Drosophila innate immunity and response to fungal infections
Summary
The fruit fly Drosophila melanogaster is an important model for the analysis of the interaction between host immune systems and fungal pathogens. Recent experiments have extended our understanding of the Toll-based signalling pathway critical to response to fungal infections, and identified new elements involved in cellular and humoral-based defences. The fly immune system shows remarkable sophistication in its ability to discriminate among pathogens, and the powerful genetics available to researchers studying the adult fly response, and the ability to manipulate cultured phagocytic cell lines with RNAi, are allowing researchers to dissect the molecular details of the process.
Introduction
The interactions between insects and fungi are complex and fascinating, and include such intriguing phenomena as the cultivation of fungi as food sources by ants (Martin, 1970) and the existence of yeast-like endosymbionts living within cells of specialized structures of some beetles and scale insects (Noda and Kodama, 1996). Several fungal species are successful pathogens of insects; these include generalists such as Beauveria bassiana and Metarhizium anisopliae, and specialists such as Furia ithacensis infecting snipe flies (Kramer, 1981) and Entomophaga grylli infecting grasshoppers (Ramoska et al., 1988). The identification and use of specific fungal pathogens for biological control of insects that are economic or health concerns of humans has become an increasingly important endeavour.
In addition to investigations on fungal pathogens of insects as biocontrol agents, the research community has used the relationships between fungal pathogens and insects to probe basic biological questions. In particular, the interaction between the fruit fly Drosophila melanogaster and bacterial or fungal pathogens has been extensively studied on a molecular level, and these investigations have led to fundamental insights into the insect and subsequently the mammalian immune system. There have been a number of excellent recent reviews that cover the use of Drosophila in studies on insect–microbial interactions (Hetru et al., 2003; Naitza and Ligoxygakis, 2004; Mylonakis and Aballay, 2005; Cherry and Silverman, 2006; Fuchs and Mylonakis, 2006); here we will focus on recent developments in this field with respect to fungal pathogenesis.
Important insight into insect immunity arose through the identification of Drosophila-encoded peptides such as drosomycin (Fehlbaum et al., 1994) and metchnikowin (Levashina et al., 1995). Some of these insect-produced molecules had clear similarity to plant-derived defence molecules, and were subsequently shown to have antipathogen activity in their own right. The observation that these peptides were induced in response to particular pathogens directed the identification of the signalling pathways leading to the defence-molecule induction (Lemaitre et al., 1995). These studies, in turn, led first to the realization that components of the fly dorsal-ventral patterning system were critical for the fungal-induced expression of the host defence molecules (Lemaitre et al., 1996), and then to the fact that Toll-like receptors were key components of the pathogen recognition systems of both insects and mammals (Akira et al., 2006). In mammals the immune response consists of both innate and acquired components; these act in synergy to defend the organism against infection. The fruit fly, on the other hand, lacks the classic acquired immune system, and is thus inherently a useful model to study innate immune responses in the absence of antibody-based acquired immunity. This insect innate immune system is composed of both humoral and cellular constituents, and is sophisticated enough to be able to distinguish among different classes of pathogens; in particular fungi and Gram-positive bacteria are dealt with differently from Gram-negative bacteria. The humoral components are concerned with biosynthesis of elements such as various antimicrobial peptides (AMPs) (Meister et al., 1997), whereas cellular reactions involve blood cells or haemocytes. These two responses act in concert (Elrod-Erickson et al., 2000) with phagocytosis of pathogens ultimately serving as an important part of the defence mechanism.
Fly-based analysis
Advances in our understanding of Drosophila response to fungi have been made using both natural fungal pathogens, as well as artificial infections using fungi that are normally human pathogens. Both B. bassiana and M. anisopliae, two generalist fungal pathogens, have been used to probe the immune response of Drosophila. Although the basic pattern of response to fungal pathogens involved the Toll receptor and the induction of the AMP drosomycin, many significant details of the upstream signalling pathway have been uncovered by recent studies in the fly (Fig. 1). Infection studies using B. bassiana suggested that the Persephone protease was critical to the activation of the Toll receptor in response to fungal infection. Persephone (psh) (Ligoxygakis et al., 2002) was itself initially identified as a suppressor of the constitutive melanization and early death exhibited by Drosophila mutants of the Necrotic (nec) gene; nec mutants have a constitutively activated Toll pathway due to loss of a nec-encoded serine protease inhibitor or serpin (Levashina et al., 1999). These results implied that the nec and psh gene products played active roles in the Toll-mediated response to fungal pathogens, but did not identify the specific pathogen recognition machinery involved although such specificity was expected as the innate immunity networks were able to induce directed responses to different classes of pathogens.
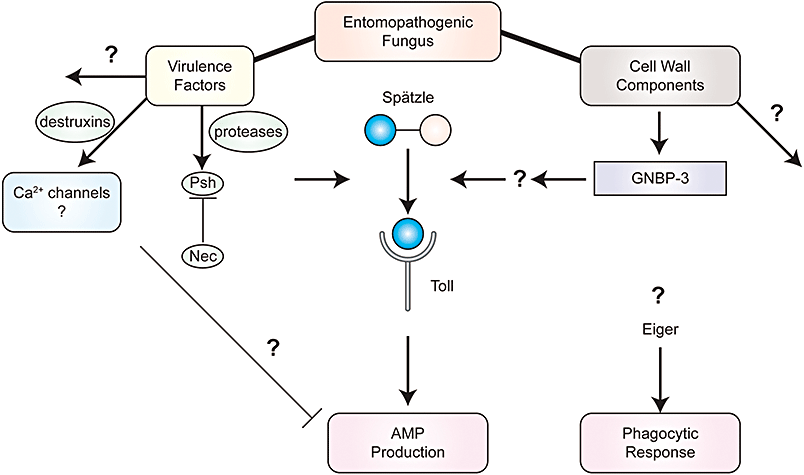
Drosophila immune response to fungal pathogens. Two major components of fly immunity are the production of antimicrobial peptides (AMPs) and the activation of a phagocytic response. The tumour necrosis factor homologue Eiger is implicated in the activation of the phagocytic response, while the Toll receptor plays a major role in activation of AMP production in response to fungal pathogens. The Toll pathway is activated by interaction with the product of proteolytic cleavage of the ligand spätzle; this can occur in response to the recognition of fungal wall components through the pattern recognition receptor GNBP-3, or through detection of protease virulence factors through activation of the Persephone gene product. Other virulence factors such as cyclic peptides of the destruxin family serve to inhibit AMP production.
Recently, identification of a pattern recognition receptor for fungal pathogens has added intriguing layers of complexity in the fungal pathogen response pathway (Fig. 1). GNBP-3, a member of a class of β-glucan recognition proteins that includes Gram-negative binding protein-1 (GNBP-1), was shown to act as a recognition factor for fungal surface components (Gottar et al., 2006). Because the related GNPB-1 served as a component of the Toll pathway-inducing recognition element for Gram-negative bacteria (Gobert et al., 2003), the connection of GNBP-3 to Toll pathway activation in response to fungal pathogens had a logical molecular symmetry. When mutant flies defective in GNBP-3 were challenged with fungal cell wall components like β1-3 glucans or with heat-killed Candida albicans cells or extracts from Aspergillus nidulans cells, they were unable to properly induce drosomycin expression (Gottar et al., 2006). Surprisingly, infections with B. bassiana were not particularly lethal in gnbp-3-defective flies, although the mutant flies were highly sensitive to infections from live C. albicans, and Toll pathway-defective flies were quite susceptible to B. bassiana infection. However, loss of both Persephone and GNBP-3 function created flies that were lethally sensitive to entomopathogenic fungal infection (Gottar et al., 2006); this overlap in GNPB-3 and psh appears to arise because psh is implicated in responding to the direct influence of fungal virulence factors generated by entomopathogenic fungi, while GNBP-3 acts to activate the Toll response in response to opportunistic fungal infections and cell surface markers.
Investigations using flies defective in the eiger gene, the D. melanogaster tumour necrosis factor homologue, suggest this gene also plays a role in pathogen recognition (Schneider et al., 2007). In eiger mutant flies, extracellular pathogens such as B. bassiana and Staphylococcus aureus were more lethal, while there was no heightened sensitivity to intracellular pathogens such as Salmonella typhimurium. This suggests that in addition to pattern recognition systems that classify pathogens on the basis of cell surface components, the fly innate immune system can differentiate pathogens on the basis of the interaction of the pathogen with the haemocyte system. In addition, fungal products, such as the peptide destruxin A produced by M. anisopliae, appear to have the ability to suppress humoral responses in flies, and this suppression can lead to non-pathogenic organisms such as Escherichia coli, becoming pathogenic (Pal et al., 2007). Thus overall the relationship between the host immune system and the fungal pathogen is multifaceted, and much work remains to be done to fully establish the links between natural fungal pathogens and the fly response.
Several lines of evidence show that the Toll pathway also serves to defend flies against artificially induced infections with fungal pathogens that are normally limited to mammalian hosts. Because these pathogens have not evolved to deal with the insect cuticle, it is necessary to infect Drosophila by injecting the fungi into the fly by pricking with a pathogen-coated needle. Initial infections with Aspergillus fumigatus (Lemaitre et al., 1996) and subsequently with C. albicans (Alarco et al., 2004) and Cryptococcus neoformans (Apidianakis et al., 2004) established that human pathogens could be lethally injected into Drosophila adults, and that the lethality of these infections was influenced by the Toll pathway. This ability to infect the genetically tractable fly with human pathogens has led to efforts to expand the use of the Drosophila model to investigate antifungal drugs. Mutations that affect the virulence of the human fungal pathogen C. albicans can reduce virulence in a Drosophila infection model (Alarco et al., 2004; Chamilos et al., 2006), suggesting that mechanisms of virulence may be related in mammals and insects. In addition, Aspergillus infections of Drosophila Toll mutants were influenced by the virulence state of the pathogen (Lionakis et al., 2005). It was possible to reduce the severity of Aspergillus infections with voriconazole treatment (Lionakis et al., 2005), and to treat C. albicans infections with fluconazole added to the fly food, although infection from the naturally resistant Candida krusei was not affected by the drug treatment (Chamilos et al., 2006). This opens up the possibility of using the fly model in screens for new antifungal drugs, or in tests of function of new candidate compounds (Tournu et al., 2005).
Cell-based analysis
An alternative to working with the whole organism is to scale down to a smaller model. There are a number of Drosophila cell lines derived from mixed embryonic tissues including the most common Schneider 2 (alternative names S2, SL2 and L2) and Kc cells. Recently, cell lines from specific tissues, larval central nervous system and imaginal discs have become available as well. Gorr et al. (2004) studied the Drosophila hypoxia-inducible factor (HIF), the key regulator of survival and adaptation during oxygen deprivation. In this work S2 cells were used to study the ability of flies to sustain oxygen deprivation as opposed to the highly oxygen-dependent organs and tissues of mammals. As these cells can function in low oxygen, an environment preferred by many pathogens, they represent a good tool to study host–pathogen interactions. Although the cell lines exhibit similar properties, they are not identical in their responses to various treatments and conditions (Cherbas and Cherbas, 2000). For example, when S2 and KC cell lines were compared, only the former exhibited scavenger receptor-mediated endocytosis, an activity observed in mammalian macrophages (Abrams et al., 1992).
The Schneider 2 cells are frequently used as a tool to study the Drosophila defence response (Echalier, 1997). In Drosophila, 95% of blood cells are a specific type of haemocyte, termed the plasmatocyte, which fulfil the functions of mammalian neutrophils and macrophages (Tepass et al., 1994). The S2 cells are Drosophila embryonic haemocytes (Schneider, 1972) that can phagocytose invading microbes and cell debris (Ramet et al., 2001; 2002). These cells have been established as a model to study host–pathogen interactions primarily due to the ability to genetically manipulate these cells with RNAi; various Drosophila plasmatocytes such as S2, KC, BG2-C6 and Shi are sensitive to double-stranded RNAi and have been successfully used to study pathogenesis of various microbes. For example, a systematic functional genomic screen was used to pinpoint the genes involved in the uptake and growth of Mycobacterium fortuitum (Philips et al., 2005), and researchers have used S2 cells in genome-wide RNAi screens for factors required by the host during infections of the cytosolic pathogen Listeria monocytogenes as well as M. fortuitum, a vacuolar pathogen (Agaisse et al., 2005).
S2 cells have recently been used as a model to study cell-mediated innate immunity of Drosophila against fungal pathogens such as C. albicans, as it was shown that S2 cells are capable of engulfing Candida and its close relative Saccharomyces cerevisiae (Stroschein-Stevenson et al., 2006; Levitin et al., 2007). Stroschein-Stevenson et al. have specifically investigated phagocytosis of C. albicans through an RNAi-based screen to identify genes involved in engulfment of Candida by Drosophila S2 cells. They found 184 genes representing a variety of functions to be important for Candida phagocytosis. The study further concentrated on one of the findings, involving the Macroglobulin complement-related (Mcr) gene product (Stroschein-Stevenson et al., 2006). The Mcr gene is closely related to a family of four Drosophila thioester proteins (Tep). Mcr was found to be secreted by S2 cells and to be preferentially and tightly bound to C. albicans, which promoted subsequent Candida phagocytosis (Fig. 2). The study illustrated the specificity of different members of this conserved group of Tep genes for different pathogens including Gram-negative E. coli and Gram-positive S. aureus.
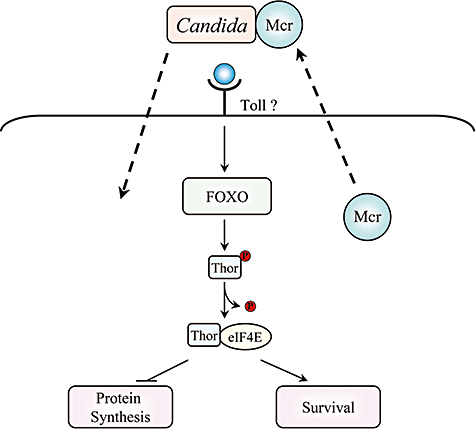
Drosophila S2 cells response to Candida albicans. Drosophila Mcr protein is required for Candida recognition and promotes subsequent phagocytosis of the pathogen. The engulfment of Candida by S2 cells triggers expression of Thor gene, regulated by a transcriptional activator, FOXO. Thor plays a role in host survival during Candida infections in Drosophila flies by interacting with the member of translation-initiation machinery, eIF4E.
Another aspect of C. albicans engulfment by Drosophila S2 cells was recently investigated through a microarray analysis that identified a number of genes differentially expressed as a result of Candida internalization by S2 cells. Candida infection was shown to trigger a production of Thor (Levitin et al., 2007), a translational regulator previously shown to be involved in starvation and oxidative stress resistance in Drosophila (Tettweiler et al., 2005), as well as to resistance to bacterial infection (Bernal and Kimbrell, 2000). Using the live Drosophila model, Thor was found to be involved in fly survival in response to Candida infection, suggesting a significant component of the fruit fly's cell-based immunity may involve regulation of translation (Fig. 2) (Levitin et al., 2007). This validation of the results derived from Drosophila macrophage-like cells by using the whole fly helps to confirm S2 cells as a useful model to study Drosophila–Candida interactions (Levitin et al., 2007).
Conclusions
Therefore, both derived cell lines and the fruit fly itself have proven to be impressive tools for the investigation of insect–fungi relationships. These studies have illuminated key components of the innate immune system that apply even to mammals, and promise to provide useful approaches for investigations into antifungal drugs. The recent application of transcriptional profiling and of RNAi to insect cell lines interacting with fungal pathogens has added powerful new tools to these studies, and should provide both further fundamental insights and new practical approaches to questions of fungal pathogen function and treatment. In the future these technologies will allow researchers to probe deeply into the interactions between the insect host and fungal pathogens; likely some of these interactions will prove specific to the insect case, while others will highlight general functions. A major need is a greater molecular understanding of the processes controlling aspects of innate immunity, such as phagocytosis, melanization and clotting, that are not yet as advanced as those that control production of antimicrobial proteins. Further use of RNAi will provide greater information about the engulfment process in phagocytic cell lines, while identification of cell lines specialized in other immune processes would provide novel tools, and exploiting the multiple Drosophila genome sequences with standardized infection assays and comparative genomics should provide a powerful screening approach. However, the development of assays for immune functions and the identification of mutants affected in these processes, the approach exploited brilliantly in the dissection of the AMP production process, is perhaps the most powerful strategy to gain insight into these functions in the whole organism. Ultimately we need to understand how the multiple processes are coordinated to provide such an impressive defence against fungal pathogens, and for this we will have to make good use of all the advantages of the fly as an experimental organism.
Acknowledgements
We thank Andre Migneault for help with the figures. This work was supported by CIHR grants, and is NRC publication 49547.