).
Table 1 summarizes patient characteristics by IDU status: 6269 patients (14%) had a history of IDU. These patients were less likely to be female (23.8 vs. 27.9%, respectively; P<0.001), and started therapy earlier (median July 1999 vs. November 2000, respectively; P<0.001) compared with non-IDUs. There was little evidence of differences in the proportion of individuals with AIDS at baseline (22.4 vs. 23.2% in IDUs and non-IDUs, respectively; P=0.15). The median baseline CD4 count was slightly higher for IDUs compared with non-IDUs [218 cells/μL (IQR 97–360 cells/μL) vs. 214 cells/μL (IQR 88–341 cells/μL), respectively]. By contrast, at 6 and 36 months after initiation of cART, the median (IQR) CD4 count was lower in IDUs compared with non-IDUs [at 6 months, 297 (IQR 160–469) cells/μL vs. 323 (IQR 186–488) cells/μL, respectively; at 36 months, 405 (IQR 249–605) cells/μL vs. 462 (IQR 310–660) cells/μL, respectively]. The proportions of patients with undetectable viral load (defined as HIV-1 RNA ≤500 copies/mL) at 6 and 36 months after initiation of cART were also lower for IDUs compared with non-IDUs (at 6 months, 71.2 vs. 79.6%, respectively; P<0.001; at 36 months, 70.2 vs. 78.5%, respectively; P<0.001). In a subset of 15 238 patients with data on coinfection with hepatitis C virus at baseline, there was a strong association between IDU status and a positive test result: 2204 (88%) of IDUs were coinfected compared with 1518 (12%) of non-IDUs (P<0.001).
| Variable | Non-IDUs (n=37 774) | IDUs (n=6269) | P-value |
|---|---|---|---|
| At start of cART | |||
| Male | 27 255 (72.2) | 4777 (76.2) | <0.001 |
| Age | |||
| 16–29 years | 6885 (18.2) | 756 (12.1) | <0.001 |
| 30–39 years | 15 775 (41.8) | 3635 (58.0) | |
| ≥ 50 years | 5653 (15.0) | 179 (2.9) | |
| CD4 count | |||
| <25 cells/μL | 3879 (10.3) | 588 (9.4) | <0.001 |
| 25–49 cells/μL | 2461 (6.5) | 373 (6.0) | |
| 50–99 cells/μL | 3906 (10.3) | 640 (10.2) | |
| 100–199 cells/μL | 7385 (19.6) | 1262 (20.1) | |
| 200–349 cells/μL | 11 059 (29.3) | 1741 (27.8) | |
| ≥350 cells/μL | 9084 (24.1) | 1665 (26.6) | |
| Plasma HIV RNA | |||
| <4 log10 copies/mL | 4900 (13.0) | 964 (15.4) | <0.001 |
| 4–4.99 log10 copies/mL | 14 933 (39.5) | 2582 (41.2) | |
| ≥ 5 log10 copies/mL | 17 941 (47.5) | 2723 (43.4) | |
| AIDS | 8771 (23.2) | 1404 (22.4) | 0.15 |
| Regimen | |||
| PI-based | 22 422 (59.4) | 3923 (62.6) | <0.001 |
| NNRTI-based | 12 351 (32.7) | 1745 (27.8) | |
| Three NRTIs | 2898 (7.7) | 585 (9.3) | |
| Other | 103 (0.3%) | 16 (0.3%) | |
| Year of first therapy | |||
| 1996–1999 | 15 092 (40.0) | 3495 (55.8) | <0.001 |
| 2000–2002 | 12 077 (32.0) | 1836 (29.3) | |
| 2003–2006 | 10 605 (28.1) | 938 (15.0) | |
| During follow-up | |||
| Deaths | 1564 (4.1) | 533 (8.5) | <0.001 |
| AIDS | 3221 (8.5) | 681 (10.9) | <0.001 |
| Median (IQR) person-years of follow-up for death | 3.7 (1.7–6.2) | 4.0 (1.7–6.3) | 0.006 |
| Median (IQR) person-years of follow-up for AIDS | 3.2 (1.3–5.8) | 3.4 (1.3–5.9) | 0.03 |
- Values are number (%) of patients, except where otherwise shown.
- cART, combination antiretroviral therapy; IQR, interquartile range; PI, protease inhibitor; NRTI, nucleoside reverse transcriptase inhibitor; NNRTI, nonnucleoside reverse transcriptase inhibitor.
A total of 533 deaths (8.5%) were recorded in patients with a history of IDU, compared with 1564 (4.1%) among non-IDUs over the follow-up period: mortality rates were 2.08 [95% confidence interval (CI) 1.91–2.26] vs. 1.04 (95% CI 0.99–1.09), respectively, per 100 person-years (P<0.001). Rates of AIDS were also higher in IDUs than in non-IDUs [2.91 (95% CI 2.70–3.13) vs. 2.33 (95% CI 2.25–2.41), respectively, per 100 person-years; P<0.001]. The unadjusted mortality rate ratio (RR), comparing IDUs with non-IDUs, was higher for patients with baseline CD4 counts ≥200 cells/μL than for those with CD4 counts <200 cells/μL [2.67 (95% CI 2.26–3.15) vs. 1.76 (95% CI 1.55–2.00), respectively; P-value for homogeneity 0.0001]. Mortality RRs increased with time since start of cART, from 1.28 (95% CI 0.98–1.65) in the first 6 months to 1.48 (95% CI 1.08–1.99) in months 6–12 and 2.41 (95% CI 2.11–2.75) in years 1–5 (P-value for homogeneity <0.0001).
Table 2 shows hazard ratios for the association of patient characteristics at baseline with progression to death and AIDS (mutually adjusted for other variables in the table and stratified by cohort) in patients who were and were not infected via IDU, together with P-values for interaction (differences in hazard ratios in IDUs and non-IDUs). Lower baseline CD4 cell count, higher baseline HIV viral load, clinical AIDS at baseline, and later year of cART initiation were associated with disease progression in both groups, consistent with associations reported previously [12,28]. However, the inverse association of baseline CD4 cell count with subsequent rates of AIDS (interaction P<0.0001) and death (interaction P=0.092) appeared to be stronger in IDUs than in non-IDUs. By contrast, the positive association of baseline HIV-1 RNA with subsequent AIDS appeared stronger in non-IDUs than IDUs (interaction P=0.006). While the positive association of a diagnosis of AIDS before starting cART with mortality appeared stronger in non-IDUs than IDUs (interaction P=0.003), the association with AIDS appeared stronger in IDUs (interaction P=0.013). The association of baseline age with AIDS appeared stronger in non-IDUs than IDUs (interaction P=0.056).
| Death | AIDS | |||||
|---|---|---|---|---|---|---|
| Adjusted HR (95% CI) | Adjusted HR (95% CI) | |||||
| IDUs | Non-IDUs | P (interaction)* | IDUs | Non-IDUs | P (interaction)* | |
| Male (vs. female) | 1.05 (0.85, 1.29) | 1.20 (1.05, 1.38) | 0.28 | 1.03 (0.86, 1.24) | 1.05 (0.97, 1.14) | 0.84 |
| Age | ||||||
| 16–29 years | 1 | 1 | 0.24 | 1 | 1 | 0.056 |
| 30–39 years | 1.28 (0.92, 1.78) | 1.28 (1.06, 1.55) | 1.09 (0.84, 1.42) | 1.05 (0.94, 1.17) | ||
| 40–49 years | 1.70 (1.21, 2.41) | 1.72 (1.41, 2.09) | 0.91 (0.68, 1.22) | 1.09 (0.97, 1.23) | ||
| ≥50 years | 2.09 (1.24, 3.52) | 3.17 (2.61, 3.85) | 0.94 (0.54, 1.64) | 1.24 (1.09, 1.40) | ||
| CD4 count | ||||||
| <25 cells/μL | 1 | 1 | 0.092 | 1 | 1 | <0.0001 |
| 25–49 cells/μL | 0.83 (0.57, 1.21) | 0.89 (0.75, 1.06) | 1.07 (0.81, 1.42) | 0.78 (0.69, 0.87) | ||
| 50–99 cells/μL | 0.91 (0.66, 1.25) | 0.78 (0.66, 0.92) | 0.80 (0.61, 1.05) | 0.67 (0.60, 0.74) | ||
| 100–199 cells/μL | 0.81 (0.61, 1.09) | 0.72 (0.61, 0.84) | 0.68 (0.53, 0.87) | 0.39 (0.35, 0.44) | ||
| 200–349 cells/μL | 0.68 (0.52, 0.92) | 0.52 (0.43, 0.61) | 0.51 (0.40, 0.67) | 0.24 (0.21, 0.27) | ||
| ≥350 cells/μL | 0.48 (0.35, 0.67) | 0.38 (0.31, 0.47) | 0.32 (0.24, 0.43) | 0.18 (0.16, 0.21) | ||
| Plasma HIV-1 RNA | ||||||
| <4 log10 copies/mL | 1 | 1 | 0.21 | 1 | 1 | 0.006 |
| 4–4.99 log10 copies/mL | 0.98 (0.73, 1.34) | 0.87 (0.72, 1.05) | 1.22 (0.90, 1.66) | 1.08 (0.93, 1.24) | ||
| ≥ 5 log10 copies/mL | 1.34 (1.00, 1.81) | 1.09 (0.90, 1.31) | 1.92 (1.43, 2.58) | 1.35 (1.17, 1.55) | ||
| Clinical AIDS | ||||||
| Yes (vs. no) | 1.57 (1.29, 1.91) | 2.23 (1.98, 2.51) | 0.003 | 1.55 (1.31, 1.84) | 1.22 (1.12, 1.32) | 0.013 |
| Year of first therapy | ||||||
| 1996–1999 | 1 | 1 | 0.66 | 1 | 1 | 0.076 |
| 2000–2002 | 0.86 (0.70, 1.06) | 0.78 (0.69, 0.88) | 0.83 (0.69, 0.99) | 0.89 (0.82, 0.96) | ||
| 2003–2006 | 0.61 (0.41, 0.93) | 0.81 (0.68, 0.96) | 0.43 (0.30, 0.62) | 0.62 (0.56, 0.69) | ||
- CI, confidence interval.
- * Interaction P-values from Wald tests with 1 degree of freedom.
Cause of death information was available for 1879 deaths: 452 (84.8%) of 533 deaths in patients infected via IDU and 1427 (90.4%) of 1564 deaths in non-IDU patients. Among these, causes of death could be assigned for 1600 (85%) deaths (379 IDUs and 1221 non-IDUs). Figure 1 shows percentages of deaths from specific causes in patients who were and were not infected via IDU. The risk of death from each cause was higher in IDUs than non-IDUs, with particularly marked increases in the risks of liver-related deaths, and deaths from violence and non-AIDS infection.
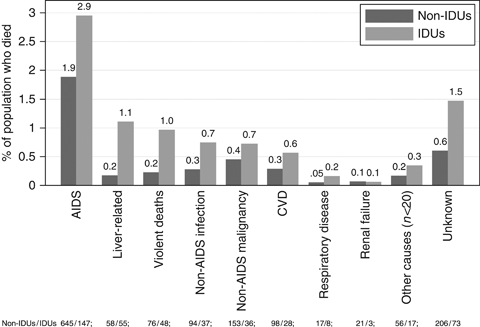
Numbers (below graph) and percentages (above bars) of specific causes of death in patients who were and were not infected via injecting drug use (IDU). Liver-related deaths include hepatitis and liver failure; violent deaths include accident, suicide and overdose; cardiovascular disease (CVD) includes myocardial infarction, ischaemic heart disease, stroke, heart failure/unspecified and other heart disease; ‘Other’ includes causes with fewer than 20 deaths overall. Unknown deaths are those for which there was insufficient information to assign a cause of death.
Figure 2 shows the estimated cumulative incidence of deaths from AIDS, liver-related disease (including hepatitis), violence (including suicide and overdose) and other causes up to 8 years after starting cART, separately for IDUs and non-IDUs. By 8 years after initiation of cART, the cumulative incidence of death was 16.3% in patients infected via IDU, compared with 7.3% in other patients. By the end of follow-up, the largest differences in the cumulative incidence of cause-specific death between IDUs and non-IDUs were in deaths resulting from hepatitis [0.72 vs. 0.08%, respectively; adjusted hazard ratio (AHR) 8.8; 95% CI 5.0–15.5], liver disease (0.38 vs. 0.09%; AHR 4.6; 95% CI 2.5–8.7) and substance abuse (0.54 vs. 0.04%; AHR 6.7; 95% CI 3.4–13.4). Mortality of unknown cause (1.46 vs. 0.60%; AHR 3.1; 95% CI 2.3–4.1) was also higher in the IDU group than in the non-IDU group. In the subset of patients with information on both HCV coinfection and causes of death (n=13 203), the hazard ratio for death from liver disease was attenuated from 4.08 (95% CI 2.24–7.44) to 1.02 (95% CI 0.50–2.09) on adjustment for coinfection with HCV.
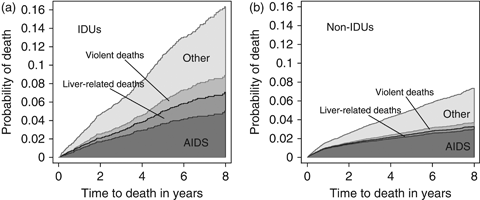
Total cumulative mortality from the start of combination antiretroviral therapy (cART) in patients who were (a) and were not (b) infected by injecting drug use (IDU), partitioned by cause of death grouped as AIDS, liver-related, violent and other.
Discussion
In this analysis involving 14 cohort studies and 44 043 participants, individuals infected via IDU experienced higher rates of death and AIDS, compared with other patients, from the time that they started cART. Although associations for patient characteristics at initiation of cART with subsequent disease progression were largely similar between the two groups, the inverse association of baseline CD4 with subsequent disease progression appeared weaker in patients infected via IDU. By contrast, associations of baseline HIV-1 RNA and AIDS diagnosis before baseline with subsequent rates of AIDS appeared stronger in patients infected via IDU. Compared with other patients, those infected via IDU were at greater risk of all of the specific causes of death we examined, with the greatest differences seen for deaths as a result of hepatitis and liver failure and deaths as a result of substance abuse. The differences we observed were not explained by differences in baseline characteristics between IDUs and non-IDUs. However, the association with liver-related death appeared to be explained by coinfection with HCV.
Strengths of our study include its large size and wide geographic representation across Europe and North America, the consistency of methods used to ascertain AIDS events across the different cohorts, and the common methods used to assign causes of death [29]. This study also has a number of limitations, foremost among them being the lack of data on continuing IDU among individuals whose presumed transmission route for HIV acquisition was IDU; and adherence after starting cART, which may mediate some of the differences observed. Participating cohort studies in the ART-CC do not collect information on treatment adherence in a standardized manner. Unmeasured confounders may also account for some of these differences in progression rates in IDUs compared with non-IDUs. Further, a greater proportion of IDU deaths were of unknown cause, which may have biased our assessment of the relative importance of different causes of death.
Consistent with our results, most previous studies have shown higher rates of mortality in IDUs than in non-IDUs [10,12]; although some have not [6,14,15]. The IDU group was more likely to start cART in the earliest treatment period, an era that has been previously associated with an increased risk for mortality [30]; however, even with adjustment for this difference, higher rates of death and AIDS were seen among the IDUs.
The most important factors and behaviours contributing to the differences in disease progression we have observed are likely to be adherence to therapy and HCV coinfection. As explained above, we did not have data on adherence, but the poorer immunological and virological responses at 6 and 36 months after starting cART in IDUs compared with non-IDUs are consistent with a role for adherence. Previous studies have shown more rapid disease progression as a result of lower rates of virological response seen in IDUs [31]. Further studies have reported that poor virological outcomes and increased immunological failure on cART among IDUs are often attributable to lack of adherence to therapy [14,17,22]. When not actively using drugs, former IDUs have been shown to have the ability to be adherent to therapy and to achieve comparable benefits to non-IDUs on cART [13,14,17,22,32]. IDUs were also at increased risk for deaths from many diseases not typically thought to be related to HIV infection, such as heart and vascular disease and non-AIDS-related malignancies. Given that excesses of these deaths have been demonstrated in untreated individuals [33], it is also possible that these deaths relate to suboptimal treatment of HIV infection in IDUs, as they may be more likely in some settings to remain off therapy for an extended period of time or be less likely to adhere to therapy. In British Columbia, however, IDUs who do adhere have similar outcomes to non-IDUs [15].
IDUs are at increased risk of HCV coinfection [10,12,34], which appeared to explain the excess of liver-related deaths in IDUs compared with non-IDUs. More than half of the approximately 5% difference in mortality between the groups can be attributed to three aetiologies, two of which could be attributed to HCV. Liver failure and hepatitis together accounted for a mortality rate of 1.1% in IDUs vs. 0.17% in non-IDUs (a difference of almost 1% between the two groups). Also, substance abuse-related deaths accounted for a 0.5% difference in mortality, and infection (both AIDS-related and -unrelated) accounted for a further 1.13% difference in mortality. In addition, there was a 0.84% difference between the two groups with respect to death from unknown causes. It is thus possible that the above-mentioned causes of death are in fact underrepresented in these numbers.
In summary, HIV-positive individuals with a history of IDU experienced higher rates of death and AIDS after starting cART, compared with individuals without a history of IDU. While liver-related disorders and deaths from the direct effects of substance abuse appeared to explain much of the excess mortality in IDUs, it also appeared that they were at increased risk for many other causes of death which are not typically thought to be related to IDU. These differences may relate to the suboptimal management of HIV disease in these individuals.
Acknowledgements
We are grateful to all patients, doctors and study nurses who were involved in the participating cohort studies. The ART Cohort Collaboration is supported by the UK Medical Research Council grant G0700820. Sources of funding of individual cohorts include the Agence Nationale de Recherche sur le SIDA (ANRS), the Institut National de la Santé et de la Recherche Médicale (INSERM), the French, Italian, Spanish and Swiss Ministries of Health, The Swiss HIV Cohort Study, supported by the Swiss National Science Foundation (Grant No. 33CSC0-08787), the Stichting HIV Monitoring (Academic Medical Center, University of Amsterdam), the European Commission, the British Columbia and Alberta Governments, the Michael Smith Foundation for Health Research, the Canadian Institutes of Health Research, the VHA Office of Research and Development and unrestricted grants from GlaxoSmith Kline, Roche and Boehringer-Ingelheim. The study was supported in part by the Spanish Network for AIDS Research (RIS; ISCIII-RETIC RD06/006).




