Homology: a synthetic concept of evolutionary robustness of patterns
Nikolaus U. Szucsich, Department of Evolutionary Biology, University of Vienna, A-1090 Vienna, Austria. E-mail: [email protected]
Christian S. Wirkner, Institut für Spezielle Zoologie und Evolutionsbiologie, Friedrich-Schiller-Universität Jena, D−07743 Jena, Germany. E-mail: [email protected]
Abstract
The history of the homology concept is a history of attempts to conceive the basis of sameness in biology. Since it was formulated in the middle of the 19th century, the concept has had to fit an ever growing number of scientific fields and purposes. These different demands have resulted in diverging, sometimes, incompatible definitions. The inconsistencies are mostly due to the lack of a clear separation of hypotheses of maintenance from hypotheses of transformation.
A synthetic approach to define homology thus has to consider the following pivotal points: (i) hypotheses of evolutionary maintenance should be kept separate from hypotheses of evolutionary transformation; (ii) the definition of homology should provide the foundation for exact specifications of what is hypothesised to be homologous and (iii) restrictions to particular levels of observation or specific scientific purposes, and the exclusion of iterative homology should be avoided.
We suggest that patterns should be delineated by characterizing components of traits, and by describing connections and interactions between these components. A shared pattern of compared traits where the characterization shows 1 : 1 correspondence may then be homologised. Homology is equivalent to a hypothesis that the pattern, starting from a single starting point, was transmitted robustly along diverging branches of a genealogical tree, that is, the homologised pattern was never changed by any transformation.
The proposed definition of homology is thus, ‘A pattern corresponding in a set of compared traits is homologous, if after a common evolutionary origin, the pattern was maintained along diverging lineages by robust pattern transmission’.
After justifying the terminological use in our definition, we discuss the interplay of our definition with the pivotal points mentioned above in comparison to other definitions.
Since our homology definition is a concept of pattern maintenance, it is clearly demarcated from transformation hypotheses, which are covered by the character concept. Robustness is understood as evolutionary maintenance of correspondence in objects linked by genealogical relations. The characterization of the pattern suffices to provide the necessary conditional phrase by specifying what is hypothesised to be homologous. Allowing development to be conceptualised as a pattern formation process makes it easier to deal with traits that are transmitted indirectly to the next generation. Patterns can be characterized on all observational levels, but the components and the quality of connections and interactions used for the characterization may differ. The replacement of the reference to an ancestor–descendant relationship by a reference to robust pattern transmission allows for the inclusion of iterative homology into the concept.
In the final part of the paper, detailed reformulations of the ‘criteria’ for the corroboration of homology hypotheses as proposed by Remane (1952) are given.
Short history of homology: strengths and shortcomings of prevailing concepts
Early observations of nature soon revealed that different organisms show striking similarities, allowing them to be categorised. The earliest explanations of categorical similarity mainly encompassed idealistic and typological causes (e.g., the archetype used by Owen (1843) who first defined homology). The main problem of such typological homology concepts is that the causes themselves lie beyond the field of science.
Darwin (1859), described traits that correspond between ancestors and their descendants, but do not correspond between unrelated individuals. Based on this, the hypothesis was set up that common descent is the cause for certain correspondences. It is this hypothesis that gave rise to evolutionary definitions of homology, which provide a historic explanation of sameness. However, by referring to ancestor–descendant relationships (e.g., Mayr 1982), most of these historic definitions exclude iterative homology (Wagner 1994). To avoid this exclusion, Wagner (1989a, 1989b) proposed mechanistic causes as explanations of sameness. It is interesting to note that it provides a proximal explanation for sameness of traits between ancestor and descendants. However, it is unclear how developmental constraints are historically maintained. Additionally, references to shared developmental constraints restrict homology to morphology. To advance the testability of historic homology hypotheses, phylogenetic reformulations defined homology as traits that characterize monophyla (Patterson 1982; De Pinna 1991). However, not only does iterative homology continue to be excluded, but also the equivalence of homology and synapomorphy in most of the phylogenetic concepts had recently been perceived as problematic (e.g., Grant & Kluge 2004; Richter 2005).
Mechanistic concepts avoid historic explanations by referring transformations in terms of mathematical relations. All transformational concepts, however both historic and non-historic, entail problems due to the mixing of hypotheses of evolutionary maintenance and evolutionary transformation. This result in a vagueness as to what extent homologised traits have to be the same and how much may have changed, thus making it hard to specify precisely what is homologous in compared traits. Many homology hypotheses, regardless of the definition used, state that the traits as a whole are homologous. In a set of traits, pairwise comparisons will result in different correspondences for each pair of traits. Thus an exact specification is necessary of what is homologous for the whole set of compared traits. This is reflected in the demands for a conditional phrase for every homology statement (Ghiselin 1966; Bock 1973; Roth 1991; Nelson 1994). Some authors tried to deal with different degrees of correspondence between different pairs of traits in a set of compared traits by proposing a concept of partial homology (e.g., Roth 1984; Minelli & Peruffo 1991; Sattler 1994; Minelli 1998).
An integrative approach attributing homology to a transmission of the same information has been proposed by various authors (Osche 1973; Van Valen 1982; Dohle 1989; Haszprunar 1992; Roth 1994; Richter 2005). However, the term information is highly problematic (Wagner 1989b; Brigandt 2002). The information concept, if taken from the mathematical communication theory, requires a decoding rule dependent on the signal to be decoded (Wagner 1981). The definition of information used by Sterelny (2004) as covariation between a signal and a source seems to fit somewhat better. Difficulties occur however, with non-linear relations, most obvious in examples where the phenotype is unchanged despite variance in the genetic source, and where the phenotype varies despite genetic invariance. Thus the relation of the object of homologisation to the object of transmission (in this case information) is unclear in some cases.
In conclusion, the most common problems associated with the various homology concepts include:
- 1
Problems with the separation of hypotheses of evolutionary maintenance from hypotheses of evolutionary transformation, or to put it differently of homology from the character concept (which includes transformation hypotheses, e.g., Hennig 1966).
- 2
Difficulties in specifying exactly what is hypothesised to be homologous (i.e., the lack of a conditional phrase). Two different ways for specifying the conditional phrase can be distinguished. In the first way, a homology hypothesis must, to be meaningful, specify precisely which aspects of a character have been conserved (e.g., Roth 1991). The second way alternatively specifies a taxonomic level as the conditional phrase (Bock 1973) — e.g., the wings of birds and forelimbs of mammals are homologous as tetrapod limbs — which is problematic, since it is already dependent on a phylogenetic hypothesis and it excludes iterative homologies.
-
Furthermore, a clear specification of what is homologised has to be consistent with both what is known to be transmitted to the next generation, and with what can be perceived by the observer.
- 3
Restriction of the concept to particular levels of observation or specific scientific purposes and the exclusion of iterative homology. Some definitions are restricted to either the molecular or the morphological level and the vast majority of concepts exclude iterative or serial homology from their definition. While Wagner (1989b) differentiated iterative homology from phylogenetic homology, Haszprunar (1992) distinguished four types of homology (iterative, ontogenetic, polymorphic and supraspecific homology), of which ontogenetic and polymorphic homology types address not only the robust aspect, but also aspects of transformation and variation of traits.
We think a proper definition of homology has to account for all these problems. After proposing our homology definition, the various aspects of its content and its implications for the above mentioned problems will be discussed.
A new, synthetic concept of homology
Many authors think that a single definition of homology cannot apply to all elements and levels in the hierarchy of biological complexity (Hall 1994), or state that different scientific goals need different homology definitions (Wagner 1994). We do believe in the possibility of a common definition however, and therefore propose a synthetic evolutionary approach that covers all potential demands.
Our proposal is a homology concept of evolutionary robustness of pattern after a common evolutionary origin:
A pattern corresponding in a set of compared traits is homologous, if after a common evolutionary origin, the pattern was maintained along diverging lineages by robust pattern transmission.
The use of pattern in the definition of homology
A pattern can be defined as a system of connections and interactions among components. This means that for homologisation both the components and the interactions, and connections have to be characterized. Different qualities of connections (e.g., articulations, innervations, muscle insertions, cell adhesion, molecular bonds, etc) and interactions (connection temporally restricted: induction, activation, inhibition, signalling, etc) can be chosen to characterize a pattern.
The term pattern as used here is not restricted to morphology but encompasses spatial, temporal and spatio-temporal patterns. Processes in particular, often opposed to patterns (e.g., Hall 1992 in Roth 1994) are understood by us as spatio-temporal patterns (see also Gilbert & Bolker 2001; Scholtz 2004, 2005; Sanetra et al. 2005).
A pattern is corresponding in a set of compared traits if the characterization of components and their connections and interactions fully holds true for the whole set of compared traits.
A thorough characterization of the pattern corresponding in a set of compared traits is necessary, since it represents the ‘level’ or conditional phrase of homology. For example, the forelimbs of a human and a cat are not homologous as a whole, but homology can be hypothesised for their bone pattern; a proximal humerus (stylopodium) articulated with a median zygopodium consisting of a parallel radius and ulna, followed by a distal autopodium. The pattern can be extended further by characterizing the connections between metacarpalia, carpalia and digits, by including the point of articulation of the forelimb and by including muscular components of the system. Note that a pattern does not have to encompass all the connections and interactions, nor all components of a trait.
Likewise the pattern does not have to be a modular unit of pattern formation, thus meeting the demand of developmental individuality (Wagner 1989a, 1989b) or to meet the demand of evolutionary context-insensitivity (see Schlosser & Wagner 2004).
Since human perception is highly based on pattern recognition, the reference to patterns additionally allows homology hypotheses to be strongly based on observations.
The set of compared traits
According to our definition a homology hypothesis among compared traits refers to a corresponding pattern. The number and extent of the components, interactions and connections that can be included in the characterization of a shared pattern, however, depends on which and how many traits are included in the comparison. The higher the number of compared traits, usually lower the number of components, interactions and connections that correspond in the whole set of compared traits.
Common evolutionary origin
Homologous patterns, according to our definition, can be traced back to a common evolutionary origin or to put it differently following Grant & Kluge (2004), homology refers to the relation between parts that resulted from the same evolutionary origin. Since this real criterion of historical identity — the common evolutionary origin of the pattern — cannot be determined directly, it has to be inferred indirectly on the basis of observable variation (Grant & Kluge 2004 and references therein). Encompassing a common evolutionary origin in the definition distinguishes homologies from patterns that correspond despite multiple evolutionary origins, homoplasies in other words (e.g., Farris 1983; Kluge 1999). Possible causes of homoplasies are: (i) correspondence by chance; (ii) shared extrinsic causes (selective pressure) and (iii) shared intrinsic causes (developmental constraints).
The more complex and extensive a compared pattern, the less likely it is to be a homoplasy caused by pure chance. Increasing complexity also decreases the likeliness of shared selective pressure as the sole cause of correspondence, in particular if weak interactions or connections are included in the pattern hypothesised to be homologous (see the weak linkages of Kirschner & Gerhart 1998). In our view, complexity is not solely dependent on the number of participating components, but more on the qualitative dimension of different (inter)dependencies. The qualitative dimension increases with the diversity of the connections and interactions used for characterization of the shared pattern. The diversity of connections and interactions is often reflected in different degrees of (inter)dependence of the components.
Some authors speak of convergence if like selective pressure is the only shared cause of correspondence, which can then be distinguished from parallelism where shared intrinsic (developmental) constraints are the cause of correspondence (for a terminological overview see, e.g., Wiens et al. 2003; Desutter-Grandcolas et al. 2005). It is important to note that neither shared extrinsic nor shared intrinsic causes are sufficient to refute homology hypotheses, since a common origin logically implies both like selective pressure and shared developmental constraints during origination. Similar selective pressures in the entire set of compared traits, however, increases the need for the homology hypothesis to be well corroborated through conjecture and congruence with other patterns. The necessary condition for a homoplasy hypothesis, which is the complement relation to homology (Kluge 1999) is the multiple origination of a pattern.
Robust pattern transmission
As noted by various authors the persistence, development, growth and reproduction of an organism is a continuity of pattern not substance (Williams 1992; Roth 1994; Wagner 1994). Therefore, a concept dealing with evolutionary robust transmission has to account for patterns.
Problems with the consistency of the object of homologisation often occur due to inconsistencies between the object of transmission to the next generation and the object of perception by the observer.
We are aware that continuity of patterns between ancestors and descendants is achieved in different ways. Some patterns, for example, sequential molecular patterns, are transmitted more or less directly between generations (for the concept of replicators see Dawkins 1978; Hull 1980; Roth 1994). While others are transmitted indirectly and built anew in the course of an iterated pattern formation process in every generation (see, e.g., Wagner 1989a, 1989b; Sattler 1994 and citations therein). Thus in patterns indirectly transmitted between generations, pattern formation processes and the robustness of the outcome to changes in the pattern formation processes have to be taken into consideration when addressing the process of pattern transmission (e.g., Sander 1996; Meinhardt 2001; Nijhout 2001; Salazar-Cuidad et al. 2003). Knowledge about pattern stability in the organismal lifespan (e.g., repairing mechanisms and morphostatic mechanisms, see Wagner & Misof 1993) also has to be taken into account.
We distinguish pattern robustness from pattern stability in the following way. Pattern robustness as used in our definition requires the reappearance of a shared pattern in different generations of an iterated process of pattern transmission. Pattern stability is differentiated from robustness by addressing the continuity of a pattern during an organismal lifespan. While pattern stability is dependent on an unbroken continuity, the genealogy of robust patterns is interrupted by iterated processes of pattern duplication or iterated processes of prepattern duplication and pattern formation.
Diverging lineages of pattern transmission
As mentioned earlier, the pattern is transmitted to a new generation of traits by the iterated process of (pre)pattern duplication (and pattern formation). Note that this does not necessarily imply a new generation of organisms but the iteration can likewise take place within a single organism.
Multiple duplication events from a single source result in diverging lineages of pattern transmission. Homology hypotheses imply a single starting point of the lineages of pattern transmission that terminate in the traits of the set.
Like Rieppel (1994), we believe that homology is not a relation of material identity, but in contrast to Rieppel, we hold that homology can nevertheless be a historical concept, since patterns are transmitted directly or indirectly between generations. Wagner (1994) criticized the fact that sameness is not answered by historical homology concepts and raised the possibility of circular reasoning. In his Figure 1, he criticized that sameness in compared species is again solely explained by sameness, namely by sameness with a common ancestor. Our concept overcomes this circularity as pattern transmission is unidirectional in time and a single starting point is ensured by reference to a common evolutionary origin (Fig. 1).
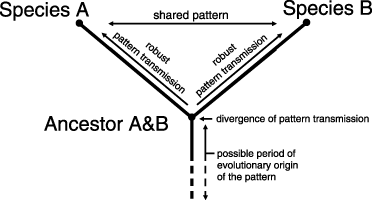
Homology. Sameness is caused by robust pattern transmission along diverging lineages, after a common evolutionary origin of the corresponding pattern. Pattern transmission is unidirectional in time, thus the explanation of sameness is not circular (compare Figure 1 of Wagner 1994).
Implications of our definition for the problems listed above (see Short history of homology: strengths and shortcomings of prevailing concepts)
Demarcation of homology from the character concept (synapomorphy)
Failure to clearly demarcate hypotheses of maintenance from hypotheses of transformation results in vagueness with regard to what exactly is the same in homologised traits. The equation of homology with synapomorphy is such a mixing of hypotheses. However, synapomorphy statements can be distinguished from homology hypotheses as unlike the latter they include: (i) hypotheses of transformation assumed to have taken place between two character states; (ii) a subsequent polarisation of this transformation series and (iii) the assignment of the transformation to a discrete period in a hierarchical genealogy. Since synapomorphy is defined as a shared derived character state, a synapomorphy statement necessarily implies the transformation of a ‘plesiomorphic’ into an ‘apomorphic’ state. A hypothesis of synapomorphy requires at least two homology hypotheses, one for the plesiomorphic and the apomorphic state, respectively, and other, a hypothesis of a single transformation changing the plesiomorphic into the apomorphic state. A third homology hypothesis concerning the character frame may be formulated to corroborate the plausibility of the assumed transformation hypothesis.
Note that to our understanding, homology is an evolutionary concept, and not an exclusively phylogenetic one. Homology is thus independent of speciation and traits can be homologised between organisms that still have interdependent pattern transmission. Therefore, homology statements are meaningful in reticulate and divergent genealogies, while apomorphy statements are restricted to divergent or hierarchical genealogies (for the difference between reticulate and divergent genealogies see, e.g., Goldstein & DeSalle 2000).
Homology and apomorphy statements also differ in their testability by observation. Insect wings, for example, as flattened outgrowths of the meso- and metathoracic segment (note that the pattern can certainly be characterized in a more inclusive way) are a synapomorphy of all pterygote orders. This synapomorphy statement is equivalent to the notion that pattern transmission of different lineages of pterygote insects became independent after the evolutionary origin of insect wings. As representatives of many groups lack wings, however, this pattern is not homologous among all pterygote orders, but only among a subset sharing the characterization of the pattern. In some groups the pattern was secondarily (with respect to pattern origination) changed or even totally reduced. So the presence of the apomorphic state in all terminal taxa is not a necessary condition for an apomorphy statement. This is best reflected in multiple state characters. So while the homologised pattern has to be observable in the whole set of compared traits covered by the homology statement, the pattern of the apomorphic state is not necessarily present in all representatives covered by the apomorphy statement.
As implied by our definition, homology hypotheses are restricted to a comparative context, since a corresponding pattern of a set of compared traits is hypothesised to be the same due to diverging robust pattern transmission after a common evolutionary origin. Thus we speak of evolutionary origins of novel patterns, not of origins of homologues (but see, e.g., Müller 2003).
Difficulties in specifying exactly what is hypothesised to be homologous (the lack of a conditional phrase)
What is needed for homology statements is a thorough characterization of the pattern shared by the whole set of compared traits. This pattern then represents the level of homology. The characterization of the pattern already represents the conditional phrase claimed to be necessary for homology statements (Ghiselin 1966; Bock 1973; Nelson 1994).
Furthermore, if homology only addresses the conservational aspects of evolution, it makes no sense to speak of partial homology (e.g., Roth 1984; Minelli & Peruffo 1991; Sattler 1994; Minelli 1998; Mindell & Meyer 2001; Rutishauser & Moline 2005), since a change in the inclusiveness of the shared pattern changes the extent of homology per definitionem.
Restriction of the concept to particular observational levels or specific scientific purposes and the exclusion of iterative homology
Wagner (1989b) following Mayr (1982) and De Beer (1971) claimed that one implication of a strictly historical definition of homology is that any form of iterative homology has to be excluded. In our view this can be overcome by replacing the reference to a common ancestor with a reference to robust pattern transmission after a common evolutionary origin, which can take place in compared traits within, as well as between organisms. Of the four types of homology listed by Haszprunar (1992), both the iterative and supraspecific types are covered by a reference to robust pattern transmission. Since ontogenetic and polymorphic homology includes transformational aspects, they can be covered by a biological character concept. The same is true for the interenvironmental homology of Pigliucci (2001).
Note, however, that the evolutionary history or fate of serial homologues is potentially still coupled (see also Ghiselin 2005).
Patterns can be characterized on all observational levels (morphological, molecular and developmental traits). Only the subunits and the quality of interactions and connections chosen for the characterization of the pattern will be different on different observational levels.
Characterization of spatio-temporal patterns in developmental genetics may use genes and gene products as components and transcription, activation and repression (inhibition) as interactions, opening our homology concept to an Evo-Devo perspective. Components may likewise be extended to stages of organ development, interactions to processes such as induction. Importantly, as in all other traits (e.g., morphological), it is not development as a whole which is homologous among compared organisms, but a spatio-temporal pattern of interacting components.
Corroboration of homology hypotheses
The following chapter deals with the testability of homology hypotheses. Shubin (1994) suggested to distinguish three distinct but interrelated steps in the analysis of homology (for a slightly different subdivision see De Pinna 1991). The first step (see Assessment of homology hypotheses section below) is the assessment of the homology hypothesis, in our concept based on the characterization of a pattern. In the second step, the hypothesis is tested on the basis of congruence with other patterns (see Corroboration of homology hypotheses section). The last step (see Mechanistic explanations section) is dedicated to mechanistic explanations of homology.
Since the nature of the hypothesis does not change with a test, we do not follow the distinction between primary and secondary homology as made by De Pinna (1991).
Assessment of homology hypotheses
There is much debate as to whether similarity or correspondence is the operational clue for the proposal of homology hypotheses (compare, e.g., Rieppel & Kearney 2002; Ghiselin 2005). Homology statements in our view address hypotheses of evolutionary maintenance of patterns and thus are equivalence relations. Since correspondence addresses an equivalence relation while similarity does not, we speak of correspondence (see also Ghiselin 2005). Note that when our definition is applied, correspondence may be restricted to the characterized pattern. Correspondence that goes beyond the characterization of the pattern can be used as an indication to corroborate the proposed homology hypothesis (see below). Note that indications can be used to corroborate homology hypotheses for subsets of the traits that are compared.
Corroboration of homology hypotheses
More disagreement exists about how homology hypotheses can be ‘tested’.
Most often, a homology hypothesis is not corroborated on the basis of an analysis of the pattern itself, but on the basis of its relation to other patterns (see Rieppel 1994; emphasis by us). Congruence and conjunction with other patterns of different qualities and levels are thus the main factors in corroboration (see Richter 2005).
Remane (1952; translated and formalised by Riedl 1978) summarized a set of possibilities for the corroboration of homology hypotheses, listing three main and three auxiliary criteria of homology. The term criterion is somewhat unfortunate, since we believe that none of the so-called ‘criteria’ are either sufficient or even necessary conditions of homologous patterns, but represent indications to corroborate homology hypotheses (Ax 1988). The only necessary condition is robust pattern transmission starting from a common evolutionary pattern origination. Since the origin of most traits cannot be perceived, nearly all homologies remain hypotheses (see Rieppel 1994; Kluge 1997).
Neither do we follow the distinction which Remane (1952) makes between main and auxiliary ‘criteria’, with the auxiliary criteria intended for simple structures only. The following list of indications represents Remane's ‘criteria’, extended and modified to fit the use of patterns in our homology definition.
- 1
Indication of congruent integration: Most patterns, regardless of the degree of individualization, are nested into the frame of a more general pattern. A homology hypothesis of a pattern of compared traits is corroborated by congruent integration in a more general pattern (criterion of position, Remane 1952).
- 2
Indication of congruent subpatterns: In most patterns it is likewise possible to differentiate subpatterns and properties not included in the characterization of the pattern itself (e.g., shared only by a subset of the traits compared). Thus the homology hypothesis can be corroborated by congruence of shared subpatterns or additional properties of the originally homologised pattern (criterion of special structure, Remane 1952).
- 3
Extension to transformation series: The first two indications point at the possibility of corroboration of a homology hypothesis by congruence with patterns which themselves are hypothesised to be homologous. However, differences may occur in both the more general pattern and in subpatterns which do not allow them to be homologised. If these differences can be hypothesised to the state of a unique transformation series (character) in the course of evolution, the original homology hypothesis is still corroborated (modified criterion of intermediate forms, Remane 1952). As other authors have noted, the third criterion does not establish sameness but rather indicates a continuum or an injunction (Sattler 1994 and references therein), due to the inclusion of a transformation hypothesis.
- 4
Indication of congruent development: A homology hypothesis concerning a pattern is corroborated by congruence with the spatio-temporal pattern of its processes of pattern formation and pattern stabilization. No developmental ‘criterion’ was formulated by Remane, but intermediate forms in the course of evolution have been viewed as examples of his ‘third criterion’ (e.g., Zachos & Hoßfeld 2006). Since ontogeny, itself a spatio-temporal pattern, represents the process of pattern formation for most patterns, we would prefer to assign a separate point to it, opening the homology concept to an Evo-Devo perspective. Like in other indications, congruence with developmental patterns is not a necessary condition for homology hypotheses. Thus differences in development do not equate non-homology.
- 5
Indication of maintenance in a more extensive set of compared traits: A homology hypothesis is corroborated by the occurrence of the pattern in a large number of related species (first auxiliary criterion of Remane 1952; general conjunctional criterion according to Riedl 1978). This indication can be seen mainly to be a corroboration of the evolutionary robustness of the pattern.
- 6
Indication of phylogenetic distribution: A homology hypothesis is corroborated by the presence of other hypothesised homologous patterns with the same distribution among closely related species (second auxiliary criterion of Remane 1952; special conjunctional criterion according to Riedl 1978). Many authors have regarded this last of Remane's criteria as the most important, and have modified it in order to use it as a basis for restricting homology to a solely phylogenetic concept, redefining homologous similarities as those that define natural or monophyletic groups of organisms (e.g., Patterson 1982; De Pinna 1991; Nelson 1994; Lauder 1994; Greene 1994). In our view this indication is different from all the others, but not because it represents a necessary condition for the corroboration of homology hypotheses. While the other indications are based on corroboration by at least possibly dependent patterns, the fifth indication increases in validity the more independent patterns (states of characters) are incorporated into the analysis. However, it should be noted that phylogenetic inference can only corroborate the homology hypotheses of character states, leaving the homology of the characters (character frames) untested. Seen from a stricter view-point, hypotheses of uniqueness of transformations are tested by phylogenetic inference. Thus homology hypotheses are tested against complementary hypotheses of homoplasy (multiple origin).
Remane (1952) also included a negative criterion in his list, stating that the probability of homology decreases with the frequency of the occurrence of a trait among species which are certainly not related (third auxiliary criterion, Remane 1952; negative conjunctional criterion according to Riedl 1978). It is, however, questionable whether the certainty of a double convergent evolution of a pattern, for example, increases the probability of multiple convergent evolutions. Wagner (1989a) states in a comparable context that ‘the origin of an individualised complex of traits must be sufficiently rare’.
Some scientists tend to fuse the first two indications into a single complexity criterion (Riedl 1978; Dohle 1989; Wägele 2001; Scholtz 2005).
However tempting this idea may be, we would like to draw attention to two significant problems.
First, every attempt to quantify complexity is problematic. Attempts in biology are reviewed by Donoghue & Sanderson (1994). Most of them use direct or indirect modifications of the algorithmic complexity concept (e.g., Richter & Rost 2002), where the number of terms used for description (Schopf et al. 1975), the number of cell types (Bonner 1988) or the number of components and their distribution (McShea 1991, 1992) provides a quantification of complexity. All these attempts are interesting, but all of them ignore the fact that in any algorithmic complexity concept complexity is a property of the description, not of the object. All quantifications of complexity thus share the weakness that they ignore the qualitative dimension of complexity by restricting the quantification to one or a few levels of description (see also Rieppel & Kearney 2002). This fact is reflected in the circumstance that complex patterns do not necessarily need to have complex causes of pattern formation (e.g. Newman & Müller 2000).
Second, the encaptic (nested) organization of general patterns (frames), patterns and subpatterns is loosened by this fusion. In some cases at least there seem to be differences in evolutionary robustness between levels. This is reflected in the concepts of burden (Riedl 1978) and generative entrenchment (e.g., Wimsatt & Schank 2004).
Conflicts with indications
Müller (2003) mentions ‘Cross-Level Justification’ as one of the major problems of homology statements. Despite the conflicts which surround the issue, we believe that patterns of different levels are useful tools to test homology hypotheses. Indeed we recommend attempting to corroborate homology hypotheses by testing congruence with patterns of as many different qualities of connections and interactions as possible. It should be noted that not every conflict is a falsifier of a homology hypothesis. Conflicts in compared traits that share a pattern at one hierarchical level but have different patterns at another level may be indicative of (i) mistakes in at least one of the proposed homology hypotheses; (ii) a different degree of context insensitivity in the homologised pattern and the pattern used to indicate homology (see Schlosser & Wagner 2004) or (iii) a transformational process that occurred in the course of evolution. Note that the latter is crucial for the formulations of transformation hypotheses necessary for character conceptualisation in the course of phylogenetic inference.
That no indication is a necessary condition of homology is best reflected in the indication of congruent development, since there is no linear mapping of changes in the course of pattern formation and changes in the resulting pattern (e.g., Janies & DeSalle 1999; Scholtz 2004, 2005; Sanetra et al. 2005).
Mechanistic explanations
In our view, homology is a hypothesis regarding the cause of correspondence (robust pattern transmission after a common evolutionary origin). Since it is a concept of pattern robustness, it is neither meant to explain the intrinsic and extrinsic causes of pattern origination, nor the causes of transformation. However, we strongly believe that explanations and models of pattern formation, stabilization, origination and transformation play a crucial role in the understanding of evolution by providing the mechanistic understanding of both evolutionary and developmental pattern formation and stabilization and an understanding of robust pattern transmission from generation to generation.
Conclusion
Our definition, ‘A pattern corresponding in a set of compared traits is homologous, if after a common evolutionary origin, the pattern was maintained along diverging lineages by robust pattern transmission’, is consistent with the following demands placed on a synthetic homology concept:
- 1
A clear separation of hypotheses of maintenance (homology concept) from hypotheses of transformation (character concept) is achieved.
- 2
The conditional phrase necessary to specify exactly what is homologised is covered by the characterization of a shared pattern (components and their interactions and connections).
- 3
The characterization of patterns is consistent with (i) what is known to be transmitted in genealogies and (ii) with human perception (what can be observed).
- 4
Patterns may be characterized on all observational levels, including spatial, temporal and spatio-temporal.
- 5
Since development may be conceptualised as a pattern formation process, an Evo-Devo perspective is integrated into the concept.
We think that the concept of homology should not become an end in itself, but should be defined in order to pave the way for a better understanding of the robust aspects of evolution, thus providing the basis for insights into transformational aspects of evolution. Phylogenetic inference is but one of the tasks the concept has to fit.
Defining homology in an evolutionary sense is perhaps one way to answer other questions thrown up on the road to understanding the history of biological complexity.
Acknowledgements
We would like to thank Günther Pass, Stefan Richter, and their teams for helpful discussions, and Gerhard Scholtz and two anonymous reviewers for useful suggestions that helped to improve earlier versions of the manuscript. Special thanks to Lucy Cathrow for linguistic help. NUS is supported by grant number P17038-B03 of the FWF. CSW by the ‘Deutsche Forschungsgemeinschaft DFG’, grant number Ri 837/6–1.




