Motility allows S. Typhimurium to benefit from the mucosal defence
Summary
The mammalian intestine is colonized by a dense bacterial community, called microbiota. The microbiota shields from intestinal infection (colonization resistance). Recently, we have shown that enteropathogenic Salmonella spp. can exploit inflammation to compete with the intestinal microbiota. The mechanisms explaining the enhanced pathogen growth in the inflamed intestine are elusive. Here, we analysed the function of bacterial flagella in the inflamed intestine using a mouse model for acute Salmonella Typhimurium enterocolitis. Mutations affecting flagellar assembly (Fla-) and chemotaxis (Che-) impaired the pathogen's fitness in the inflamed intestine, but not in the normal gut. This was attributable to a localized source of high-energy nutrients (e.g. galactose-containing glyco-conjugates, mucin) released as an element of the mucosal defence. Motility allows Salmonella Typhimurium to benefit from these nutrients and utilize them for enhanced growth. Thus, nutrient availability contributes to enhanced pathogen growth in the inflamed intestine. Strategies interfering with bacterial motility or nutrient availability might offer starting points for therapeutic approaches.
Introduction
Bacterial pathogenesis has affected the evolution of host defences. Mammals have evolved multiple mechanisms to interfere with colonization and deter invading pathogens. These include chemical barriers (antimicrobial peptides, bile salts, lysozyme, lipases, pH), mechanical barriers (epithelium, mucus) and innate and adaptive immune responses. In addition, the intestinal lumen is colonized by a dense bacterial community called the ‘microbiota’ (Xu and Gordon, 2003). The microbiota protect efficiently against intestinal colonization by most enteric pathogens (‘colonization resistance’). This phenomenon is still not entirely understood. Recently, we have shown that Salmonella subspecies I serovar Typhimurium (S. Typhimurium) can exploit intestinal inflammation to out-compete the microbiota (Stecher et al., 2007). Inflammation was necessary and sufficient to shift this competition in favour of the pathogen. However, the underlying mechanisms have remained elusive. It is unknown which elements of the host defence (e.g. antimicrobial compounds, increased release of high-energy nutrients like glycoproteins, etc.) define the hallmarks of this altered ecosystem and which virulence factors enable the pathogen to exploit them. Here, we used a mouse model for acute Salmonella Typhimurium enterocolitis to study the function of bacterial motility in the inflamed intestine.
Salmonella Typhimurium is a Gram-negative pathogen causing enterocolitis in humans and livestock. Pathogenesis of these medically and economically important infections can be studied in a mouse model for Salmonella enterocolitis. In this model, the commensal microflora is transiently reduced by streptomycin treatment allowing S. Typhimurium to efficiently colonize the intestine and elicit colitis, a pronounced inflammation of the cecum and colon (Barthel et al., 2003; Coombes et al., 2005; Hapfelmeier and Hardt, 2005; Stecher et al., 2005; Becker et al., 2006; Suar et al., 2006).
Multiple S. Typhimurium virulence factors contribute to the induction of colitis, including the two-type three-secretion systems (referred to as TTSS-1 and TTSS-2 or T1- and T2- in case of the respective mutants throughout this paper) and flagella (Stecher et al., 2004; Coburn et al., 2005; Hapfelmeier et al., 2005). Flagella are surface structures propelling bacteria towards favourable environments. Directional movement (‘chemotaxis’) towards nutrient reservoirs requires chemosensors (‘chemotaxis receptors’), signal integration and regulated flagellar rotation.
Flagella are key virulence factors of many bacterial pathogens. Often, they have pleiotropic phenotypes including chemotaxis, adherence, host cell invasion, colonization and innate immune signalling (Josenhans and Suerbaum, 2002). This has prohibited unequivocal conclusions about flagellar function in host infection. In the mouse model for S. Typhimurium colitis, mutations disrupting flagellar biosynthesis or chemotactic movement attenuate disease and reduce bacterial fitness in competitive infection experiments (Stecher et al., 2004). However, the functional role of flagella during the acute enteric infection has remained unclear.
Here, we used the streptomycin mouse model to study why flagella enhance S. Typhimurium fitness in the inflamed intestine. We analysed the function of flagella in the presence and absence of intestinal inflammation. Our findings establish that mucosal inflammation provides a localized source of high-energy nutrients. Motility in turn enhances the pathogen's fitness because it allows accessing these nutrients resulting in faster replication. This implies that nutrient availability (and efficient access to these nutrients) plays an important role in gut colonization by S. Typhimurium in the infected host.
Results
S. Typhimurium requires flagella for colonizing the inflamed but not the non-symptomatic intestine
Flagella are important virulence factors of enteropathogenic bacteria. We showed earlier, that S. Typhimurium requires flagella for efficient replication in the lumen of an inflamed intestine (Stecher et al., 2004). However, the selective pressure favouring motile bacteria in the gut lumen is still unclear. We speculated that some feature of the inflamed intestine (e.g. neutrophil influx, altered antibacterial defences or nutrient availability) which may be lacking in the non-symptomatic intestine might drive this selection. To test this hypothesis we used the streptomycin-pretreated mouse model and applied two different experimental approaches.
First, we studied the role of motility in the genetic background of the attenuated S. Typhimurium strain T1-T2-[M557; deficient in type three secretion via TTSS-1 and TTSS-2; Table 1 (Hapfelmeier et al., 2005)]. T1-T2- efficiently colonizes the intestinal lumen for 3 days but is incapable of triggering colitis by itself (Hapfelmeier et al., 2005; Stecher et al., 2007). T1-T2-Che- (M965; Table 1) is an isogenic variant of T1-T2- which lacks a key element of the signalling cascade controlling chemotaxis. To measure the selective advantage (competitive index, CI), five mice were infected with a 1:1 mixture of T1-T2- and T1-T2-Che- (5 × 107 cfu intragastrically (i.g.)). As expected, no intestinal inflammation was detected (Fig. S1A) and T1-T2- did not have a competitive advantage over T1-T2-Che- in the normal gut (P > 0.05; Fig. 1A). In contrast, in the presence of inflammation [infection with isogenic wild type strains M964 (Che-) versus SB300 (wild type)], Che- was out-competed by wild type (P = 0.008). Competitive infection experiments with a strain completely lacking flagella [M933 (Fla-T1-T2-) versus M557 (T1-T2-); M913 (Fla-) versus SB300 (wild type)] yielded identical results (Fig. S1B and C). Thus, flagella-based movement strongly enhances fitness of S. Typhimurium in the intestinal lumen – but only in the inflamed gut.
| Strain | Designation | Relevant genotype | Resistance | Induction of Inflammation | Reference |
|---|---|---|---|---|---|
| Escherichia coli SM10 λpir | thi thr leu tonA lacY supErecA::RP4-2-Tc::Mu | KmR | n.a. | Miller and Mekalanos (1988) | |
| SB300 | Wild type | S. Typhimurium SL1344, hisG | SmR | Yes | Hoiseth and Stocker (1981) |
| SB161 | T1- | ΔinvG | SmR | Yes;Not in MyD88−/− mice | Kaniga et al. (1994) |
| M913 | Fla- | fliGHI::Tn10 | SmR TetR | Stecher et al. (2004) | |
| M583 | T1–Fla- | ΔinvG; fliGHI::Tn10 | SmR TetR | Yes;Not in MyD88−/− mice | This study |
| M944 | T1-Che- | ΔinvG; cheY::Tn10 | SmR TetR | Yes | This study |
| M557 | T1-T2- | ΔinvG; sseD::aphT | SmR KmR | No | Hapfelmeier et al. (2004) |
| M933 | T1-T2-Fla- | ΔinvG; sseD::aphT; fliGHI::Tn10 | SmR KmR TetR | No | This study |
| M946 | T1-T2–Che– | ΔinvG; sseD::aphT; cheY::Tn10 | SmR KmR TetR | No | This study |
| M984 | T1-T2-ampR | ΔinvG sseD::aphT; chromosomal insertion of pM1491 downstream of pagC | SmR AmpR KmR | No | This study |
| M951 | T1-T2-tetR | ΔinvG sseD::aphT; BCB4 tetR | SmR KmR TetR | No | This study |
| M318 | T1-T2- | invC::aphT; ssaV::cat | SmR KmR CmR | No | Hapfelmeier et al. (2005) |
| M957 | Che- | ΔcheY::cat | SmR CmR | n.a. | This study |
| M958 | T1-T2-Che- | ΔinvG sseD::aphT; ΔcheY::cat | SmR KmR CmR | No | This study |
| ST4/74 | Wild type | S. Typhimurium ST4/74 | SmR | n.a. | Hoiseth and Stocker (1981) |
| M960 | T1-T2- | histidin-prototrophic transductant of M557 | SmR KmR | No | This study |
| M962 | Che- | ΔcheY | SmR | n.a. | This study |
| M963 | T1-T2-Che- | ΔinvG sseD::aphT; ΔcheY | SmR KmR | No | This study |
| M964 | Che- | ΔcheY; BCB4 tetR | TetR | n.a. | This study |
| M965 | T1-T2-Che- | ΔinvG sseD::aphT; ΔcheY; BCB4 tetR | SmR KmR TetR | No | This study |
- n.a., not applicable.
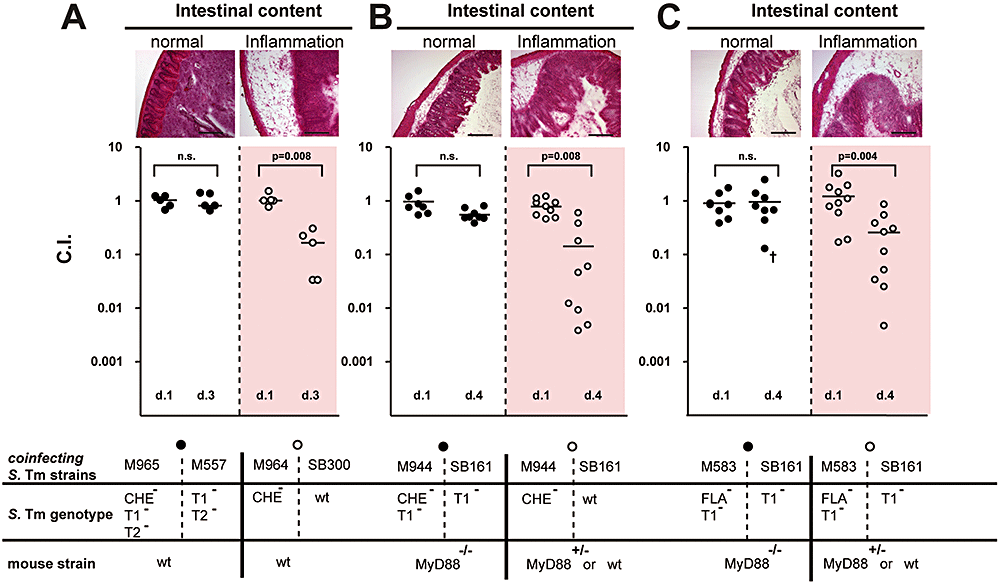
S. Typhimurium Che- and Fla- mutants are defective in colonizing the inflamed but not the non-symptomatic intestine.A. Streptomycin-pretreated wild-type C57BL/6 mice (n = 5) infected for 3 days with 1:1 mixtures (total 5 × 107 cfu intragastrically (i.g.)) of S. Typhimurium strains T1-T2-Che- (M965) and T1-T2- (M557; closed circles ●) or Che- (M964) and wild type (SB300 open circles ○).B. Streptomycin-pretreated MyD88−/− (n = 9; closed circles ●) or C57BL/6/MyD88+/− mice (n = 8; open circles ○) were infected (5 × 107 cfu i.g.) for 4 days with a 1:1 mixture of S. Typhimurium strains T1–Che– (M944) and T1– (SB161).C. Streptomycin-pretreated MyD88−/− (n = 10; closed circles ●) or C57BL/6/MyD88+/− mice (n = 9; open circles ○) were infected (5 × 107 cfu i.g.) for 4 days with a 1:1 mixture of S. Typhimurium strains T1-Fla- (M583) and T1– (SB161). Competitive indices (CI) for the pairs of isogenic Che-/Che+ or Fla-/Fla+ strains were defined at day 1 in the feces and at days 3 or 4 p.i. in the cecal content. Cecal tissues were embedded and inflammation (or lack thereof) was confirmed by histopathological evaluation of HE-stained tissue sections (Figs S1 and S2). Representative images are shown above. Scale bar = 200 μm. Red-shaded background indicates cecal inflammation (total pathological score >5, severe inflammation). The symbol ‘†’ indicates unusual MyD88–/– animal with colitis (cecal pathological score of 6, Fig. S2F). n.s., not statistically significant (P = 0.05); P = difference of CI between days 1 and 3/4 p.i.; dotted line, limit of detection; black bar, median.
This was confirmed in an alternative approach using knockout mice (MyD88−/−) lacking a central element of the innate immune system (Takeda and Akira, 2005). A T1- strain (SB161; ΔinvG) cannot trigger colitis in MyD88−/− mice. In contrast, wild-type C57BL/6 mice (MyD88+/− or MyD88+/+) develop colitis after ≥ 3 days of infection with T1- (Hapfelmeier et al., 2005). This allowed us, using the same pair of bacterial strains, to compare the fitness of Che+ and Che-S. Typhimurium strains in the presence or absence of inflammation. In competitive infection experiments, groups of MyD88−/− and wild-type C57BL/6 mice were infected with a 1:1 mixture of T1- and T1-Che- (M944; Tables 1 and 5 × 107 cfu i.g.). Total S. Typhimurium fecal and systemic loads were comparable to previous experiments (Fig. S1). As expected, wild-type C57BL/6 mice developed colitis and T1- out-competed T1-Che- in these animals by day 4 post infection (p.i.; P = 0.008; Fig. 1B). In contrast, MyD88−/− mice did not develop colitis and the ratio of T1- versus T1-Che- did not change significantly between days 1 and 4 p.i. (P > 0.05; Fig. 1B; Fig. S2A–C).
Similar results were obtained in competitive infection experiments with T1- and T1-Fla- (M583; Table 1), an isogenic non-flagellated mutant (Fig. 1C; Fig. S2D–F).
Additional control experiments confirmed that the results were not skewed by the choice of the antibiotic resistance cassettes tagging the different strains (Fig. S1D). In conclusion, it did not make a difference whether bacterial motility was disrupted at the level of flagellar expression or directed movement. These data confirm that bacterial motility enhances S. Typhimurium fitness in the intestinal lumen but only in case of an inflamed gut. Thus, the inflamed intestine must display features favouring motile over non-motile strains of the pathogen.
Motility increases the S. Typhimurium growth rate in the inflamed intestine
Next, we sought to identify the principal mechanism explaining the improved fitness of flagellated, chemotactic S. Typhimurium strains in the inflamed intestine. Superior fitness could be explained by (i) enhanced growth rates of the flagellated, chemotactic strains, (ii) reduced resistance of non-flagellated or non-chemotactic strains to defensive mechanisms (e.g. reactive O- or N-intermediates, phagocytosis, antimicrobial peptides) and (iii) resisting wash-out through exacerbated peristaltic transport in the inflamed bowel.
We used the temperature-sensitive plasmid pHSG422 (Benjamin et al., 1990) and its variant pM1419 (lacking chloramphenicol and kanamycin resistance markers) to analyse bacterial growth rates and to distinguish between these mechanisms. At 27°C pHSG422 and pM1419 can replicate (i.e. in vitro) and each daughter bacterium harbours the plasmid after cell division. At 37°C (upon inoculation of the mouse) the plasmids cannot replicate and after cell division only one of the two daughter bacteria retains the plasmid. Pilot experiments using pHSG422 indicated that S. Typhimurium strains triggering colitis (i.e. SB300 pHSG422) grew at higher rates than strains failing to trigger colitis (i.e. T1-T2- pHSG422; data not shown). Competitive infection experiments were performed to compare growth rates of the motile strain T1-T2-ampR (M984; Table 1) and the non-motile strain T1-T2-Fla- (M933; tetracycline resistant; Table 1) in vivo side by side. Mice were infected for 1 day with 1:1 mixtures of T1-T2-ampR (M984 pHSG422) and T1-T2-Fla- (M933 pHSG422; in total 5 × 107 cfu i.g.) in the presence (inflammation) or absence (no inflammation) of wild-type S. Typhimurium (15 × 107 cfu i.g.). In mice with inflammation, T1-T2-ampR out-competed the isogenic non-motile strain and lost pHSG422 at a significantly higher frequency (Fig. 2A). However, in the absence of inflammation growth rates of both strains did not differ significantly.
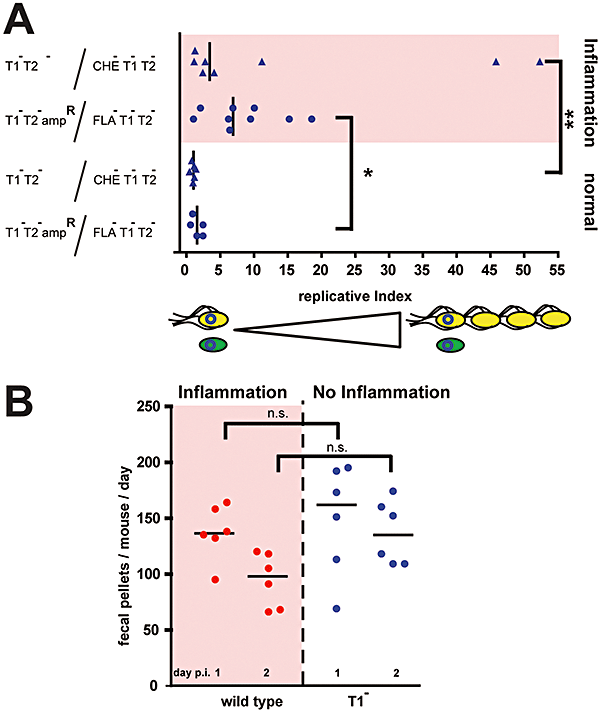
Decreased growth rate of a S. Typhimurium Fla- and Che- mutants in the inflamed intestine.A. Analysis of the replicative index. Four groups of streptomycin-pretreated mice were infected with 1:1 mixtures of isogenic S. Typhimurium strain pairs: (i) M984 (T1–T2–ampR; kanR ampR) and M933 (T1-T2-Fla-; kanR tetR; 5 × 107 cfu in total; circles) harbouring pHSG422 (cmR) at time of infection (> 99%). (ii) The motile, chemotactic strain T1-T2- (M318; kanR cmR) and the non-chemotactic strain T1-T2-Che– (M965; kanR tetR; 5 × 107 cfu in total; triangles) bearing the temperature-sensitive plasmid pM1419 (ampR). If indicated, colitis was generated in trans (pink) by co-infecting with 15 × 107 cfu S. Typhimurium wild type (kmS ampS cmS tetS). The replicative index was determined by differential plating as described in Experimental procedures. Asterisks indicated a significant difference between the respective groups: *P = 0.019; **P = 0.029.B. Frequency of defecation. Groups of six mice were pretreated with streptomycin, housed in separate cages and infected with either wild-type S. Typhimurium (SB300; triggers inflammation) or the isogenic T1- mutant (SB161; no inflammation by day 2 p.i.). The numbers of fecal pellets were counted at days 1 and 2 p.i.; n.s. no statistically significant difference.
These results were confirmed in competitive infection experiments using the motile, chemotactic strain T1-T2- (M318; kanR cmR; Table 1) and its isogenic chemotaxis-deficient variant T1-T2-Che- (M965; kanR tetR; Table 1) bearing the temperature-sensitive plasmid pM1419 (ampR). Again, growth of T1-T2- was significantly enhanced in the presence of inflammation (Fig. 2A). Therefore, bacterial motility was required for enhancing pathogen growth in the inflamed intestine.
To study possible effects of the fecal transit time in the large intestine, we analysed the frequency of defecation in inflamed and non-inflamed mice. The number of fecal pellets shed per mouse did not differ significantly between both groups of mice at day 1 and day 2 p.i. (Fig. 2B). If anything, the inflamed mice tended to shed even slightly reduced numbers of fecal pellets by day 2 p.i. This indicated that intestinal contents were travelling with approximately equal rates in both groups of mice, at least in the large bowel. Thus, preferential ‘wash-out’ of the non-motile mutants cannot explain the reduced fitness of these mutants in the inflamed gut. This led us to conclude that the competitive advantage in the inflamed intestine can be attributed to faster growth of flagellated, motile strains (Fig. 2A).
Flagellated subpopulations accumulate proximal to the mucosa
Next we analysed mechanisms contributing to the superior intestinal growth rates of flagellated S. Typhimurium strains. Movement into nutrient-rich gut regions could provide an explanation. To test this hypothesis we analysed the localization of wild-type S. Typhimurium, non-flagellated and non-chemotactic mutants in the cecal lumen. The strains harboured the reporter plasmid pFLIC, expressing GFPmut2 under control of the fliC promoter pfliC. pfliC is, as a ‘class III promoter’ of the flagella regulon, only expressed during final steps of flagella biosynthesis (Aldridge and Hughes, 2002). In line with previous reports (Cummings et al., 2006) only a fraction of the bacteria in a given LB culture expressed flagella as indicated by the percentage of GFP+ bacteria [wild type (pFLIC): 61 ± 20%; T1-T2- (pFLIC): 56 ± 19%; T1-T2-Che- (pFLIC): 45 ± 24%]. Streptomycin-pretreated mice were infected for 1 day with pFLIC-carrying strains and we analysed the localization of GFP+ and GFP-S. Typhimurium in the cecum by immunofluorescence microscopy (Fig. 3; Fig. S3). Deep inside the cecal lumen, the fraction of GFP+ wild type (pFLIC; triggers colitis) was low (40–60%). The proportion of GFP+ bacteria increased near the epithelium and was maximal in close proximity (< 200 μm) to the cecal epithelium (92 ± 9%; Fig. 3). This suggested that a substantial part of the flagellated fraction of the wild-type S. Typhimurium population accumulated at the epithelium.
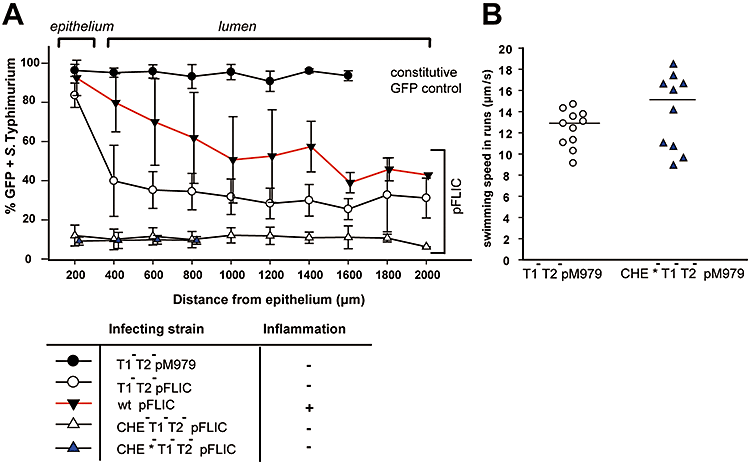
Chemotaxis-mediated accumulation of pfliC-expressing bacteria at the cecal mucosa.A. Accumulation of GFP-expressing bacteria in regions close to the cecal epithelium (x-axis). Cecal tissue samples from streptomycin-pretreated mice infected with T1-T2- (M557 pFLIC; 4 mice; open circles ○), wild type (SB300 pFLIC; 7 mice; closed triangles ▾, red line), S. Typhimurium T1-T2-Che- (M946 pFLIC; 3 mice; open triangles ▵), S. Typhimurium T1-T2-Che- (M963 pFLIC; 3 mice; blue triangles ▴) and T1-T2-[M557 pM979 (constitutive ribosomal promoter pRPSM); 3 mice; closed circles ●] were analysed with respect to GFP expression in individual bacteria located at the indicated distance from the epithelium.B. Speed of swimming of GFP-expressing bacteria isolated from cecal contents analysed by time-lapse microscopy. Cecal contents were extracted from mice infected for 1 day with T1–T2– (M557 pM979; open circles ○) or T1-T2-Che- (M963pM979; blue triangles ▴) and analysed bacterial motility by time-lapse microscopy in HBSS supplemented with chloramphenicol to prevent de novo flagellar protein synthesis. Time-lapse movies were recorded and the speed of swimming of bacteria in ‘run’ phases was determined.
Infection with T1-T2- (pFLIC) did not yield inflammation and approximately 40% of the bacteria expressed the reporter deep inside the intestinal lumen (Fig. 3A; Fig. S3). In the small zone adjacent to the cecal epithelium, GFP-expressing T1-T2- (pFLIC) Salmonella were highly enriched. Control experiments verified that this was not attributable to enhanced fluorophore maturation (see T1-T2- with pM979; constitutive GFP expression; Fig. 3A) or to increased expression levels of the pFLIC reporter in oxygen- and nutrient-rich zones near the gut epithelium (Fig. S4). Thus, our data suggest that motility allows S. Typhimurium to home towards the epithelium of the non-inflamed gut.
To explore the role of chemotaxis in homing to the gut epithelium, we have analysed two different T1-T2-Che- strains [M946(pFLIC) and M963(pFLIC); Table 1]. With both strains, we did not detect enrichment of the pFLIC-expressing bacteria near the epithelial surface (Fig. 3A). This indicated an important role for chemotaxis. However, both T1-T2-Che- strains yielded only ∼10% of GFP-positive bacteria in the gut lumen. This was in contrast to data obtained in LB medium in vitro (45 ± 24% GFP-positive bacteria). Therefore, we performed a control experiment to verify functional expression of flagella by the T1-T2-Che- strains in the gut. We extracted cecal contents from mice infected for 1 day with T1-T2-[M557(pM979)] or T1-T2-Che-[M963(pM979)] and analysed bacterial motility by time-lapse microscopy (GFP-fluorescence). Chloramphenicol was added to prevent de novo flagellar protein synthesis. T1-T2- and T1-T2-Che- bacteria were swimming at the same speed (∼12–15 μm s−1 in ‘run’ phases; P > 0.05; Fig. 3B). This suggested that T1-T2-Che- strains were fully motile (but non-chemotactic). The reason for the low expression (10% versus 45%) of the GFP-reporter by T1-T2-Che-(pFLIC) in vivo has remained unclear and represents a subject for further investigation. Nevertheless, these data suggest that the S. Typhimurium population in the gut lumen homes to the epithelium via chemotaxis. Therefore, gradients of some type of chemo-attractant (i.e. nutrients) must emanate from the intestinal wall.
Glycoconjugate distribution in the murine cecum
Nutrient gradients could drive S. Typhimurium chemotaxis towards the epithelium. Oxygen, amino acid or sugar gradients might also be sensed. Intestinal mucins are one likely source of nutrient gradients (Wadhams and Armitage, 2004): Mucins (i.e. Muc2) are produced in large quantities by the ‘goblet cells’ of the large intestine. They cover the intestinal epithelium at a high density, are highly glycosylated (up to 50 Mol% sugar modifications, i.e. galactose, fucose) and they are a known energy source for commensal bacteria as well as for S. Typhimurium (Ketyi, 1988; McCormick et al., 1988; Sonnenburg et al., 2005; 2006; Stecher et al., 2006). Moreover, insults such as mechanical forces (or inflammation) are known to increase mucus secretion (Deplancke and Gaskins, 2001; Miyake et al., 2006; McAuley et al., 2007). Several observations indicate that Salmonella-induced colitis results in increased mucin secretion by the cecal mucosa:
- i.
The inflamed cecal mucosa harboured similar goblet cell numbers per optical field than the normal cecal mucosa. This was found by immunofluorescence staining for a goblet cell-specific Muc2 precursor (non-O-glycosylated Muc2; Fig. S5).
- ii.
Each goblet cell in the normal intestine harbours multiple mucous storage vesicles. In contrast, goblet cells of the inflamed mucosa harboured virtually none. This pointed to increased mucin secretion rates.
- iii.
Increased amounts of glyco-conjugates were detected in the lumen of the inflamed intestine (see intense UEA-1 and CGL-2 stains in Fig. 4A). The lectins Ulex europaeus agglutinin (UEA-1) and galectin (CGL-2; Walser et al., 2005) bind terminal α-1-2-linked fucose and galactose, which are abundant glycosyl-modifications of the mucins (Thomsson et al., 2002). Thus, mucosal inflammation encompassed increased release rates of glycoconjugates, including mucins.
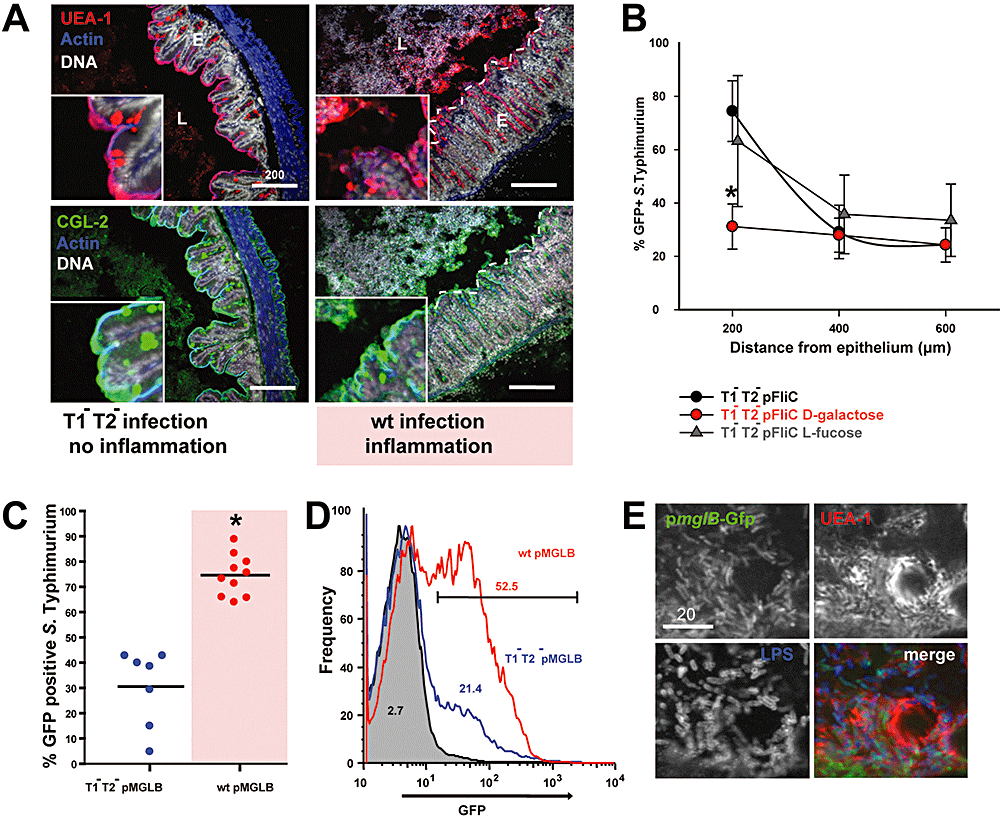
Role of fucose/galactose-rich glyco-conjugates in the inflamed cecum.A. Glyco-conjugate distribution in the S. Typhimurium infected cecum at 1 day p.i. Sugars were stained with the TRITC-labelled lectin UEA-1 (red; Fuc-linked α1–2; upper panels) and FITC-labelled CGL-2 (green; Gal-linked β1–4; lower panels). Actin was visualized with phalloidin-Alexa647 (blue) and DNA with DAPI (grey). T1-T2- infected cecum (left panels), wild type-infected cecum (right panels). Scale bar: 200 μm. Dotted line: epithelial border.B. Galactose supplementation alleviates chemotaxis. Streptomycin-pretreated mice were infected with T1-T2- (pFLIC) and treated repeatedly per os with 100 mM d-galactose (red circles), 100 mM l-fucose (grey triangles) or left untreated (black circles; 3 mice per each condition) between 24 and 28 h of infection. At 29 h p.i. the fraction of GFP-expressing bacteria was determined in situ (in fixed tissue sections) at the indicated distance from the epithelium (Experimental procedures).C. Upregulation of the high-affinity galactose transporter pmglB in the inflamed cecum. Streptomycin-pretreated mice were infected with 5 × 107 cfu T1-T2- (pMGLB) or wild type (pMGLB), killed at day 1 p.i. Cecal tissues were embedded and stained and the fraction of pmglB-expressing S. Typhimurium within 200 μm distance from the cecal epithelium was analysed (Experimental procedures). Blue circles: T1-T2- (pMGLB; no inflammation). Red circles: wild type (pMGLB; inflammation). * signifies statistical significant difference.D. FACS analysis of pmglB expression in the murine cecum. S. Typhimurium recovered from cecal contents of streptomycin-pretreated mice infected for 1 day with 5 × 107 cfu T1-T2- (grey, neg. control), T1-T2- (pMGLB; blue; no inflammation) or wild type (pMGLB; red; inflammation) was stained with α-Salmonella LPS Fab fragments and APC-labelled anti-rabbit Fab (Experimental procedures). Numbers indicate percentageGFP+ bacteria; gate was manually set on negative control.E. pmglB expression by bacteria located within mucus-rich areas. pmglB expression by S. Typhimurium wild type (pMGLB) in the cecal lumen at day 1 p.i. by fluoresecence microscopy. Colocalization of GFP-positive S. Typhimurium (LPS; blue) and UEA-1 stained glycoconjugates (red). Scale bar: 20 μm.
There was a striking enrichment of the S. Typhimurium subpopulations expressing flagella (pFLIC reporter) in regions intensively stained by UEA-1/CGL-2 (Fig. 4B; see also 3, 4). This suggested that intestinal mucins (or other glycoproteins) might represent a source for the nutrient gradients attracting chemotactic S. Typhimurium strains to regions close to the intestinal epithelium.
Effect of galactose-feeding and expression of the mglBAEC operon by mucus-associated S. Typhimurium
Next we analysed the chemotactic cues attracting S. Typhimurium towards the cecal mucosa. Galactose residues were present in high abundance at the cecal mucosa (Fig. 4A) and S. Typhimurium is known to express genes encoding galactose uptake and utilization systems in the cecal lumen (Becker et al., 2006). Thus, a galactose concentration gradient might represent one of many possible chemotactic cues attracting S. Typhimurium towards the intestinal epithelium. Indeed, we found that T1-T2- (pFLIC) failed to accumulate at the epithelium if d-galactose was supplied in excess to the intestinal lumen by repeated gavage (untreated versus mice treated three times with 100 μl 100 mM d-galactose per os; P < 0.001; Fig. 4B). We verified that galactose supplementation had no effect on pfliC expression in vitro (Fig. S6A). In contrast, repeated gavage with l-fucose, which is a growth substrate for S. Typhimurium (J. Guntern, K. Ehrbar and W.-D. Hardt, unpubl. results) but does not elicit chemotactic responses by various bacteria including S. Typhimurium (Koshland, 1979; Adler, 1969; our own observations) did not yield this effect (untreated versus l-fucose P > 0.5; Fig. 4B). This suggested that galactose may belong to the cues forming the chemotactic gradient attracting S. Typhimurium towards the mucosal surface.
To verify galactose utilization by the mucus-associated bacteria we used the reporter plasmid pMGLB. This plasmid expresses GFP via the promoter of the mglBAEC operon which encodes a high-affinity ABC transporter for galactose uptake (Muller et al., 1985). We verified that gfp expression of pMGLB is induced in the presence of d-galactose in vitro (Fig. S6B). In general, infection with wild-type S. Typhimurium (pMGLB; inflammation; red symbols) yielded much higher levels of reporter expression than infection with T1-T2- (pMGLB; no inflammation; Fig. 4C and D; blue symbols). This correlated with the increased mucin levels in the inflamed intestine (UEA-1 and CGL-2 stain; Fig. 4A). Moreover, mucus-associated bacteria showed particularly high levels of mglB promoter activity (Fig. 4E). These data indicated that S. Typhimurium can sense d-galactose gradients emanating from the cecal mucosa and benefit from this local nutrient source via chemotaxis. The inflamed mucosa secretes particularly high levels of mucus glycoproteins. Thus, efficient access to this localized source of high-energy nutrients can explain the fitness benefit of flagellated and chemotactic S. Typhimurium strains in the inflamed murine intestine.
Discussion
We used an in vivo infection model to study how flagella enhance S. Typhimurium colonization of the host's intestine. This work revealed two fundamental principles explaining why flagella can improve pathogen fitness during an acute mucosal infection: (i) mucosal inflammation encompasses elevated, localized nutrient availability, particularly near the infected mucosa, and (ii) motility allows efficient access to these localized high-energy nutrients. This explains the increased growth rates of flagellated S. Typhimurium strains in the inflamed intestine.
Nutrients in the inflamed intestine
We identified localized nutrient availability in the inflamed intestine as an important factor in enteric S. Typhimurium infection. How is this linked to inflammation? Our data indicate that inflammation has two different fundamental effects on the pathogen. First of all, inflammation serves to limit infection. This effect is well established and several mouse lines with genetic defects in inflammatory response pathways are hyper-susceptible to systemic spread of the pathogen (Vazquez-Torres et al., 2000; Eckmann and Kagnoff, 2001; Weiss et al., 2004; Hapfelmeier et al., 2005). A second effect has been identified, recently: Inflammation provides S. Typhimurium with a means to colonize the host's intestine by successfully competing with the indigenous microbiota (Stecher et al., 2007). Here, we found that the enhanced fitness of S. Typhimurium in the inflamed intestine is attributable at least in part to the local release of high-energy nutrients by the mucosa as a result of the inflammatory response. The latter explains elevated growth rates of S. Typhimurium (inflamed versus normal intestine) and the increased fitness of flagellated, chemotactic bacteria. Glycoconjugates seem to play a key role in this as indicated by lectin-staining and upregulation of galactose utilization operons by the pathogen.
Serum leakage into the intestinal lumen, debris from transmigrating PMN, remains of shed epithelial cells and in particular the highly glycosylated proteins (mucins) forming the mucous layer may contribute to this energy-rich glyco-conjugate pool. Mucus is stored in vesicles of the goblet cells and secreted into the intestinal epithelium. Epithelial damage, as observed in S. Typhimurium colitis (Barthel et al., 2003), is known to trigger mucus secretion (Miyake et al., 2006). Normally, this response serves tissue protection and repair. But at the same time the mucins represent a local source of high-energy nutrients. Mucins are highly modified by O-linked oligosaccharides harbouring approimately 50% N-actetyl glucosamine, sialic acid, fucose and galactose residues. Our data show that the localized nature of these nutrients yields a selective growth advantage for virulent, motile S. Typhimurium strains. Non-motile S. Typhimurium strains are attenuated and cannot benefit to the same extent. Thus, the acute inflammatory response of the host's intestinal mucosa does not simply limit the infection. At the same time it provides a rich source of nutrients and generates a selection pressure favouring highly virulent, motile bacteria.
Chemotaxis versus enhanced growth at the mucosal surface
The accumulation of wild-type S. Typhimurium at the mucosal surface might be explained by two different mechanisms. One may argue that directed and non-directed motility equally allow access to this nutrient-rich environment. There, bacteria may grow faster than in the nutrient-poor gut lumen. Thus, the pFliC-expressing bacteria may grow and accumulate right at the mucosal surface (enhanced growth on site; Fig. 3A). However, two observations seem to argue against this ‘growth only’ explanation. First of all, cheY strains can get close to the mucosal surface ‘by chance’, but they fail to accumulate there (Fig. 3A). Second, the feeding experiment with l-fucose (energy source, but no chemoattractant; Fig. 4B) created high nutrient levels throughout the gut lumen. According to the ‘growth only’ hypothesis, this should have alleviated the accumulation of pFliC-GFP+ bacteria at the mucosal surface. However, it did not. We concluded that chemotaxis was necessary and that the ‘growth only’ hypothesis was insufficient for explaining our results. However, we could not exclude that enhanced growth at the mucosal surface also contributes to some extent.
Sequence of events: normal → inflamed intestine: a vicious circle
Upon oral infection, the disease develops in several consecutive steps (Fig. 5). Initially, S. Typhimurium faces a non-inflamed intestine. At this stage, high-energy nutrients, i.e. galactose, are scarce in the cecal lumen and only a very limited reservoir is available within the thin mucus layer covering the epithelium. Even though chemotaxis allows S. Typhimurium to access this source of nutrients, this does not seem to enhance growth rates significantly if the pathogen fails to trigger inflammation (Stecher et al., 2007). Presumably, the total amount of high-energy nutrients available at the normal mucosal surface is simply too small. Mutants incapable of triggering intestinal inflammation get ‘stuck’ at this stage of the infection. In contrast, wild-type bacteria initiate a second phase of the acute infection by engaging the mucosa itself which triggers acute colitis. The resulting host defence includes massive mucus secretion. Enhanced mucus secretion fosters a robust infection via the following positive feedback loop: increased mucus production provides more high-energy nutrients; these nutrients are accessed via chemotaxis and allow further growth of the pathogen; high densities of virulent S. Typhimurium reaching the mucosal surface trigger sustained intestinal inflammation; this keeps up massive mucus secretion. This positive feedback loop allows massive intestinal colonization by S. Typhimurium (Barthel et al., 2003). Data obtained from bovine infection models (Bispham et al., 2001; Coombes et al., 2005) suggest that this may also apply to enteric Salmonella infections in other animal species and in humans. In normal, healthy humans, S. Typhimurium infection generally causes self-limiting enterocolitis (equivalent to stage 2; Fig. 5) and immune responses clear the infection within less than a week.
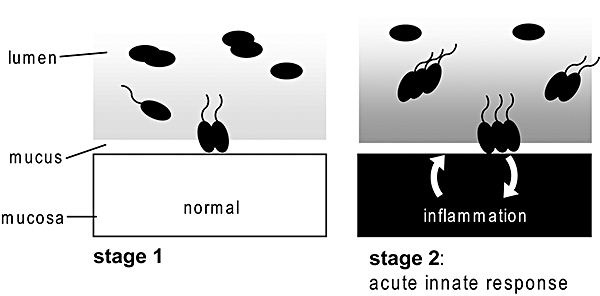
‘Positive feedback model’ explaining the function of flagella during S. Typhimurium colits. Stage 1. Virulent S. Typhimurium reaches the mucosal surface of the murine large intestine by chemotactic movement along sugar gradients. At this stage, glycoconjugates are confined to a thin layer lining the epithelial border. Stage 2. The resulting host defence induced via Salmonella virulence factors (i.e. TTSS-1 and TTSS-2) includes massive mucus secretion. Enhanced mucus secretion fosters a robust infection via the following positive feedback loop: increased mucus production provides more high-energy nutrients; these nutrients are accessed via chemotaxis and allow further growth of the pathogen; high densities of virulent S. Typhimurium reaching the mucosal surface trigger sustained intestinal inflammation; this keeps up massive mucus secretion.
Flagellin and innate immunity
Selection pressures for/against flagellar expression differ significantly between various niches within the same infected host. Within host tissues, the receptors of the innate immune system recognize pathogen associated ‘molecular patterns’ (PAMPs). PAMPs are evolutionarily conserved structures important for microbial fitness but are absent in the host. Flagellin, the structural building block of the flagella, is recognized by a specific Toll-like receptor (TLR5; Smith et al., 2003; Andersen-Nissen et al., 2005) and possibly also by additional intracellular pattern recognition molecules (Nod/Naip/Nalp; Delbridge and O'Riordan, 2006). In principle, the flagellin-triggered defences should select against flagellated strains within host tissues. Indeed, S. Typhimurium can replicate more efficiently within the internal organs of MyD88−/− animals which have a severely reduced innate immune response to most TLR ligands (Weiss et al., 2004; Hapfelmeier et al., 2005; Takeda and Akira, 2005; Fig. S2B and E; B. Stecher and W.-D. Hardt, unpubl. obs.). However, flagellin is poorly expressed at systemic sites (Cummings et al., 2006) and other PAMPs (i.e. LPS) are more potent elicitors of systemic innate immune defences than flagellin. This is corroborated by the equivalent competitive fitness of M583 (no flagella at all; Fig S2D) and M944 (no chemotaxis; normal flagellin expression in vitro (Fig. S2A; Stecher et al., 2004); detectable flagellin expression in 10% of the gut luminal bacteria) at systemic sites of MyD88−/− and C57BL/6 wild-type mice. Similarly, recent studies on TLR5−/− and TLR4−/−TLR5−/− knockout mice revealed that the LPS–TLR4 interaction dominates the innate defences against Gram-negative pathogens while flagellin-binding to TLR5 contributes little (Feuillet et al., 2006). Nonetheless, flagella-specific defences can reduce pathogen fitness within host tissues and should lead to the accumulation of mutations within flagellar genes in the long term. On the other hand side, the localized nutrient availability within the inflamed intestine enhanced the fitness of virulent, chemotactic S. Typhimurium strains. Thus, the conditions in the inflamed gut represent a rectifying selective pressure eliminating functional mutations in the flagellar apparatus and the chemotaxis signalling systems. This is in line with the observation that the vast majority of human S. Typhimurium isolates from enteric and systemic infections express flagella (Table S1). In conclusion, the fitness benefits from ‘motility functions’ of flagella, namely access to nutrients and the epithelial surface, seem to have a greater impact in the acute S. Typhimurium infection cycle, than possible fitness costs attributable to flagella-specific innate immune defences.
Conclusions
In this work we identified a key role of motility and nutrient access in enteric salmonellosis. Conceptually, ‘stimulation’ of nutrient release represents one important mechanism explaining how pathogens can use virulence factors to sabotage defensive responses of the host for their own benefit. Other mechanisms may also contribute and represent an important subject for future research.
The eminent role of motility might offer a basis for novel anti-infective strategies: Chemotaxis inhibitors or non-metabolizable nutrient analogues binding to chemotaxis receptors may allow preventing the onset of inflammation or breaking the feed-forward loop fuelling S. Typhimurium growth during the acute infection.
Experimental procedures
Bacterial strains and plasmids
Bacterial strains and plasmids were generated using standard techniques and are listed in Tables 1 and 2. M963, the ΔcheY in-frame mutant, was generated using the Red-recombinase technique (Datsenko and Wanner, 2000). Briefly, PCR primers were designed bearing homology to the 5′ and 3′ boarders of the cheY coding region and the chloramphenicol-resistant cassette on pKD3 (cheY-rev-ko: 5′-gcct tcatcagcag gcttgataga tggttgcatc atcatcgcatcc tgt gta ggc tgg agc tgc ttc-3′ and cheY-fwd-ko: 5′-cagtg ccggacaggc gatacgtatt tgaaccagga gtagtatttt ata tga ata tcc tcc tta gtt-3′). PCR products were transformed into S. Typhimurium SL1344 wildtype (SB300) and transformants were selected with chloramphenicol (M957). Insertions into the cheY coding region were confirmed by PCR using external primers. The mutation was transducted via P22 transduction into the invG sseD::aphT strain background yielding M958. The chloramphenicol resistance cassette was removed using via flp recombinase on pCP20 yielding strains M962 (cheY) and M963 (cheY invG sseD::aphT). Correct excision of the gene was confirmed by PCR. The strains were further marked by insertion of a tetracycline resistance [BCB4 (Hensel et al., 1999)] by P22 transduction yielding strains M964 and M965 respectively (see Table 1). pM1419, a variant of the temperature-sensitive plasmid pHSG422 (Benjamin et al., 1990) was constructed by removal of the chloramphenicol (cat) and kanamycin (aphT)-resistant cassettes by digestion, blunting of the site and relegation.
| Plasmids | Relevant genotype | Resistance | Reference |
|---|---|---|---|
| pLB02 | oriR6K; carrying promoterless gene for firefly luciferase and β-galactosidase coding region | AmpR | Gunn and Miller (1996) |
| pM1491 | flhC promoter region in pLB02 | AmpR | This study |
| pM968 | promoterless gfpmut2 in promoterless pBAD24 vector | AmpR | |
| pFLIC | fliC promoter region in pM968 | AmpR | This study |
| pMGLB | mglB promoter region in pM968 | AmpR | This study |
| pHSG422 | Temperature sensitive vector for measuring S. Typhimurium replication rate (pSC101 ori). Plasmid is defective for replication at mouse body temperature | AmpR KmR CmR | Benjamin et al. (1990) |
| pM979 | rpsM promoter region in pM968 | AmpR | Stecher et al. (2004) |
| pM1419 | Variant of pHSG422 lacking KmR CmR resistances | AmpR | This study |
Reporter plasmids pMGLB and pFLIC were generated in the context of a promoter trap library in pM968 (carrying promoterless gfpmut2 gene; B. Stecher and W.-D. Hardt, unpubl.). The regions (> 300 bp) upstream of gfpmut2 were sequenced using an internal gfpmut2 sequencing primer and assigned to promoter regions of mglBACD and fliC operons according to the S. Typhimurium LT2 genome sequence (Accession No. NC_003197). Specifically, upstream of the gfpmut2, pFLIC contained the sequence corresponding to position 2,049146–2,049433 in the LT2 genome (fliC coding sequence: complement 2,047659–2,049145), pMGLB contained the sequence corresponding to position 2,287415–2,287872 (mglB coding sequence: complement 2,286618–2,287616). We determined that the stability of all vectors derived from pM968, including pMGLB used in this study is over 95% at day 1 p.i.
Recovery of S. Typhimurium from cecal contents for flow cytometry
Mice were dissected cecal contents (100–200 mg per mouse) were suspended in ice-cold PBS and kept at 4°C throughout the following procedure. Contents were filtered through sterile 40 μm cell sieves (Milian) and loaded onto a discontinuous percoll/PBS gradient (100–80–60–40% percoll; centrifugation at 15 000 g, 4°C for 30 min). The fraction containing S. Typhimurium (border between 80% and 100% percoll) was recovered. Bacteria were washed with PBS and an appropriate dilution was used for FACS analysis. For specific FACS of S. Typhimurium, samples were stained with Fab fragments prepared from α-Salmonella O antigen group B serum (factors 1, 4, 5 and 12; Difco) by papain digest (Sambrook et al., 1989). Allophycocyanin (APC)-labelled-goat-α-rabbit-Fab Fab fragments (Invitrogen) were used as secondary antibodies.
Animals
Specified pathogen-free (SPF) C57BL/6 mice (6–10 weeks old) were from Harlan (Horst, Netherlands) or Janvier (Le Genest Saint Isle). MyD88−/− mice (Adachi et al., 1998) (6–10 weeks old) on C57BL/6 background were maintained at the BZL Zurich under barrier conditions.
Infection experiments in the streptomycin-pretreated mouse model were approved by the Swiss authorities and carried out as described previously (Hapfelmeier et al., 2005). Briefly, mice were pretreated by gavage with 20 mg of streptomycin. Twenty-four hours later the mice were intragastrically inoculated with single strains or strain mixtures, as indicated. In co-infection experiments, the strain ration of the inoculum was determined by plating. Infections were performed for 18 h (day 1 p.i.), 42 h (day 2 p.i.), 66 h (day 3 p.i.) or 90 h (day 4 p.i.) if not stated otherwise.
Analysis of S. Typhimurium loads in cecal content, mesenteric lymph nodes, spleen and liver
Mesenteric lymph nodes, spleen and liver were removed aseptically and homogenized in cold PBS (0.5% tergitol, 0.5% BSA). Cecum content was suspended in 500 μl cold PBS. The bacterial loads were determined by plating on MacConkey agar plates (50 μg ml−1 streptomycin) as described recently (Hapfelmeier et al., 2005). Total colonization levels of mutant (carrying an appropriate antibiotic marker) and wild-type bacteria were determined by plating (50 μg ml−1 streptomycin) and the mutant/wild-type ratio was defined by replica-plating on media containing tetracycline (12.5 μg ml−1). Competitive indices were calculated according to the formula CI = ratiooutput/ratioinput.
Determination of the replicative index
pHSG422 and pM1419 were used to compare replication rates between two different S. Typhimurium strains. Streptomycin-pretreated mice were co-infected i.g. with 1:1 mixtures (5 × 107 cfu in total) of T1-T2-ampR (M984; ampR; kanR; LacZ+) and T1-T2-Fla- (M933; tetR; kanR). Both strains carried the cmR temperature-sensitive plasmid pHSG422 at the time of infection (> 99%). If indicated, colitis was induced ‘in trans’ by co-infecting with 15 × 107 cfu wild type (SB300; kanS.). Loss of pHSG422 at 24–26 h after infection was determined by plating on MacConkey agar harbouring four different combinations of antibiotics: (i) 50 μg ml−1 kanamycin + 100 μg ml−1 ampicillin (all M984), (ii) 50 μg ml−1 kanamycin + 125 μg ml−1 tetracycline (all M933), (iii) 50 μg ml−1 kanamycin + 100 μg ml−1 ampicillin + 50 μg ml−1 chloramphenicol (M984 harbouring pHSG422), and (iv) 50 μg ml−1 kanamycin + 125 μg ml−1 tetracycline + 50 μg ml−1 chloramphenicol (M933 harbouring pHSG422). Furthermore, T1-T2-ampR (lacZ+) was distinguished from T1-T2-Fla- (lacZ-) via the bright red colour of the lacZ+ colonies. The presence of the lacZ gene did not alter bacterial growth rate in the intestine during inflammation as tested via competitive infection of T1-T2-ampR (ampR; kanR; LacZ+) with a tetR variant of T1-T2- (M951; tetR; kanR; data not shown). The replicative index is defined as the ratio of #[pHSG422+M933]/[#total M933] and #[pHSG422+M984]/#total [M984]. When pM1419 was used, loss of pM1419 at 24–26 h after infection was determined by plating on MacConkey agar harbouring four different combinations of antibiotics: (i) 50 μg ml−1 kanamycin + 30 μg ml−1 chloramphenicol (all M318), (ii) 50 μg ml−1 kanamycin + 125 μg ml−1 tetracycline (all M965), (iii) 50 μg ml−1 kanamycin + 30 μg ml−1 chloramphenicol (all M318 haboring pM1419), and (iv) 50 μg ml−1 kanamycin + 125 μg ml−1 tetracycline (all M965 harbouring pM1419). The replicative index is defined as the ratio of #[pM1419+M965]/[#total M965] and #[pM1419+M318]/#total [M318].
Histopathological evaluation
If not otherwise stated, tissues were embedded in OCT (Sakura, Torrance, CA) and snap-frozen in liquid nitrogen. Cryosections (5 μm; cross-sectional) were stained with haematoxylin and eosin (HE). Cecum pathology was evaluated by a pathologist in a blinded manner using a histopathological scoring scheme as previously described (Stecher et al., 2004; Hapfelmeier et al., 2005).
Immunofluorescence microscopy
For detecting GFP expression (pFLIC or pMGLB) in situ, cecal tissues were recovered and treated as described recently (Stecher et al., 2004). Briefly, the tissues were fixed in paraformaldehyde (4% in PBS, pH 7.4 overnight, 4°C), washed with PBS, equilibrated in PBS (20% sucrose, 0.1% NaN3 overnight, 4°C), embedded in OCT (Sakura, Torrance, CA), snap-frozen in liquid nitrogen and stored at −80°C. Cryosections (7 μm) were air-dried for 2 h at room temperature, fixed in 4% paraformaldehyde (5 min), washed and blocked in 10% (w/v) normal goat serum in PBS for 1 h. The sections were immunostained for 1 h with a polyclonal rabbit α-Salmonella O antigen group B serum [factors 1, 4, 5 and 12, Difco; 1:500 in PBS, 10% (w/v) goat serum].
Non-O-glycosylated Muc2-precursors were detected with a polyclonal rabbit α-Muc2 gpda PH497 (1:100; kind gift of Professor Gunnar Hansson). We used FITC-, Cy5- or Cy3-conjugated secondary goat α-rabbit antibodies [Milan; 1:300 in PBS, 10% (w/v) goat serum] as indicated. DNA was stained with DAPI (4′6′-diamidino-2-phenylindole, 0.5 μg ml−1; Sigma). F-Actin was visualized by staining with Alexa-647-conjugated phalloidin, as indicated (Molecular Probes). Glyco-conjugates were stained with the labelled lectins UEA-1-rhodamine (1:100; Reactolab) or CGL-2-fluoresceine (1:100; Walser et al., 2005). Sections were mounted with Vectashield hard set (Vector laboratories) and sealed with nail polish.
The spatial distribution of GFP+ and GFP- bacteria in the cecal lumen was determined by imaging series of adjacent optical fields (200 × 200 μm) using a Perkin Elmer Ultraview confocal imaging system and a Zeiss Axiovert 200 microscope. Red, green and cyan fluorescence was recorded confocally while DAPI fluorescence was imaged by epifluorescence microscopy. Images were assembled using Adobe Photoshop version 7.0.1. The fraction of GFP-expressing bacteria was determined by enumerating the total number of Salmonella (red α-LPS stain) and the number of GFP+Salmonella (GFP fluorescence and red α-LPS stain) in each optical field counting at least 150 originating from three different areas of equivalent distance to the epithelium. The fraction of bacteria expressing the reporter was calculated as: GFP+red+[%] = #GFP+red+/(#red+) × 100.
For measuring fluorescence intensity of GFP expressing S. Typhimurium, image stacks were acquired using a confocal system (Zeiss/Perkin Elmer) and Plan Neofluar ×63 oil objective. Fluorescence intensity was measured and corrected for background fluorescence using Ultraview imaging software (Perkin Elmer).
Time lapse microscopy
For time lapse microscopy, infected mice were killed and cecal contents were quickly removed and re-suspended in 5 volumes (100 μg = 100 μl) HBSS containing 10% BSA and 30 ug ml−1 chloramphenicol. Contents were mounted onto a heated specimen holder (37°C) on a Zeiss Axiovert 200 m inverted microscope. Time series of GFP-fluorescence signals were recorded with an Ultraview confocal head (PerkinElmer), a krypton argon laser (643-RYB-A01, Melles Griot, Didam, Netherlands) and a Plan Neofluar 20× objective (Zeiss, Jena, Germany) at 6 frames/s. Motile GFP-expressing bacteria in the run phase were manually tracked using Volocity 4 software (Improvision, UK).
Detection of Salmonella O- and H-antigens
Isolated S. Typhimurium strains were subject to purity control by growth on appropriate Salmonella-selective media (Endo agar, Oxoid). Morphological examination was followed by serological identification of S. Typhimurium specific O-antigen (4, [5]). Rough forms were excluded by the trypaflavine test (Pampana, 1933). H-antigenic phase was examined by agglutination using H1 and H1,2 antiserum (SIFIN). To detect the second H phase, phase inversion was selected by growth on Sven Gard agar swarm plates (20% proteose peptone, 1% glucose, 3% yeast extract, 3% meat extract, 8% agar, 5 g l−1 NaCl, 2 g l−1 Na2HPO4 × 2 H2O, pH 7.3–7.4) containing respective H-antiserum (corresponding to the phase already determined, i.e. Hi or H1,2). S. Typhimurium was allowed to swarm at 37°C. After formation of a swarming zone, material was used from the outer region of the zone and subject to agglutination using the second H-specific antiserum.
Statistical analysis
Statistical analysis was performed using the exact Mann–Whitney U-test and the SPSS Version 14.0 software, as described before (Barthel et al., 2003). Values of P < 0.05 were considered as statistically significant. Box plots were created using GraphPad Prism 4 version 4.03.
Acknowledgements
The authors are grateful to Mathias Heikenwälder and Benjamin Misselwitz for discussion, Kristin Ehrbar for construction of S. Typhimurium M583, Gunnar Hansson for the gift of α-Muc2 antiserum, Markus Künzler for CGL-2-fluoresceine and Siegfried Hapfelmeier and Emma Slack for critically reading the manuscript. We are grateful to Jörg Fehr, Susanne Freedrich, Thomas Weber and the other members of the RCHCI team for excellent support of the animal experiments. B.S. and W.D.H. designed research, B.S., M.B., M.S. and L.H. performed research, M.K. and W.R. analysed the data, B.S. and W.D.H. wrote the paper. This work was supported by grants to WDH from the Swiss National Science Foundation (No. 3100A0-100175/1 and 310000-113632/1).