In vivo imaging of NF-κB activity during Escherichia coli-induced mammary gland infection
Summary
In mammary gland infections, the contribution of NF-κB is not well defined, and was therefore investigated following intramammary inoculation of Escherichia coli. Non-invasive real-time in vivo imaging of the transcription factor activation was performed in mammary glands of transgenic mice expressing luciferase under the control of NF-κB. Bacterial inoculation resulted in a major increase in luminescence as compared with control glands. This activation was confirmed by immunohistochemical nuclear staining of the NF-κB p65 subunit in mammary epithelium of infected glands, while nuclear p50 was not detected. The systemic response to the intramammary inoculation of Escherichia coli was also studied. NF-κB activation in the liver increased over time, and a relatively mild but longer-lasting response was observed as compared with the acute hepatic response of mice receiving lipopolysaccharide. This systemic reaction was confirmed by increased circulating levels of the acute phase protein serum amyloid A, tumour necrosis factor-α and interleukin-6. In addition, high concentrations of both cytokines in the mammary gland inoculated with bacteria showed that the infection was also well established at the local level. These results indicate that in vivo monitoring of NF-κB activation is an attractive novel approach to study mammary gland inflammation, and that this transcription factor is imperative in the early stages of the host immune response towards coliform intramammary infections, both at the local and systemic level.
Introduction
Immune and inflammatory responses are often orchestrated by the transcription factor nuclear factor-κB (NF-κB) (Li and Verma, 2002). In resting cells, NF-κB family members are sequestered in the cytoplasm, bound to their inhibitors, which belong to the IκB family. Upon cell activation, IκB proteins become phosphorylated, ubiquitinated and degraded by the proteasome. Subsequently, NF-κB can enter the nucleus and bind to the DNA in order to promote transcription (Baeuerle and Baltimore, 1996). NF-κB can be activated by many distinct stimuli, including pro-inflammatory cytokines, bacteria and bacterial toxins such as lipopolysaccharide (LPS). NF-κB alters the transcription of a large number of target genes of which the proteins are involved in host response processes to foreign challenge or tissue injury (Pahl, 1999).
Mastitis is defined as an inflammation of the mammary gland mainly caused by microbial pathogens, such as Staphylococcus aureus (S. aureus) and Escherichia coli (E. Coli) (Watts, 1988). In breastfeeding women, mastitis is quite common (Fetherston, 2001), and in the dairy industry, intramammary infections are of great economical importance (Hillerton and Berry, 2005). Consequently, the control and pathogenesis of this infectious disease have been studied intensively. However, molecular studies regarding the role of NF-κB in mastitis are scarce. Only a few in vitro studies demonstrate NF-κB activation in murine or bovine mammary gland cells following incubation with E. coli, LPS or tumour necrosis factor-α (TNF-α) (Safieh-Garabedian et al., 2004; Strandberg et al., 2005; Szperka et al., 2006; Yang et al., 2006; Fitzgerald et al., 2007). In addition, isolated milk cells of cows with mastitis mainly caused by S. aureus contained increased levels of activated NF-κB (Boulanger et al., 2003). These active NF-κB complexes frequently consisted of the p65 and p50 subunits, which are considered to be the most relevant NF-κB family members.
To assess NF-κB activity, various methods have been used, covering the measurement of mRNA levels of genes containing NF-κB binding elements in their promoter or in vitro reporter gene expression under the control of NF-κB, the determination of free NF-κB complexes able to bind a specific oligonucleotide using the classical electrophoretic mobility shift assay (EMSA) or enzyme-linked immunosorbent assay (ELISA)-based methods, and the quantification of phosphorylated proteins in nuclear extracts by Western blot or immunohistochemical analysis (Carlsen et al., 2004).
For the first time, real-time in vivo imaging of the NF-κB activity was performed in mammary glands of intact animals following challenge with E. coli bacteria. Transgenic mice expressing luciferase under the control of NF-κB were used for this purpose. This novel approach to detect mammary gland NF-κB activity in vivo allowed us to investigate the kinetics of inflammation during bacterial challenge. The results thus obtained underline the importance of this transcription factor in the pathogenesis of E. coli-induced mammary gland infections.
Results
In vivo NF-κB activity during intramammary E. coli challenge: time-course in mouse mammary glands
To study the intramammary kinetics of NF-κB activation, six transgenic female mice were challenged with 104 colony-forming units (cfu) of E. coli in the right fourth (R4) mammary gland and PBS in the left fourth (L4) mammary gland. Inoculated mice were imaged at the ventral and lateral sides at different time points during the course of infection (0, 4, 6, 8, 10 and 24 h post inoculation). At the time of inoculation, low activities were found in both the right and the left mammary gland. At later time points, luminescence of glands challenged with E. coli was significantly increased as compared with control glands receiving PBS (P < 0.05 for 4, 6, 8 and 10 h post inoculation, n = 6). Maximal activities were seen at either 6 or 8 h post inoculation. Luminescence recorded in ventral view was higher than the activity observed at the lateral sides of the body. At 24 h following inoculation, NF-κB activity of the R4 glands nearly regained basal levels, thereby approaching the activity of the L4 control glands (1, 2).
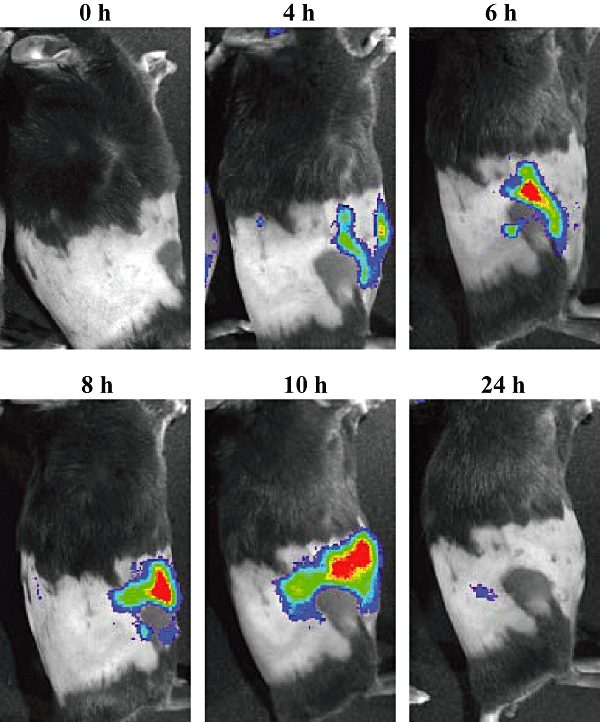
In vivo imaging of NF-κB activity following intramammary inoculation. Transgenic mice received 104 cfu of E. coli in the R4 mammary gland, and were then positioned on their left lateral side to image luciferase activity at different time points (0, 4, 6, 8, 10 and 24 h) following challenge. A transient increase in luminescence was located in the infected gland. Images are representative for all challenged mice.
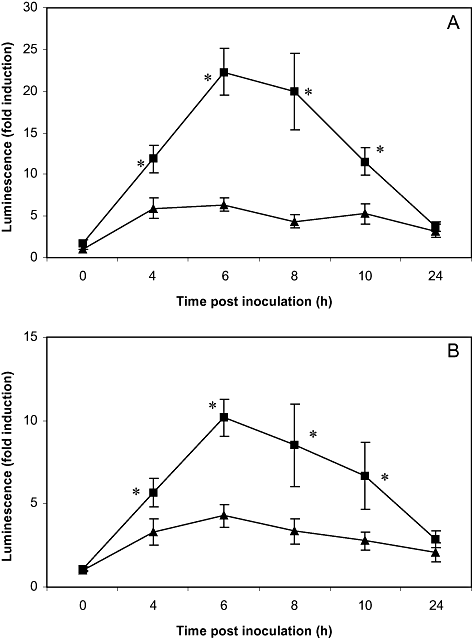
Average luminescence obtained during in vivo imaging of NF-κB activity of the mammary glands following intramammary inoculation. Six transgenic mice received 104 cfu of E. coli in the R4 mammary gland and PBS in the L4 mammary gland. They were positioned dorsally (A) and on their lateral sides (B) to image luciferase activity at different time points (0, 4, 6, 8, 10 and 24 h) following challenge. The symbol ▪ represents the mammary gland inoculated with E. coli, while ▴ denotes the control gland receiving PBS. Luminescence of the infected gland was transiently increased as compared with the control gland. An asterisk indicates the significant difference between both glands (P < 0.05).
In vivo NF-κB activity during intramammary E. coli challenge: time-course in the liver
To study the reaction of the liver towards bacterial inoculation, two mice were challenged with 104 cfu of E. coli, and chemiluminescence originated in the liver was determined at the indicated time points. As an alternative, two additional mice received 5 μg of LPS in the R4 mammary gland. At the time of inoculation, low NF-κB activities were found in the liver of both groups of mice. From 4 h post inoculation on, luminescence increased for all mice. Mice challenged with LPS showed a stronger response than mice receiving E. coli. Inoculation with endotoxin resulted in a maximal liver chemiluminescence at 6 h post inoculation, returning to basal levels at 24 h (Fig. 3). NF-κB activity following bacterial challenge reached maximal levels at 8 h post inoculation and remained high up to 24 h post inoculation (Fig. 4).
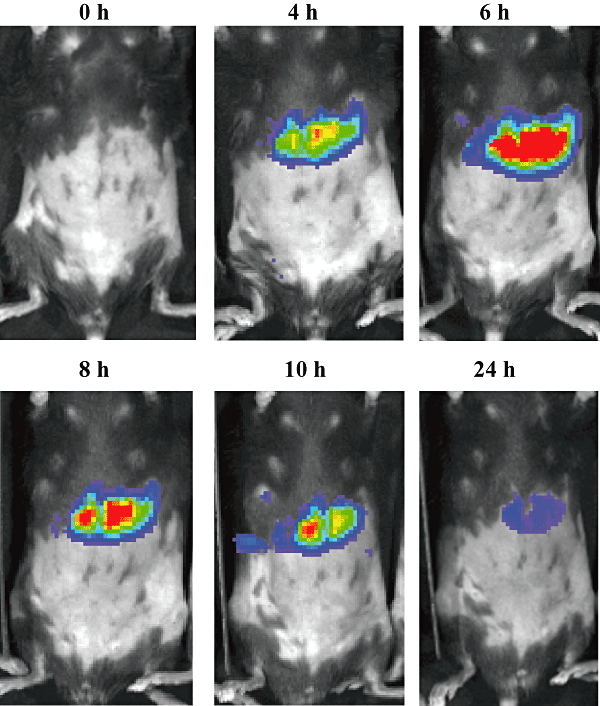
In vivo imaging of NF-κB activity following intramammary inoculation. Transgenic mice received 5 μg of LPS in the R4 mammary gland, and were then positioned dorsally to image luciferase activity at different time points (0, 4, 6, 8, 10 and 24 h) following challenge. A transient increase in luminescence was located in the liver. Images are representative for all challenged mice.
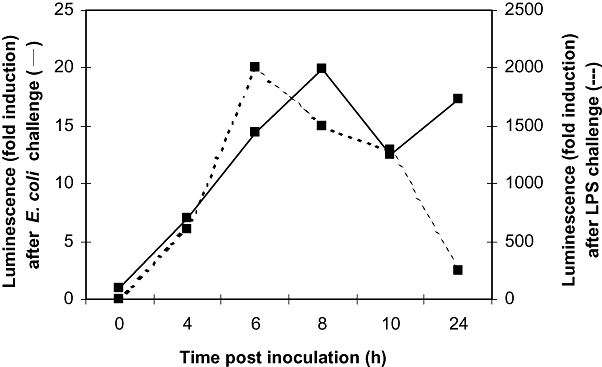
Average luminescence obtained during in vivo imaging of NF-κB activity of the liver following intramammary inoculation. Two transgenic mice received 104 cfu of E. coli in the R4 mammary gland (solid line), while two other transgenic mice were inoculated with 5 μg of LPS (dashed line). They were positioned dorsally to image luciferase activity at different time points (0, 4, 6, 8, 10 and 24 h) following challenge. A very high and transient activity was seen in the liver following LPS challenge, while a more modest and longer-lasting increase was apparent after inoculation of E. coli.
Ex vivo NF-κB activity during intramammary E. coli challenge: chemiluminescence in mouse mammary glands
In order to evaluate the NF-κB activity in exposed and isolated mammary glands following E. coli challenge, six transgenic female mice were inoculated, and in vivo luminescence was followed over time until maximal levels of activity were reached. Similar observations as the time-course in Fig. 2 were made up to 8 h post inoculation. At that time point, glands challenged with bacteria showed significantly higher NF-κB activities as compared with control glands upon necropsy or excision (P < 0.05, n = 6) (Fig. 5A and B).
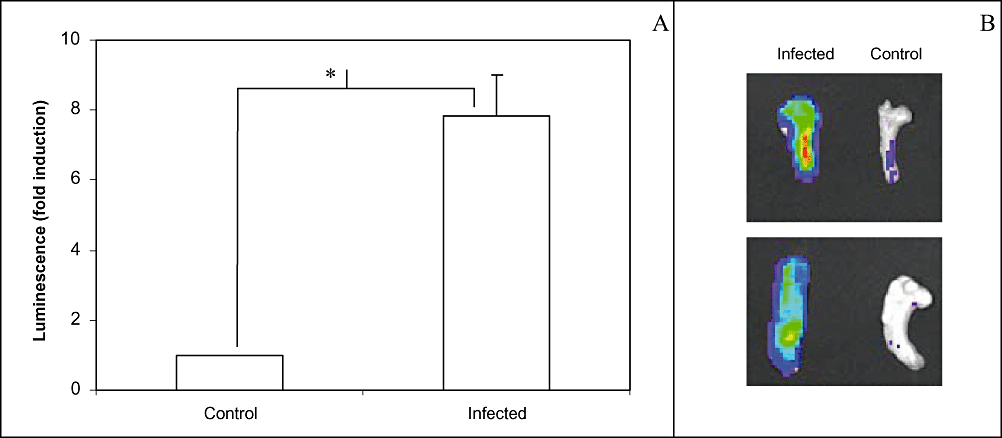
Ex vivo imaging of NF-κB activity following intramammary inoculation. Six transgenic mice received 104 cfu of E. coli in the R4 mammary gland and PBS in the L4 mammary gland. At 8 h post inoculation, they were sacrificed and dissected to expose their mammary glands.A. Average luminescence obtained during ex vivo imaging of NF-κB activity of the mammary glands upon necropsy. Mice were positioned dorsally to image luciferase activity following challenge. Luminescence of the infected gland was increased as compared with the control gland. An asterisk indicates the significant difference between both glands (P < 0.05).B. Ex vivo imaging of NF-κB activity of two representative pairs of mammary glands upon excision. Luminescence of the infected gland was increased as compared with the control gland.
Ex vivo NF-κB activity during intramammary E. coli challenge: immunohistochemical analysis of p65 and p50 subunits in mouse mammary glands
In order to identify cells with activated NF-κB, sections of mammary glands isolated at 8 h post inoculation were analysed histologically for the presence of p65 and p50, as well as the precursor of the latter, p105. Activated NF-κB was detected by the nuclear localization of p65 only in mammary epithelial cells of the gland challenged with bacteria, while no nuclear signal was obtained for cells in control glands (Fig. 6). In contrast, p50 was not observed in the nucleus of cells in either gland (Fig. 7).
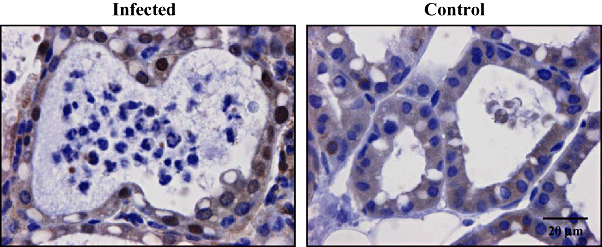
Immunohistochemical staining of the NF-κB p65 subunit following intramammary inoculation. Transgenic mice received 104 cfu of E. coli in the R4 mammary gland and PBS in the L4 mammary gland. At 8 h post inoculation, they were sacrificed and mammary glands were harvested. Control glands showed no signs of inflammation while in infected glands, neutrophil recruitment was apparent in many alveolar spaces which are lined up by the epithelial cells. Inactive NF-κB p65 was visualized in the cytoplasm of all cells of both glands, while a strong activity was only observed in the nuclei of mammary epithelial cells of the infected gland. Images are representative for all challenged mice.
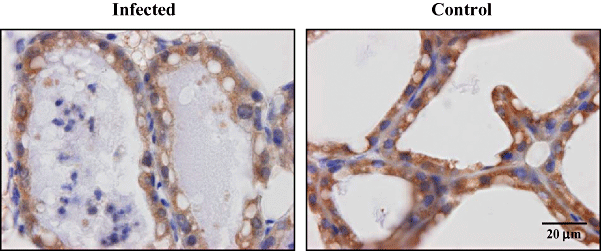
Immunohistochemical staining of the p105/p50 subunit following intramammary inoculation. Transgenic mice received 104 cfu of E. coli in the R4 mammary gland and PBS in the L4 mammary gland. At 8 h post inoculation, they were sacrificed and mammary glands were harvested. Control glands showed no signs of inflammation while limited neutrophil recruitment was apparent in infected glands. Inactive NF-κB p105/p50 was detected in the cytoplasm of all cells of both glands. In addition, activity was absent in the nuclei of all cells of both infected and control glands. Images are representative for all challenged mice.
Acute phase reaction
To confirm the systemic reaction to the intramammary inoculation of E. coli bacteria, the acute phase protein serum amyloid A (SAA) was quantified in plasma samples of the six challenged mice sacrificed at 8 h post inoculation. Plasma concentrations were significantly increased (on average 387 ± 148 μg ml−1, n = 6) as compared with pre-challenge values (on average 10.03 ± 0.61 μg ml−1, n = 4, P = 0.01).
Cytokine production
To additionally confirm the inflammatory reaction, plasma samples and mammary gland homogenate supernatants were also analysed for the presence of cytokines. In challenged mice sacrificed at 8 h post inoculation, TNF-α was detected in plasma at an average concentration of 32.24 ± 5.31 pg ml−1 (n = 6), while this cytokine was nearly absent in blood of control mice (3.52 ± 1.33 pg ml−1, n = 4, P = 0.01). Furthermore, TNF-α levels were significantly increased in the supernatants of infected glands as compared with those of control glands (on average 3.18 ± 0.36 ng ml−1 for R4 and 1.28 ± 0.13 ng ml−1 for L4, n = 6, P < 0.05).
Regarding interleukin (IL)-6, elevated concentrations (on average 1.98 ± 0.24 ng ml−1, n = 6) were measured in all plasma samples of challenged mice as compared with pre-challenge values (on average 0.25 ± 0.25 ng ml−1, n = 4, P = 0.01). In addition, high concentrations were detected in the R4 mammary glands (on average 81.76 ± 18.46 ng ml−1, n = 6), while amounts of IL-6 as low as 0.59 ± 0.29 ng ml−1 were detected in the L4 glands (n = 6, P < 0.05).
Clinical and post-mortem observations
The six mice killed at 8 h post inoculation showed no or only minor indications of illness: animals were active but a significant loss in body weight was observed (on average 7.0 ± 2.0%, P < 0.05, n = 6). Upon necropsy, early indications of inflammation were present: the right mammary glands inoculated with E. coli were pink and mildly hardened, while the left control glands were white, soft and full of milk. Infected glands also weighed more than control glands with an average difference of 57.0 ± 21.1 mg (0.1 < P < 0.05, n = 6).
The six mice followed up in time until 24 h post inoculation showed obvious signs of infection: they were less active and had slightly swollen inoculated glands. Body weights were significantly decreased (on average 12.0 ± 1.7%, P < 0.05, n = 6). Following dissection of the mice, challenged glands were enlarged, hardened, darkened and sometimes bloody in appearance. Again, control glands looked normal in size, colour and morphology. The average difference in weight between both glands was 121 ± 47.1 mg (P < 0.05, n = 6).
Discussion
In the present study, a clear activation of the transcription factor NF-κB has been demonstrated under in vivo conditions in mouse mammary glands inoculated withE. coli bacteria. The transgenic mice used in our experiments have already proved their utility in the assessment of NF-κB activity in various tissues and under diverse physiological and pathological conditions (Carlsen et al., 2002). For the first time, we now show that this model is undoubtedly valuable to the study of mammary glands, and intramammary infections as well. The major advantage of this non-invasive molecular imaging technique is the visualization of both the temporal and spatial regulation of NF-κB activity within the natural environment of an intact living animal, as it allows repetitive assessments within the same mouse. In addition, light generated upon injection of D-luciferin is directly correlated to the amount of transcriptionally active NF-κB. Alternative methods, such as the traditionally used EMSA, are not able to prove transcription, as modifications of NF-κB, such as phosphorylation and acetylation, additionally regulate transcription after binding to the DNA (Carlsen et al., 2004).
The importance of the transcription factor NF-κB in the early phase of the pathogenesis of mastitis is clearly established with this in vivo imaging approach. Until now, indications on its critical role were only obtained from in vitro experiments focusing on murine or bovine mammary tissue cells incubated with E. coli, LPS or TNF-α (Safieh-Garabedian et al., 2004; Strandberg et al., 2005; Szperka et al., 2006; Yang et al., 2006; Fitzgerald et al., 2007). Importantly, NF-κB activation was apparent within the first hours of culture. In addition, one ex vivo study demonstrated increased levels of activated NF-κB in milk cells isolated from natural cases of ruminant mastitis mainly caused by S. aureus (Boulanger et al., 2003). These results were obtained when the infection was already well established, as demonstrated by the culmination of neutrophil infiltration. In the current in vivo study, maximal NF-κB activity was achieved at 6–8 h post inoculation. The contribution of neutrophils to this activity is minimal, as their influx into the mammary gland this early in the inflammation process is still limited. In fact, the immunohistochemical analysis of infected mouse mammary glands showed that particularly epithelial cells are responsible for the high NF-κB activity observed by imaging. These findings are in agreement with the early NF-κB activation in bovine mammary epithelial cells following in vitro stimulation (Safieh-Garabedian et al., 2004; Strandberg et al., 2005; Yang et al., 2006; Fitzgerald et al., 2007). Still, NF-κB activation in other cell types at later time points, as observed in milk neutrophils by Boulanger et al. (2003), can not be excluded. However, we believe that this activation is very minimal in comparison with the strong activation observed in epithelial cells, as also been demonstrated in our previous work (Notebaert et al., 2008).
Kinetic studies of experimentally induced mastitis in cows show that the inflammatory response is not initiated until bacterial concentrations reach a certain level in milk (Shuster et al., 1996). Bacterial growth is accompanied by the release of microbial products that can be recognized by the host as a danger signal. The LPS membrane component of Gram-negative bacteria such as E. coli is known to be recognized by Toll-like receptor 4 (TLR4) (Miyake, 2004). Mammary tissue expresses mRNA for TLR4, and this expression is increased in udders of cows suffering from infections (Goldammer et al., 2004). Signalling mediated by TLR4 leads to the activation of NF-κB which results in the expression of several proteins involved in inflammatory processes (Doyle and O'Neill, 2006). Interestingly, a recent report showed that LPS and TLR4 signalling in mammary epithelium are not required to elicit the inflammatory response towards E. coli intramammary infections (Gonen et al., 2007). These authors suggest that other components of E. coli bacteria might play a role in the pathogenesis of coliform mastitis, such as lipoproteins, peptidoglycans, flagellins and CpG DNA, which are recognized by TLR1, 2, 5 and 9 respectively (Albiger et al., 2007). Although the specific molecular interaction between E. coli and the mammary epithelium remains to be elucidated, the NF-κB signalling pathway is activated in response to all TLRs, thereby resulting in the expression of inflammatory mediators.
Mammary epithelial cells thus generate cytokines and chemokines targeting neutrophils and mononuclear cells (Rainard and Riollet, 2006). These secondary mediators include TNF-α and IL-6, of which the expression is known to be dependent upon NF-κB (Hayden et al., 2006). In the current study, both cytokines are clearly upregulated in infected glands as compared with control glands. We previously also observed high local concentrations of TNF-α and IL-6 upon E. coli intramammary infection in wild-type mice (Notebaert et al., 2008). Following in vitro culture with LPS or E. coli, several cytokines and chemokines are produced by bovine mammary epithelial cells, as shown at the mRNA and protein level (Boudjellab et al., 1998; Okada et al., 1999; Wellnitz and Kerr, 2004; Pareek et al., 2005; Strandberg et al., 2005; Lahouassa et al., 2007). In mammary epithelial cells from cows experimentally infected with E. coli, these inflammatory proteins are increased (McClenahan et al., 2006) and secreted in milk as well (Riollet et al., 2000). In addition, NF-κB activation seemed to be essential for the production of bactericidal peptides following in vitro culture of bovine and murine mammary epithelial cells with E. coli (Yang et al., 2006).
Interestingly, in the current study only the NF-κB p65 subunit was activated in epithelial cells of infected mouse mammary glands, while p50 did not seem to contribute to the observed NF-κB activation. We suggest an induction of p65 homodimers in mouse mammary gland infections instead of an involvement of the classical p65/p50 heterodimers. This hypothesis is in agreement with previous findings in epithelial cells of different origins and derived from various species. In bovine mammary epithelial cells, p65 homodimers were induced in response to TNF-α (Fitzgerald et al., 2007). In addition, bronchial epithelial cells from heaves-affected horses contained p65 homodimers as activated NF-κB complexes (Bureau et al., 2000). In human gastric epithelial cells infected with Helicobacter pylori (Wada et al., 2001), and in colon or cervical epithelium incubated with enteropathogenic bacteria or their toxins (Philpott et al., 2000; Schulte et al., 2000; Kim et al., 2006), these homodimers were observed as well. This uncommon NF-κB complex is thought to be crucial for the transcription of a limited number of genes, such as those encoding for IL-8 and the intracellular adhesion molecule-1 or ICAM-1 (Kunsch and Rosen, 1993; Ledebur and Parks, 1995). These two molecules are known to participate in neutrophil chemotaxis, diapedesis and transmigration (Kunsch and Rosen, 1993; Rahman et al., 1999). In early inflammatory conditions arising from bacterial infections, p65 homodimers thus seem to be imperative for subsequent neutrophil participation.
Our clinical and post-mortem observations show that the intramammary infection was well established in the transgenic mice. To investigate the severity of infection, NF-κB activity was additionally examined in the liver by in vivo imaging. A clear reaction of the liver following E. coli intramammary inoculation was observed. It is generally suggested that cytokines produced during the inflammatory process are the primary cause of the systemic effects observed during intramammary infections. We demonstrated increased concentrations of IL-6 and TNF-α in blood of infected mice in comparison with control mice. In addition, the same observation was previously made for wild-type animals (Notebaert et al., 2008). As a potent inducer of NF-κB, at least TNF-α can target this transcription factor in cells throughout the body. Elevated plasma levels of the positive acute phase protein SAA were also demonstrated in the present study in analogy with E. coli-infected wild-type mice (Notebaert et al., 2008). NF-κB controls the biosynthesis of SAA, which is dramatically upregulated in the liver during inflammation (Uhlar and Whitehead, 1999). Interestingly, the liver response towards E. coli challenge was relatively mild, slow and longer-lasting as compared with the very strong reaction in mice inoculated with LPS. The LPS dose chosen is of the same order of magnitude as used in other studies with mice (Gonen et al., 2007). In cows, the intramammary challenge with LPS is frequently employed to study inflammation of the bovine mammary gland because of its resemblance to E. coli challenge (Lohuis et al., 1988a,b). Still, the more dynamic release of LPS during bacterial growth and killing in the E. coli mastitis model is in clear contrast with the single-dose administration during endotoxin challenge, and might explain the observed differences in the liver response curves.
In conclusion, we demonstrate the elegant possibility to monitor NF-κB activity in mammary glands in real time. During the early hours of bacterial infection, activation of this transcription factor was apparent and was localized within the mammary epithelium. We identified p65 as being the key player in NF-κB-mediated transcription. In addition, a systemic reaction was confirmed through the molecular imaging of the NF-κB activity within the liver and by measurement of circulating levels of NF-κB-dependent acute phase proteins and cytokines. This first in vivo study regarding the role of NF-κB in the pathogenesis of mastitis emphasizes the importance of investigating early events in infected mammary glands. As most research within the field of mammary gland infections has been carried out upon diagnosis, it has mainly focused on the role of neutrophils, thereby relatively underestimating the active contribution of mammary epithelial cells. We demonstrate that mammary epithelium is not just a passive physical barrier, but a critical regulator within the establishment of the immune response towards mastitis, via major activation of the transcription factor NF-κB.
Experimental procedures
Bacteria
For the intramammary inoculation, E. coli strain P4:O32 was used as previously described (Notebaert et al., 2008). Bacteria were grown overnight at 37°C in Brain Heart Infusion medium (Oxoid, Hampshire, UK). To verify their purity, bacteria were streaked onto a blood agar plate (Oxoid). Bacterial concentration of the culture was determined using a standard curve plotting cfu as a function of the absorbance at 550 nm. Sterile PBS (Invitrogen, Merelbeke, Belgium) was applied to further dilute the culture until the desired concentration of 2 × 105 cfu ml−1. The actual number of cfu injected was 2.2 ± 0.6 × 104 as determined by spreading the inoculum onto a blood agar plate and counting the colonies after overnight incubation.
Intramammary inoculation of E. coli in transgenic mice
All animal experiments were approved by the Ethical Committee of the Faculty of Veterinary Medicine, Ghent University, Belgium. The 3x-κB-luc transgenic mice were generated as previously described (Carlsen et al., 2002). Mice were housed in a controlled light, humidity and temperature environment and were provided with food and water ad libitum. Female mice were challenged 5–10 days after parturition. The intramammary inoculation technique was carried out as previously described for wild-type mice (Notebaert et al., 2008). In brief, mice were anaesthetized using a mixture of ketamin (80–100 mg kg−1 mouse) (Eurovet, Heusden-Zolder, Belgium) and xylazin (10 mg kg−1 mouse) (VMD, Arendonk, Belgium). After disinfecting the teats of the fourth pair of mammary glands, the very near end of the teats was cut with small scissors. A 32 G needle with a blunt end was then inserted into the teat canal and a volume of 50 μl was slowly injected. Challenged mice received 104 cfu of E. coli in the R4 mammary gland and an equal volume of sterile PBS in the L4 mammary gland. As an alternative, mice received LPS from E. coli (5 μg in 50 μl; Sigma-Aldrich, Bornem, Belgium) in the right mammary gland. The offspring was removed before mice returned to their cage to recover.
In vivo and ex vivo imaging of NF-κB activity
Before imaging, mice were anaesthetized by inhalation of isoflurane (Abbott Laboratories, Kent, UK) and shaved on their ventral and lateral sides. Mice were injected intraperitoneally with d-luciferin (Biosynth, Staad, Switzerland; 120 mg kg−1 dissolved in PBS, pH 7.4), and placed in a light-sealed imaging chamber connected to an ultra-sensitive CCD camera of the IVIS imaging device (Caliper Life Sciences, Hopkinton, USA). Grey scale images were obtained before luminescence imaging to localize the fourth pair of mammary glands. A small fraction of the gland is observed at the ventral side of the body while the largest part is visible at the lateral sides. Luminescence was recorded 5 min after the injection of d-luciferin. Exposed or excised mammary glands were imaged in the same way immediately after dissection. Images were analysed with the Living Image software (Caliper Life Sciences).
Immunohistochemical analysis of NF-κB activity
Excised mammary glands were fixed in 3.5% phosphate-buffered formaldehyde (VWR International, Leuven, Belgium) immediately after imaging. Histological paraffin sections with a thickness of 5 μm were prepared. After deparaffinization and hydration, antigens were unmasked using a pressure cooker and a microwave oven. Endogenous peroxidase activity was quenched with hydrogen peroxide in methanol for 5 min. After rinsing in Tris-buffered saline (TBS), non-specific binding sites were blocked by goat serum for 30 min. The sections were then incubated overnight at 4°C with rabbit anti-p65 (sc-372; Santa Cruz, Heidelberg, Germany) or rabbit anti-p105/p50 (ab7971; Abcam, Cambridge, UK) diluted in TBS with bovine serum albumin. The samples were washed with TBS and then incubated with a biotinylated goat anti-rabbit secondary antibody (Dako, Heverlee, Belgium) for 1 h. After another wash, a mixture of avidin and biotinylated horseradish peroxidase (HRP) or streptavidin coupled to HRP (both from Dako) was used for the detection of p65 and p105/p50 respectively. Subsequently, the samples were stained with diaminobenzidine solution (Dako) for 5 min and rinsed with distilled water. After counterstaining with hematoxylin, the slides were again washed, dehydrated and mounted. Inactive NF-κB complexes were visualized by brown staining of the cytoplasm while brown nuclei reflected active complexes.
Clinical and post-mortem observations
Following intramammary inoculation, mice were examined for generalized and local reactions. The latter included redness of the teat, swelling of the mammary gland and the appearance of a sunken abdomen. Generalized reactions, such as awareness of the environment, activity and grooming, weakness and mortality, were observed as well. In addition, food and water uptake and body weight were monitored with the same frequency. After sacrificing, mice were dissected to expose their mammary glands. The fourth pair of glands was examined for signs of infection such as swelling, discolouration, rigour and abscess formation.
Blood sampling and plasma preparation
Mice were anaesthetized using ketamin and xylazin. Following cardiac puncture with a 26 G needle (Terumo, Medical Corporation, Leuven, Belgium), blood was slowly withdrawn into a syringe filled with approximately 50 U of heparin (LEO Pharma, Wilrijk, Belgium). Subsequently, mice were killed by cervical dislocation. Plasma was prepared by centrifugation of the blood at 2300 g and 4°C for 15 min.
Preparation of mammary gland samples
After sacrificing, mice were dissected and mammary glands were removed, weighed and homogenized in PBS (Invitrogen) supplemented with a cocktail of protease inhibitors (Calbiochem, La Jolla, USA). The homogenate was then centrifuged at 16 000 g and 4°C for 30 min. After removing the creamy top layer, the supernatant was frozen at −80°C until further analysis.
Serum amyloid A quantification
Serum amyloid A proteins were quantified by means of an ELISA (Tridelta Development, Maynooth, Ireland) according to the manufacturer's protocol. Briefly, a 96-well plate coated with an anti-mouse SAA was used. Standards with a known SAA concentration and plasma samples were incubated for 1 h together with a secondary antibody labelled with HRP. After extensive washing, an HRP substrate was added and a coloured product was formed which allowed a colorimetric measurement at 450 nm.
Cytokine analysis
Tumour necrosis factor-α was detected and quantified using a sandwich ELISA (eBioscience, San Diego, USA) according to the manufacturer's protocol. In brief, 100 μl of standards and plasma and mammary gland samples were incubated overnight at 4°C in a 96-well plate containing a capture anti-mouse antibody for TNF-α. Then, a biotin-conjugated detection antibody was added for 1 h. After incubation with avidin-bound HRP for 30 min, the addition of a HRP substrate solution allowed a colorimetric read-out at 450 nm.
Interleukin-6 was measured in a bio-assay (Landegren, 1984) as a hybridoma growth factor for mouse 7TD1 cells. Briefly, cells were cultured for 72 h in medium with different dilutions of the plasma and mammary gland samples. A colorimetric hexosaminidase enzyme reaction reflects the number of cells which is related to the amount of IL-6 in the media. Reference standards were included in the assay. Analysis was based on the half-maximal proliferation of the cells.
Statistical analyses
Data are expressed as the mean ± SEM. By using the non-parametric Wilcoxon matched pairs test, comparisons between the E. coli-inoculated gland (R4) and the control gland receiving PBS (L4) were made, and changes in weight were analysed. Differences between challenged and control mice were statistically examined by the non-parametric Mann–Whitney U-test. The spss software (SPSS Belux, Brussels, Belgium) was used for all analyses.
Acknowledgements
The authors gratefully acknowledge the technical assistance of K. Demeyere. The E. coli strain was a kind gift from C. Burvenich. The authors also wish to thank K. Chiers and W. Van den Broeck for the immunohistochemical analyses. This work was financially supported by the Research Foundation-Flanders (Research Program B/06931/0101).




