Host cell processes that influence the intracellular survival of Legionella pneumophila
Summary
Key to the pathogenesis of intracellular pathogens is their ability to manipulate host cell processes, permitting the establishment of an intracellular replicative niche. In turn, the host cell deploys defence mechanisms that limit intracellular infection. The bacterial pathogen Legionella pneumophila, the aetiological agent of Legionnaire's Disease, has evolved virulence mechanisms that allow it to replicate within protozoa, its natural host. Many of these tactics also enable L. pneumophila's survival and replication inside macrophages within a membrane-bound compartment known as the Legionella-containing vacuole. One of the virulence factors indispensable for L. pneumophila's intracellular survival is a type IV secretion system, which translocates a large repertoire of bacterial effectors into the host cell. These effectors modulate multiple host cell processes and in particular, redirect trafficking of the L. pneumophila phagosome and mediate its conversion into an ER-derived organelle competent for intracellular bacterial replication. In this review, we discuss how L. pneumophila manipulates host cells, as well as host cell processes that either facilitate or impede its intracellular survival.
Introduction
The intracellular bacterial pathogen Legionella pneumophila is responsible for the severe pneumonia Legionnaire's disease and the less severe flu-like disease Pontiac fever (McDade et al., 1977; Kaufmann et al., 1981). L. pneumophila is found ubiquitously in freshwater environments, where it replicates within protozoan hosts (Fields, 1996). However, modern technologies, such as air conditioning systems, that cause water aerosolization have enabled L. pneumophila to become an opportunistic human pathogen. Following inhalation of L. pneumophila-contaminated water droplets into the human lung, L. pneumophila infects alveolar macrophages (Horwitz, 1983a). L. pneumophila then establishes a replicative niche within an ER-derived compartment protected from endolysosomal destruction (Horwitz, 1983b). This ability to establish an intracellular replicative niche is vital to L. pneumophila's pathogenesis, and its mechanistic basis is under active investigation.
Evolution with protozoa
Legionella pneumophila's natural habitat is freshwater environments, where it infects a wide range of protozoa (Fields, 1996). Phagocytosed bacteria are generally killed in lysosomes, where an acidic pH and lysosomal enzymes digest the bacteria, thus releasing nutrients for use by the protozoan host. Several bacteria, including L. pneumophila, have evolved strategies to prevent lysosome-mediated destruction and persist within amobae. An intracellular symbiont of Amoeba proteus, X-bacteria (renamed Candidatus Legionella jeonii), is a relative of L. pneumophila (Park et al., 2004). Therefore, L. pneumophila and its pathogen and symbiont relatives have acquired genes that enable their persistence within protozoan hosts. However, L. pneumophila has also obtained virulence factors essential for its pathogenesis in protozoa. Because L. pneumophila can infect a diversity of protozoan species, it is thought that L. pneumophila has compiled a large repertoire of virulence factors to deal with the unique allelic protein differences and intracellular conditions of each particular species.
The sequenced genomes of three L. pneumophila isolates, Philadelphia, Paris and Lens, revealed that the L. pneumophila genome is highly plastic and encodes a multitude of proteins containing eukaryotic protein motifs (Cazalet et al., 2004; Chien et al., 2004). Such genes may have been acquired either through horizontal gene transfer from a host cell or other bacteria or convergent evolution during L. pneumophila's coevolution with its protozoan hosts. Human to human transmission has not been reported, indicating that L. pneumophila has not yet evolved to overcome the strong selection pressure imposed by human immune responses. Given that many eukaryotic cellular processes are highly conserved, it is unsurprising that L. pneumophila uses similar strategies to establish infection in both amoeba and mammalian phagocytes (Gao et al., 1997).
L. pneumophila's type II secretion system
Legionella pneumophila encodes a type II secretion system (Lsp) critical for intracellular replication (Hales and Shuman, 1999; Liles et al., 1999; Rossier et al., 2004; Cianciotto, 2005). Type II secretion involves a two-step process initiated by protein translocation across the bacterial inner membrane by the Sec or Tat pathway, followed by protein transport from the periplasm out to the exterior by an outer membrane secretin (Cianciotto, 2005). At least 20 Lsp-translocated substrates have been identified and display a variety of enzymatic activities (Hales and Shuman, 1999; Liles et al., 1999; Aragon et al., 2000; 2001; 2002; Flieger et al., 2002; Rossier et al., 2004; Banerji et al., 2005; DebRoy et al., 2006). However, L. pneumophila lacking individual Lsp substrates have a slight or no defect during infection (Szeto and Shuman, 1990; Moffat et al., 1994; Aragon et al., 2001; 2002; Flieger et al., 2002; Banerji et al., 2005), indicating functional redundancy or that additional unidentified type II substrates also contribute to L. pneumophila pathogenesis. A L. pneumophila mutant lacking a Lsp-transported chitinase, ChiA, was competent for intracellular replication but was significantly attenuated during mouse infection (DebRoy et al., 2006). ChiA may degrade a mammalian compound with similarity to chitin, but the precise target of ChiA remains to be identified.
L. pneumophila's type IV secretion system
Following host cell entry, the L. pneumophila-containing phagosome bypasses the early and late endocytic pathway (Horwitz, 1983b; Horwitz and Maxfield, 1984). Essential to this process is a type IV secretion system encoded by the dot/icm genes (Marra et al., 1992; Berger and Isberg, 1993; Segal et al., 1998; Vogel et al., 1998). Wild-type (WT) L. pneumophila initially resides in a Lamp-1-negative compartment surrounded by smooth host vesicles (Tilney et al., 2001). After 4 h, the Legionella-containing vacuole (LCV) is studded with ribosomes (Horwitz and Silverstein, 1980; Horwitz, 1983a; Swanson and Isberg, 1995; Abu Kwaik, 1996; Tilney et al., 2001; Kagan and Roy, 2002; Robinson and Roy, 2006) and contains resident ER markers (Tilney et al., 2001; Kagan and Roy, 2002; Robinson and Roy, 2006). This ability to remodel its phagosome is essential to L. pneumophila's ability to replicate intracellularly (Coers et al., 1999).
It was predicted that L. pneumophila utilizes the Dot/Icm system to translocate bacterial effectors into the host cell. These effectors were envisaged to manipulate eukaryotic processes, enabling the establishment of a replicative vacuole and promoting intracellular survival. Over 70 Dot/Icm-translocated proteins have been identified through multiple approaches involving bioinformatics, genetic screens and biochemistry (Nagai et al., 2002; Conover et al., 2003; Luo and Isberg, 2004; Campodonico et al., 2005; de Felipe et al., 2005; Ninio et al., 2005; Shohdy et al., 2005; Laguna et al., 2006; Machner and Isberg, 2006; Murata et al., 2006; VanRheenen et al., 2006; Zusman et al., 2007; Altman and Segal, 2008; Kubori et al., 2008). The biochemical activities of several effectors have been elucidated and will be discussed below.
Uptake into host cells and avoidance of the endocytic pathway
It is thought that uptake of L. pneumophila occurs by host-driven phagocytosis. However, the Dot/Icm system may modulate endocytic events as dot/icm mutants show significantly decreased uptake compared with WT L. pneumophila (Hilbi et al., 2001), and uptake of WT but not dot/icm mutant L. pneumophila is independent of phosphatidylinositol 3-kinase activity (Khelef et al., 2001; Weber et al., 2006) (Fig. 1). Immediately following uptake, a Dot/Icm-dependent event prevents fusion with the endolysosomal pathway (Roy et al., 1998). These data indicate that upon host cell contact, Dot/Icm-translocated effectors promote uptake and prevent endolysosomal fusion. Studies with L. pneumophila mutants lacking IcmS and IcmW, which comprise a putative chaperone complex necessary for Dot/Icm-mediated translocation of a subset of effectors, have provided some insight into this process (Bardill et al., 2005; Ninio et al., 2005; Cambronne and Roy, 2007). icmS and icmW mutant LCVs recruit early secretory vesicles and can support limited replication (Coers et al., 2000). However, icmS and icmW mutants eventually fuse with lysosomes (Coers et al., 2000), indicating that some of the translocated effectors guided by IcmS/W are essential for blocking lysosomal fusion.

L. pneumophila uptake and replicative vacuole biogenesis. (a) A PI(3)kinase-dependent pathway mediates uptake of dot/icm mutants, which then traffic to the endosomal pathway. (b) WT L. pneumophila uptake is independent of PI(3)kinase activity. The LCV then immediately avoids endosomal fusion. (c) The Dot/Icm effectors RalF and DrrA (SidM) recruit the host factors Arf1 and Rab1 to the LCV. The t-SNARE Sec22b is also recruited. The LCV then intercepts ER-derived vesicles that normally traffic to the Golgi. (d) The Dot/Icm effectors SdhA and SidF appear to inhibit host cell death and (e) the Dot/Icm effector LubX ubiquitinates the host cell factor Clk1. (f) Eventually, the LCV becomes studded with ribosomes and contains resident ER markers. The Dot/Icm effector LepB removes Rab1 from the LCV. (g) L. pneumophila then begins to replicate. Following multiple rounds of replication, (h) L. pneumophila then exits the host cell with the aid of the Dot/Icm effectors LepA and B, allowing L. pneumophila to infect neighbouring cells. Dot/Icm-translocated effectors implicated in these processes are in green.
The Dot/Icm-translocated proteins VipA, VipD and VipF may participate in blocking lysosomal fusion. These proteins were identified in a yeast screen for L. pneumophila proteins that cause vacuolar protein missorting (Shohdy et al., 2005). VipA, VipD and VipF inhibit yeast lysosomal protein trafficking (Shohdy et al., 2005). However, vipA/vipD mutant L. pneumophila replicate normally inside cells (VanRheenen et al., 2006). Therefore, it is uncertain whether these proteins prevent lysosomal fusion or whether the absence of these genes is masked by other functionally redundant effectors.
Legionella pneumophila virulence determinants other than the Dot/Icm transporter or Dot/Icm-translocated substrates also contribute to early trafficking events. The flagellar sigma factor FliA, heat shock protein Hsp60, the locus rtxA and the tetratricopeptide repeat-containing proteins LpnE, EnhC and LidL influence host cell entry and/or trafficking to the endolysosomal pathway (Garduno et al., 1998; Cirillo et al., 2000; Molofsky et al., 2005; Newton et al., 2006; 2007).
Remodelling the LCV into an ER-derived replicative organelle
The LCV intercepts early secretory vesicles prior to their transport to the ER-Golgi intermediate compartment (ERGIC) and Golgi (Kagan and Roy, 2002). Analysis of host factors recruited to the LCV revealed that Arf1 and Rab1, which are involved in regulating ER-Golgi traffic, are found on WT but not dot/icm mutant LCVs (Kagan and Roy, 2002; Derre and Isberg, 2004a; Kagan et al., 2004). This suggested that Dot/Icm-injected substrates are responsible for the recruitment of these host factors.
Arf1 (ADP ribosylation factor-1), a small GTP-binding protein, is an important regulator of vesicle traffic from the ER and Golgi (Donaldson and Jackson, 2000). A search of the L. pneumophila genome revealed a gene, ralF, with a predicted Sec7-homology domain (Nagai et al., 2002). Sec7 domains are found in eukaryotic Arf-specific guanine nucleotide exchange factors (GEFs), which catalyse nucleotide exchange on Arfs, thereby converting Arfs from the inactive, GDP-bound form into the active, GTP-bound form. RalF is a Dot/Icm-translocated effector that localizes to the LCV, is required for Arf1 recruitment to the LCV, and biochemically behaves as an Arf1-GEF (Nagai et al., 2002).
Rab GTPases organize membranes and recruit effectors that facilitate the transport, tethering and fusion of vesicles (Zerial and McBride, 2001). It was hypothesized that another Dot/Icm effector was responsible for Rab1 recruitment to the LCV. One approach to identify such an effector searched for Rab1-binding partners using a Rab1 affinity column (Machner and Isberg, 2006). The other approach utilized a visual screen to identify L. pneumophila mutants that no longer recruit Rab1 (Murata et al., 2006). This led to the simultaneous identification of the Dot/Icm-translocated effector DrrA (SidM). Biochemical analyses revealed that DrrA is a highly specific Rab1-GEF that catalyses the exchange of GDP for GTP on Rab1 (Machner and Isberg, 2006; Murata et al., 2006). However, this did not entirely account for how DrrA could mediate Rab1 recruitment to the LCV.
Inactive GDP-bound Rabs are removed from membranes and maintained in the cytosol by GDP association inhibitor (GDI), which prevents spontaneous activation of Rab1 (Sasaki et al., 1990; Ullrich et al., 1993). It is believed that Rabs must be released from GDI by GDI displacement factors (GDFs) prior to Rab recruitment to the membrane and activation by GEFs (Soldati et al., 1994; Sivars et al., 2003). To complete the cycle, Rabs are inactivated by GTPase-activating proteins (GAPs) and extracted from the membrane by GDI. Thus, DrrA's GEF activity was insufficient to explain its ability to recruit Rab1 to the LCV. Further studies revealed that DrrA is a bifunctional enzyme (Ingmundson et al., 2007; Machner and Isberg, 2007). One region in DrrA is required for Rab1 recruitment to membranes and functions as a GDF, whereas the second region stimulates Rab1 activation by functioning as a GEF (Ingmundson et al., 2007; Machner and Isberg, 2007).
An additional Dot/Icm effector also binds Rab1 (Ingmundson et al., 2007). LepB inactivates Rab1 by stimulating GTP hydrolysis, indicating that LepB is a GAP that regulates removal of Rab1 from membranes (Ingmundson et al., 2007). It is thought that accumulation of translocated LepB on the LCV membrane allows LepB's GAP activity to facilitate the removal of Rab1. Further studies will likely reveal how the activities of DrrA and LepB are temporally co-ordinated to regulate Rab1 function.
Another Dot/Icm effector required for efficient replicative vacuole formation is LidA (Conover et al., 2003; Derre and Isberg, 2005). Interestingly, LidA also binds Rab1, although LidA does not possess GEF or GAP activity (Machner and Isberg, 2006). Unlike DrrA and LepB, which show strict Rab1 binding and demonstrate nucleotide preference, LidA binds multiple Rabs regardless of the bound nucleotide (Machner and Isberg, 2006). How LidA contributes to replicative vacuole biogenesis is unknown. Perhaps it serves as a factor that is recruited to the LCV through its association with Rabs and assists in tethering ER vesicles to the LCV.
Legionella pneumophila lacking the Dot/Icm-translocated protein SidJ are temporally delayed in the recruitment of ER proteins to the LCV (Liu and Luo, 2007). Unlike many effector mutants, sidJ mutants have a significant replication defect in both macrophages and protozoa, but the mechanistic basis is unknown. Interestingly, ralF or drrA mutants replicate normally despite an inability to recruit Rab1 or Arf1 to the LCV (Nagai et al., 2002; Machner and Isberg, 2006; Murata et al., 2006). This indicates that L. pneumophila simultaneously targets multiple functionally redundant pathways, leading to the absence of a phenotype when any single pathway is disrupted. This is supported by data demonstrating that although ER-derived secretory vesicles are important for remodelling of the LCV, replicative vacuole biogenesis is incompletely blocked when ER vesicular traffic is inhibited (Kagan and Roy, 2002; Robinson and Roy, 2006). Additionally, RNA interference (RNAi) studies demonstrated that knock-down of multiple regulators of secretory transport, including the transport protein particle (TRAPP) complex, was required to inhibit bacterial replication (Dorer et al., 2006). The TRAPP complex is localized to the ERGIC and Golgi and may participate in ER-derived vesicle tethering and Rab1 activation. Thus, either the TRAPP complex or membrane traffic through the ERGIC may also contribute to replicative vacuole formation. Finally, L. pneumophila may have evolved multiple redundant effectors that autonomously mimic the activities of host cell factors.
It is expected that recruitment of ER-derived vesicles to the LCV requires factors necessary for the tethering and fusion of ER-derived vesicles. Membrane fusion requires the binding of target soluble N-ethylmaleimide-sensitive factor attachment protein receptors (t-SNAREs) on the target membrane to a v-SNARE on the vesicular membrane. Sec22b is a v-SNARE found on ER-derived vesicles. During ER-derived vesicle trafficking to the ERGIC, Sec22b normally binds its cognate t-SNARE, composed of Membrin, Syntaxin 5 and Bet1. Sec22b is recruited to the LCV in a Dot/Icm-dependent manner (Derre and Isberg, 2004a; Kagan et al., 2004) and contributes to efficient replicative vacuole formation (Kagan et al., 2004). It is anticipated that a cognate t-SNARE on the LCV binds Sec22b, thus tethering ER-derived vesicles to the LCV. Perhaps L. pneumophila Dot/Icm effectors mimic t-SNARE function. Alternatively, a t-SNARE complex present at the plasma membrane may be incorporated into the LCV during uptake.
Legionella pneumophila also appears to exploit phosphoinositide (PI) lipids during the establishment of the replicative vacuole. PI(4) phosphate [PI(4)P] is found on LCVs in a Dot/Icm-dependent manner (Weber et al., 2006). The Dot/Icm-translocated substrate SidC binds to PI(4)P in vitro, and this affinity for PI(4)P influences SidC's recruitment to the LCV in vivo (Weber et al., 2006). This may represent a mechanism by which L. pneumophila anchors Dot/Icm effectors to the LCV.
ER-associated degradation machinery
The intimate association of the ER with the LCV during its remodelling and subsequent expansion suggests that other aspects of ER biology may contribute to establishment and maintenance of the replicative vacuole (Fig. 2). Indeed, recent RNAi studies have revealed that components of the ER-associated degradation (ERAD) machinery and the proteasome are required for intracellular L. pneumophila replication (Dorer et al., 2006). The ERAD pathway eliminates misfolded proteins from the ER by targeting them to the ER surface, where these proteins are ubiquitinated, removed by the Cdc48/p97 AAA ATPase and degraded by the proteasome. These studies also revealed that Cdc48/p97 and unidentified polyubiquitinated proteins associate with the LCV in a Dot/Icm-dependent manner.
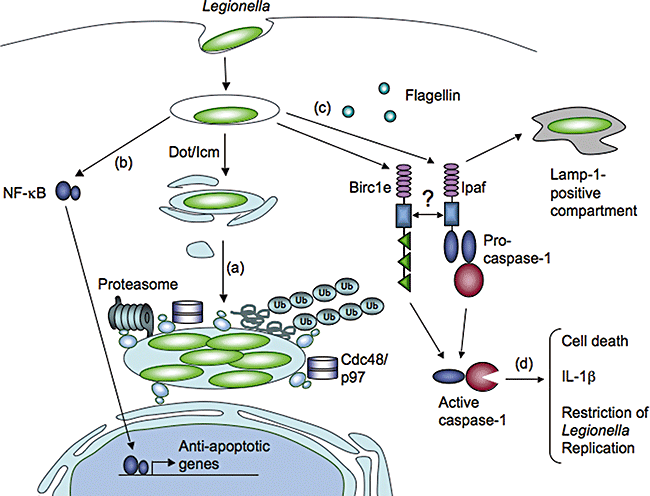
Host cell processes that influence L. pneumophila replication. (a) The proteasome, Cdc48/p97, and ubiquitinated proteins are recruited to the LCV and are required for efficient L. pneumophila replication. (b) L. pneumophila modulates host cell survival by activating the NF-κB signalling pathway, leading to the induction of anti-apoptotic genes. (c) The Nod-LRR proteins Birc1e and Ipaf signal in response to Dot/Icm activity and lead to caspase-1 activation. Ipaf responds to macrophage detection of cytosolic flagellin. (d) Caspase-1 then proteolytically cleaves substrates that cause cell death, IL-1β production, trafficking of the LCV to Lamp-1 positive compartments, and the restriction of L. pneumophila replication.
How the ERAD machinery promotes L. pneumophila replication is unclear. Cdc48/p97 may behave as a mammalian chaperone that aids in translocating Dot/Icm effectors into the cytosol. Alternatively, Cdc48/p97 and the proteasome may direct the temporal turnover of Dot/Icm effectors, thus regulating LCV maturation. Intriguingly, the L. pneumophila genome encodes proteins containing homology to F- or U-box domains (Cazalet et al., 2004; Chien et al., 2004). F- and U-box domains are found in eukaryotic E3-ubiquitin ligases, which covalently attach ubiquitin to proteins, thus targeting them for degradation. Perhaps these putative F-or U-box proteins participate in the ubiquitination of proteins on the LCV. One such U-box-containing L. pneumophila effector, LubX, was shown to contain ubiquitin ligase activity and displayed specificity for Cdc2-like kinase 1 (Clk1; Kubori et al., 2008). Clk kinases phosphorylate serine/arginine-rich proteins, which are involved in alternate splicing. Pharmacological inhibition of Clk kinases inhibited L. pneumophila intracellular replication, indicating that Clk kinases contribute to L. pneumophila's intracellular life cycle (Kubori et al., 2008). The precise role of this interaction during L. pneumophila infection remains to be determined.
ERAD is upregulated by ER stress and the unfolded protein response (UPR). The accumulation of unfolded proteins within the ER induces the UPR, which controls three key processes: (i) increased elimination of unfolded proteins by upregulation of ERAD machinery, (ii) downregulation of protein synthesis and (iii) cell death, if the UPR fails to eliminate ER stress. Given that L. pneumophila subverts ER function by redirecting ER to Golgi traffic, L. pneumophila may activate the UPR and in turn, contain effectors that manipulate this pathway. However, preliminary studies did not detect ER stress in L. pneumophila-infected cells (Dorer et al., 2006).
Modulation of host cell survival
Many pathogens manipulate host signalling in order to prevent host cell apoptosis and the premature termination of pathogen replication. The obligate intracellular bacteria Chlamydia trachomatis and Coxiella burnetii inhibit host cell apoptosis (Byrne and Ojcius, 2004; Luhrmann and Roy, 2007; Voth et al., 2007). L. pneumophila may also modulate host survival, as L. pneumophila infection induces host cell death (Kirby et al., 1998; Gao and Abu Kwaik, 1999a; Derre and Isberg, 2004b; Abu-Zant et al., 2005; Molofsky et al., 2006; Ren et al., 2006; Zamboni et al., 2006). It was reported that L. pneumophila induces Dot/Icm-dependent, caspase-3-dependent apoptosis (Hagele et al., 1998; Gao and Abu Kwaik, 1999b; Abu-Zant et al., 2005) required for replicative vacuole biogenesis (Zink et al., 2002; Molmeret et al., 2004). However, caspase-3-deficient macrophages show no defect in supporting L. pneumophila replication, indicating caspase-3 is not required for replicative vacuole formation (Zamboni et al., 2006). Microarray analysis revealed that L. pneumophila-infected cells transcriptionally upregulate genes involved in NF-κB signalling as well as genes with anti-apoptotic function (Losick and Isberg, 2006; Abu-Zant et al., 2007). Accordingly, L. pneumophila-infected cells activate NF-κB signalling in a Dot/Icm-dependent manner, and NF-κB signalling promotes host survival (Losick and Isberg, 2006; Abu-Zant et al., 2007). This led to the proposal that Dot/Icm-translocated substrates modulate NF-κB signalling and host cell survival.
Intriguingly, two Dot/Icm-translocated proteins may fulfill a role in promoting host cell survival. L. pneumophila lacking the Dot/Icm-translocated protein SdhA are severely attenuated in intracellular replication (Laguna et al., 2006). sdhA mutant-infected cells undergo nuclear DNA fragmentation, a hallmark of caspase-3-driven apoptosis, and display caspase activation and disrupted mitochondrial morphology (Laguna et al., 2006). SidF, another Dot/Icm-translocated protein, may also promote host survival (Banga et al., 2007). sidF mutant-infected cells are more susceptible to apoptosis than WT L. pneumophila-infected cells (Banga et al., 2007). SidF interacts with BNIP3 and Bcl-rambo, proapoptotic members of the Bcl2 protein family (Banga et al., 2007). The replication defect observed for the sidF mutant is not as severe as that of sdhA, indicating that SidF and SdhA differ either in function or in the pathways they target.
Interestingly, NF-κB signalling and many components of apoptosis, such as BNIP3 and Bcl-rambo, are specific to higher eukaryotes and absent in protozoa. This raises the question of whether there is selection pressure on L. pneumophila by a mammalian host to evolve effectors that target higher eukaryotic pathways. Alternatively, L. pneumophila effectors might have specificity for protozoan proteins that bear some resemblance to mammalian proteins with mammalian-specific functions, thus resulting in the accidental targeting of mammalian pathways.
Birc1e, Ipaf and flagellin
Although L. pneumophila promotes its intracellular survival and replication, mouse macrophages have developed cell-autonomous mechanisms to restrict intracellular infection. Macrophages from virtually all mouse genetic backgrounds, with the exception of A/J mice, are refractory to intracellular L. pneumophila replication (Yamamoto et al., 1988). Lgn1, an autosomal recessive locus that maps to the gene Birc1e, is responsible for this mouse strain-specific susceptibility (Beckers et al., 1995; Dietrich et al., 1995; Diez et al., 2003; Wright et al., 2003). Birc1e is a cytosolic protein that contains a nucleotide-binding oligomerization domain (Nod) and a C-terminal leucine-rich repeat (LRR) domain (Fortier et al., 2005). This homology to Nod-LRR proteins suggested that Birc1e plays a role in the cytosolic innate immune detection and restriction of microbial infection. Birc1e also contains three N-terminal baculovirus inhibitors of apoptosis repeat (BIR) domains, which typically bind and regulate caspases (Fortier et al., 2005).
The permissive A/J and non-permissive C57BL/6 Birc1e proteins differ at 14 amino acid residues (Wright et al., 2003). A/J macrophages express less Birc1e relative to C57BL/6 macrophages (Diez et al., 2000), and silencing of Birc1e renders macrophages more susceptible to L. pneumophila replication (Wright et al., 2003). Therefore, differences in Birc1e sequence and/or expression appear to influence macrophage susceptibility to L. pneumophila infection.
Birc1e-dependent signalling in response to Dot/Icm activity restricts intracellular L. pneumophila replication in macrophages (Zamboni et al., 2006). Birc1e restriction involves Dot/Icm-dependent caspase-1 activation. C57BL/6 Casp-1−/− macrophages and mice are permissive for L. pneumophila replication; in contrast, A/J Casp-1−/− macrophages are equally as permissive as A/J Casp-1+/+ macrophages (Zamboni et al., 2006). This epistatic effect suggests that Birc1e and caspase-1 function together to prevent L. pneumophila replication. However, another study concluded that Birc1e restricted L. pneumophila growth independently of caspase-1 (Lamkanfi et al., 2007). Thus, the relationship between Birc1e and caspase-1 may be quite complex.
Recent work has shown that caspase-1 activation requires the assembly of multimeric complexes known as inflammasomes, which consist of adaptor proteins containing various homotypic protein–protein interaction domains that are utilized to recruit inactive caspase-1 (Mariathasan and Monack, 2007). The Nod-LRR protein Ipaf, an adaptor that recruits caspase-1 into inflammasomes, is required for restricting L. pneumophila replication (Zamboni et al., 2006). Interestingly, the inflammasome adaptor protein Asc is unnecessary for restricting L. pneumophila replication but is required for caspase-1-dependent processing of the inflammatory cytokine IL-1β downstream of L. pneumophila infection (Zamboni et al., 2006). This indicates that functionally distinct inflammasomes are formed in response to L. pneumophila.
Macrophage detection of flagellin also participates in the control of L. pneumophila infection. Flagellin-deficient L. pneumophila do not induce caspase-1 activation and can replicate in macrophages or mice expressing the non-permissive Birc1e allele (Molofsky et al., 2006; Ren et al., 2006). The innate immune receptor Toll-like receptor (TLR) 5, which senses flagellin, and the TLR signalling adaptor MyD88 are not required for cell-autonomous restriction of L. pneumophila (Molofsky et al., 2006; Ren et al., 2006), indicating that another signalling pathway is responsible. Detection of flagellin requires the presence of a functional Dot/Icm translocator (Molofsky et al., 2006; Ren et al., 2006), but whether flagellin is translocated by Dot/Icm is unclear. Ipaf is required for caspase-1 activation in response to the flagellin of several bacterial pathogens, including L. pneumophila (Amer et al., 2006; Franchi et al., 2006; Miao et al., 2006). However, it is unknown whether Ipaf directly recognizes flagellin or is a signalling adaptor downstream of the flagellin sensor. Interestingly, it has been shown that another Legionella species, L. longbeachae, is able to replicate in C57BL/6 macrophages and does not activate caspase-1 despite being flagellated (Izu et al., 1999; Asare et al., 2007). However, despite the presence of dot/icm genes, indicating that L. longbeachae contains a type IV secretion system, L. longbeachae does not have the same type of in vitro pore-forming activity exhibited by L. pneumophila, leading the authors to propose that perhaps the flagellin of L. longbeachae does not have access to the host cytosol (Asare et al., 2007). Further comparison of these two strains will likely reveal insight into how flagellin causes caspase-1 activation.
Exactly how Birc1e, Ipaf and caspase-1 restrict L. pneumophila replication is uncertain, but multiple mechanisms have been implicated. C57BL/6 macrophages die more rapidly than A/J macrophages following L. pneumophila infection (Derre and Isberg, 2004b). Given that caspase-1 induces cell death in response to many pathogens, caspase-1-induced cell death may prematurely terminate L. pneumophila replication. Alternatively, caspase-1 may cleave host factors critical for L. pneumophila replication. Birc1e, Ipaf and caspase-1 also influence trafficking and maturation of the LCV. In non-permissive macrophages, LCVs display increased acquisition of lysosomal markers (Derre and Isberg, 2004b; Fortier et al., 2007). Conversely, a higher percentage of LCVs avoid lysosomes in caspase-1-deficient or Ipaf-deficient macrophages (Amer et al., 2006).
Given that both Birc1e and Ipaf control caspase-1 activation and cell-autonomous restriction of L. pneumophila, one must wonder whether Birc1e and Ipaf function together or in two separate pathways. Ipaf and Birc1e can form a complex in vitro (Zamboni et al., 2006); whether this occurs in vivo is unknown. As described above, Ipaf is required for caspase-1 activation in response to flagellin, however, it is unknown whether Ipaf, Birc1e or another protein is the flagellin sensor. As Birc1e restriction requires Dot/Icm activity, Birc1e may sense a Dot/Icm substrate or a cellular perturbation induced by Dot/Icm activity. Additionally, type I interferon and TNFα signalling cooperate with Birc1e and Ipaf to restrict L. pneumophila replication (Coers et al., 2007), indicating that multiple signalling pathways intersect to restrict L. pneumophila infection.
Autophagy
Autophagy is a metabolic pathway conserved from yeast to mammals whereby cells capture cytosolic material into membrane-bound vesicles that eventually fuse with lysosomes, where the cargo is degraded (Xie and Klionsky, 2007). Accumulating evidence indicates that cells utilize autophagy to control intracellular pathogens such as Mycobacterium tuberculosis (Gutierrez et al., 2004). Some pathogens have therefore evolved mechanisms to evade autophagy. Shigella flexneri encodes an effector, IcsB, that prevents the autophagic protein Atg5 from recognizing another Shigella effector, VirG, and initiating autophagy (Ogawa et al., 2005). Considering that autophagy is so highly conserved, it is feasible that L. pneumophila contains effectors that manipulate autophagy. It has been suggested that autophagy contributes to replicative vacuole biogenesis, as LCV maturation resembles the autophagic pathway (Swanson and Isberg, 1995; Swanson and Hammer, 2000; Dorn et al., 2002; Swanson and Fernandez-Moreira, 2002). However, studies utilizing Dictyostelium strains lacking autophagy components indicate that autophagy is dispensable for intracellular L. pneumophila replication (Otto et al., 2004). Additionally, systematic recruitment of autophagy components to the LCV was not observed (Otto et al., 2004). Other data suggests that mouse macrophages utilize autophagy to control L. pneumophila infection. WT but not dotA mutant LCVs recruit the autophagy conjugation enzyme Atg7 and a second enzyme Atg8 (Amer et al., 2005). It was proposed that this represents a host defence response, as non-permissive C57BL/6 macrophages induce autophagy in response to L. pneumophila with higher frequency than permissive A/J macrophages (Amer et al., 2005).
Signal transduction and innate immunity
Many pathogens have evolved virulence mechanisms to enhance or disrupt signal transduction in cells. It has not been established whether L. pneumophila manipulates host signal transduction. As described above, it has been proposed that L. pneumophila manipulates NF-κB signalling to promote host cell survival. Interestingly, the L. pneumophila genome encodes putative serine/threonine protein kinases (Cazalet et al., 2004; Chien et al., 2004). It is attractive to postulate that they are Dot/Icm effectors that phosphorylate host cell proteins and thus modulate host signalling and downstream processes.
Protozoa contain several homologues of innate immune signalling proteins (Chen et al., 2007). In particular, two proteins contain Toll/interleukin-1 receptor (TIR) domains, which are found in highly conserved innate immune proteins that play a key role in signal transduction in response to detection of microbes. Dictyostelium lacking one of these proteins are more susceptible to killing by L. pneumophila (Chen et al., 2007), suggesting both that these proteins have a role in protozoan resistance to bacterial pathogens and that L. pneumophila may have evolved effectors to subvert innate immune signalling in protozoa. As TIR domains are evolutionarily conserved in higher eukaryotes, it is conceivable that such effectors could target innate immune signalling in mammalian cells as well.
Egress
Following completion of intracellular replication, L. pneumophila must exit the host cell in order to infect neighbouring cells. How this process occurs is not completely understood. Mutants defective in egress appear to be defective in pore-formation (Alli et al., 2000; Molmeret et al., 2002), leading to the proposal that L. pneumophila forms, in addition to the Dot/Icm transporter pore, a second cytolysin/egress pore required for host cell lysis (Molmeret and Abu Kwaik, 2002). Two Dot/Icm effectors, LepA and LepB, were implicated in the active egress of L. pneumophila from protozoa, but not mammalian cells, through a non-lytic process (Chen et al., 2004). LepA and LepB were initially identified based on their weak homology to SNAREs (Chen et al., 2004), but how LepA and B promote egress is unknown. LepB is a Rab1-GAP involved in replicative vacuole biogenesis (Ingmundson et al., 2007). However, LepB may contain other functional domains that contribute to egress.
Conclusions
It is clear that L. pneumophila has evolved multiple mechanisms for subverting host cell function in order to promote its own intracellular survival. Recent studies have uncovered several Dot/Icm-translocated proteins which promote replicative vacuole biogenesis, appear to prevent host cell death and promote egress from the host cell. Additionally, studies have identified several eukaryotic processes that influence intracellular L. pneumophila replication. Additional host cell processes are likely to also be manipulated by L. pneumophila. The fact that L. pneumophila deficient in many of the known Dot/Icm effectors are minimally affected in their ability to replicate within host cells indicates that there are redundant effectors or host cell processes involved in the formation and maintenance of the LCV. Future studies will elucidate the contribution of additional host cell processes and virulence factors to intracellular L. pneumophila replication and provide insight into L. pneumophila pathogenesis.
Acknowledgements
We would like to thank members of the Roy lab for helpful discussions and Dr Igor Brodsky for insightful discussions and critical reading of the manuscript. This work was supported by the NIH (C.R.R.) and the Irvington Institute Postdoctoral Fellowship Program of the Cancer Research Institute (S.S.). We apologize to those whose work we were unable to cite due to space limitations.




