Pregnancy may be followed by an inflexion of the immune reconstitution in HIV-infected women who receive antiretroviral drugs before conception
*See Appendix A.
Abstract
Background
Whether pregnancy has an impact on the evolution of CD4 cell counts in women treated with highly potent antiretrovirals before conception remains largely unknown.
Methods
Among patients enrolled in the ANRS CO8 (APROCO/COPILOTE) cohort, we selected all women aged between 18 and 50 years at initiation of combination antiretroviral therapy (cART). Slopes of CD4 cell counts during follow-up were estimated using mixed longitudinal models with time-dependent indicators for pregnancy and delivery.
Results
Of the 260 selected HIV-infected women, a pregnancy occurred in 39 women in a median follow-up time of 66 months. Women who became pregnant had higher CD4 cell count at baseline but this difference progressively lessened during follow-up because they had a slower increase than women who did not become pregnant. The estimated slope of CD4 cell count decreased significantly from +2.3 cells/μL/month before pregnancy and in women who did not become pregnant to −0.04 cells/μL/month after delivery (P=0.0003).
Conclusion
A significant increase in CD4 cell count may be preferable before pregnancy in women treated with cART, in order to overcome the evolution observed after pregnancy.
Introduction
Pregnancy is common in HIV-infected women treated with antiretroviral (ARV) drugs. It has been shown that the first trimester of pregnancy is temporarily associated with a decline in CD4 cell counts as a result of a decrease in total lymphocyte count and that these modifications reverse soon after delivery in HIV-infected women [1]. Moreover, several studies have shown that pregnancy has no unfavourable impact on HIV disease progression in developed countries [2–6]. However, data remain sparse about the impact of pregnancy on CD4 cell count changes in women treated with ARV before conception. We studied the association between pregnancy and evolution of CD4 cell counts in a cohort of 260 HIV-infected women treated with combination antiretroviral therapy (cART).
Patients and methods
The ANRS CO8 cohort study (APROCO/COPILOTE) enrolled 1281 HIV-1-infected patients when they initiated a protease inhibitor-containing regimen in 1997–1999 [7]. Patients are prospectively followed every 4 months. In the present analysis, we selected all women of childbearing age, i.e. aged between 18 and 50 years at baseline. Slopes of CD4 cell count changes during follow-up were estimated using mixed longitudinal models with time-dependent indicators for pregnancy and delivery. The evolution of CD4 cell count was modelled by two slopes: before and after the first 4 months of treatment, based on previous analyses of the same cohort [8]. Modelling with time-dependent covariates further allowed the estimation of three CD4 cell count slopes after the first 4 months: (1) before pregnancy and in women who did not become pregnant during follow-up, (2) during pregnancy, and (3) after delivery.
We then performed the same analysis with censoring of data at 36 months of follow-up. This sensitivity analysis was performed because CD4 cell count increase reached a plateau at 36 months after initiation of cART in patients of the cohort as a whole who had a persistent complete virological response [9]. Proportions of women having CD4 counts >500 cells/μL or plasma HIV RNA <500 HIV-1 RNA copies/mL after 6 years of follow-up were compared according to the occurrence of a pregnancy during the preceding follow-up period using multivariate logistic regression. All statistical analyses were performed using statistical analysis system software 9.1 (SAS Institute Inc., Cary, NC, USA).
Results
A total of 260 women aged between 18 and 50 years were enrolled in the cohort. During a median follow-up time of 66 months, 39 women had a total of 43 pregnancies (incidence: 3.4 per 100 person-years). The median delay between baseline and first pregnancy was 31 months [interquartile range (IQR) 15–53 months]. At baseline, compared with other women enrolled in the cohort, those who became pregnant at least once during follow-up were younger (median age 29 vs. 34 years; P<0.0001), had a higher median CD4 count (364 vs. 275 cells/μL; P=0.02) and tended to be more frequently born in Africa (31 vs. 19%; P=0.12) but were not different according to presumed risk factor for HIV (infection through injecting drug use: 26%), coinfection with hepatitis C virus (30%) and plasma HIV RNA (median 4.2 log10 copies/mL). The median proportion of follow-up spent on antiretroviral therapy (ART) was 99% in women who became pregnant and 99% in those who did not (P=0.69). ART was temporarily interrupted during the first and second trimesters during only seven pregnancies. Of all measurements of plasma HIV RNA performed during follow-up, 73% were <500 copies/mL in women who became pregnant and 79% were <500 copies/mL in the other women (P=0.57). Although women who became pregnant had higher CD4 cell counts at baseline, this difference progressively lessened during follow-up because they had a slower increase than women who did not become pregnant (Fig. 1). Based on modelling, CD4 cell count remained stable but did not increase after delivery. The estimated CD4 cell count slope after delivery [−0.04 cells/μL/month; 95% confidence interval (CI) −1.5 to +1.4] was not significantly different from 0 and was significantly (P=0.003) lower than the slope estimated before pregnancy and in women who did not become pregnant (+2.3 cells/μL/month; 95% CI +1.7 to +2.9) (Table 1). Results were similar after adjustment for baseline clinical stage and plasma HIV RNA, after exclusion of the seven women who interrupted ART at any time during pregnancy and when data were censored at the second pregnancy (data not shown). The results were also similar when data were censored at 36 months of follow-up (data not shown). This latter sensitivity analysis included 22 pregnancies with a median follow-up time of 10 months (IQR 2–32) between delivery and the censoring date. In the subgroup of 116 women who had both CD4 cell count and plasma HIV RNA measured after 6 years of follow-up, among whom 22 had a pregnancy during the preceding follow-up period, the proportion of women with plasma HIV RNA <500 copies/mL or CD4 count >500 cells/μL did not differ according to whether they had a pregnancy during the first 6 years of follow-up (Table 2). No progression to AIDS or death after pregnancy was reported among women who became pregnant.
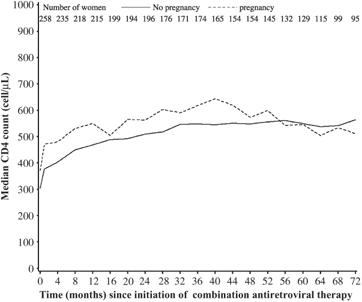
Mean CD4 cell counts during follow-up according to the occurrence of a pregnancy in 260 women aged 18–50 years at enrolment in the ANRS CO8 (APROCO/COPILOTE) cohort study.
| Estimated values(cells/μL/month) | 95% CI | |
|---|---|---|
| CD4 at M4 in women who became pregnant | 488 | 414; 561 |
| CD4 at M4 in women who did not become pregnant | 420 | 387; 452 |
| CD4 slope between M4 and before pregnancy in women who became pregnant and after M4 in women who did not become pregnant | +2.3 | +1.7; +2.9 |
| CD4 slope during pregnancy | −0.80 | −7.8; +6.3 |
| CD4 slope after delivery | −0.04 | −1.5; +1.4 |
- CD4, CD4 cell count; CI, confidence interval; M4, after first 4 months of follow-up.
| Response at 6 years | Pregnancy duringfollow-up (%)(n=22) | No pregnancyduring follow-up(%) (n=138) | Adjustedodds ratio* | P |
|---|---|---|---|---|
| Plasma HIV RNA <500 copies/mL | 73 | 73 | 1.09 | 0.88 |
| CD4 count >500 cells/μL | 51 | 52 | 0.91 | 0.86 |
- * Odds ratios were estimated with multiple logistic regression models adjusted for whether patients were naïve to antiretroviral drugs at baseline, baseline CD4 cell counts, and prescription of nelfinavir at baseline for virological response, and for whether patients were naïve to antiretroviral drugs at baseline and cumulative duration of antiretroviral therapy during follow-up for immunological response.
Discussion
In this cohort of 260 women of childbearing age treated with cART, CD4 cell counts were stable but did not increase after delivery. Nevertheless, this inflexion of the CD4 cell count slope, i.e. this interruption in CD4 cell count increase after delivery, did not translate to the long-term immunological response, as women who became pregnant had higher CD4 cell count at the initiation of protease inhibitor therapy. The long-term virological response was not affected by the occurrence of a pregnancy during follow-up.
A clear biological explanation for this apparent interruption of immune reconstitution following pregnancy in cART-treated women is lacking. It has been shown that cART partially reverses the T-helper type 1 (Th1) to Th2 cytokine shift induced by pregnancy [10]. Conversely, it may be hypothesized that pregnancy has an unfavourable effect on the Th2 to Th1 shift induced by cART in responding patients, but this hypothesis requires in vitro confirmation.
We acknowledge that our study has potential biases as a consequence of its observational design and the relatively small number of women included. Moreover, part of the results we obtained may be explained by the plateau in CD4 cell count increase after 3–4 years of complete virological response reported by ourselves and others [9]. Our sensitivity analyses in which data were censored at 36 months of follow-up, however, suggest that this plateau is not the only explanation for our findings. Consequently, our findings do not imply a causal relationship between this apparent inflexion of CD4 cell count increase and pregnancy and confirmatory analyses with a longer follow-up time and in other cohorts and settings are needed. Although the quite small differences we observed between slopes of CD4 cell counts according to occurrence of pregnancy might have little clinical relevance, we believe that our findings, along with other cautionary notes concerning the foetal risk associated with cART [11,12], contribute to balancing the benefits and risks of pregnancy in HIV-infected women. More specifically, women who need ARV drugs for their own health should be informed that a significant increase in CD4 cell count, for example above 350 cells/μL, may be preferable before beginning a pregnancy.
Appendix
Appendix A: the APROCO/COPILOTE (ANRS CO8) study group
Scientific committee
Steering committee: C. Leport, F. Raffi, G. Chêne, R. Salamon, J.-P. Moatti, J. Pierret, B. Spire, F. Brun-Vézinet, H. Fleury, B. Masquelier, G. Peytavin, R. Garraffo, D. Costagliola, P. Dellamonica, C. Katlama, L. Meyer, M. Morin, D. Salmon, A. Sobel, L. Cuzin, M. Dupon, X. Duval, V. Le Moing, B. Marchou, T. May, P. Morlat, C. Rabaud, A. Waldner-Combernoux, F. Collin, P. Bursachi, J. F. Delfraissy, J. Dormon, M. Garré, C. Lewden
Investigators: Amiens (Prof. J. L. Schmit), Angers (Dr J. M. Chennebault), Belfort (Dr J. P. Faller), Besançon (Prof. J. L. Dupond, Dr J. M. Estavoyer, Prof. P. Humbert), Bobigny (Prof. A. Krivitzky), Bordeaux (Prof. M. Dupon, Prof. Longy-Boursier, Prof. P. Morlat, Prof. J. M. Ragnaud), Bourg-en-Bresse (Dr P. Granier), Brest (Prof. M. Garré), Caen (Prof. R. Verdon), Compiègne (Dr Y. Domart), Corbeil Essonnes (Dr A. Devidas), Créteil (Prof. A. Sobel), Dijon (Prof. H. Portier), Garches (Prof. C. Perronne), Lagny (Dr P. Lagarde), Libourne (Dr J. Ceccaldi), Lyon (Prof. D. Peyramond), Meaux (Dr C. Allard), Montpellier (Prof. J. Reynes), Nancy (Prof. T. May), Nantes (Prof. F. Raffi), Nice (Prof. J. P. Cassuto, Prof. P. Dellamonica), Orléans (Dr P. Arsac), Paris (Prof. E. Bouvet, Prof. F. Bricaire, Prof. P. Bergmann, Prof. J. Cabane, Dr G. Cessot, Prof. P. M. Girard, Prof. L. Guillevin, Prof. C. Leport, Prof. S. Herson, Prof. M. C. Meyohas, Prof. J. M. Molina, Prof. G. Pialoux, Prof. D. Salmon), Poitiers (Prof. B. Becq-Giraudon), Reims (Prof. R. Jaussaud), Rennes (Prof. C. Michelet), Saint-Etienne (Prof. F. Lucht), Saint-Mandé (Prof. T. Debord), Strasbourg (Prof. J. M. Lang), Toulon (Dr J. P. De Jaureguiberry), Toulouse (Prof. B. Marchou), Tours (Prof. J. M. Besnier).
Monitoring and data analysis
C. Alfaro, F. Alkaied, S. Boucherit, A. D. Bouhnik, C. Brunet-François, M. P. Carrieri, M. Courcoul, F. Couturier, J. L. Ecobichon, M. François, L. Iordache, V. Journot, P. Kurkdji, J. P. Legrand, E. Lootvoet, E. Pereira, M. Préau, C. Protopopescu, C. Roy, J. Surzyn, A. Taieb, F. Tourteau, V. Villes, H. Zouari.
Promotion
Agence Nationale de Recherches sur le Sida et les hépatites virales (ANRS, Action Coordonnée no. 7).
Other support: Collège des Universitaires de Maladies Infectieuses et Tropicales, Sidaction Ensemble contre le Sida, and the laboratories of Abbott, Boehringer Ingelheim, Bristol-Myers Squibb, GlaxoSmithKline and Roche.




