Importance of EGF receptor, HER2/Neu and Erk1/2 kinase signalling for host cell elongation and scattering induced by the Helicobacter pylori CagA protein: antagonistic effects of the vacuolating cytotoxin VacA
Summary
Helicobacter pylori is the causative agent of gastric pathologies ranging from chronic gastritis to peptic ulcers and even cancer. Virulent strains carrying both the cag pathogenicity island (cagPAI) and the vacuolating cytotoxin VacA are key players in disease development. The cagPAI encodes a type IV secretion system (T4SS) which forms a pilus for injection of the CagA protein into gastric epithelial cells. Injected CagA undergoes tyrosine phosphorylation and induces actin-cytoskeletal rearrangements involved in host cell scattering and elongation. We show here that the CagA-induced responses can be inhibited in strains expressing highly active VacA. Further investigations revealed that VacA does not interfere with known activities of phosphorylated CagA such as inactivation of Src kinase and cortactin dephosphorylation. Instead, we demonstrate that VacA exhibits inactivating activities on the epidermal growth factor receptor EGFR and HER2/Neu, and subsequently Erk1/2 MAP kinase which are important for cell scattering and elongation. Inactivation of vacA gene, downregulation of the VacA receptor RPTP-α, addition of EGF or expression of constitutive-active MEK1 kinase restored the capability of H. pylori to induce the latter phenotypes. These data demonstrate that VacA can downregulate CagA's effects on epithelial cells, a novel molecular mechanism showing how H. pylori can avoid excessive cellular damage.
Introduction
Helicobacter pylori is a highly successful bacterial pathogen that can persistently colonize the human stomach of about half the human world population. This bacterium has been recognized as the causative agent of chronic gastric inflammation, which can further progress to a variety of other diseases such as peptic ulcer, mucosa-associated lymphoid tissue (MALT) lymphoma or even gastric cancer (Cover and Blaser, 1999; Blaser and Berg, 2001; Montecucco and Rappuoli, 2001; Peek and Blaser, 2002; Atherton, 2006). Infection with H. pylori results in mucosal release of chemotactic factors that attract neutrophils and mononuclear cells, which enhance the inflammatory response (Bodger and Crabtree, 1998; Covacci et al., 1999; Zarrilli et al., 1999). In spite of the accumulating knowledge about mechanisms involved in H. pylori-induced pathogenesis, it is not well understood why infected individuals develop one or the other disease. This enigma emphasizes the importance of multiple bacterial factors, differences among strains, differences in the host response to the bacteria, differences in host–microbe interaction and/or genetic susceptibility of the host in determining the course and outcome of H. pylori infections (Blaser and Atherton, 2004; Cover and Blanke, 2005; Wilson and Crabtree, 2007; Amieva and El-Omar, 2008; Backert and Selbach, 2008).
Virulent H. pylori isolates are characterized by the presence of major disease-associated components: namely the vacuolating cytotoxin (VacA) and the CagA protein. The gene encoding vacA is present in virtually all of the H. pylori strains, which suggests that the production of VacA plays an important role in the colonization or persistence of H. pylori in the human stomach (Blaser and Atherton, 2004; Cover and Blanke, 2005). Previous studies reported that the intragastric administration of VacA into mice induced erosions of the gastric epithelia (Telford et al., 1994). When added to epithelial cells in vitro, VacA acts as a pore-forming toxin and induces both structural and functional alterations in the cells, the most prominent being mitochondrial damage, apoptosis and the formation of cytoplasmic vacuolation (Telford et al., 1994; Galmiche et al., 2000; Blaser and Atherton, 2004; Cover and Blanke, 2005). Interestingly, mice deficient in protein tyrosine phosphatase receptor type Z (called RPTP-ζ or RPTP-β) do not show mucosal damage by VacA, although VacA is incorporated into the gastric epithelial cells to the same extent as in wild-type (wt) mice (Fujikawa et al., 2003). Both RPTP-α and RPTP-β (Fujikawa et al., 2003; Yahiro et al., 2003; Nakayama et al., 2006) as well as sphingomyelin (Gupta et al., 2008) act as receptors for VacA in gastric epithelial cells. Several reports indicate that VacA can also bind to other surface molecules such as heparan sulfate (Utt et al., 2001), RACK1 (Hennig et al., 2001), fibronectin (Hennig et al., 2005) and epidermal growth factor receptor (EGFR) (Seto et al., 1998), but the importance of the latter interactions for toxin function are widely unclear. The vacuoles induced by internalized VacA contain both a late endosomal marker (Rab7) and a lysosomal marker (Llgp110) but they do not contain any markers for early endocytic compartments, thus suggesting that they are hybrids of late endosomes and lysosomes (Papini et al., 1994; 1997; Molinari et al., 1997). A current model of vacuole formation is that VacA binds to the plasma membrane, is internalized by cells and forms anion-selective membrane channels, and the vacuoles then arise due to swelling of the endosomal compartments (Blaser and Atherton, 2004; Cover and Blanke, 2005).
Besides VacA, the protein encoded by the cytotoxin-associated gene A (CagA) receives great attention. Epidemiological studies have demonstrated a strong correlation between the presence of CagA and development of gastric diseases (Montecucco and Rappuoli, 2001; Peek and Blaser, 2002; Atherton, 2006; Backert and Meyer, 2006). Virulent H. pylori isolates harbour the cag (cytotoxin-associated genes) pathogenicity island (cagPAI), a 40 kb stretch of DNA, which encodes CagA and components of a sophisticated type IV secretion system (T4SS). The T4SS forms a pilus for the injection of virulence factors into host target cells such as CagA (Blaser and Atherton, 2004; Backert and Meyer, 2006; Amieva and El-Omar, 2008). This is accomplished by a specialized adhesin of the pilus surface, the CagL protein, which binds to and activates host cell integrin β1 for subsequent delivery of CagA across the host cell membrane (Kwok et al., 2007; Backert et al., 2008). Several models how H. pylori can establish contact with integrins at the basolateral surface of polarized epithelial cells were proposed (Wessler and Backert, 2008). Injected CagA becomes tyrosine-phosphorylated (CagAPY) in its Glu–Pro–Ile–Tyr–Ala (EPIYA) repeat region by Src and Abl family kinases and mimics a host cell protein in binding and activation of multiple signalling factors (Selbach et al., 2002a; Stein et al., 2002; Poppe et al., 2007; Tammer et al., 2007).
Both phosphorylation-dependent and phosphorylation-independent signalling activities of CagA and the T4SS have been identified in vivo and in vitro which include the induction of membrane dynamics, actin-cytoskeletal rearrangements and the disruption of cell-to-cell junctions as well as proliferative, pro-inflammatory and antiapoptotic nuclear responses (Blaser and Atherton, 2004; Backert and Selbach, 2008; Hatakeyama, 2008). Non-phosphorylated CagA can interact with multiple host cell proteins such as cell adhesion proteins E-cadherin and ZO-1, the hepatocyte growth factor receptor c-Met, the adaptor protein Grb2 and the kinase Par1 (Mimuro et al., 2002; Amieva et al., 2003; Churin et al., 2003; Murata-Kamiya et al., 2007; Saadat et al., 2007; Zeaiter et al., 2007). While phosphorylation-independent interactions of CagA induce disruption of tight and adherens junctions, loss of cell polarity, pro-inflammatory and mitogenic responses, CagAPY mainly signals to the actin cytoskeleton (Backert and Selbach, 2008). Three SH2 domain-containing host cell proteins were found to bind CagA in a phosphorylation-dependent manner: the tyrosine phosphatase Shp-2, the carboxy-terminal Src kinase (Csk) and the adaptor protein Crk (Higashi et al., 2002; Tsutsumi et al., 2003; Suzuki et al., 2005; Brandt et al., 2007). CagAPY can induce cellular elongation in vitro by causing a cell retraction defect in focal adhesions (Bourzac et al., 2007). In line with the latter conclusion, CagAPY inactivates proteins controlling cell-to-matrix adhesion, including Shp-2-dependent dephosphorylation of focal adhesion kinase FAK (Tsutsumi et al., 2005) and Csk-dependent inactivation of Src resulting in tyrosine-dephosphorylation of the actin-binding protein cortactin, vinculin and ezrin (Selbach et al., 2003; 2004; Tsutsumi et al., 2003; Selbach and Backert, 2005; Moese et al., 2007). In addition, H. pylori induces several T4SS-dependent but CagA-independent signalling events such as the activation of epidermal growth factor receptor EGFR (Keates et al., 2001; 2007; Wallasch et al., 2002; Du et al., 2007) and the small Rho GTPase Rac1 (Churin et al., 2001; Suzuki et al., 2005; Brandt et al., 2007). Despite the significant research progress made both in vitro and in animal models, the pathogenic role of CagA and the cagPAI in disease development remains to be fully understood.
The finding that H. pylori rapidly induces elongation and disruption of cell-to-cell junctions in gastric epithelial cells in vitro makes CagA an attractive model system to study molecular mechanisms contributing to cancer development. However, there is some debate whether CagAPY alone can induce the elongation phenotype in AGS cells (Backert et al., 2001; Higashi et al., 2002; 2004; Moese et al., 2004). Here we demonstrate by genetic exchange and other experiments that phosphorylation of CagA is necessary but not sufficient for inducing the latter phenotype which requires additional T4SS-dependent signalling such as that of growth factor receptors EGFR and HER2/Neu. Even more complexity arises from evidence that there is substantial cross-talk between CagAPY and VacA signalling in infected gastric epithelial cells. In fact, we show here that VacA can downregulate EGFR, HER2/Neu and Erk1/2 requiring the RPTP receptor of VacA and subsequent internalization in sphingolipid-cholesterol-rich membrane microdomains (so-called ‘lipid rafts’) but not cell vacuolation itself. Thus, our data demonstrate that VacA can downregulate CagA's effects on epithelial cells, a novel mechanism showing how H. pylori can control and avoid excessive cell damage.
Results
Phosphorylation of CagA is not sufficient to induce AGS cell elongation during infection with H. pylori
The H. pylori T4SS encoded by the cagPAI is thought to play an essential role in host–pathogen interactions. A 4 h infection of AGS gastric epithelial cells with typical cagPAI+ wt H. pylori strains such as P1 induces severe responses such as tyrosine phosphorylation of CagA and the CagA-dependent cellular scattering and elongation, also known as the ‘hummingbird’ phenotype (Fig. 1). This phenotype is characterized by the formation of 20–70 μm thin needle-like structures in 60–80% of infected cells which is visible as soon as 2–3 h after infection (Segal et al., 1999; Selbach et al., 2002b; Moese et al., 2004). During a screen of clinical H. pylori isolates (Backert et al., 2004), we detected two isolates (P310 and P277) which carry a functional cagPAI but do not induce the elongation phenotype in infected AGS cells. Infection with strains P310 and P277 exhibited CagAPY as indicative for a functional T4SS in the cagPAI, and CagAPY was found in amounts similar to our control strain P1 (Fig. 1A) or the TIGR strain 26695 (data not shown). However, under identical experimental conditions, only strain P1 and 26695 induced the elongation phenotype, while P310 and P277 did not (1, 4). This was a surprising observation, since tyrosine phosphorylation of injected CagA is commonly believed to be sufficient for H. pylori to induce host cell elongation. A second bacterial factor being involved in this phenotypical outcome is yet unknown. In order to better understand CagA-induced actin-cytoskeletal rearrangements, we aimed to investigate the molecular mechanism involved in the blocking effect described above.

Different effects of H. pylori infection on phosphorylation of CagA and the elongation phenotype.A. AGS cells were infected with the indicated H. pylori wt strains for 4 h using an moi = 100. Tyrosine phosphorylation of CagA was analysed by Western blotting using an α-phosphotyrosine antibody (α-PY99). Western blotting using an α-CagA antibody demonstrates that equal amounts of proteins are loaded on the gel.B. Phase-contrast microscopy of AGS cells before and after infection with the indicated H. pylori wt strains. Note that the elongation and scattering phenotype are only expressed during infection with wt strain P1, but not with P310 and P277.C. The number of elongated AGS cells in each experiment was quantified in 10 different 0.25 mm2 fields. The results are representative from at least three independent experiments. P-values < 0.005 (**) were considered as significant.
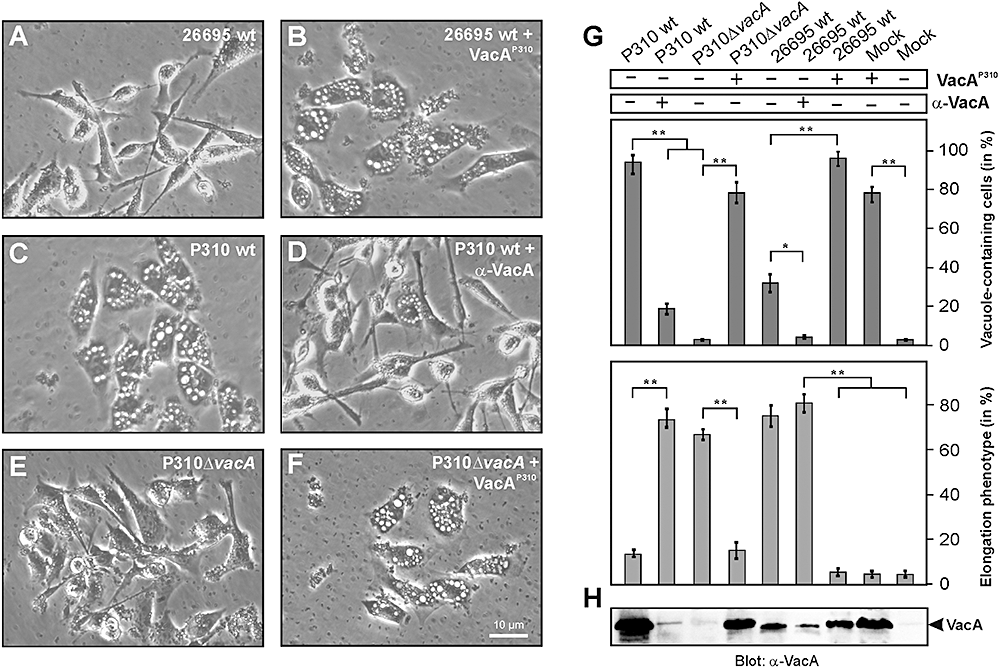
The VacA protein of strain P310 is responsible for the inhibitory effect on AGS cell elongation.A. Phase-contrast microscopy of AGS cells infected with wt H. pylori strain 26695 for 4 h using an moi = 100.B. Phase-contrast microscopy of AGS cells infected with 26695 in the presence of 10 μg ml−1 VacA prepared from strain P310. Note that the infected cells exhibit VacA-induced vacuoles while the elongation phenotype is inhibited.C. Phase-contrast microscopy of AGS cells infected with H. pylori strain P310 for 4 h using an moi = 100.D. Phase-contrast microscopy of AGS cells infected with P310 in the presence of 50 ng ml−1α-VacA antibody. Note that the number of VacA-induced vacuoles is largely suppressed while the elongation phenotype is restored.E. Phase-contrast microscopy of AGS cells infected with P310ΔvacA mutant strain for 4 h using an moi = 100. Note that there are no vacuoles in the cells and the elongation phenotype is largely restored.F. Phase-contrast microscopy of AGS cells infected with P310ΔvacA mutant strain in the presence of 10 μg ml−1 VacA prepared from wt strain P310. Note that the infected cells exhibit VacA-induced vacuoles while the elongation phenotype is inhibited.G. Quantification of the cell elongation and vacuolation phenotypes in AGS cells in response to the presence or absence of VacA protein of strain P310 and α-VacA antibodies. The number of vacuole-containing AGS cells (top) and elongated AGS cells (bottom) was quantified in each infection experiment in 10 different 0.25 mm2 fields. Note that presence of VacAP310 induces vacuoles while the elongation phenotype is inhibited. In contrast, when the vacA gene is inactivated in the P310ΔvacA mutant or VacAP310 is inactivated in the presence of 50 ng ml−1α-VacA antibody, the formation of vacuoles is largely suppressed while the elongation phenotype is restored. The results are representative from at least three independent experiments. P-values < 0.005 (**) or P < 0.05 (*) were considered as significant.H. Confirmation of VacA presence or absence in each experiment by Western blotting of total-cell lysates using an α-VacA antibody.
Genetic exchange of cagA genes among H. pylori strains confirms functional activity of CagAPY proteins
To investigate whether this inhibitory effect is due to strain-specific differences in the CagA proteins, we exchanged the cagA genes among the isolates. For this purpose, we produced cagA deletion mutants in each of the strains and complemented them with cagA genes which were cloned in a shuttle plasmid as described (Backert et al., 2001; Brandt et al., 2005). Expression and phosphorylation of individual CagA proteins during infection were confirmed by Western blotting (Fig. 2A). As expected, we found that expression of cagA from the TIGR strain 26695 (cagA26695) in P1ΔcagA restored the elongation phenotype (Fig. 2B). Surprisingly, expression of cagA26695 in P310ΔcagA and P277ΔcagA did not restore the elongation phenotype (Fig. 2B). As next, we expressed cagA of P310 (cagAP310) and P277 (cagAP277) in P1ΔcagA (Fig. 2A). As shown in Fig. 2B, expression of the latter CagA proteins in P1ΔcagA restored the elongation phenotype. These data demonstrate that the H. pylori strains P310 and P277 have functional CagA proteins when expressed in the genetic background of strain P1 but not in strains P310 and P277.
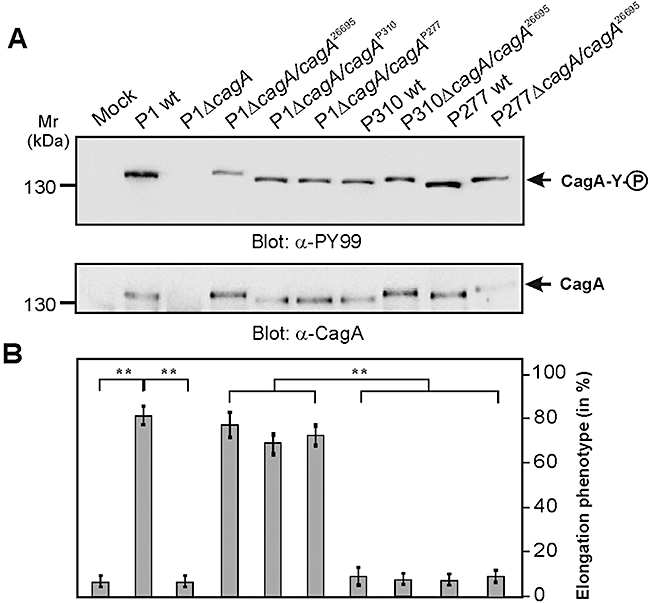
Genetic exchange of cagA genes among H. pylori strains confirms functional activity of CagAPY proteins.A. H. pylori cagA deletion mutants in strains P1, P310 and P277 were produced and complemented with different indicated cagA genes as cloned in a shuttle plasmid. AGS cells were infected with the indicated H. pylori strains for 4 h using an moi = 100. Tyrosine phosphorylation of CagA was analysed by Western blotting using an α-phosphotyrosine antibody (α-PY99) and expression of CagA in the strains was confirmed using an α-CagA antibody.B. The number of elongated AGS cells in each experiment was quantified in 10 different 0.25 mm2 fields. Note that P310 and P277 have functional CagA proteins when expressed in strain P1 but not in P310 and P277. The results are representative from at least three independent experiments. P-values < 0.005 (**) were considered as significant.
Inhibition of AGS cell elongation does not involve altered Src and cortactin signalling
We have recently shown that H. pylori induces the CagA-dependent inactivation of Src kinase between 2 and 3 h of infection and subsequently the tyrosine dephosphorylation of the actin-binding protein cortactin, both of which are important to induce host cell elongation and scattering (Selbach et al., 2003; Backert et al., 2004). H. pylori wt strain P310 induces the inactivation of Src (indicated by strongly reduced levels of phosphorylation at Y-418 in the kinase domain) and the tyrosine dephosphorylation of cortactin to the same extent as seen for complemented P310ΔcagA/cagA26695 (Fig. 3A–C) or P1 wt (Selbach et al., 2003; Backert et al., 2004). Similar results were obtained with strain P277 (data not shown). Thus, the observed blocking effect of the H. pylori-induced actin-cytoskeletal rearrangements involved in the elongation phenotype is not due to altered signalling to Src kinase and cortactin but may account for one or more P310- and P277-specific factors independently of CagA.

CagA of H. pylori strains P310 and 26695 are functional with respect to described activities on inactivation of Src tyrosine kinase and dephosphorylation of the actin-binding protein cortactin.A. AGS cells were infected with the indicated H. pylori strains for 4 h using an moi = 100. P310 wt strain, P310ΔcagA and P310ΔcagA complemented with cagA gene from strain 26695 were used in this experiment. Tyrosine phosphorylation of CagA and cortactin in total-cell lysates was analysed by Western blotting using an α-phosphotyrosine antibody PY99.B. Immunoprecipitation (IP) of cortactin from infected and non-infected cells as indicated. Western blotting of the IPs using α-phosphotyrosine antibody PY99 reveals distinct differences in the phosphorylation status of cortactin depending on the infected strain (top). Presence of equal amount of cortactin in each lane was confirmed using an α-cortactin antibody (middle). Quantification of cortactin tyrosine phosphorylation status (bottom) was performed using the luminescence image analyser. P-values < 0.005 (**) were considered as significant.C. Western blotting of total-cell lysates using an activation-specific Src antibody (α-Src-PY-418, top). Presence of equal amount of Src in each lane was confirmed using an α-Src antibody (middle). Quantification of Src kinase activity (bottom) was performed using the luminescence image analyser. P-values < 0.005 (**) were considered as significant.
H. pylori VacA protein is involved in inhibition of AGS cell elongation
A remarkable feature of H. pylori strains P310 and P277 during infection of AGS cells is that both strains exhibited a very strong vacuolation phenotype while strain P1 or 26695 did not (1, 4). Since this vacuolation phenotype is caused by VacA (Blaser and Atherton, 2004; Cover and Blanke, 2005), and was even visible without addition of vacuolation enhancing reagents such as NH4Cl, we postulated that VacA from these strains may be involved in the observed phenotypical blocking effect. To test this hypothesis, we purified the VacA protein from P310 (VacAP310) as described recently (Sundrud et al., 2004). Indeed, addition of purified VacAP310 to infections with H. pylori wt strains 26695 or P1 induced AGS cell vacuolation which was accompanied by drastic reduction of the elongation phenotype (Fig. 4A and B and data not shown). In line with these observations we found that addition of α-VacA antibodies to infections with P310 largely inhibited cellular vacuolation and restored the elongation phenotype to substantial amounts (Fig. 4C and D). To provide further evidence that VacA is involved in the inhibitory process, we produced vacA gene deletion mutants in both strains, P310 and P277. Infection with P310ΔvacA indeed restored the elongation phenotype (Fig. 4E) while addition of purified VacAP310 to infections with P310ΔvacA reversed the effect as expected and confirmed the absence of polar effects in the mutant (Fig. 4F). Similar results were obtained with strain P277ΔvacA (data not shown). All quantification data of these experiments are shown in Fig. 4G and H. The results of these studies suggest that H. pylori strains P310 and P277 express functional VacA proteins which induce signalling in AGS cells that inhibits the CagA-induced elongation phenotype. Similar results were obtained with the H. pylori strains 60190 and NCTC11638 which also express highly active VacA (Table 1). To exclude a cell type-specific effect in infected AGS cells, we have tested two other cell lines, MKN-28 and MCF-7. Interestingly, P310ΔvacA and 60190ΔvacA induced more pronounced cell scattering and elongation during infection of these two cell lines as compared with their corresponding wt strains (data not shown). Thus, the observed findings are not restricted to AGS cells but are a common phenomenon.
| H. pylori strain | Diagnosis | Geographictype | CagA EPIYA typea | VacAgenotypea | Downregulation of AGS cell elongation by VacAb | Reference |
|---|---|---|---|---|---|---|
| 26695 | Gastritis | Western | ABC | s1m1 | + | Tomb et al. (1997) |
| P1 | Duodenal ulcer | Western | ABC | s1m2 | + | Haas et al. (1993) |
| P277 | Gastritis | Western | AB | s1m1 | +++ | Backert et al. (2004) |
| P310 | Gastric cancer | Western | ABC | s1m1 | +++ | Backert et al. (2004) |
| 60190 | n.p. | Western | ABC | s1m1 | ++ | Leunk et al. (1988) |
| NCTC11637 | Gastritis | Western | ABCCC | s1m1 | ++ | Yamazaki et al. (2005) |
- a. CagA EPIYA and VacA typing were performed in the present study as described in Experimental procedures.
- b. Suppression of the CagA-induced elongation phenotype in infected host cells by VacA as observed in this study: – (no suppression, < 10%), + (suppression by 10–30%), ++ (suppression by 30–60%), +++ (suppression by 60–95%); for more details see text.
- n.p., not provided in the Leunk et al. (1988) paper.
VacA-dependent membrane signalling but not vacuolation itself is involved in inhibition of AGS cell elongation
The VacA cytotoxin is recognized by its receptor RPTP-α and RPTP-β in lipid rafts of gastric epithelial cells, which can then induce the internalization of the toxin followed by cellular vacuolation as well as the activation of the p38 MAP kinase signalling pathway (Blaser and Atherton, 2004; Nakayama et al., 2004; Cover and Blanke, 2005). As illustrated in Fig. 5A, each of these events can be inhibited by specific pharmacological compounds as recently described (Nakayama et al., 2006). Consequently, we aimed to investigate whether the production of VacA-dependent vacuoles, activation of p38 or more upstream signalling in the lipid rafts are important for the blocking effect on the CagA-induced elongation phenotype. Addition of inhibitors effectively blocking V-ATPase and formation of VacA-induced vacuoles (Bafilomycin A1) or p38 MAP kinases (SB203580) did not restore the elongation phenotype (Fig. 5B). Next, we added methyl-β-cyclodextrin (MCD) or 5-nitro-2-(3-phenylpropylamino)-benzoic acid (NPPB), two well-described inhibitors of functional lipid rafts (Ricci et al., 2000; Patel et al., 2002; Schraw et al., 2002; Nakayama et al., 2006). Surprisingly, the presence of MCD or NPPB restored the elongation phenotype induced by H. pylori (Fig. 5B). These findings suggest that the cellular vacuolation or p38 MAP kinase signalling per se does not suppress the elongation phenotype but membrane-linked processes associated with binding and internalization of VacA in the lipid rafts are involved in the phenomenon.
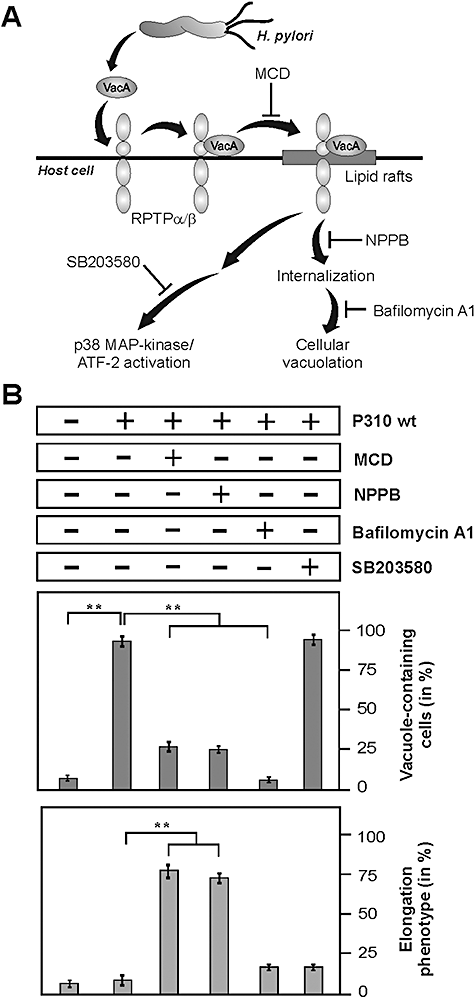
VacA-dependent membrane signalling, but not vacuolation itself or p38 MAP kinase activation, is involved in inhibition of AGS cell elongation as indicated by infection studies in the presence of pharmacological inhibitors.A. Multiple actions of VacA on cell surface of gastric epithelial cells infected with H. pylori. After clustering of VacA and RPTP receptor in membrane lipid rafts, cytotoxic effects of VacA include the toxin uptake in the endocytic compartment resulting in vacuolation and stimulation of pro-inflammatory signalling, such as p38 MAP kinase/ATF-2 cascade activation. Different inhibitors interfering with VacA signalling at different steps are indicated as described by Nakayama et al. (2006).B. AGS cells were treated with several inhibitors 30 min prior to infection at concentrations described in Experimental procedures. Infection with H. pylori strain P310 was performed for 4 h using an moi = 100. The number of vacuole-containing AGS cells (top) and elongated AGS cells (bottom) was quantified in each infection experiment in 10 different 0.25 mm2 fields. Note that inhibitors MCD or NPPB blocked VacA-induced vacuolation and restored AGS cell elongation, while the presence of Bafilomycin A1 or SB203580 did not. The results are representative from at least three independent experiments. P-values < 0.005 (**) were considered as significant.
Knock-down of the VacA receptor RPTP-α by siRNA restores AGS cell elongation
AGS cells express the VacA receptor RPTP-α but not RPTP-β (De Guzman et al., 2005). To test the hypothesis that VacA binding to RPTP-α and resulting membrane signalling are involved in the above described blocking effect, we transfected AGS cells with siRNA against RPTP-α or a scrambled control siRNA. Western blotting of total-cell lysates using an anti-RPTP-α confirmed downregulation of RPTP-α protein expression with the respective siRNA but not in the control (Fig. 6A). The transfected cells were then infected with H. pylori strains P310 or P1 and the number of vacuole-containing and elongated AGS cells was quantified. Indeed, downregulation of RPTP-α inhibited the vacuolation and restored AGS cell elongation significantly in infections with both strains P310 and P1 (Fig. 6B and C).
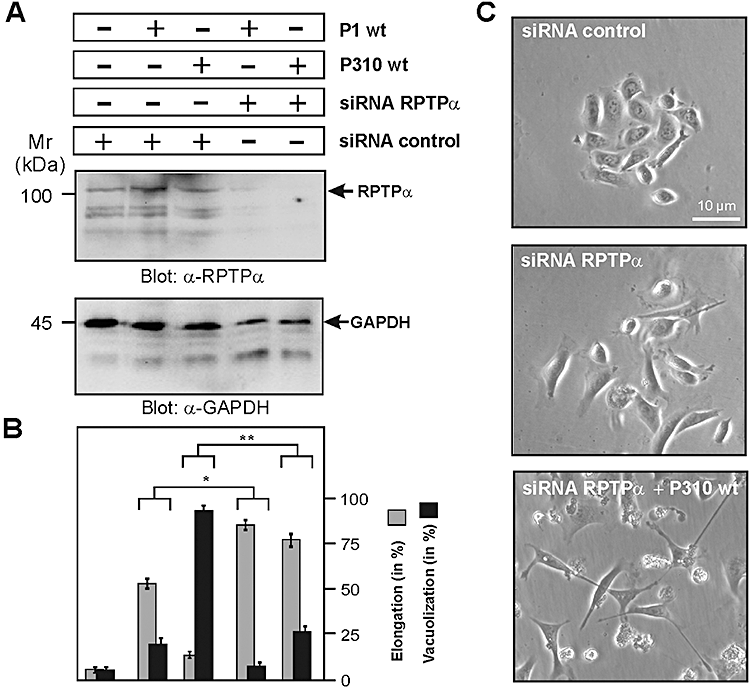
Knock-down of the VacA receptor RPTP-α by siRNA blocks cellular vacuolation and restores AGS cell elongation during infection with H. pylori strain P310.A. AGS cells were treated with siRNA against RPTP-α or control siRNA, and then infected with H. pylori strains P310 or P1 for 4 h using an moi = 100 as indicated. Western blotting of total-cell lysates using an anti-RPTP-α confirms downregulation of RPTP-α protein expression. The α-GAPDH blot serves as loading control (bottom).B. The number of vacuole-containing and elongated AGS cells was quantified in each infection experiment in 10 different 0.25 mm2 fields. Note that downregulation of RPTP-α inhibited the vacuolation and restored AGS cell elongation significantly. The results are representative from at least three independent experiments. P-values < 0.05 (*) and P < 0.005 (**) were considered as significant.C. Phase-contrast microscopy of AGS cells treated with control siRNA or siRNA against RPTP-α in the presence or absence of H. pylori P310 wt strain.
H. pylori VacA negatively regulates EGF receptor, HER2/Neu and Erk1/2 signalling
Recent immunoprecipitation and inhibitor studies in HeLa cells indicated that VacA may bind EGFR for subsequent uptake of the toxin; however, the importance of this putative interaction for EGFR activity was not investigated (Seto et al., 1998). Interestingly, cagPAI+H. pylori can activate the EGFR→Ras→Erk pathway leading to IL-8 expression and pro-inflammatory responses (Keates et al., 2001). Since activation of Erk1/2 MAP kinase also plays an important role in the H. pylori-induced elongation phenotype (Higashi et al., 2004; Moese et al., 2004), we investigated whether there are differences in the activation of EGFR and Erk1/2 between the strains. For this purpose, AGS cells were infected for 3 h with the H. pylori strains P1, 26695, P310 and P277; and Western blots were probed with an activation-specific antibody of EGFR. The results show that whereas P1 and 26695 induced profound EGFR activation, strains P310 and P277 were remarkably attenuated in this response (Fig. 7A, top panel). We also tested whether other EGFR family members such as HER2/ErbB2/Neu (also called HER2/Neu) are affected during infection. Similarly, HER2/Neu is also activated by H. pylori strains P1 and 26695 with much less activity obtained during infection with strains P310 and P277 (Fig. 7A, second panel), both resulting in attenuated activity of the downstream effector MAP kinase Erk1/2 (Fig. 7A, third panel). The quantification data of these kinase activities are shown in Fig. 7B. Taken together, high activities of EGFR, HER2/Neu and Erk1/2 kinases during infection with wt strains P1 and 26695 correlate remarkably with the induction of the elongation phenotype whereas inhibition of these kinases by P310 and P277 results in blocking of host cell elongation (Fig. 7C).
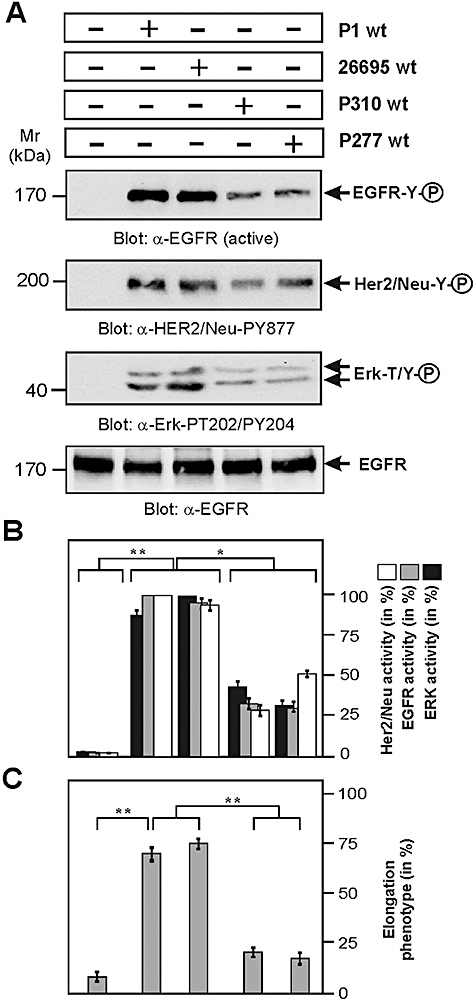
Infection of AGS cells with H. pylori and activation status of EGF receptor, HER2/Neu and Erk1/2 kinases.A. AGS cells were infected with the indicated H. pylori wt strains for 3 h using an moi = 100. Western blotting of total-cell lysates using an activation-specific anti-EGFR (α-EGFR active), anti-HER2/Neu (α-HER2/Neu-PY877) and anti-Erk1/2 (α-Erk1/2-PT202/PY204) antibodies. Equal amounts of EGFR in each lane was confirmed using an α-EGFR antibody (bottom). Note that wt H. pylori strains P1 or 26695 activate EGF receptor, HER2/Neu and Erk1/2 signalling, while infection with strains P310 or P277 does not.B. Quantification of EGFR, HER2/Neu and Erk1/2 kinase activity was performed using the luminescence image analyser. P-values < 0.05 (*) and P < 0.005 (**) were considered as significant.C. The number of elongated AGS cells was quantified in each infection experiment in 10 different 0.25 mm2 fields. The results are representative from at least three independent experiments. P-values < 0.005 (**) were considered as significant.
Infection with VacA-expressing H. pylori affects the cellular distribution of EGFR
Next, we investigated the subcellular localization of EGFR before and after infection with H. pylori. For this purpose, non-infected control AGS cells or cells infected with P310 were stained with phalloidin (F-actin) or anti-EGFR antibody followed by epifluorescence microscopy (Fig. 8). As expected, non-stimulated AGS control cells revealed cell membrane-associated EGFR signals in a spotted pattern, probably due to localization of EGFR receptor in lipid rafts (top, arrows). In contrast, infection of AGS cells with P310 showed that large portions of EGFR were internalized in the cytoplasm and were located around the VacA-dependent vacuoles (bottom, arrows). Similar results were obtained when purified VacAP310 proteins were added to AGS cells (data not shown). This suggests that internalization of VacA during infection with H. pylori is associated with the internalization of EGFR, possibly by the endocytic pathway, which in turn could explain that large portions of this receptor are inactivated.

Infection of AGS cells with the VacA-expressing H. pylori strain P310 affects the cellular distribution of the EGF receptor (EGFR). Non-infected control AGS cells (top) or cells infected with P310 (bottom) were stained with phalloidin (F-actin) or anti-EGFR antibody as indicated and investigated by epifluorescence microscopy. Transmitted light microscopy of the sections is shown in the right panels. Note that the pattern of cell surface-associated EGFR in the non-infected control changes to intracellular localization after infection (arrows).
Inactivation of vacA gene is sufficient to restore EGFR signalling and AGS cell elongation
In the next set of experiments, we aimed to investigate whether suppression of EGFR and Erk1/2 activity by P310 and P277 can be restored by inactivation of the vacA gene in these strains. Indeed, infection of AGS cells with P310ΔvacA and P277ΔvacA rescued the activation of these kinases (Fig. 9A and B), and this correlated with the ability of the ΔvacA mutant strains to induce AGS cell elongation (Fig. 9C). These results suggest that the VacAP310 and VacAP277 proteins interfere with EGFR signalling resulting in the inhibition of Erk1/2 kinase activity and actin-cytoskeletal rearrangements leading to host cell scattering and elongation.
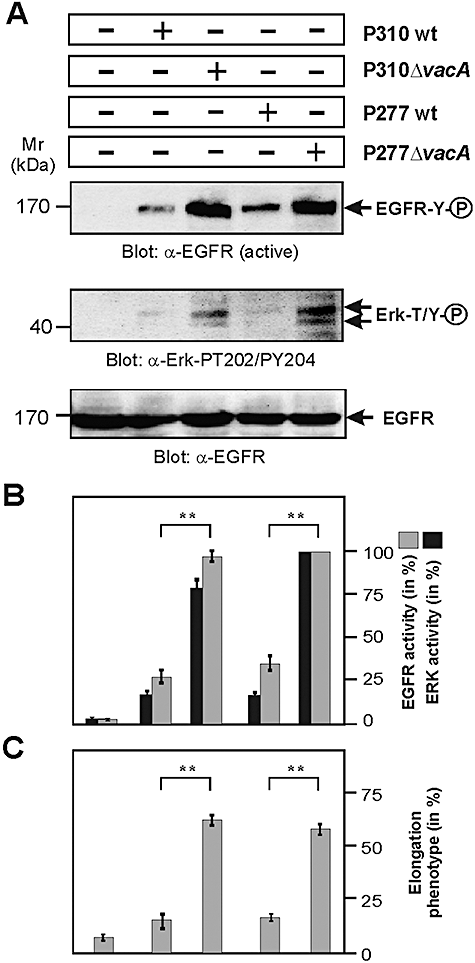
Inactivation of vacA gene in H. pylori strains P310 and P277 is sufficient to restore EGFR and Erk1/2 signalling and AGS cell elongation.A. AGS cells were infected with the indicated H. pylori wt strains for 3 h using an moi = 100. Western blotting of total-cell lysates using an activation-specific anti-EGFR (α-EGFR active) and anti-Erk1/2 (α-Erk1/2-PT202/PY204) antibodies is shown. Equal amounts of EGFR in each lane was confirmed using an α-EGFR antibody (bottom). Note that inactivation of vacA gene in the strains restored activation of EGFR and Erk1/2 signalling.B. Quantification of EGFR and Erk1/2 kinase activity was performed using the luminescence image analyser. P-values < 0.005 (**) were considered as significant.C. The number of elongated AGS cells was quantified in each infection experiment in 10 different 0.25 mm2 fields. The results are representative from at least three independent experiments. P-values < 0.005 (**) were considered as significant.
Addition of EGF or expression of active MEK1 is sufficient to restore Erk1/2 signalling and AGS cell elongation
Finally, we wanted to examine whether the AGS cell scattering and elongation phenotype in infections with P310 or P277 can be restored by activating EGFR externally by addition of EGF or internally by transient expression of an active MEK1 kinase variant which can constitutively activate Erk1/2. For this purpose, AGS cells were infected with P310 for 2 h. As expected, the activity of EGFR and Erk1/2 was weak (Fig. 10A, second lane). At this time point, cells were stimulated with EGF for another hour. Addition of EGF resulted in pronounced activation of EGFR and Erk1/2 (Fig. 10A and B, third lane), which subsequently induced the elongation phenotype in infected AGS cells which was visible as soon as 30 min after EGF treatment and strongly exposed after 1 h (Fig. 10C). Similar effects were observed when we expressed constitutive-active MEK1 which did not significantly affect EGFR phosphorylation but strongly enhanced Erk1/2 activity (Fig. 10A and B, fourth lane) and subsequently rescued the induction of the elongation phenotype by P310 (Fig. 10C). These results suggest that the VacAP310 and VacAP277 proteins inhibit EGFR-HER2/Neu and Erk1/2 kinase signalling, and this effect interferes with CagA-induced signalling leading to cell scattering and elongation. Erk1/2 activation is indeed important for phenotypical outcome because the EGF-mediated response is blocked in the presence of the Erk inhibitor PD98059 as indicated (Fig. 10A–C, last lane).
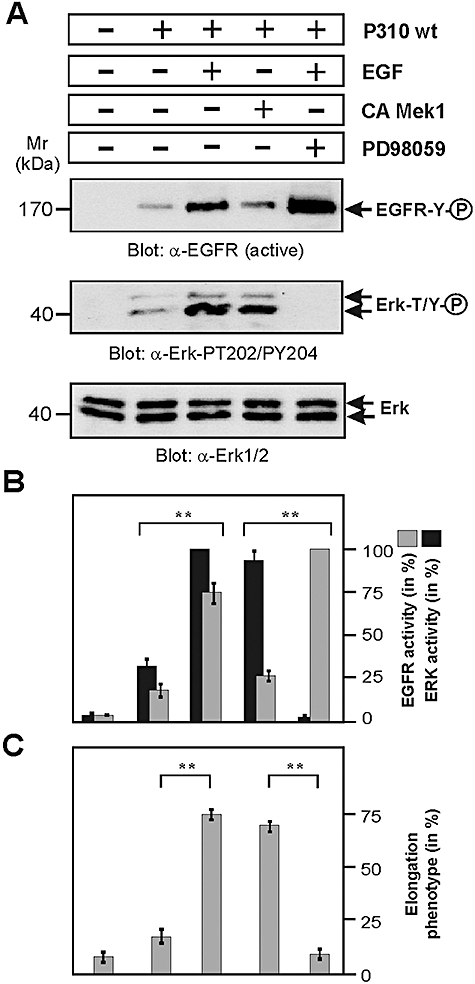
Treatment of AGS cells with EGF or expression of active MEK1 is sufficient to restore Erk1/2 signalling and cellular elongation in infections with H. pylori strains P310 and P277.A. AGS cells [non-transfected or transfected with constitutive-active (CA) MEK1 kinase] were infected with H. pylori strain P310 for 2 h using an moi = 100. After 2 h 10 ng ml−1 EGF (Sigma) was added in the indicated samples or left untreated. Incubation of the cells continued for another hour. Western blotting of total-cell lysates using an activation-specific anti-EGFR (α-EGFR active) and anti-Erk1/2 (α-Erk1/2-PT202/PY204) antibodies is shown. Equal amounts of Erk1/2 in each lane were confirmed using an α-Erk1/2 antibody (bottom). Note that addition of EGF or expression of MEK1 restored Erk activity and AGS cell elongation. The latter response was blocked in the presence of the Erk inhibitor PD98059 (25 μM) as indicated.B. Quantification of EGFR and Erk1/2 kinase activity was performed using the luminescence image analyser. P-values < 0.005 (**) were considered as significant.C. The number of elongated AGS cells was quantified in each infection experiment in 10 different 0.25 mm2 fields. The results are representative from at least three independent experiments. P-values < 0.005 (**) were considered as significant.
Discussion
Helicobacter pylori colonizes the human gastric mucosa and has been identified as an aetiological factor of different disorders such as chronic gastritis, duodenal and gastric peptic ulcer, gastric adenocarcinoma and MALT lymphoma (Cover and Blaser, 1999; Montecucco and Rappuoli, 2001; Peek and Blaser, 2002; Atherton, 2006). Infection with this bacterium can persist lifelong in the stomach and the mechanisms responsible for the diversity of clinical outcomes are still not well established. Both bacterial and host genetic factors and their specific interactions are suspected to play a role in the development of H. pylori-related diseases (Blaser and Atherton, 2004; Cover and Blanke, 2005; Wilson and Crabtree, 2007; Amieva and El-Omar, 2008; Backert and Selbach, 2008). Two of the major bacterial virulence factors, VacA and CagA, are of special interest because epidemiological and functional studies have shown that their expression correlates with disease outcome. We show here that there is substantial cross-talk between the molecular signalling induced by VacA and CagA in infected gastric epithelial cells. We demonstrate that VacA suppresses the activity of the growth factor receptors EGFR and HER2/Neu leading to downregulation of Erk1/2 MAP kinase signalling and subsequently to suppression of H. pylori-induced AGS cell elongation. These data provide direct mechanistic evidence that VacA can downregulate CagA's effects on epithelial cells.
Helicobacter pylori uses a T4SS to induce global actin-cytoskeletal rearrangements in infected AGS cells (Backert et al., 2002; Blaser and Atherton, 2004). CagA, encoded by the cagPAI, is injected into target cells in a integrin β1-dependent manner and is the only described effector protein of this T4SS (Kwok et al., 2007). Several lines of evidence have recently emerged that provide insights into the mechanisms by which CagAPY modulates the host actin cytoskeleton to trigger cellular elongation and cell scattering (Blaser and Atherton, 2004). Numerous signalling pathways are activated in the latter response involving important host cell factors such as Src, Abl, Shp-2, Crk, Grb2, cortactin and others (Suzuki et al., 2005; Tammer et al., 2007; Backert and Selbach, 2008; Hatakeyama, 2008). It is clear that phosphorylation of CagA is an important prerequisite for the induction of host cell elongation as reported by several groups; however, the molecular mechanisms involved in remodelling the actin cytoskeleton are still not fully understood. It also remained unclear whether CagAPY is the sole bacterial factor triggering AGS cell elongation. In fact, induction of cellular elongation is usually very low for transfected CagA comprising 7.5–23% (Higashi et al., 2004) whereas 60–80% of the cells commonly elongate during infection with CagA-expressing H. pylori (Segal et al., 1999; Backert et al., 2001; Brandt et al., 2007; Argent et al., 2008). Interestingly, infection of a ΔcagA knockout mutants did not result in AGS cell elongation while infection of CagA-transfected cells with ΔcagA efficiently restored the phenotype almost to wt levels of up to 80% (Backert et al., 2001). This suggests that there are bacterial factors other than CagA which positively regulate this phenotype. Further evidence for CagAPY being important but not the sole factor in mediating AGS cell elongation is provided in the present study. Our genetic exchange experiments show that the H. pylori strains P310 and P277 have functional CagAPY proteins in the genetic background of strain P1 but not when expressed in strains P310 or P277 respectively. These findings highlight the fact that bacterial factors other than CagA can also negatively regulate H. pylori-induced AGS cell elongation.
We verified that the T4SS is functional in P310 and P277 as judged from injection of CagA and production of CagAPY levels which were similar to that of strains P1 and 26695. In addition, well-known CagAPY-mediated downstream signalling to Src and cortactin, which is important for phenotypical outcome (Selbach et al., 2003; Backert et al., 2004), is not altered in infections with H. pylori strains P310 and P277. Instead, we could provide several lines of evidence that VacA of multiple H. pylori strains including P310, P277, 60190 and NCTC11637 exhibit important suppressive effects on CagA-induced AGS cell elongation. In particular, we have shown that inactivation of vacA gene in P310 and P277 or pre-incubation of cells with α-VacA antibodies inhibited cellular vacuolation resulting in restoration of the AGS elongation phenotype. Finally, addition of purified VacAP310 to infections with H. pylori strains 26695 or P1 induced host cell vacuolation, accompanied by drastic reduction of the elongation phenotype. Thus, VacA represents an antagonist of CagA signalling. This assumption is in line with earlier observations showing that CagA and VacA have opposing effects in T cells, for example, on the activation of transcription factor NFAT (Yokoyama et al., 2005). In another study, ΔcagA and ΔcagE mutants were found to significantly increase VacA-induced vacuolation of epithelial cells, and the ΔvacA mutants significantly increased CagA-induced AGS cell elongation, as compared with their wt strains 60190 and 84-183 (Argent et al., 2008). These reports and our present data clearly show that there is substantial cross-talk between the different H. pylori virulence factors, a phenomenon that was previously underestimated. In addition, we demonstrate here that this cross-talk is not restricted to AGS cells but was also observed in infected MKN-28 and MCF-7 cells.
Next, we were interested in characterizing the signalling pathway involved in the VacA-triggered blocking effect on cell elongation. Studies with inhibitors SB203580 or Bafilomycin A1 clearly demonstrated that VacA-induced downstream signalling such as activation of p38 MAP kinase or cell vacuolation per se does not suppress the elongation phenotype. However, recent reports indicated that the integrity of lipid rafts is essential for VacA-induced vacuolation (Ricci et al., 2000; Patel et al., 2002; Schraw et al., 2002; Nakayama et al., 2006). Interestingly, we found that membrane-linked processes associated with binding and internalization of VacA in the lipid rafts are involved in the phenomenon. First, pre-treatment of AGS cells with MCD, a compound that can selectively extract cholesterol from the plasma membrane without damaging cellular viability (Ohtani et al., 1989), which inhibits translocation of the VacA–RPTP complex to lipid rafts (Nakayama et al., 2006) as well as VacA-induced vacuolation (Patel et al., 2002) and even has suppressive effects on CagA injection (Lai et al., 2008), restored the elongation phenotype. Second, pre-treatment of AGS cells with NPPD, a compound that does not inhibit translocation of VacA to lipid rafts or p38 activation but disrupts anion channels, thus abrogating VacA internalization and vacuolation (Gauthier et al., 2004; Nakayama et al., 2006), also restored the elongation phenotype. Taken together, these data suggest that VacA inserted into the host cell membrane, rather than internalized VacA in the vacuoles or VacA-triggered p38 signalling, induces signals leading to abrogation of the H. pylori-induced elongation phenotype.
The induction of EGF and EGFR tyrosine kinase as well as other factors plays an important role in continuous cell proliferation, renewal of the gastric mucosa, repair of mucosal injury and healing of mucosal ulcerations (Konturek et al., 1988; Romano et al., 1998; Zarrilli et al., 1999). Earlier in vitro studies have shown that EGFR is activated by H. pylori in a T4SS-dependent manner but independent of CagA and VacA; however, the actual bacterial factor involved remained unknown (Keates et al., 2001; Wallasch et al., 2002; Du et al., 2007). We have shown here that T4SS-positive H. pylori strains P1 and 26695 profoundly activate EGFR (as well as its related receptor HER2/Neu), while T4SS-positive H. pylori strains P310 and P277 having strong VacA vacuolation activity did not. Interestingly, inactivation of vacA gene in P310 and P277 rescued the activation of EGFR and HER2/Neu by these strains. These results are in good agreement with recent publications showing that purified VacA or VacA-containing cell culture supernatants exhibited suppressive effects on distinct cellular effects including proliferation, cell migration or wound healing of gastric epithelial cells in vitro (Ricci et al., 1996; Pai et al., 1998; 2000; Tabel et al., 2003). Immunoprecipitation experiments in HeLa cells indicated that VacA may bind EGFR for subsequent uptake of the toxin (Seto et al., 1998). These findings suggest that EGFR could act as alternative receptor for VacA, and subsequent endocytosis of EGFR provides a possible mechanism how significant portions of EGFR are inactivated by internalization of this receptor. Our immunofluorescence data showing internalized EGFR in the infection with VacA-expressing strain P310 are in agreement with the latter hypothesis. However, further investigation is necessary to reveal whether this is indeed the case or whether another mechanism is involved in VacA-dependent inactivation of EGFR and HER2/Neu.
Taken together, our findings in this study clearly demonstrate that VacA can directly influence the phosphorylation/activation status of EGFR/HER2/Neu and downstream signalling leading to suppression of Erk1/2 kinase activity. Thus, this is the first study showing directly that VacA can inactivate growth factor receptor tyrosine kinases during infection. Our study further provides evidence for substantial cross-talk between CagAPY and VacA signalling in gastric epithelial cells infected with H. pylori. In fact, we could demonstrate that VacA, by expressing inhibitory activity on EGFR, HER2/Neu and Erk1/2 kinases, can downregulate CagA's effects on epithelial cells, a novel mechanism showing how H. pylori can control and avoid excessive cell damage. It is well known that the determination of disease development is highly complex; however, additional complexity arises from substantial allelic variation in cagA and vacA genes as shown in our study with T4SS-positive and VacA-positive strains P1, 26695, P310, P277, NCTC11637 and 60190. Our data therefore considerably extend the findings of other groups and shed new light on the role of the H. pylori T4SS, CagA and VacA during infection with this important pathogen. Future work should be directed towards this exciting field of pathogen–host interactions.
Experimental procedures
H. pylori isolates
The H. pylori wt strains used in this study and their characteristics are summarized in Table 1. All strains are typical virulent isolates with a functional T4SS encoded by the cagPAI. Mutagenesis of cagA, vacA and genetic complementation in the strains was performed with a pHel vector derivate as described (Backert et al., 2001; Brandt et al., 2005). H. pylori was grown in thin layers on horse serum agar plates supplemented with vancomycin (10 μg ml−1), nystatin (1 μg ml−1) and trimethoprim (5 μg ml−1) as described (Backert et al., 2000; Moese et al., 2002; Selbach et al., 2002b). All antibiotics were obtained from Sigma-Aldrich. Incubation of the bacteria was performed at 37°C for 2 days in an anaerobic jar containing a campygen gas mix of 5% O2, 10% CO2 and 85% N2 (Oxoid).
PCR-based genotyping of cagA and vacA genes
Determination of the number and types of EPIYA motifs A, B and C was carried out by PCR using primers as described (Argent et al., 2005). Briefly, a reaction mixture containing 0.2 mM concentrations of each dNTP, a 0.4 μM concentration of the forward primer, a 0.08 μM concentration of the reverse primer, 0.05 U μl−1Taq DNA polymerase (Qiagen) and 50 ng of genomic DNA was incubated at 95°C for 90 s, followed by 35 cycles at 95°C for 30 s, 57°C for 60 s and 72°C for 30 s and a final extension at 72°C for 5 min.
Typing of the vacA gene was determined by PCR amplification of genomic DNA as described previously (van Doorn et al., 1998). In brief, to amplify the vacA s and m regions, described primer sets were used, resulting in fragments of 176 bp (type s1), 203 bp (type s2), 401 (type m1) or 476 bp (type m2) respectively. All PCR mixtures consisted of dNTPs at concentrations of 0.2 mM each, 0.5 μM of the forward and reverse primers, 0.05 U μl−1Taq DNA polymerase (Qiagen) and 50 ng of genomic DNA and were incubated at 94°C for 9 min, followed by 40 cycles at 94°C for 30 s, 50°C for 45 s and 72°C for 45 s and a final incubation at 72°C for 5 min.
Cell culture, transient transfection assays and inhibitor studies
AGS (ATCC CRL 1739, a human gastric adenocarcinoma epithelial cell line), MKN-28 (another human gastric cancer cell line) and MCF-7 cells (a human breast cancer cell line) were grown in six-well plates containing RPMI 1640 medium (Invitrogen) supplemented with 25 mM Hepes buffer and 10% heat-inactivated fetal bovine serum (FBS, purchased from Biochrom) for 2 days to reach monolayers of approximately 70% cell confluence. For transient transfection assays, plasmids carrying constitutive-active MEK1 kinase (2 μg) were transfected into 8 × 105 AGS for 36 h using GeneJammer according to the instructions of the supplier (Stratagene). For pharmacological inhibitor studies, all chemical compounds (or their respective resolvent controls) were added to AGS cells 30 min prior to infection: 5 mg ml−1 MCD (Sigma-Aldrich), 100 μM NPPB (Sigma-Aldrich), 20 nM Bafilomycin A1 (Calbiochem), 10 μM SB203580 (Calbiochem) or 25 μM PD98059 (Calbiochem).
Elongation phenotype, motility assays and cell vacuolation
AGS cells were grown in six-well plates as monolayers of about 70% cell confluence as described above. Infections were performed using a multiplicity of infection (moi) of 100. The number of elongated AGS cells in each experiment was quantified in 10 different 0.25 mm2 fields using an Olympus IX50 phase-contrast microscope. The elongation phenotype is characterized by the production of thin needle-like cell protrusions of 20–70 μm in length (Backert et al., 2001; 2004). Cells exhibiting smaller protrusions (< 10 μm) were occasionally also seen in the uninfected control but were not counted. The number of infected cells containing vacuoles was also quantified in the same experiments in the phase-contrast microscope, and verified by neutral red uptake assay (Papini et al., 1994). All experiments were performed in triplicates.
Immunofluorescence microscopy
Non-infected AGS cells or cells infected for 3 h were washed with PBS to remove non-adherent bacteria and fixed with 3.8% paraformaldehyde in PBS. Fluorescence staining procedures using phalloidin (F-actin) and monoclonal anti-EGFR antibody (Santa Cruz) were described previously (Brandt et al., 2005; 2007). Specimens were analysed using a fluorescence microscope (Leica DMRE7) equipped with a CCD camera (Spot RT, Diagnostic Instruments) and a 63/1.4 objective.
Preparation of VacA
Helicobacter pylori strains were grown in brain–heart infusion (BHI) medium at 150 r.p.m. overnight until OD550 = 0.6. VacA was purified in an oligomeric form from culture supernatants of H. pylori, as described previously (Sundrud et al., 2004). Purified VacA preparations were routinely acid activated before addition of VacA to cultured AGS cells (Sundrud et al., 2004). The VacA concentration was 10 μg ml−1 for all experiments.
Determination of Src activity
Src activity depends on autophosphorylation of the protein at Y-418 in the kinase domain (Hunter, 1987). Src activity during infection of AGS cells was determined by Western blot experiments using a rabbit polyclonal anti-Src-PY-418 antibody (NEB Cell Signaling). As loading control, the total amount of Src was detected using a monoclonal anti-Src antibody (Santa Cruz). Densitometric measurements of band intensities revealed the percentage of Src activity (Lumi-Imager F1, Roche).
Phosphorylation status of cortactin
Phosphorylated cortactin can be detected in non-infected AGS cells as a predominant band at 80 kDa using a mouse monoclonal anti-phosphotyrosine antibody PY99 (Santa Cruz) and a monoclonal anti-cortactin antibody (BD Biosciences). The phosphorylation status of cortactin was verified by immunoprecipitation experiments as described (Selbach et al., 2003). Briefly, infected or non-infected AGS cells were washed with cold PBS and lysed for 30 min at 4°C in lysis buffer (20 mM Tris pH 7.2, 150 mM NaCl, 5 mM EDTA, 1% Triton X-100, 10% glycerol, 1 mM Na3VO4, COMPLETETM inhibitor mix from Roche). Lysates were pre-cleared with protein G-Sepharose (Pharmacia) for 2 h at 4°C. Two micrograms of the polyclonal α-Cortactin antibody (Santa Cruz) were added to the supernatant and incubated overnight. Immune complexes were precipitated by addition of protein G-sepharose for 2 h, washed three times in 0.5× PBS and mixed with equal amounts of 2× SDS-PAGE buffer. Precipitates were analysed by SDS-PAGE and immunoblotting.
SiRNA knock-down, SDS-PAGE and immunoblot analysis
Cell pellets with attached bacteria were mixed with equal amounts of 2× SDS-PAGE buffer and boiled for 5 min. Proteins were separated by SDS-PAGE on 6–12% polyacrylamide gels and blotted onto PVDF membranes (Immobilon-P, Millipore). Before addition of the antibodies, membranes were blocked in TBS-T (140 mM NaCl, 25 mM Tris-HCl pH 7.4, 0.1% Tween-20) with 3% BSA or 5% skim milk for 1 h at room temperature. Phosphorylated and non-phosphorylated CagA proteins were detected by incubation of the membranes with a mouse monoclonal anti-phosphotyrosine antibody PY99 (Santa Cruz) and a rabbit polyclonal anti-CagA antibody (Austral Biologicals). Monoclonal antibody recognizing activated EGFR was purchased from BD Biosciences and rabbit polyclonal anti-EGFR, anti-Erk1/2, anti-phospho-HER2/Neu-Y877 as well as anti-phospho-Erk1/2-T202/Y204 antibodies were from NEB Cell Signaling. Two polyclonal rabbit anti-VacA antibodies were used: rabbits were immunized with a VacA peptide, C-EGGYKDKPKDKPSN (corresponding to amino acids 328–341 of VacA protein from H. pylori TIGR strain 26695). Anti-VacA antibody produced against the whole VacA protein was kindly provided by Tim Cover (Sundrud et al., 2004). Knock-down of RPTP expression in AGS cells was performed using siRNA transfection specific for RPTP-α according to the protocol of the supplier (Santa Cruz) and was verified by Western blotting using a rabbit anti-RPTP-α antibody (Santa Cruz). As secondary antibodies, horseradish peroxidase-conjugated anti-mouse or anti-rabbit polyvalent sheep immunoglobulin was used (Amersham Pharmacia Biotech). Antibody detection was performed with the ECL Plus chemoluminescence Western Blot system for immunostaining (Amersham Pharmacia Biotech).
Statistical analysis
Al data were evaluated using Student's t-test with SigmaStat statistical software (version 2.0). P-values < 0.05 (*) and P < 0.005 (**) were considered as statistically significant.
Acknowledgements
We are very grateful to Tim Cover (Vanderbilt University Nashville, TN) for providing us with anti-VacA antibody and critical comments on the manuscript. We are also grateful to Dawn Israel and Richard Peek (Vanderbilt University Nashville, TN) for providing us with the strains 60190 and NCTC11637. We also thank Bianca Bauer (Max Planck Institute for Infection Biology, Berlin, Germany) for her kind advise on EGFR immunofluorescence staining and Wolfgang König (University Magdeburg, Germany) for his generous support of the project. This work was supported through Priority Program SPP1150 of the Deutsche Forschungsgemeinschaft to S.B. (Ba1671/3-1) and another DFG grant (Ba1671/8-1).