Gingipains from Porphyromonas gingivalis synergistically induce the production of proinflammatory cytokines through protease-activated receptors with Toll-like receptor and NOD1/2 ligands in human monocytic cells
Summary
Gingipains (HRgpA, RgpB and Kgp) are cysteine proteinases and virulence factors of Porphyromonas gingivalis, the major causative bacterium of periodontal disease. To study synergistic effects of gingipains and signalling via Toll-like receptors (TLRs) and NOD1/2, we investigated effects of a gingipain on the secretion of proinflammatory cytokines from monocytic THP-1 cells in the presence of pathogen-associated molecular patterns (PAMPs). Gingipains stimulated interleukin (IL)-8's secretion from THP-1 cells, which was completely inhibited by proteinase inhibitors of gingipain and increased in the presence of PAMPs. Synergistic effects of gingipains and PAMPs were also seen in the secretion of IL-6 and MCP-1 and reduced to about 50% the secretion of IL-8 from THP-1 cells treated with siRNA targeting either protease-activated receptor (PAR)-1, -2 or -3. PAR agonist peptides mimicked the synergistic effects of gingipains with PAMPs. These results indicate that gingipains stimulate the secretion of cytokines from monocytic cells through the activation of PARs with synergistic effects by PAMPs. This is the first report of synergism of signalling via PARs, and TLRs or NOD1/2. The host defence system against P. gingivalis may be triggered through the activation of PARs by gingipains and augmented by PAMPs from this pathogen via TLRs or NOD1/2.
Introduction
Periodontal disease is chronic gingival inflammation and causes periodontal tissue destruction, loss of alveolar bone, and eventually, tooth loss. Porphyromonas gingivalis is the causative pathogen not only for adult periodontitis but also for rapidly progressive periodontitis (Holt and Bramanti, 1991). P. gingivalis possesses a number of putative virulence factors, such as lipopolysaccharide (LPS), fimbriae, toxic products of metabolism and proteinases, all of which stimulate host cells to release inflammatory mediators and promote this infectious disease. We have studied virulence activities of two types of cysteine proteinases (Potempa et al., 1995); arginine-specific gingipains (Rgp) of 50 and 95 kDa that cleave peptide bonds specifically at Arg residues (Chen et al., 1992) and a lysine-specific gingipain (Kgp) of 105 kDa that cleaves peptide bonds specifically at Lys residues (Pike et al., 1994). The 95 kDa high molecular mass Rgp (HRgpA) and kgp are complexes of a catalytic domain and a hemagglutinin/adhesion domain, whereas the 50 kDa Rgp (RgpB) lacks the latter domain. Gingipains enhance vascular permeability through activation of the kallikrein/kinin pathway (Imamura et al., 1994; 1995a), disrupt plasma clotting (Scott et al., 1993; Imamura et al., 1995b; 1997), activate components of the complement system (Wingrove et al., 1992) and modify neutrophil functions (Jagels et al., 1996). The conversion of profimbrilin to mature fimbrilin by gingipains is indispensable for the expression of P. gingivalis fimbriae (Kadowaki et al., 1998), an important cell surface structure of this bacterium for adhesion, colonization and invasion (Amano, 2007). Furthermore, we have shown that gingipains cleave CD14 on human monocytes (Sugawara et al., 2000) and gingival fibroblasts (Tada et al., 2002), and ICAM-1 on human oral epithelial cells (Tada et al., 2003), inhibiting LPS-elicited defensive responses of these cells to this pathogen and interaction between epithelial cells and leukocytes, respectively, which help P. gingivalis to evade the innate immune responses.
Protease-activated receptors (PARs) are G protein-coupled receptors, characterized by signal transduction triggered through proteolytic cleavage at each N-terminal peptide (Déry et al., 1998; Coughlin, 2000; O'Brien et al., 2001). PAR-1, PAR-3 and PAR-4 are activated mainly by thrombin, while PAR-2 is activated by trypsin and mast cell tryptase as well as the coagulation factors VIIa and Xa but not by thrombin (Déry et al., 1998; Coughlin, 2000; O'Brien et al., 2001). We added neutrophil serine proteinase 3 (PR3) as another activator of PAR-2 on human oral epithelial cells and human gingival fibroblasts (Uehara et al., 2002; 2003). PARs are expressed on a wide variety of cell types, and suggested to play important roles in pathophysiological processes, such as growth, development, inflammation, tissue repair and pain (Déry et al., 1998; Coughlin, 2000; O'Brien et al., 2001). RgpB activated PAR-2 on human neutrophils (Lourbakos et al., 1998), and induced interleukin (IL)-6's secretion by activating PAR-1 and PAR-2 on human oral epithelial KB cells (Lourbakos et al., 2001a) and platelet aggregation via PAR-1 and PAR-4 (Lourbakos et al., 2001b). Furthermore, RgpB induced the release of neuropeptide from dental pulp cells via PAR-2 signalling (Tancharoen et al., 2005). Recently, we revealed that Rgps (HRgpA and RgpB) stimulated production of hepatocyte growth factor (HGF) through PAR-1 and PAR-2 in human gingival fibroblasts (Uehara et al., 2005a), which may be associated with both inflammatory and reparative processes in periodontal tissues. Therefore, PARs are important molecules that mediate gingipains' stimulatory effects on cells.
The innate immune system recognizes microorganisms through a series of pattern recognition receptors that are highly conserved and bind specifically to common motifs, designated pathogen-associated molecular patterns (PAMPs), present in microorganisms but not in eukaryotes. Representative PAMPs are the lipid A moiety of LPS, lipopeptides, peptidoglycans (PGNs), bacterial DNA, viral double-stranded and single-stranded RNA. Several studies have demonstrated that in mammals, these PAMPs are recognized specifically by the respective Toll-like receptor (TLR) (Akira et al., 2006). In addition, the NOD-like receptor (NLR) family were demonstrated to be intracellular receptors for a partial structure of PGN; NOD1 and NOD2 recognize diaminopimelic acid (DAP) containing a peptide moiety and muramyl peptide respectively (Fritz et al., 2006).
We revealed that a combination of chemically synthesized TLR agonists with muramyldipeptide (MDP) or DAP-containing desmuramylpeptides synergistically induced production of IL-8 in a NOD2- or NOD1-dependent manner, respectively, in human monocytic THP-1 cells in culture (Uehara et al., 2005b). Furthermore, we recently reported that anti-PR3 Abs primes human monocytic cells through PAR-2 for TLR- and NOD-dependent enhanced cell activation (Uehara et al., 2007). These results suggest an interaction of signalling triggered PARs and TLRs or NODs. Therefore, we investigated the possible synergistic effect of a PAR agonist peptide (AP) (PAR-1AP, -2AP or -3AP) and a TLR/NLR ligand agonist on the production of IL-8 in human monocytic cells. Then, we examined whether gingipains as PAR agonists exert a synergistic effect in combination with a TLR/NLR agonist to present a novel activity of gingipains.
Results
Increase of IL-8 secretion from cultured human monocytic THP-1 cells by PAR-APs
First, we investigated the effect of PAR signalling (PAR-1, -2 and -3) on the production of IL-8 by THP-1 cells. PAR-APs significantly promoted IL-8's secretion from the cells over an incubation period of 8–24 h, after which there appeared to be a slight decrease (Fig. 1A). PAR-APs increased the amount of IL-8 secreted in a dose-dependent manner from 10 μM to at least 100 μM (Fig. 1B). The secretion was augmented about fourfold by PAR-APs at 100 μM and the three types of PAR agonists had similar stimulatory effects.
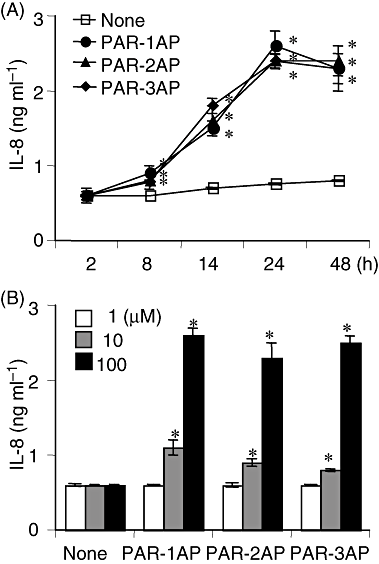
The time-course and dose response of PAR-AP-induced IL-8 production.A. THP-1 cells were incubated with 100 μM of PAR-1AP, PAR-2AP or PAR-3AP for a range of different time periods.B. THP-1 cells were incubated with one of the PAR-APs at various concentrations for 24 h. The concentrations of IL-8 released into supernatants were measured by ELISA and values are the means ± SD for triplicate assays. *P < 0.01 versus the culture medium alone. Results representative of three different experiments are shown.
Synergistic effect of PAR-APs and synthetic TLR or NOD ligands on secretion of IL-8 from cultured THP-1 cells
As reported (Uehara et al., 2005b), synthetic PAMPs induced the secretion of IL-8 in THP-1 cells in a dose-dependent manner (data not shown). To elucidate the possible synergistic effects of PAR-APs (PAR-1AP, -2AP and -3AP) and various TLR or NOD agonists, we examined IL-8's secretion from THP-1 cells in the presence of both a PAR-AP and a synthetic PAMP. Clear synergistic effects were observed with all combinations of PAR-APs and PAMPs and the secretion of IL-8 evoked by a PAR-AP increased about four- to fivefold in the presence of any of the PAMPs (Fig. 2).
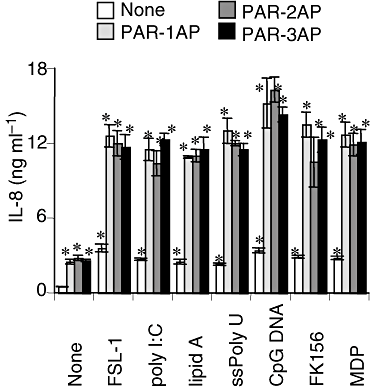
Synergistic effect of PAR-APs and synthetic TLR and NOD ligands on IL-8 secretion from cultured THP-1 cells. THP-1 cells were stimulated with 100 μM of a PAR-AP in the presence of either FSL-1 (1 nM), poly I:C (1 μg ml−1), lipid A (10 ng ml−1), ssPoly U (10 μg ml−1), CpG DNA (1 μM), FK156 (100 μg ml−1) or MDP (100 μg ml−1) for 24 h. Concentrations of IL-8 in the culture supernatants were measured by ELISA, and values are means ± SD for triplicate assays. *P < 0.01 versus the culture medium alone and the respective control respectively. Results representative of three different experiments are shown.
Increase in secretion of IL-8 caused by gingipains in cultured human monocytic THP-1 cells
We demonstrated that Rgps activate PAR-1 and PAR-2, stimulating the production of hepatocyte growth factor in cultured human gingival fibroblasts (Uehara et al., 2005a). We therefore studied the effects of gingipains (Kgp, HRgpA and RgpB), as PAR agonists, on the secretion of proinflammatory cytokines from THP-1 cells. Gingipains increased production of IL-8 in a time-dependent manner (Fig. 3A). A significant increase in secretion was observed over an incubation period of 14–24 h, and Kgp and HRgpA were more effective than RgpB (Fig. 3A). Gingipains augmented IL-8's secretion in a dose-dependent manner starting at a gingipain concentration of 20 nM (Fig. 3B). FPR-cmk and z-FK-cmk, inhibitors specific to Rgps and Kgp respectively (Potempa et al., 1997), completely inhibited the gingipain-induced secretion of IL-8, indicating its dependency on the enzymatic activities (Fig. 3C).
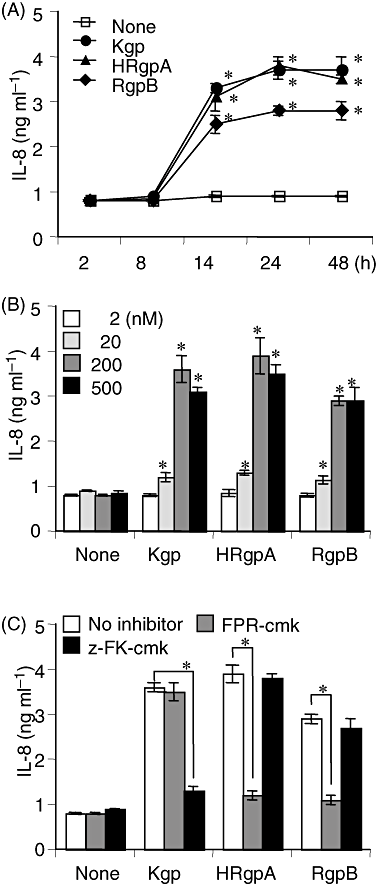
Effects of gingipains on IL-8 production by THP-1 cells.A. THP-1 cells were incubated with 200 nM of gingipains for various periods.B. THP-1 cells were incubated with various concentrations of gingipains for 24 h.C. THP-1 cells were incubated with gingipains, pretreated with 10 μM of FPR-cmk or z-FK-cmk for 15 min at 37°C, for 24 h. The solution for gingipain activation was used as a control. The concentrations of IL-8 in the supernatant were measured by ELISA and values are the means ± SD for triplicate assays. *P < 0.01 versus the culture medium alone or respective counterpart. Results representative of three different experiments are shown.
Synergistic effect of gingipains and synthetic TLR or NOD ligands on secretion of proinflammatory cytokines from THP-1 cells
Next, we examined the synergistic effects of gingipains and the synthetic PAMPs on cytokine production. Among PAMPs, Poly I:C and CpG DNA induced definite secretion of the three cytokines in the absence of gingipains (Fig. 4). Similar to PAR-APs, the three gingipains exhibited synergistic effects with these PAMPs on IL-8's secretion from THP-1 cells, as was seen for the secretion of IL-6, and monocyte chemoattractant protein (MCP)-1 and HRgpA were most effective in combination with any PAMP, except with FK156 in the secretion (Fig. 4).
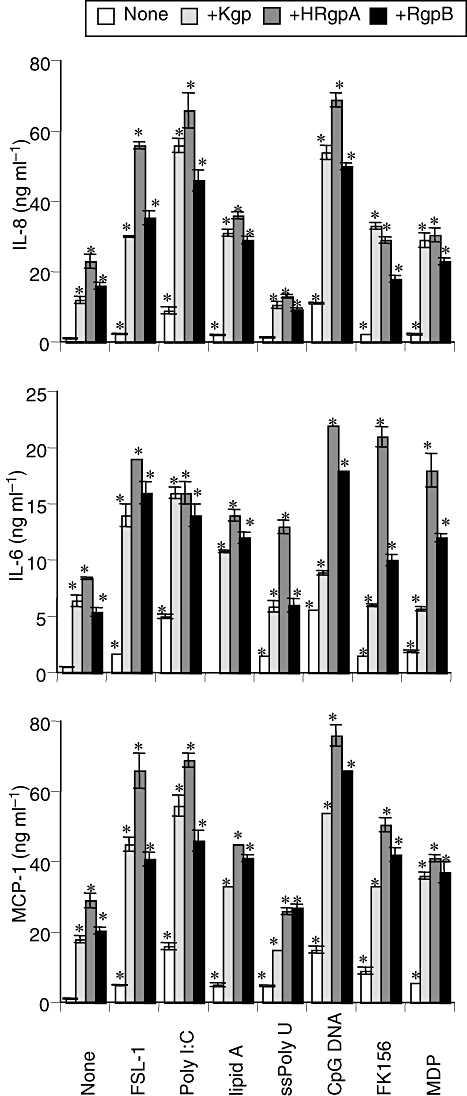
Synergistic effects of gingipains and synthetic TLR or NOD ligands on secretion of proinflammatory cytokines in cultured THP-1 cells. THP-1 cells were incubated with 200 nM of Kgp, HRgpA, or RgpB in the presence of either of FSL-1 (1 nM), poly I:C (1 μg ml−1), lipid A (10 ng ml−1), ssPoly U (10 μg ml−1), CpG DNA (1 μM), FK156 (100 μg ml−1) or MDP (100 μg ml−1) for 24 h. Concentrations of IL-8, IL-6 and MCP-1 in the culture supernatants were measured by ELISA, and values are the means ± SD for triplicate assays. *P < 0.01 versus the culture medium alone and the respective control. Results representative of four different experiments are shown.
Synergistic effect of gingipains and synthetic TLR or NOD ligands on secretion of proinflammatory cytokines from human peripheral blood mononuclear cells (PBMCs)
Next, we examined whether similar synergistic effects of gingipains and synthetic TLR or NOD ligands were observed in human PBMCs. Consistent with the results for THP-1 cells, the gingipains exhibited synergistic effects with these PAMPs on the secretion of IL-8, IL-6 and MCP-1 from PBMCs (Fig. 5).
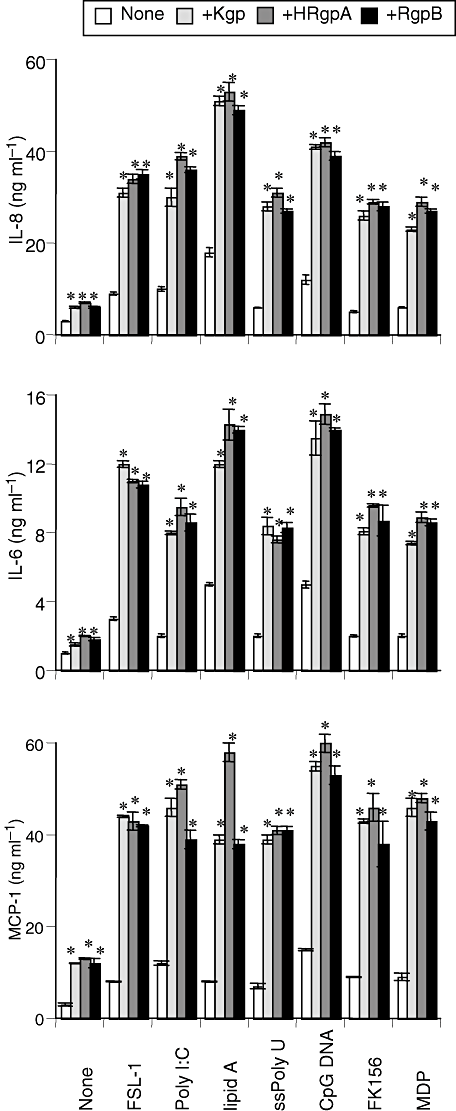
Synergistic effects of gingipains and, synthetic TLR or NOD ligands on secretion of proinflammatory cytokines in cultured PBMCs. PBMCs were incubated with 200 nM of Kgp, HRgpA, or RgpB in the presence of either of FSL-1 (1 nM), poly I:C (1 μg ml−1), lipid A (10 ng ml−1), ssPoly U (10 μg ml−1), CpG DNA (1 μM), FK156 (100 μg ml−1) or MDP (100 μg ml−1) for 24 h. Concentrations of IL-8, IL-6 and MCP-1 in the culture supernatants were measured by ELISA, and values are the means ± SD for triplicate assays. *P < 0.01 versus the culture medium alone and the respective control. Results representative of four different experiments are shown.
Involvement of PARs in synergistic effects of gingipains on IL-8 secretion from THP-1 cells
To confirm the involvement of PARs in the synergistic effects of gingipains and PAMPs, TLR agonists or NOD agonists, we used short-interfering RNA (siRNA) to block the expression of PAR-1, -2 or -3. Protein levels of PAR-1, -2 and -3 in the cells treated with the siRNA were suppressed about 80% (Fig. 6A). As shown in Fig. 6B, synergistic effects of gingipains and synthetic PAMPs on the secretion of IL-8 were significantly inhibited to about 50% in all of the PAR-silenced THP-1 cells, but not in Lamin-silenced THP-1 cells, irrespective of PAMPs. These results clearly indicate PAR-1, -2 and -3 to be critical for the synergistic effects of gingipains and TLR or NOD agonists.
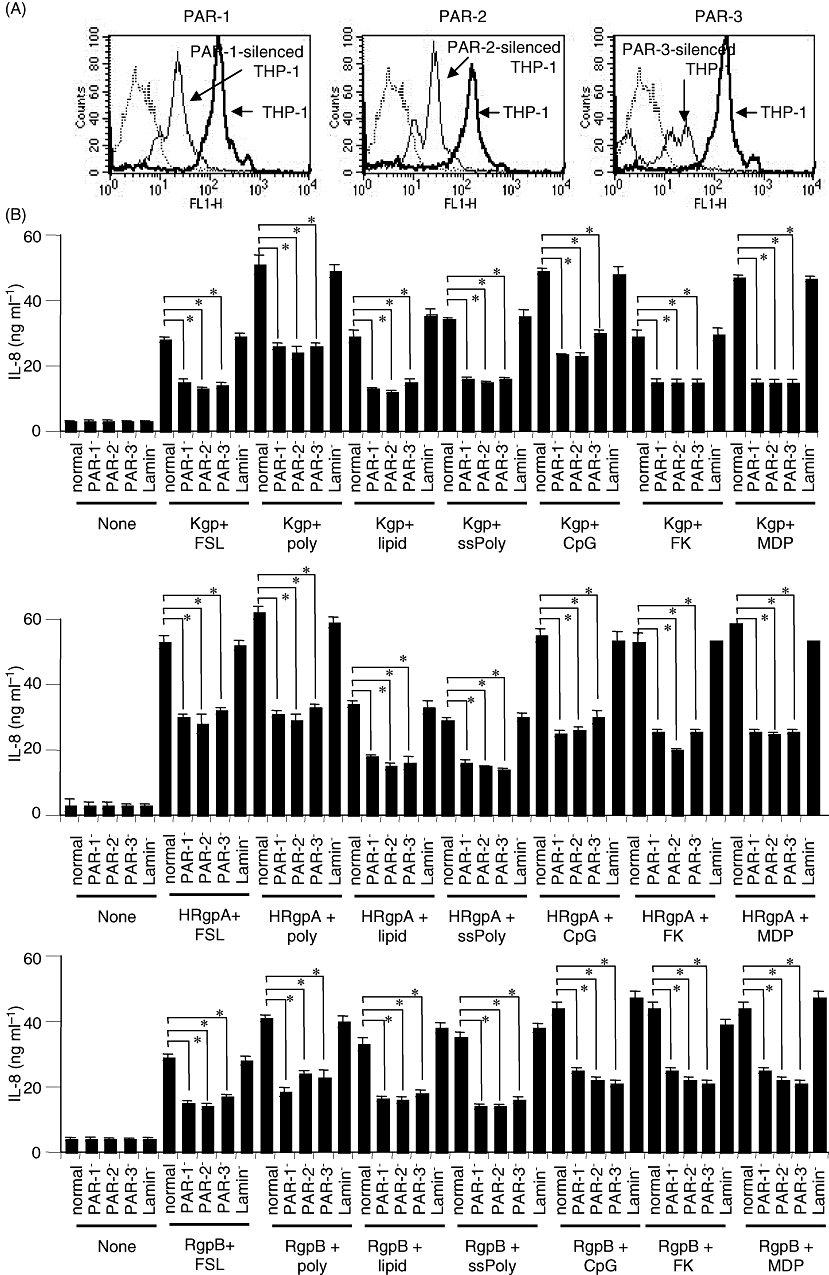
Suppression of synergistic effects of gingipains and synthetic PAMPs in THP-1 cells treated with siRNA for PAR-1, PAR-2, or PAR-3.A. THP-1 cells were transfected with siRNA targeting the PAR-1, PAR-2 or PAR-3 gene. Lamin A/C was used as a control. After 24 h, the cells were stained with Abs specific for PAR-1, PAR-2, or PAR-3 or, control IgG at 4 C for 30 min, followed by FITC-conjugated secondary Abs, and then subjected to flow cytometry.B. THP-1 cells transfected with siRNA targeting the PAR-1, PAR-2, PAR-3 or Lamin A/C gene for 24 h were stimulated with 200 nM of a gingipain with either FSL-1 (1 nM), poly I:C (1 μg ml−1), lipid A (10 ng ml−1), ssPoly U (10 μg ml−1), CpG DNA (1 μM), FK156 (100 μg ml−1), or MDP (100 μg ml−1) for 24 h. Concentrations of IL-8 in the culture supernatants were measured by ELISA, and values are the means ± SD for triplicate assays. *P < 0.01 versus respective control (normal cells). Results representative of three different experiments are shown.
Discussion
Porphyromonas gingivalis cysteine proteinases, Rgps and Kgp, synergistically increase the secretion of proinflammatory cytokines from human monocytic cells via PAR-1, -2 and -3 in combination with TLR or NOD agonists. This is the first report of synergistic effects of signalling of PARs and TLR or NOD and indicates a link between the PAR system and innate immunity. The involvement of PARs in the synergistic effects were shown by the results that (i) PAR-APs mimicked the effects of gingipains (Fig. 2), (ii) the effects were completely inhibited by treating gingipains with inhibitors specific to each proteinase (Fig. 3C), and (iii) decreasing the expression of PARs by treating cells with siRNA suppressed the effects (Fig. 6B). Activation by gingipains has been shown for PAR-1, -2 and -4 (Lourbakos et al., 2001a,b; Tancharoen et al., 2005; Uehara et al., 2005a) but no report has demonstrated PAR-3's activation. The present results clearly demonstrated that Kgp and Rgps synergistically increased the secretion of proinflammatory cytokines in combination with a TLR or NOD agonist through PAR-1, -2 and -3 (Fig. 6B). It is obvious that the effects of kgp are dependent on the cleavage of PARs by its enzymatic activity (Fig. 3C). PAR-3 is activated by cleavage at the carboxy-terminal side of the Lys residue in the tethered ligand (Déry et al., 1998; Coughlin, 2000; O'Brien et al., 2001), which is consistent with the substrate specificity of Kgp (Pike et al., 1994); however, PAR-1 and PAR-2 require cleavage after the Arg residue and are activated by Rgps. The result that the synergistic effects of gingipains and synthetic PAMPs on IL-8 secretion were inhibited by about 50% in all PAR-silenced THP-1 cells irrespective of the PAMPs indicates an involvement of Rgps and Kgp in the activation of PAR-1 and PAR-2 and PAR-3 respectively (Fig. 6B). Although in 3, 4 there seems to be some differences in IL-8 production between Kgp, HRgpA and RgpB, in Fig. 5 such differences appear to disappear by a combination of each gingipain with PAMPs. Therefore, the synergism between gingipains and PAMPs varies depending on the combination used, cells used and cytokines secreted. Anyway, the present study shows cross-talk between the PAR system, and TLR or NOD1/2 system, and suggests a new interaction between bacteria and the host defence system. However, previous studies have demonstrated that gingipains would rapidly and efficiently inactivate (Calkins et al., 1998; Baba et al., 2002; Bodet et al., 2005) or be related to decreased extracellular levels of various proinflammatory cytokines, including IL-6 and IL-8 (Steffen et al., 2000), even though their mRNA levels are increased after infection with wild-type P. gingivalis but not with its mutant deficient in gingipains (Baba et al., 2002). In addition, there is accumulating evidence that gingipains are responsible for shedding and cleavage of CD14 receptors after treatment of human macrophage-like cells with the bacterium (Sugawara et al., 2000; Duncan et al., 2004). Taken together, we cannot rule out the possibility that gingipains may produce biologically inactive fragments of these cytokines, thereby contributing to an increased capacity of the bacterium to evade the host immune system mechanisms.
Porphyromonas gingivalis has been reported to possess TLR agonistic PAMPs: TLR4 agonist lipid A (Ogawa et al., 2007), TLR2 agonist lipopeptides (Asai et al., 2007), fimbriae (Asai et al., 2001) and TLR9 agonist CpG DNA (Takeshita et al., 1999). It should be noted, however, that TLR4-agonistic activity of P. gingivalis lipid A was exceptionally weak (Ogawa et al., 2007). Furthermore, P. gingivalis PGN carries ll-DAP (Holt and Bramanti, 1991), which scarcely activated NOD1 unlike usual meso-DAP (Uehara et al., 2006). Therefore, synergism between signalling via PARs induced by gingipains and PAMPs derived from the bacterium might be responsible for definite inflammatory responses induced by the bacterium in relation to pathogenesis of periodontitis. In addition, we may also speculate of the role of these processes in enhancement of host defence mechanisms.
Experimental procedures
Reagents
We used chemically synthesized PAMPs to avoid the influence of minor components in microbial preparations. The synthetic MDP, a NOD2 agonist, and Escherichia coli type lipid A (LA-15-PP), a synthetic TLR4 agonist, were purchased from the Protein Research Foundation Peptide Institute (Osaka, Japan). The Mycoplasma salivarium type diacyl lipopeptide FSL-1, a TLR2/6 agonist, was obtained from RMC microcollections (Tübingen, Germany). Poly I:C, a TLR3 agonist, and ssPoly U, a TLR7 agonist, were purchased from Sigma-Aldrich (St Louis, MO, USA). A conventional CpG DNA, CpG DNA 1826 [TCCATGACGTTCCTGACGTT (CpG motif is underlined)], a TLR9 agonist, was purchased from SIGMA Genosys (Tokyo, Japan). FK156 (d-lactoyl-l-Ala-γ-d-Glu-meso-DAP-Gly) (Kitaura et al., 1982), a NOD1 agonist, was supplied by Astellas Pharmaceutical (Tokyo, Japan). Synthetic PAR-1AP (SFLLRN), PAR-2 AP (SLIGKV) and PAR-3AP (TFRGAP) were purchased from Takara (Otsu, Japan). All other reagents were obtained from Sigma-Aldrich, unless otherwise indicated.
Cells and cell culture
The human monocytic leukaemia cell line THP-1, supplied by the Health Science Research Resources Bank (Osaka, Japan), was cultured in RPMI 1640 medium (Nissui Seiyaku, Osaka, Japan) with 10% heat-inactivated fetal calf serum (FCS) at 37°C in a humidified CO2 atmosphere. The THP-1 cells were maintained in a logarithmic phase of growth (2 × 105 to 2 × 106) by passage every 3–4 days.
Human PBMCs were isolated from heparinized peripheral blood of healthy adult donors by Lympholyte-H (Cedarlane Laboratories, Hornby, Ontario, Canada) gradient centrifugation at 800 g for 20 min at room temperature. The isolated PBMCs were washed three times with PBS and suspended in RPMI1640 medium.
Purification and activation of gingipains
HRgpA, RgpB and Kgp were purified from P. gingivalis HG66 culture supernatant, as described previously (Pike et al., 1994; Potempa et al., 1998). The purity of each enzyme was checked by SDS-PAGE. In a 10% Tricine gel (Von Jaggow), RgpB showed a single band with an apparent molecular weight of 48 kDa and the purity was > 95% as determined by laser densitometric scanning of the gel. HRgpA was composed of four major and one minor band on SDS-PAGE and each protein band was identified as a HRgpA component by N-terminal sequence analysis (Pike et al., 1994). The amount of active enzyme in each purified gingipain was determined by active site titration using FPR-cmk and Z-FK-cmk for Rgps and Kgp respectively (Potempa et al., 1997). The concentration of fully activated gingipains with cysteine was calculated from the amount of inhibitor needed for complete inactivation of the proteinases. Therefore, gingipain concentrations indicate active enzyme concentrations. The gingipains were activated in 0.2 M HEPES, 5 mM CaCl2 and 10 mM cysteine, pH 8.0, at 37°C for 10 min, and then diluted with the medium or buffer. To block enzymatic activity, the activated gingipains were incubated with FPR-cmk or Z-FK-cmk for 10 min at room temperature before use.
Measurement of cytokines
The cells were collected and washed twice in PBS. They (2 × 105 cells per ml) were cultured in RPMI 1640 medium, supplemented with 10% FCS, in the presence or absence of a stimulant for 24 h in 96-well culture plates. Then, the culture supernatants were collected and the levels of IL-6, IL-8 and MCP-1 were measured with an enzyme-linked immunosorbent assay (ELISA) kit (OptEIA ELISA, BD Pharmingen, San Diego, CA, USA). The concentrations of a cytokine in the supernatants were calculated using the LS-Platemanager 2004 data analysis program (Wako Pure Chemical Industries, Osaka, Japan).
RNA interference
siRNAs (200 nM, the final concentration) for targeting the genes of PAR-1, PAR-2, PAR-3 or Lamin A/C were purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA) and introduced into cells using Lipofectamine 2000 (Invitrogen, Carlsbad, CA, USA) according to the manufacturer's instructions. The viability of the transfected cells was more than 95% when assessed with the Trypan blue exclusion test, and the cells did not change morphologically after the transfection.
Flow cytometry
Flow cytometric analyses were performed using a FACSCalibur flow cytometer and CELLQuest software (BD Biosciences, San Diego, CA, USA). Washed THP-1 cells were stained with mouse monoclonal antibodies specific for PAR-1, PAR-2 or PAR-3 (Santa Cruz), or isotype-matched control IgG at 4°C for 30 min, followed by fluorescein isothiocyanate (FITC)-conjugated anti-mouse IgG or goat IgG (Biosource International, Camarillo, CA, USA) at 4°C for a further 30 min. To calculate the percentage of positive cells, the baseline cursor was set at a channel that yielded less than 2% of the events as positive for the secondary Ab in the absence of the primary antibodies. Fluorescence to the right was counted as specific binding.
Data analysis
All experiments were performed at least three times to confirm the reproducibility of the results. Values are shown as the means ± SD from triplicate assays. The significance of differences was statistically evaluated by the one-way analysis of variance, using the Bonferroni or Dunnett method, and P-values less than 0.05 were considered significant.
Acknowledgements
We thank D. Mrozek (Medical English Service, Kyoto, Japan) for reviewing the paper. This work was supported by Grants-in-Aid for Scientific Research from the Japan Society for the Promotion of Science (18390484 to H.T. and 19659426 to A.U.), from the Ministry of Education, Sports, Science and Culture, Japan (18689901 to A.U.), from Naito Memorial Foundation (to A.U.) and by the Exploratory Research Program for Young Scientists from the President of Tohoku University (to A.U.).