Subtilase cytotoxin, produced by Shiga-toxigenic Escherichia coli, transiently inhibits protein synthesis of Vero cells via degradation of BiP and induces cell cycle arrest at G1 by downregulation of cyclin D1
Summary
Subtilase cytotoxin (SubAB) is a AB5 type toxin produced by Shiga-toxigenic Escherichia coli, which exhibits cytotoxicity to Vero cells. SubAB B subunit binds to toxin receptors on the cell surface, whereas the A subunit is a subtilase-like serine protease that specifically cleaves chaperone BiP/Grp78. As noted previously, SubAB caused inhibition of protein synthesis. We now show that the inhibition of protein synthesis was transient and occurred as a result of ER stress induced by cleavage of BiP; it was closely associated with phosphorylation of double-stranded RNA-activated protein kinase-like ER kinase (PERK) and eukaryotic initiation factor-2α (eIF2α). The phosphorylation of PERK and eIF2α was maximal at 30–60 min and then returned to the control level. Protein synthesis after treatment of cells with SubAB was suppressed for 2 h and recovered, followed by induction of stress-inducible C/EBP-homologous protein (CHOP). BiP degradation continued, however, even after protein synthesis recovered. SubAB-treated cells showed cell cycle arrest in G1 phase, which may result from cyclin D1 downregulation caused by both SubAB-induced translational inhibition and continuous prolonged proteasomal degradation.
Introduction
Shiga toxins 1 and 2 are both produced by Shiga-toxigenic Escherichia coli (STEC); infections with these bacteria in children may result in toxin-induced diarrhoea and haemolytic uremic syndrome (Paton and Paton, 1998; Tarr et al., 2005). Shiga toxins are members of the AB family of toxins; B oligomer consists of five identical B subunits and binds to glycolipid Gb3 on the cell surface membrane (Lingwood et al., 1987; Waddell et al., 1988); the A subunit has enzymatic activity and blocks protein synthesis by removing adenine from 28S rRNA, thereby inhibiting the binding of amino-acyl-tRNA (Endo et al., 1987). Recently, Paton et al. (2004) identified a novel subtilase cytotoxin (SubAB) from STEC. The toxin also belongs to the AB family; SubAB at ng ml−1 caused Vero cell damage at 48 h. As with other AB family toxins, the B subunit (SubB) binds to the surface receptor and the A subunit (SubA) enzymatic activity is necessary for cytotoxicity. In addition, SubAB induced vacuolation in Vero cells after an 8 h incubation at concentrations greater than 1 μg ml−1. In contrast to the cytotoxicity induced by ng ml−1 amounts of toxin, vacuolation was dependent on SubB alone; SubA was not necessary (Morinaga et al., 2007). SubA was thought to be a subtilase-like serine protease with a catalytic triad, characteristic of members of the subtilase family (Paton et al., 2004). It did not, however, cleave azocasein or several other synthetic polypeptides and was believed to have a limited range of substrate specificity (Morinaga et al., 2007). Very recently, Paton et al. (2006) showed that SubA cleaved BiP/Grp78, a chaperone resident in the endoplasmic reticulum (ER).
BiP is a master regulator of ER function. It is responsible for maintaining the permeability barrier of the ER during protein translocation. It directs protein folding and assembly, targeting misfolded proteins for retrograde translocation, so that they can be degraded by the proteasome. By sensing conditions of stress in this organelle, BiP activates the mammalian unfolded protein response (UPR) (Hendershot, 2004; Rao et al., 2004). Mutant BiP with replacement of leucine 416 with aspartic acid, BiPD416, was not cleaved by SubAB. In cells expressing BiPD416, BiP cleavage by SubAB was suppressed and cells challenged with SubAB survived (Paton et al., 2006). These data are consistent with the conclusion that BiP cleavage triggers cell damage.
Previously, we showed that SubAB inhibited protein synthesis of Vero cells, following a ∼30 min incubation, which is associated with inhibition of cell growth, and 2 days later, cell death (Morinaga et al., 2007). In the current paper, we have focused on the mechanism by which SubAB induces inhibition of protein synthesis and cell growth arrest. We show that inhibition of protein synthesis by SubAB is transient, triggered by BiP degradation and resulting from phosphorylation of eIF2α. Phosphorylation did not occur with a mutant SubAB(S272A), lacking protease activity. SubAB led to cell cycle arrest in G1 phase consistent with inhibition of DNA synthesis, which may be the result of cyclin D1 downregulation, caused by both inhibition of cyclin D1 synthesis and cyclin D1 degradation by proteasomes.
Results
Protein synthesis inhibition by SubAB was suppressed by BFA
As previously reported, protein synthesis by Vero cells was completely inhibited at ∼30 min after incubation of cells with SubAB (8). BiP cleavage was also found at ∼30 min in SubAB-treated cells. First, we examined the association of BiP cleavage and protein synthesis inhibition. Brefeldin A (BFA) is known to disrupt Golgi, therefore we investigated whether BFA treatment of cells suppressed BiP cleavage. BFA treatment for 20 min dispersed a Golgi marker, GM130, from the perinuclear region and suppressed BiP cleavage in a dose- and time-dependent manner (Fig. 1A). We next examined whether inhibition of protein synthesis by SubAB occurred in BFA-treated cells (Fig. 1B). BFA itself suppressed protein synthesis of Vero cells ∼35%, and it completely abrogated inhibition of protein synthesis by SubAB. These data suggested that SubAB internalized into cells by endocytosis and trafficked via Golgi to ER and cleaved BiP localized in lumen of ER, which may cause inhibition of protein synthesis.
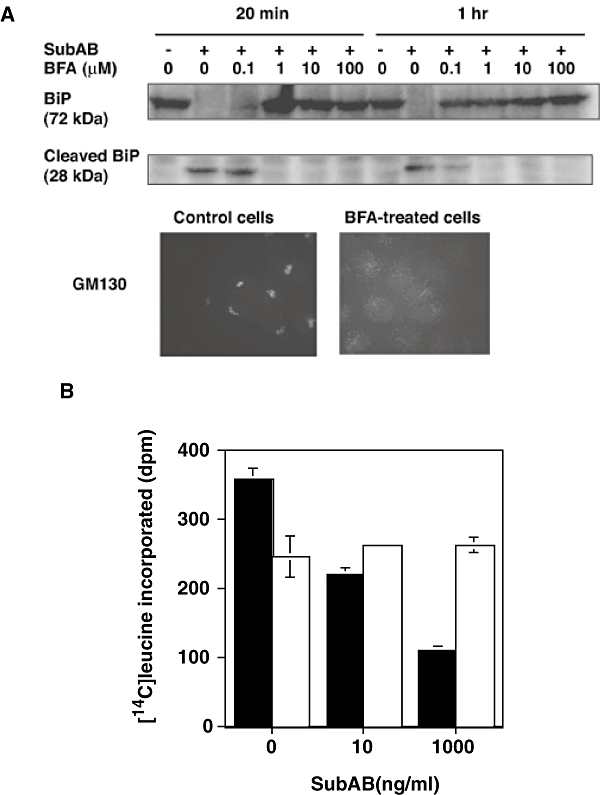
Effect of BFA on SubAB activities.A. Effect of BFA on SubAB-induced cleavage of BiP. Cells were treated with various concentrations of BFA for 20 min or 1 h followed by addition of SubAB (1 μg ml−1) and incubation at 37°C for 30 min. Upper panel shows a blot representative of three experiments and lower panel immunostaining of GM130 in control and 20 μM BFA-treated cells (20 min).B. Effect of BFA on inhibition of protein synthesis by SubAB. Cells were treated with 20 μM BFA for 20 min before addition of SubAB at indicated concentrations plus [14C]-leucine and incubation for an additional 1 h. Reaction was then terminated by addition of TCA and incorporated isotope was quantified as described in Experimental procedures. Closed bars, control cells; open bars, BFA-treated cells. Data are means ± SD of three samples. Experiment was repeated twice with similar results.
SubAB induced ER stress by cleavage of BiP
BiP cleavage is thought to induce UPR based on C/EBP-homologous protein (CHOP) induction (Paton et al., 2006). ER stress is first recognized by three transmembrane stress sensors, Ire1, ATF6 and PERK (Cox et al., 1993; Shi et al., 1998; Harding et al., 1999; Koumenis et al., 2002). When PERK is activated by phosphorylation, it then phosphorylates eIF2α and UPR-mediated repression of translation occurs (Kaufman et al., 2002; Hendershot, 2004; Rao et al., 2004;). We therefore investigated whether SubAB-induced inhibition of protein synthesis results from phosphrylation of eIF2α. Phosphorylation of PERK was detected during a 30–60 min incubation with SubAB and was decreased at 120 min (Fig. 2A). eIF2α phosphorylation was also detected, which was maximal at 30–60 min and gradually decreased to control levels at 180 min (Fig. 2B). SubAB(S272A) failed to increase phosphorylation of either PERK or eIF2α. If protein synthesis inhibition by SubAB was a result, in part, of phosphorylation of eIF2α, protein synthesis is predicted to recover after a 3 h incubation. To investigate this hypothesis, protein synthesis at various time points after incubation with SubAB was quantified using a 20 min-pulse label with [14C]-leucine. As shown in Fig. 2C, protein synthesis was suppressed at 1 and 2 h after SubAB treatment, and after 3 h, it recovered gradually. Accumulation of unfolded proteins in the ER triggers increased expression of genes encoding ER chaperones like BiP (Lee, 1987; Kozutsumi et al., 1988; Tirasophon et al., 1998). Even after protein synthesis returned to near control levels, intact BiP was not detected. Even 6 h after incubation with SubAB, only a cleaved form of BiP was observed (Fig. 3A), suggesting that intracellular SubAB was still active. Expression of an ER stress marker, CHOP (Lee, 1992; Wang et al., 1996), was elevated after a 120 min incubation with SubAB but not with SubAB(S272A), and continued for 6 h, indicating that ER stress induced by SubAB was observed even after protein synthesis was restored (Fig. 3B).
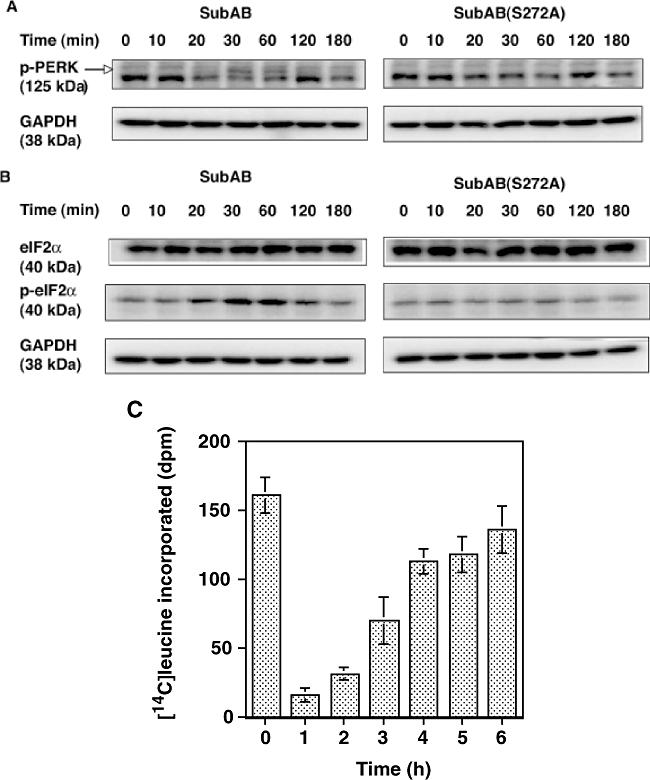
SubAB-induced phosphorylation of PERK and eIF2α and suppressed protein synthesis transiently. Vero cells were incubated with 100 ng ml−1 of SubAB for indicated times. The cells were lysed in SDS sample buffer and lysates were subjected to SDS-PAGE followed by Western blotting.A and B. Membranes were probed with antiphospho-PERK (A) and eIF2α or phospho-eIF2α (B) antibodies respectively. The results are representative of three experiments.C. Protein synthesis by SubAB-treated cells. Cells (2 × 105 well−1) were treated with SubAB (100 ng ml−1, 0.5 ml) for indicated times, followed by addition of [14C]-leucine and incubation for an additional 20 min before addition of 0.25 ml of 30% TCA to stop the reaction. [14C]-leucine incorporation by the cells was quantified as described in Experimental procedures. Data are means ± SD of three samples.

BiP cleavage (A) and CHOP induction (B) by SubAB. Cells were incubated with 100 ng ml−1 of SubAB or SubAB(S272A) and the reaction was terminated at the indicated times by the addition of SDS sample buffer. Samples were analysed by SDS-PAGE and transferred to PVDF membranes. BiP or CHOP was detected by anti-BiP or anti-CHOP antibody respectively. The results are representative of four experiments.
SubAB-induced growth arrest at G0/G1 phase in Vero cells
We reported that the SubAB-induced growth inhibition in cells (Morinaga et al., 2007) and the above data suggested that ER stress occurred in SubAB-treated cells. The activation of UPR is reported to decrease cyclin D1 expression and induce cell cycle arrest in G1 phase (Brewer and Diehl, 2000). We therefore investigated whether similar signal transduction events were responsible for growth arrest of SubAB-treated cells. FACS analysis of cells treated with SubAB for more than 20 h showed that > 80% were in the G0/G1 phase; in contrast, 67% of untreated control cells were in G0/G1 phase (Fig. 4). This cell cycle arrest was confirmed in HeLa cells, which also showed cell growth arrest by SubAB. Percentage of G0/G1 phase in HeLa cells treated and untreated with SubAB for more than 20 h were 73 and 60 respectively (data not shown). SubAB caused inhibition of BrdU incorporation into DNA of Vero cells in a time-dependent manner (Fig. 5), consistent with cell cycle arrest at G0/G1 phase. Similarly, SubAB treatment of HeLa cells suppressed BrdU incorporation into DNA (data not shown). The mechanism underlying cell cycle arrest was examined. SubAB treatment was accompanied by significant downregulation of cyclin D1 and a slight decrease of cyclin D3 (Fig. 6A) were observed. Downregulation of cyclin D1 by SubAB was dose dependent and was not induced by SubAB(S272A) (Fig. 6B). Expression of cyclin-dependent kinases, cdk4 or cdk6, was not altered by SubAB treatment. The activities of cdk4 or cdk6 are known to be regulated by the kinase inhibitors, p21warf/cip1 and p27kip1 (Morgan, 1995; Sherr and Roberts, 1995); the level of p21warf/cip1 was decreased and that of p27kip1 was slightly decreased (Fig. 6C). These data suggest that cell cycle arrest may not be caused by a decrease of cdk kinase activities. mRNA of cyclin D1 was assessed by real-time polymerase chain reaction (PCR) compared with that of GAPDH. The ratio of mRNA of cyclin D1 to that of GAPDH of cells without or with SubAB for 3 h was 2.99 and 3.3 respectively. Rapid decrease of cyclin D1 may be a result of SubAB-induced protein translation inhibition by phosphorylation of eIF2α. However, decrease in cyclin D1 continued for up to 6 h (Fig. 6D); by that time, protein synthesis had recovered, suggesting that SubAB may exert its effects through regulation of protein stability. Cyclin D1 degradation is regulated by phosphorylation-triggered, ubiquitin-dependent proteolysis in proteasomes (Diehl et al., 1997). We investigated whether phosphorylation of cyclin D1 was increased by incubation with SubAB (Fig. 7). Ratio of densities of phosphorylated-cyclin D1 versus that of cyclin D1 was increased after a 60 min incubation with SubAB and was maximum at 180 min. In contrast, no change was observed by incubation with SubAB(S272A). Further, blockade of cyclin D1 degradation by the proteasome pathway with an inhibitor, MG132, suppressed downregulation of cyclin D1 by SubAB completely, suggesting that proteasome degradation of cyclin D1 was responsible for the decrease in cyclin D1 levels (Fig. 8).
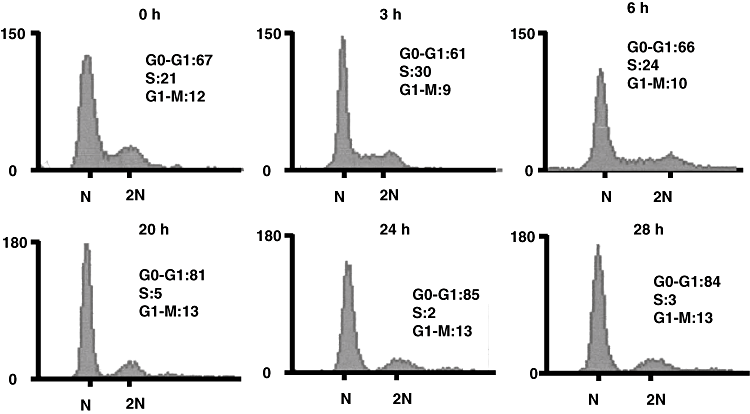
Changes in Vero cell cycle by SubAB. Cells were incubated with 100 ng ml−1 of SubAB for indicated times and then with PI as described in Experimental procedures. Cell cycle was analysed using a FACS flow cytometer. Values were determined using the ModFit LT cell cycle analysis software. The experiment was repeated three times with similar results.
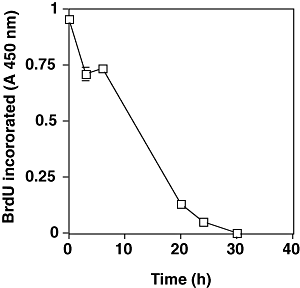
Effect of SubAB on DNA synthesis by incorporation of BrdU. Cells were incubated with SubAB for indicated times and further incubated in the presence of BrdU for 3 h. BrdU incorporated into DNA was assessed by immunoassay using cell proliferation ELISA, BrdU kit (Roche Diagnostic). Data are means ± SD of three samples.
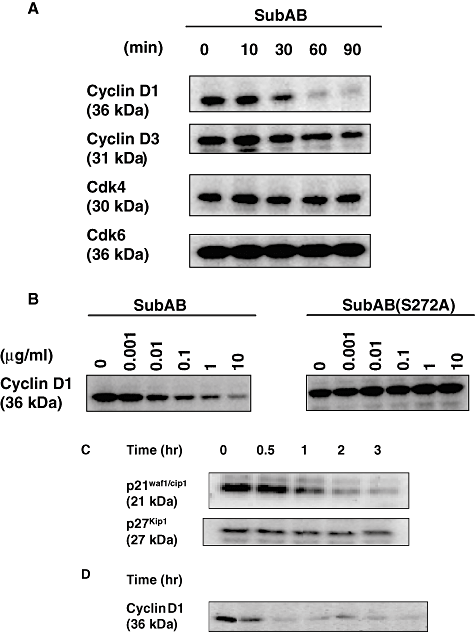
Effect of SubAB on the levels of G1 regulators. Cells (2 × 105 cells well−1) were incubated with EMEM for 30 min, and then with 10 μg ml−1 (A) or 1 μg ml−1 (C and D) SubAB for the indicated times or treated with different amounts of SubAB or SubAB(S272A) for 60 min at 37°C (B). The cells were lysed by addition of SDS sample buffer and subjected to SDS-PAGE followed by Western blotting as described in Experimental procedures. The membranes were probed with anti-cyclin D1, anti-cyclin D3, anti-cdk4, anti-cdk6, anti-p21waf1/cip1, anti-p27kip1, and anti-GAPDH antibodies. The results are representative of at least two experiments.
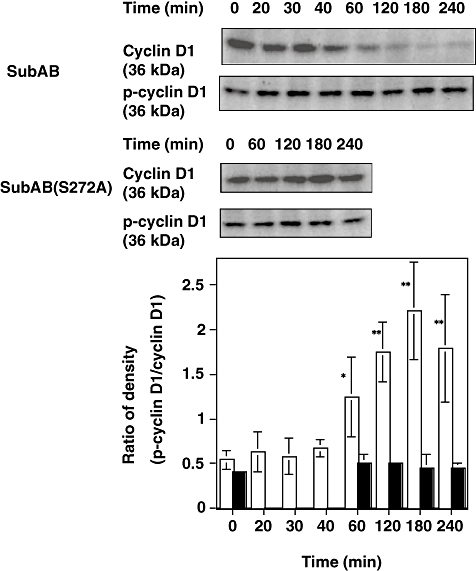
Effect of SubAB on phosphorylation of cyclin D1. Cells (2 × 105 cells well−1) were incubated with 100 ng ml−1 of SubAB or SubAB(S272A) for indicated times. After incubation, SDS sample buffer was added and lysate was subjected to SDS-PAGE, followed by Western blotting as described in Experimental procedures. The membranes were probed with anti-cyclin D1 or antiphospho-cyclin D1 antibodies. Upper panel shows a representative blot of four experiments. Western blots were analysed by densitometer and ratio of density of phospho-cyclin D1 versus cyclin D1 was calculated and is presented in lower panel. Open bars, SubAB; closed bars, SubAB(S272A). Data are means ± SD of four samples. P-values for the comparison of control and SubAB-treated cells are shown. *P < 0.05 and **P < 0.015.
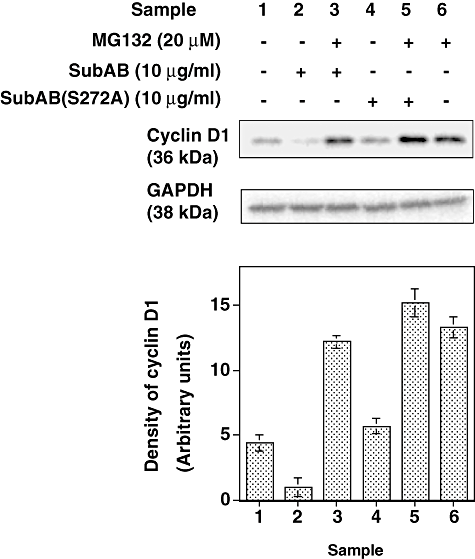
Effect of proteasome inhibitor, MG132, on SubAB-induced downregulation of cyclin D1. After incubation with or without MG132 (20 μM) for 30 min, SubAB or SubAB(S272A) 10 μg ml−1 was added and incubation continued for 60 min at 37°C. The immunoblot analysis was performed using anti-cyclin D1 or anti-GAPDH antibodies. Upper panel shows the representative blot of three experiments and lower, densitometry analysis of the Western blotting data. Data are means ± SD of three samples.
Discussion
In this report, we showed the mechanism by which SubAB induces protein synthesis inhibition and cell cycle arrest after binding to Vero cells. One of the receptors on Vero cell membranes for SubAB is thought to be integrin α2β1, at least for vacuolation (Yahiro et al., 2006). The receptor for cell damage induced by ng ml−1 SubAB, however, has not been identified. After binding and endocytosis, toxin trafficked through Golgi to the ER and cleaved BiP. Previously, Golgi localization of a toxin was not detected, perhaps owing to low efficiency of toxin labelling (Morinaga et al., 2007). BFA treatment of cells suppressed SubAB-induced BiP cleavage in a dose- and time-dependent manner, consistent with toxin trafficking via Golgi to ER. Retrograde trafficking of toxin to Golgi and ER was also required for inhibition of protein synthesis by SubAB, taking into consideration BFA suppression of SubAB inhibition of protein synthesis.
BiP is a major chaperone localized in the ER lumen. Cleavage of BiP is thought to result in loss of activity. As a result, integrity of protein folding in the ER may be lost and misfolded proteins accumulate, leading to activation of a signalling network known as the UPR (Kaufman et al., 2002; Hendershot, 2004). The molecular mechanism of how cleavage of BiP induces UPR is not known. Early signal transduction pathways induced by the UPR involve ER transmembrane kinases Ire1, PERK and transcription factor, ATF6. Ire1 and ATF6 are primarily involved in the regulation of transcription and induction of ER chaperones including BiP (Lee, 1987; Kozutsumi et al., 1988; Tirasophon et al., 1998). In SubAB-treated cells, BiP was cleaved after 30 min incubation with SubAB. Even at 6 h, native BiP was not observed, suggesting that SubAB may still be active and continues to cleave newly synthesized BiP. The other signal, PERK, contributes to the regulation of protein translation by phosphorylation of eIF2α and adaptation to ER stress (Shi et al., 1998; Harding et al., 1999; Koumenis et al., 2002). Tunicamycin disrupts proper protein glycosylation and induces UPR and phosphorylation of eIF2α (Hamanaka et al., 2005). We used tunicamycin as a control of ER stress in Vero cells; we observed that phosphorylation of eIF2α by tunicamycin occurred after 1 h and continued at least 6 h (data not shown) similar to the results of Hamanaka et al. (2005). These results differ from the rapid phosphorylation and dephosphorylation of eIF2α by SubAB shown in this report. These data suggested that the cellular response to BiP cleavage was different from that induced by improper glycosylation of protein. SubAB induced ER stress by the cleavage of BiP, and induced PERK and eIF2α phosphorylation, followed by transient inhibition of translation. Association of cleavage of BiP and protein synthesis inhibition was supported by studies with the toxin mutant SubAB(S272A). eIF2α phosphorylation was transient compared with the ongoing BiP degradation, and may be the result of dephosphorylation induced by specific phosphatases (Novoa et al., 2001). Previously, we hypothesized that SubAB cytotoxicity might be a result of protein synthesis inhibition, similar to that seen with Shiga toxin or diphtheria toxin. However, it is now evident that SubAB-induced inhibition of protein synthesis was transient and triggered by cleavage of BiP by SubAB. Therefore, inhibition of translation may be a cellular response to recovery from stress as induced by other ER stress signals, and does not result in immediate cell death. SubAB also induced CHOP, as reported by Paton et al. (2006), which is an ER stress-induced transcription factor and may be involved in apoptosis (Yamaguchi and Wang, 2004). CHOP expression by SubAB may be regulated by PERK activation, which represses translation of most mRNAs but selectively increases translation of ATF4, resulting in the induction of the downstream gene CHOP (Harding et al., 2000), or CHOP induction may be regulated by another pathway via Ire1 activation (Tirasophon et al., 1998; Wang et al., 1998), taking into consideration that there was a time interval between activation of PERK and CHOP induction.
SubAB-treated cells showed cell cycle arrest in G1 phase, which was accompanied by DNA synthesis inhibition. SubAB-induced cell cycle arrest in G1 phase was confirmed in HeLa cells. Cyclins are essential components of the cell cycle machinery. Each binds and activates specific cdk partner proteins. To progress through G1 phase requires the activities of cyclin D1-dependent cdk4 or cdk6, followed by activation of the cyclin E and A-dependent kinase cdk 2. The Cyclin–cdk complex formed during G1 phase catalyses phosphorylation of the dominant inhibitor of G1/S cell cycle progression, retinoblastoma family of tumour suppressor proteins, thereby blocking their inhibitory activity and allowing the cell to progress into S phase (Sherr and Roberts, 1995; Weinberg, 1995). Cell cycle arrest in G1 phase therefore occurs by cyclin D1 degradation and/or by a decrease in cyclin D1 kinase activities. SubAB-induced cell cycle arrest did not result from loss of cdk4 or cdk6 kinases, or from upregulation of their inhibitors, p21waf1/cip1 or p27kip1, but rather was caused by downregulation of cyclin D1 (Fig. 6). As cyclin D1 mRNA did not decrease by following SubAB treatment, the rapid decrease may result from SubAB-induced translational inhibition. It has been shown that PERK contributes to eIF2α phosphorylation and mediates cell cycle arrest by blocking cyclin D1 translation during UPR (Brewer et al., 1999; Hamanaka et al., 2005). SubAB-induced decrease in cyclin D1 for the first 1–2 h may result from inhibition of protein synthesis. However, even after translation inhibition by SubAB was no longer evident, cyclin D1 downregulation and BiP cleavage was still observed. Cyclin D1 degradation is mediated by phosphorylation-triggered ubiquitination, followed by proteolysis (Diehl et al., 1997). Increased phosphorylation of cyclin D1 in the presence of SubAB (Fig. 7) and inhibition of SubAB-induced decrease of cyclin D1 by MG132 (Fig. 8) suggested that continued reduction in cyclin D1 by SubAB may also be regulated by the proteasomal pathway.
In this report, we showed that SubAB bound to membranes of Vero cells, internalized into cells and was delivered to Golgi and then ER. In the ER, it cleaved BiP, which triggered phosphorylation of PERK and then eIF2α, resulting in transient translation inhibition. Decrease in cyclin D1 by translation inhibition for ∼2 h, and by continuous prolonged proteasomal degradation via phosphorylation of cyclin D1, may lead to cell cycle arrest in G1 phase.
Experimental procedures
Chemicals
Antibodies against eIF2α, phospho-eIF2α, cyclin D1, phospho-cyclin D1, cyclin D3, cdk4 and cdk6 were purchased from Cell Signaling Technology. Antibodies against phospho-PERK, GAPDH and Grp78 (BiP) were from Santa Cruz Biotechnology. Antibody against GM130 was from BD Biosciences. Anti-CHOP antibody was from Affinity BioReagents MG132 and BFA was purchased from Sigma.
Preparation of SubAB
Recombinant His-tagged SubAB was purified as previously reported (Morinaga et al., 2007).
Western blotting
Vero cells (1 or 2 × 105 well−1) in a 24-well plate were cultured overnight in 1 ml of EMEM plus 10% FBS and then incubated in EMEM for 30 min. Indicated amounts of SubAB were added to each well (final volume 200 μl) followed by incubations for various times at 37°C. Cells were then lysed by addition of 100 μl of SDS sample buffer (62.5 mM Tris-HCl, pH 6.8, 1% SDS, 10% glycerol, 2.5% β-mercaptoethanol, 0.01% bromophenol blue), and lysates were heated at 100°C for 5 min. Samples (15 μl) were analysed using 7% (for PERK), 10% (for BiP, cyclinD1, eIF2α and GAPDH), 12% (for p21waf1/cip1, p27kip1, cdk4, cdk6 and CHOP), and 15% (for SubAB) SDS-polyacrylamide gels, and the separated proteins were transferred to polyvinylidine difluoride (PVDF) membranes. Membranes were blocked with 5% skim milk in TTBS (20 mM Tris, pH 7.6, 137 mM NaCl, 0.1% Tween 20) for 30–60 min and probed with primary antibodies overnight and then with HRP-conjugated secondary antibodies for 1 h. HRP was detected by ECL (GE Healthcare Bio-Science) reagent.
FACS analysis
Vero cells (1 × 106 cells) were treated with 100 ng ml−1 of SubAB for various times at 37°C. Cells were then harvested following trypsinization and washed once with PBS. Cells were then fixed by addition of 70% ethanol and incubation for 30 min at 4°C, followed by incubation at 37°C for 15 min in the presence of RNase (10 μg ml−1). After washing the cells with PBS, cells were incubated with 200 μl of propidium iodide (PI) solution (20 μg ml−1) for 30 min at 4°C. Cell cycle distribution was then analysed by FACS flow cytometry. The percentage of cells in different phases of the cell cycle was determined by ModFit LT cell cycle analysis software.
Analysis of protein synthesis
Vero cells (2 × 105 well−1) in a 24-well plate (each well, 1 ml) were seeded and incubated overnight in EMEM plus 10% FBS. Medium was replaced with 0.5 ml of EMEM plus 10% FBS containing 0.625 mCi ml−1 of [14C]-leucine. After addition of SubAB at the indicated amounts (in 50 μl), the plate was incubated at 37°C for 1 h in a water bath. Protein synthesis was stopped by addition of 0.25 ml of 30% trichloroacetic acid (TCA); cells were washed three times with 1 ml of 10% TCA and lysed in 0.25 ml of 0.5 M KOH for 10 min at 37°C. The lysate was neutralized with 0.25 ml of 0.5 M acetate and protein synthesis was quantified by radioassay of [14C].
Assay of cyclinD1 mRNA by real-time PCR
RNA was extracted from Vero cells treated with or without 100 ng ml−1 of SubAB for 3 h using Isogen (Nippon gene) and RNA (1 μg) was reverse transcribed into single-stranded cDNA using random primers. Real-time PCR was performed using left primer (5′-tgtgcatctacaccgacaact-3′), right primer (5′-ccacttgagcttgttcacca-3′) and probe #17 for cyclin D1, and left primer (5′-agccacatcgctcagaca-3′), right primer (5′-gcccaatacgaccaaatcc-3′) and probe #60 for GAPDH (Roche Applied Science).
Assay of DNA synthesis
Cells were cultured overnight in a 96-well plate (2 × 104 cells well−1) and medium was changed to 1% FBS plus EMEM with SubAB 100 ng ml−1 and incubation continued for various times. Then cells were treated with BrdU for 3 h and BrdU incorporation into DNA was assessed by immunoassay using cell proliferation ELISA, BrdU kit (Roche Diagnostic).
Immunostaining of Golgi
Indirect immunostaining was performed as previously described (Morinaga et al., 2005). To detect Golgi, rabbit antibodies against GM130, followed by Alexa488 anti-rabbit IgG, was used and cells were inspected by fluorescence microscopy (Olympus GX-FLA).
Acknowledgements
This work was supported by Grants-in-Aid of Scientific Research from Ministry of Education, Science and Culture of Japan. We thank Dr Iwao Kato, the former professor of Chiba University, for useful discussions and critical review of the manuscript. Authors thank Ms. Reiko Komine for her skillful technical assistance. Joel Moss and Kinnosuke Yahiro were supported by the International Research Program, NIH/NHLBI.




