Characterization of the tuberculous granuloma in murine and human lungs: cellular composition and relative tissue oxygen tension
Summary
The granulomatous reaction is the hallmark of the host response to infection with Mycobacterium tuberculosis. Despite its apparent importance to host defence against the tubercle bacillus, the granulomatous response remains to be completely defined. The present study used histological, immunohistochemical and flow-cytometric analyses to characterize pulmonic granulomatous tissues of tuberculous mice and humans. The kinetics of recruitment of neutrophils, macrophages, dendritic cells, and T and B lymphocytes into the lungs of mice infected aerogenically with the virulent Erdman strain of M. tuberculosis was evaluated in detail in both the acute and persistent phase of infection. A hypoxia-sensing compound based on the 2-nitroimidazole structure (EF5), together with immunohistochemical studies targeting endothelial cells were used to examine the relative oxygen tension in tuberculous granulomatous tissues in mice. The results have provided evidence that: (i) the granulomatous tissues are a highly organized structure whose formation is regulated by orderly recruitment of specific immune cells exhibiting distinct spatial relationship with one another; (ii) the granulomatous reaction, at least in the mouse, may represent an exaggerated response to the tubercle bacillus that can play a role in the development of immunopathology; (iii) B lymphoid aggregates are a prominent feature in both murine and human granulomatous tissues, although the immune cells that are most prominently associated with these clusters vary among the two species; (iv) murine tuberculous granulomatous tissues are relatively aerobic, suggesting that mouse models of persistent tuberculosis may not be suitable for the study of any hypoxic response of M. tuberculosis.
Introduction
Tuberculous infection begins with the inhalation of Mycobacterium tuberculosis-containing aerosols into the pulmonary alveoli, where bacilli enter resident macrophages – the presumed first line of host defence (Dannenberg and Rook, 1994). Evidence exists that pulmonic dendritic cells (Bhatt et al., 2004) and the alveolar epithelium (Bermudez et al., 2002) also participate in the initial interaction between M. tuberculosis and the host. This host–cell/bacterium interaction results in the initiation of chemoattractant mechanisms, involving chemokines, chemokine receptors, and adhesion molecules. These immunological factors play essential roles in the recruitment to the developing granulomatous tissues of specific inflammatory cells (Orme and Cooper, 1999; Algood et al., 2003; Zhu et al., 2003). Emerging evidence suggests that certain spatial relationship between the different cell types within fully formed tuberculous granulomatous tissues exists (Gonzalez-Juarrero et al., 2001; Kaplan et al., 2003; Ulrichs et al., 2004). The bacterium–host interaction within the granulomatous tissues determines disease outcome: control of the infection and healing or disease progression; the latter process usually associated with high bacillary burden and extensive immunopathological tissue damage (Dannenberg and Rook, 1994; Rook and Bloom, 1994). Thus, it is generally thought that the granulomatous reaction plays a significant role in host defence against the tubercle bacillus in both acute and latent persistent infection. The granulomatous response, however, remains incompletely defined. The present study (i) examined the kinetics of migration of specific immune cells into the pulmonic granulomatous lesions in mice with acute and chronic tuberculosis; (ii) characterized the spatial distribution of these infiltrating leucocytes during these two phases of infection; (iii) evaluated the relative levels of anaerobicity in the lungs of mice infected with M. tuberculosis, and (iv) revealed, through parallel experiments using clinical samples from tuberculous patients, significant differences as well as common features in the pulmonic granulomas of humans and mice with tuberculosis.
Results and discussion
Infection kinetics of the low-dose aerogenic murine experimental tuberculosis model
In order to correlate the granulomatous response with bacillary burden in the lungs of mice infected with M. tuberculosis Erdman, the number of viable bacilli was assessed over time. The results depicted in Fig. 1 demonstrate that upon infection with ∼100 colony-forming units (cfu) of the virulent Erdman strain of M. tuberculosis via the respiratory route, bacterial burden in the lungs of infected C57BL/6 mice peaks between 2 and 4 weeks post aerosolization, reaching a bacillary load of ∼5 × 106 per organ. This peak pulmonic load is stably maintained thereafter for at least 6 months (Fig. 1), as the infection progresses from the acute phase into the chronic persistent phase. The infected mice remain clinically well throughout this period, exhibiting no apparent distress.
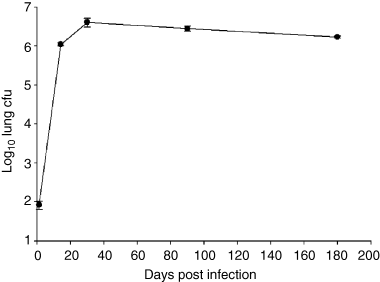
Kinetics of M. tuberculosis Erdman growth in the lungs of 8- to 10-week-old C57BL/6 mice infected aerogenically with ∼100 viable bacilli. Bacterial burden in the lungs of infected mice were assessed at the times indicated. Three mice per time point were examined. The experiment was repeated once with similar results.
Kinetics of inflammatory cell influx into the lungs of M. tuberculosis-infected mice during the acute and chronic phase of infection: correlation with pulmonic bacterial burden
In agreement with previous characterization of the progression of pulmonic tuberculous granuloma (Rhoades et al., 1997), histological studies revealed that the level of inflammation in the lungs of M. tuberculosis-infected mice continues to progress at least until 6 months post infection (Fig. 2). This progression is also evident by the continued increase in the number of CD45+ cells in the lungs of mice throughout the course of infection (Fig. 3). The level of pulmonic inflammation, as assessed by total number of CD45+ leucocytes in the lungs (Thomas, 1989), increases most rapidly in the initial 3–4 weeks post infection. This period of robust recruitment of inflammatory cells coincides with the time of rapid bacillary replication (a 4.5-log increase of bacterial load over a period of 4 weeks). During the initial 4 weeks post infection, the number of CD45+ leucocytes in the lungs of infected mice increases 10-fold from a baseline of 1.5 × 106−1.5 × 107 per organ (Fig. 3). As the infection transits from the acute phase with rapid bacterial growth into a more chronic phase with stably maintained bacillary burden, the number of pulmonic CD45+ cells in infected mice continues to increase, reaching a total of 2.1 × 107 cells by 27 weeks after inoculation. This represents a 40% increase in the lung CD45+ leucocytes compared with that present at 4 weeks post infection. These results suggest that once initiated, the granulomatous response continues despite an apparently stable antigenic load, as evidenced by a relatively constant bacterial burden. These data suggest the possibility that the progressive pattern of tissue inflammation represents an immunopathological response that may be detrimental to the host.
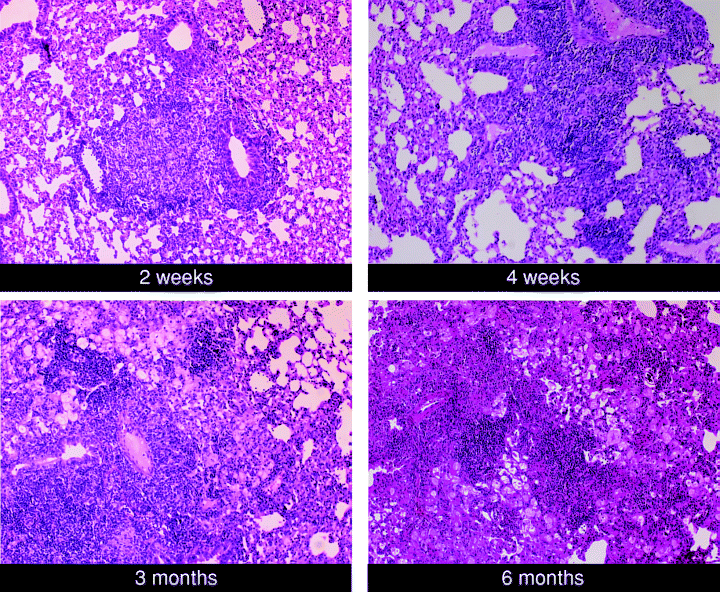
Histological studies of the lungs of C57BL/6 mice with acute and chronic tuberculosis. Mice were infected with ∼100 viable bacilli of M. tuberculosis Erdman. At the indicated times post infection, lungs were procured, formalin-fixed, and paraffin-embedded. Six micrometre sections were stained with haematoxylin and eosin. The results shown are representative of two independent studies.
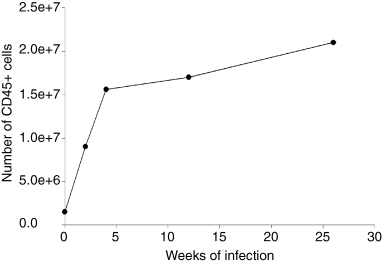
Kinetics of the accumulation of CD45+ leucocytes in the lungs of C57BL/6 mice infected aerogenically with ∼100 M. tuberculosis Erdman bacilli. CD45+ leucocytes in the lungs of mice with acute and chronic tuberculous mice were procured immunomagnetically for quantification. Cells from four to five mice were pooled together at each time point post infection for analysis. The study was repeated once with similar results.
Flow-cytometric characterization of leucocytes in the lungs of mice during the acute and chronic phase of infection revealed distinct kinetics of accumulation for specific immunocyte subsets
Flow-cytometric studies were initiated to assess the kinetics of influx of specific immune cells into the granuloma of the lungs of mice aerogenically infected with a low dose of tubercle bacilli during the acute and chronic phase of tuberculous infection. Analysis of live CD45+ leucocytes procured from the lungs of mice infected with M. tuberculosis revealed that the relative contribution of the types of immune cells to the granulomatous infiltrates varies with specific phases of infection (Table 1). During the acute phase of infection (2 weeks post inoculation), macrophages and neutrophils are the predominant cell types in the lungs of M. tuberculosis-infected mice: 36.2% and 37.5% respectively, of the pulmonic CD45+ leucocyte population (Table 1). At this early time of the infection, CD3+ T cell represents 16.7% of CD45+ lung leucocytes. Between 2 and 4 weeks post infection, the CD3+ T cell population underwent a rapid fivefold expansion (from 1.5 × 106 per lung to 7.4 × 106 per lung) (Table 1). During this same 2-week period, there is a moderate 42.4% increase in the total number of F4/80+ macrophages in the lungs of M. tuberculosis-infected mice; while the number of neutrophils decreases by 52.9% (Table 1). By 4 weeks post infection, CD3+ T cells have become the dominant CD45+ leucocyte subset in the lung of infected animals, representing 47.6% of the total population, while macrophages and neutrophils together constitute ∼40% of the total (Table 1). From 4 to 27 weeks post infection, there is a 34% increase in the total number of lung CD3+ T cells, while the F4/80+ macrophage population increases by about 62%. The number of neutrophils in the lungs of infected mice doubles between 4 and 12 weeks post infection, but by 27 weeks post infection, the number of neutrophils decreases to a quantity comparable with that at 4 weeks after inoculation.
| Cell type | 2 weeks | 4 weeks | 12 weeks | 27 weeks |
|---|---|---|---|---|
| CD3+ T cells | 1.5 × 106 (16.7) | 7.4 × 106 (47.6) | 6.0 × 106 (35.3) | 9.9 × 106 (47.1) |
| CD4+ T cells | 1.0 × 106 (11.1) [67.0] | 4.5 × 106 (28.9) [60.8] | 4.2 × 106 (24.7) [69.4] | 6.7 × 106 (32) [67.7] |
| CD8+ T cells | 2.8 × 105 (3.1) [18.2] | 2.4 × 106 (15.4) [32.7] | 1.5 × 106 (8.8) [24.2] | 1.7 × 106 (8.1) [17.0] |
| F4/80+ macrophages | 3.3 × 106 (36.2) | 4.7 × 106 (30) | 6.3 × 106 (35.6) | 7.6 × 106 (36.4) |
| Ly6G+ neutrophils | 3.4 × 106 (37.5) | 1.6 × 106 (10.3) | 3.4 × 106 (19.4) | 2.0 × 106 (9.5) |
| CD19+ B cells | 1.6 × 105 (1.8) | 1.2 × 106 (7.8) | 1.4 × 106 (8.2) | 1.2 × 106 (5.9 |
| DEC205+ dendritic cells | 3.6 × 105 (4.0) | 5.8 × 105 (3.7) | 5.7 × 105 (3.3) | 9.0 × 105 (4.3) |
- Round bracket: % of cells in CD45+ population. Square brackets (boldface): % of CD4+ or CD8+ T lymphocytes in CD3+ cells. Each datum point represents the results of flow-cytometric analysis of lung cells pooled from four to five mice.
- The experiments were repeated once with similar results.
Analysis of CD3+ T cell subsets revealed that the CD4+ T cell is the predominant subtype (60–70%) of CD3+ population during both acute and chronic infection (Table 1). During the acute phase of infection, CD8+ T cells constitute 18.2% of the CD3+ population. The relative number of CD8+ T cells peaks at 4 weeks post infection representing 32.7% of CD3+ cells. The proportion of CD8+ T cells decreases to ∼25% by 12 weeks post infection and is maintained at this level up to at least 27 weeks after inoculation. Throughout the acute and chronic phase of infection, the majority of CD3+ cells (91–96%) display αβ T cell receptor (TCR). The γδ TCR is expressed in only ∼2% of the CD3+ cell population throughout the 6-month study period (data not shown).
Examination of the CD19+ population at 2 weeks post infection revealed that B cells, totalling on average 1.6 × 105 per lung, are the least represented among pulmonic immunocytes, contributing to only 1.8% of the CD45+ leucocytes (Table 1). The number of CD19+ B cells in the lungs of infected animals has increased by about eightfold to 1.2 × 106 per organ (∼8% of pulmonic CD45+ leucocytes) by 4 weeks post infection. Thereafter until 27 weeks post infection, the total number of CD19+ B cells remains relatively stable, accounting for about 6–8% of the total CD45+ leucocyte population in the lungs of infected mice. Evaluation of the influx of DEC205+ dendritic cells revealed that there is a slow but progressive increase in the total number of this leucocyte population in the lungs of infected mice throughout the course of the 6-month study period. A limitation for the use of DEC205 (Kraal et al., 1986; Jiang et al., 1995) as a dendritic cell marker is that the expression of this glycoprotein could vary considerably among the likely heterogeneous populations of dendritic cells present in the lungs of tuberculous mice. Nevertheless, the number of DEC205+ dendritic cells increases about threefold from 3.6 × 105 (4% of the CD45+ leucocytes) at 2 weeks post infection to ∼9 × 105 at 27 weeks after inoculation.
Together, these results revealed that specific types of immune cells accumulate in distinct patterns in the lungs of mice as the tuberculous infection progresses from an acute to a chronic phase (Table 1). There is a rapid expansion of fivefold and eightfold of pulmonic CD3+ T lymphocytes and CD19+ B cells between 2 and 4 weeks post infection respectively. Thereafter, as the infection enters the chronic persistent phase, the number of CD3+ T cells progressively increases. By contrast, the steady rise of the number of CD3+ T cells in the chronic phase of infection beyond 4 weeks post inoculation was not observed with CD19+ B cells. The number of B cells in the lung of infected mice remains relatively stable from 4 to 27 weeks post infection. The DEC205+ dendritic cells and F4/80+ macrophages share a similar kinetic pattern of accumulation, characterized by a slow but steady increase in number present in the lungs throughout the 6-month course of infection. The rapid expansion seen with T and B cells, most notably during the acute phase of infection between 2 and 4 weeks after inoculation, is not observed with these antigen presenting cells. The pattern of accumulation of neutrophils in the infected lungs is distinct from the other leucocyte populations: this is the only leucocyte subset analysed that displays a peaked presence in the lungs of infected animals at 2 weeks post infection that subsequently diminishes as the infection progresses into the chronic phase. Overall, neutrophils and macrophages are the predominant cell types in the granulomatous tissues during the early acute phase of infection (2 weeks post infection: ∼75% of the total CD45+ leucocytes). As the infection progresses into chronic phase, CD3+ T cells and macrophages constitute the major cellular components of the tuberculous lungs (50–80% of CD45+ leucocytes). These results support the notion that polymorphonuclear leucocytes may contribute to anti-tuberculous activity in acute tuberculosis (Pedrosa et al., 2000; Seiler et al., 2003) and that T cells are critical in the maintenance of a chronic persistent infection (Flynn and Chan, 2001).
Immunohistochemical characterization of immune cells in the pulmonic granuloma of mice during the acute and chronic phase of M. tuberculosis infection revealed prominent B lymphocyte aggregates and their unique spatial relationship with macrophages
Evidence exists that the structural integrity of the granulomatous tissues correlates with favourable disease outcome in tuberculosis. For example, in a murine reactivation tuberculosis model based on the functional neutralization of tumour necrosis factor (TNF), disease recrudescence is associated with disorganization of the granulomatous tissues (Mohan et al., 2001). TNF blockade therapy in humans with inflammatory disorders such as rheumatoid arthritis and Crohn's Disease can lead to reactivation of latent tuberculous infection that is associated with apparent disorganization of pulmonic granulomatous tissues (Keane et al., 2001). Finally, controversy notwithstanding, results of clinicopathological studies have provided evidence suggesting that the granulomatous response in individuals with AIDS, who are at markedly increased risks for developing tuberculosis, may be compromised (Marchevsky et al., 1985; Nambuya et al., 1988; Sathe and Reichman, 1989; Fischl et al., 1992). Despite the apparent significance of the structural integrity of the tuberculous granuloma in the containment of M. tuberculosis, the nature of the infiltrating cells and their spatial relationship in the lesion are just beginning to be defined (Gonzalez-Juarrero et al., 2001; Kaplan et al., 2003; Ulrichs et al., 2004). Consequently, histological and immunohistochemical studies were undertaken to characterize the nature of the immune cells infiltrating the granulomatous tissues, as well as their intralesional location during the acute and chronic persistent phase of tuberculous infection.
Assessment of the overall organization of the granulomatous tissues using haematoxylin and eosin (H&E)-stained, formalin-fixed, paraffin-embedded lung tissues from infected mice at various times post infection revealed that inflammatory cell aggregates begin to form as early as 2 weeks after an aerogenic challenge with ∼100 viable M. tuberculosis Erdman (Fig. 2). There is a predilection for these early inflammatory cells to aggregate adjacent to blood vessels and the bronchial tree. Between 2 and 27 weeks post infection, there is a remarkable progression of the granulomatous response despite the relative stability of the total lung bacterial load (1, 2). By 1 month post inoculation, loose lymphocytic aggregates begin to form in the granulomatous tissues. Two months later, these aggregates appear more conspicuous and the cells within the aggregation become tightly apposed to one another. These lymphoid aggregates, situated in a background of histiocytic leucocytes, lymphocytes, and neutrophils, remain a prominent component in the pulmonic granulomatous tissues until 27 weeks post infection (Fig. 2), the last time interval examined in these studies. In other studies, these lymphoid aggregates maintain a prominent presence until at least 10–12 months post infection (data not shown).
Immunohistochemical study of frozen sections prepared from tuberculous lungs was initiated to further delineate the spatial relationship between the leucocyte subtypes in the granulomatous tissues. There is a T cell response detected as early as 2 weeks post infection (Fig. 4). In agreement with results of flow-cytometric analysis, the T cell infiltration intensifies rapidly between 2 and 4 weeks after infection. These light microscopic studies did not reveal any apparent specific location of T lymphocytes infiltrating the granulomatous tissues. In agreement with results obtained from flow-cytometric analyses, there is a more pronounced CD4+ T cell infiltration compared with that of CD8+ T cells throughout the 6-month study period (Fig. 4). Results obtained from experiments carried out to stain specifically for γδ and αβ TCR confirm the data yielded by flow-cytometric analyses that the latter is the predominant TCR expressed by T lymphocytes present in the tuberculous granulomas (data not shown).
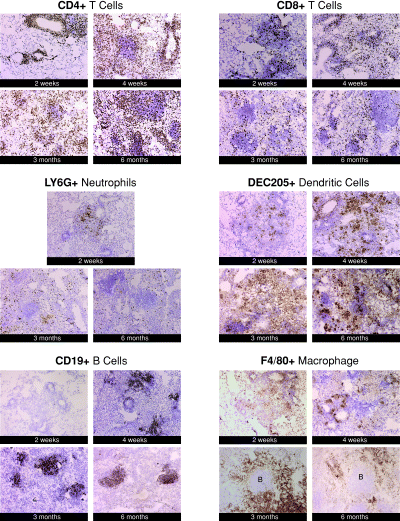
Immunohistochemical detection of specific immune cells in the lungs of C57BL/6 mice during acute and chronic tuberculous infection. Mice were infected aerogenically with ∼100 viable bacilli of M. tuberculosis Erdman. At 2 weeks, 1 month, 3 months or 6 months post infection, 6 µm frozen sections were examined to immunohistochemically detect specific immune cells. Results depicted are representative of sections obtained from three to four mice per time point. The studies were repeated once with similar results. Magnification was 40× except for the Ly6G results (10×). ‘B’ depicts aggregates of B lymphocytes.
Using anti-Ly6G antibodies, immunohistochemical staining revealed that a neutrophilic response was apparent as early as 2 weeks post infection (Fig. 4). Neutrophils are seen located in the granulomatous tissues in a diffuse pattern throughout the acute and chronic infection. There is also a vigorous dendritic cell infiltration in the lungs of mice during the acute and chronic phase of tuberculous infection as determined by immunostaining studies using DEC205-specific antibodies (Fig. 4). DEC205+ dendritic cells were present in early granulomatous aggregates examined at 2 weeks post infection. As was observed with the neutrophil studies, the dendritic cell infiltrates do not appear to favour specific location in the granulomatous tissues.
Staining using anti-CD19 antibodies showed that the conspicuous lymphoid aggregates consist predominantly of B cells (Fig. 4). Prominent B cell aggregates have been reported previously in the lungs of tuberculous mice (Gonzalez-Juarrero et al., 2001). The kinetics of the infiltration of B lymphocytes, as well as the spatial relationship of this cell type with other immune cells present in tuberculous tissues have not been examined. The results of the current study, obtained from immunohistochemical and flow-cytometric analyses, revealed that B lymphocytes are scarce in the pulmonic granulomatous tissues at 2 weeks post infection (Table 1 and Fig. 4). At this early time post inoculation, B cells are virtually absent in the clusters of inflammatory cells (Fig. 4). By 1 month post infection, however, immunohistochemical studies revealed that the B lymphocytes began to establish a prominent presence in the granulomatous tissues in the form of lymphoid aggregates. Results of studies using serial sections indicate that B cell lymphoid aggregates are surrounded by F4/80+ macrophages (data not shown). F4/80+ macrophages are found in the inflammatory site as early as 2 weeks post infection (Fig. 4). The macrophage infiltration intensifies by 4 weeks post infection. By 3 months and beyond after aerogenic challenge with M. tuberculosis, F4/80+ macrophages can be seen surrounding the lymphoid aggregates to form closely associated units (Fig. 4). The existence of the B cell-macrophage unit was directly confirmed by double immunohistochemical staining studies targeting B cells and macrophages simultaneously on the same sections (Fig. 5). Various cell types including CD4+ T cells, CD8+ T cells, and to a lesser extent, macrophages and dendritic cells, localize within the B cell aggregates, suggesting that these B lymphocytes can interact directly with different immune cells in the granulomatous tissues (Fig. 4).
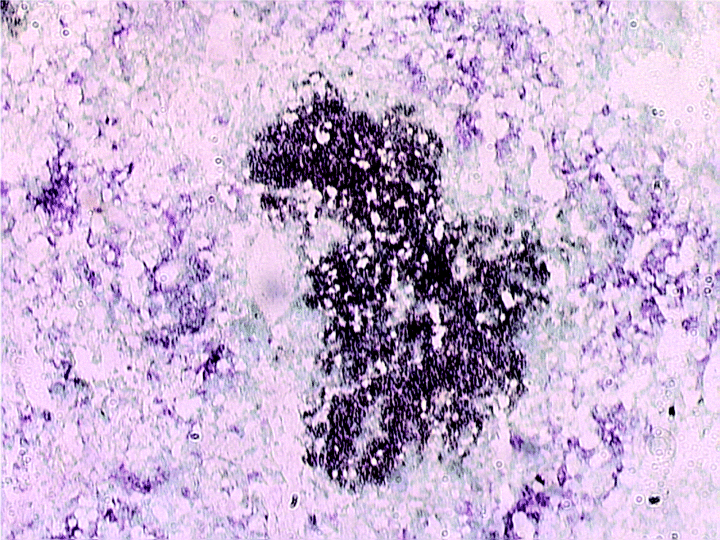
Immunohistochemical detection of F4/80+ macrophages and CD19+ B cells in the lungs of C57BL/6 mice with chronic tuberculous infection. Cryosections of lung tissues of mice aerogenically infected with ∼100 cfu of M. tuberculosis strain Erdman were examined immunohistochemically for the spatial relationship between macrophages and B cell aggregates during chronic tuberculous infection. The section was prepared from the lungs of a tuberculous mouse at 3 months post infection. Macrophages: purplish stain; B lymphocytes: dark brown stain. Magnification: 100×.
In summary, the overall results provided by light microscopic examination of immunohistochemically stained tuberculous lung sections parallel that of the flow-cytometric studies. Immunohistochemical studies revealed that T cells, neutrophils, and dendritic cells do not exhibit a readily discernible pattern of localization in the granulomatous tissues. In contrast, B lymphocytes infiltrating the tuberculous granulomas form well-organized aggregates that are surrounded by macrophages. The demonstration of the B cell-macrophage unit strongly suggests that cell recruitment into tuberculous granulomatous tissues represents specifically regulated events and there exists a specific spatial relationship between certain types of immune cells in the lesion.
Human tuberculous granulomatous tissues have features both similar to, and others distinct from that of the mouse counterpart
It is generally accepted that the granulomatous response in M. tuberculosis-infected humans is different from that in tuberculous mice. Therefore, immunohistochemical studies of human tuberculous tissues were carried out in parallel to the mouse studies described above. Sequential examination of the development of the granulomatous response in human tuberculous lungs, particularly with respect to the organization of the various infiltrating inflammatory cells, cannot be systemically carried out. Individual human specimens represent a snapshot of a specific stage of granulomatous development. Therefore, histological, biochemical or molecular parameters are lacking that can precisely predict whether a specific granulomatous lesion is progressing as a result of uncontrolled infection or a tissue-damaging immunological response, or is regressing concomitant to the elimination of an infectious focus. To further complicate matters, granulomatous lesions at various stages of development can be seen within the lung specimen obtained from a single tuberculous individual (Fayyazi et al., 2000; Ulrichs et al., 2004). We chose to focus on characterizing the organization of human lung tuberculous lesions with a necrotic centre because these specimens most likely represent a well-developed granulomatous reaction that is comparable with that encountered in the chronic phase of infection in the mouse.
Results generated by these studies revealed that although human tuberculous granulomatous tissues are different from that of the mouse (2, 6), prominent common features also exist in both species. A most remarkable common feature observed in both the human and mouse granulomatous tissues is the presence of B lymphocyte aggregates (4, 7). Interestingly, the spatial relationship between the B cell aggregates with other immune cells infiltrating the granulomatous tissues is different in the two species. Specifically, the distinct B cell-macrophage unit present in the mouse tuberculous tissues (4, 5) is not observed in human specimens. Rather, the human B cell aggregates are surrounded by CD3+ T cells (Fig. 7F). Also detected within the same specimen are subsets of B cell aggregates that harbour relatively abundant T cells that are evenly distributed within the clusters (Fig. 7C). Thus, while the T cells in the lungs of tuberculous mice do not display specific spatial location discernible by light microscopy throughout the acute and chronic phase of infection (Fig. 4), those in lungs of humans with tuberculosis exhibit two patterns of association with the B lymphoid aggregates (Fig. 7). Finally, there is a tendency for the CD3+ T cells in human granulomatous tissues to localize to the perimeter of necrotic tissues (Fig. 8).
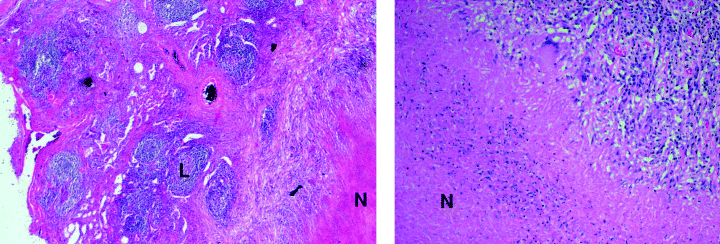
Histological examination of human tuberculous lung tissues. Six micrometre sections of formalin-fixed, paraffin-embedded human tuberculous lung tissues were H&E-stained. A total of three human tuberculous samples were examined. The section shown represents a granulomatous reaction with a classic necrotic centre. Left panel: 10×; right panel: 40×. ‘N’, necrosis; ‘L’, lymphoid aggregate.
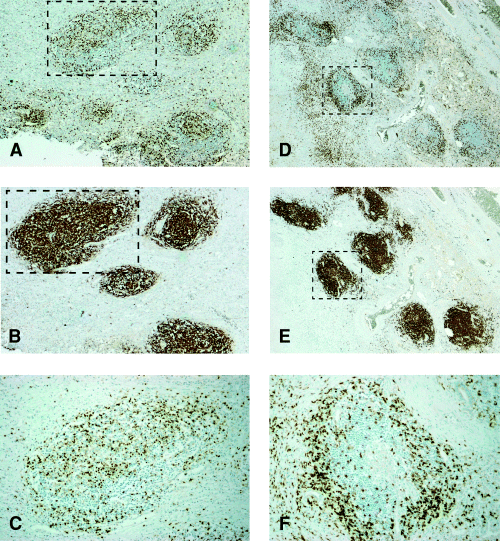
Immunohistochemical study of human tuberculous lung tissues: the relationship between B lymphoid aggregates and T cells. Six to eight micrometre sections were obtained from formalin-fixed, paraffin-embedded human tuberculous lung specimens and subjected to immunohistochemical detection of T (A,C,D,F) and B (B,E) lymphocytes. Magnification: A and D, 10×; B and E, 20×; C and F, 40×. Serial sections were obtained in order to trace the spatial relationship between infiltrating T cells and B lymphoid aggregates. The photomicrograph depicted in C represents the boxed lymphoid aggregate shown in A and B, demonstrating T cells evenly distributed within the B cell aggregate. Section F represents area of the boxed lymphoid aggregate depicted in D and E, demonstrating T cells circumscribing the B lymphoid aggregates.
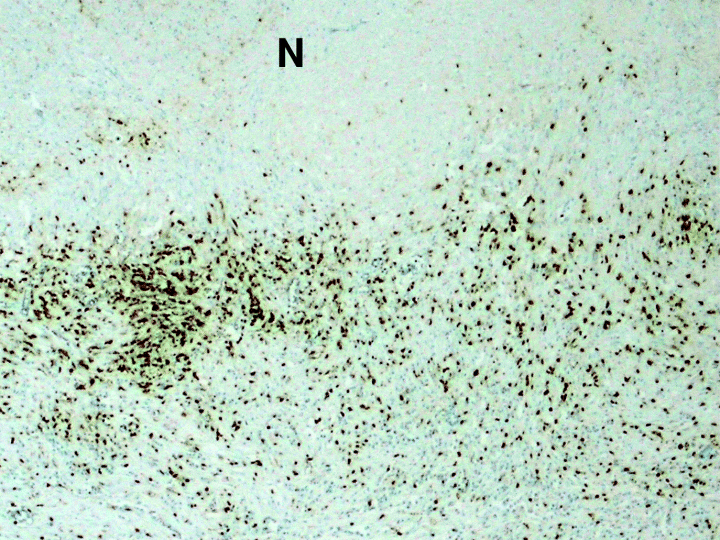
Immunohistochemical detection of T lymhocytes in human tuberculous lungs in areas of necrosis. Anti-CD3 antibodies were used to detect T lymphocytes in 6 to 8 µm sections of formalin-fixed, paraffin-embedded tuberculous human lung tissues. ‘N’: necrotic area. Magnification: 40×.
The CD68+ macrophages in the human tuberculous tissues, which, in contrast to that in the mouse, do not form a tight unit with the B lymphoid aggregates (Fig. 9), are present in a diffuse pattern. A small number of macrophages are observed within the human B aggregates. As was observed for T cells, there is a proclivity of macrophages to localize to the periphery of necrotic tissues (Fig. 9). Multinucleated giant cells, absent in the mouse tuberculous tissues, are a prominent feature of M. tuberculosis-infected human lung tissues. These multinucleated giant cells, also known as Langerhans cells, stained positively for the macrophage marker CD68, thus confirming their monocytic origin (Fig. 9). Caseation necrosis, absent in the mouse, is a prominent feature in human tuberculous lung tissues (6, 9).
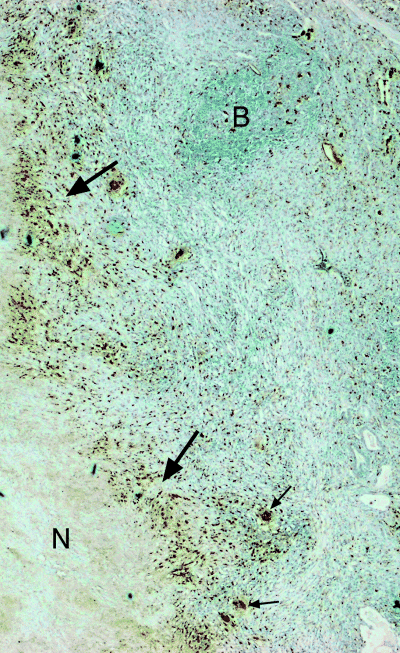
The localization of CD68+ macrophages in human tuberculous lungs. Immunodetection of macrophages were carried out using anti-CD68 antibodies. Six to eight micrometre sections of formalin-fixed, paraffin-embedded tuberculous human lung tissues were examined. ‘N’ designates area of necrosis. Large arrows: border of necrotic centre. Small arrows: multinucleated giant cells. Magnification: 40×.
The overall structure of mature human tuberculous granulomatous tissues consists of a central area of necrosis, whose border is populated with T lymphocytes and macrophages (Fig. 6). Further away from this central necrotic zone and situated in a background of apparently diffusely distributed macrophages and T lymphocytes are prominent B cell aggregates. These B cell clusters are either circumscribed with T cells at their periphery or contain T cells and a small number of macrophages that are evenly distributed within (7, 9). Thus, the cellular organization of pulmonic granulomatous tissues of tuberculous patients are significantly different from that of the mouse. Together, these studies demonstrate similarities as well as remarkable differences in the organization of the tuberculous granulomatous tissues in mice and humans.
Tissue hypoxia studies: probable anaerobicity in human granulomas but lack of evidence for hypoxia in granulomatous tissues in the lungs of mice with chronic tuberculosis
It is generally accepted that anaerobicity is a characteristic feature encountered in the tuberculous granuloma. Direct evidence to support this notion, however, has been lacking. We have used the hypoxia-sensitive compound EF5 to evaluate the relative oxygen tension in the granulomatous tissues of the lungs of M. tuberculosis-infected mice. Under anaerobic conditions, reduction of EF5 occurs, which facilitates the reaction of the compound to thiol groups of cellular proteins to form covalently bound adducts (Lord et al., 1993; Laughlin et al., 1996; Koch, 2002). In the absence of hypoxia, EF5 adducts are not formed and the compound is cleared rapidly from tissues. Immunodetection using ELK3-51, a monoclonal antibody specific for EF5 adducts (Lord et al., 1993), failed to show immunoreactivity in pulmonic granulomatous tissues from EF5-treated mice that are in the chronic persistence phase of infection (Fig. 10). This is in contrast to the intense signals readily detected in hypoxic tumour tissues used as positive controls (Fig. 10). The relative lack of anaerobicity in the lungs of mice chronically infected with M. tuberculosis is further suggested by the finding that abundant endothelial cells are present throughout the granulomatous tissues (Fig. 10). That these vasculatures are patent is supported by the demonstration of staining of the nuclei of cells in the granulomatous tissues after intravenous injection of Hoechst 33342 (data not shown). These endothelial cell studies do not, however, provide formal proof that the vascularized tuberculous granulomatous tissues are not hypoxic, particularly at the subcellular level. Not unexpectedly, results of studies using human samples revealed that areas of necrosis in tuberculous lung tissues is likely to be hypoxic, as evidenced by the lack of endothelial cell immunoreactivity (Fig. 11). In areas that are non-necrotic, the tuberculous tissues are well populated with endothelial cells (Fig. 11 and data not shown). These latter results do not, however, provide direct evidence regarding the relative oxygen tension in human tuberculous tissues because the patency of the vasculature demonstrated by endothelial cell immunoreactivity cannot be assessed. Finally, the tissues evaluated represent those from individuals with active tuberculosis. Therefore, the relative level of oxygenation in the environment within human latent tuberculous foci remains to be determined.
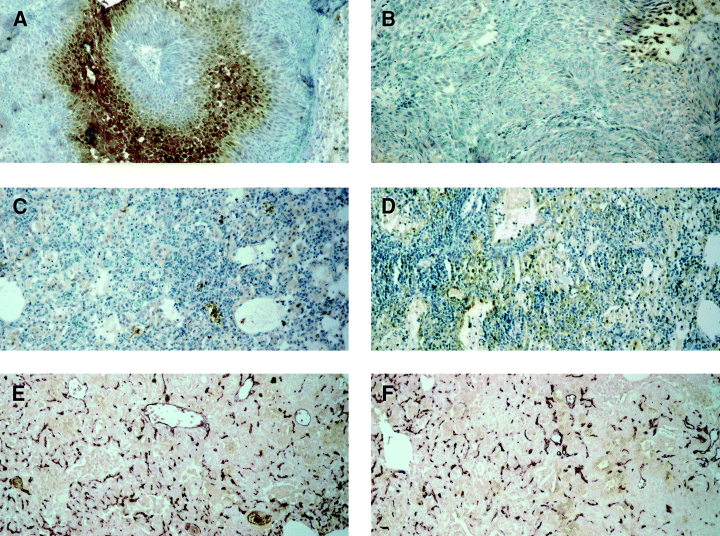
Immunohistochemical detection for tissue hypoxia and endothelial cells in tuberculous lung tissues. EF5, a nitroimidazole, in conjunction with immunohistochemical analysis, was used to detect hypoxic tissues in the lungs of tuberculous mice at 4 weeks, 1 month, 3 months and 4 months after infection. Six to eight micrometre sections of formalin-fixed, paraffin-embedded specimens were examined. The primary antibody used in this procedure is ELK3-51, which is known to be specific for adducts of EF5 and host macromolecules that are formed only under anaerobic conditions. Magnification: A, B, E, and F: 40×; C and D: 10×. A. Hypoxic tissues (stained brown) in tumour implanted subcutaneously in mice were used as positive controls. B. Immunodetection of hypoxic tumour tissues in the absence of ELK3-51 (the negative control for ‘A’). C. Immunodetection of hypoxic tissues in the lungs of tuberculous mice at 4 months post infection. Specimens obtained at 1 month, 2 months and 3 months after infection similarly did not reveal area of hypoxia (data not shown). D. Immunodetection of hypoxic tissues in the lungs of tuberculous mice in the absence of ELK3-51 (the negative control for ‘C’). E and F. Detection of endothelial cells in the lungs of tuberculous mice at 4 months post infection.
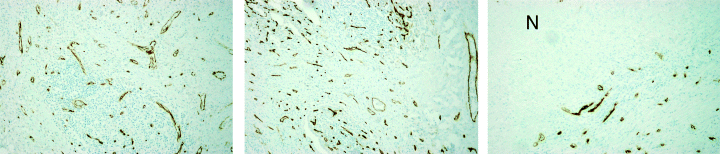
Detection of endothelial cells in human tuberculous lung tissues. Anti-CD31 antibodies were used to immunodetect endothelial cells in 6 to 8 µm sections of formalin-fixed, paraffin-embedded samples of human tuberculous lung tissues. ‘N’: necrotic area. Magnification: 40×.
Conclusion
The results of this study demonstrate that subtypes of immune cells infiltrate pulmonic granulomatous tissues of M. tuberculosis-infected mice with distinct kinetic patterns (Table 1), and that there exists specific spatial relationship between immunocyte subtypes (4, 5). Specific spatial relationship between different immune cells is also observed in human tuberculous granulomatous tissues 7-9). These results strongly suggest that the tuberculous granulomatous response represents regulated events that result in the recruitment of immune cells to specific locales in the lesion. The significance of the structural organization of the granulomatous tissues in the immune response to M. tuberculosis remains to be determined. Worthy of note, the steady increase in the number of CD45+ leucocytes (in particular, T cells, macrophages, and dendritic cells) in chronic infection when the mice exhibit no apparent illness and the bacterial burden is stable, suggests that additional recruitment of these leucocytes may represent an exaggerated immune response that can contribute to the tissue-damaging immunopathology observed in M. tuberculosis-infected hosts. It is currently unknown whether an inappropriately exaggerated immune response to the tubercle bacillus occurs in the chronic progressive phase of human tuberculosis, an infectious disease in which tissue damage plays an important role in pathogenesis (Dannenberg and Rook, 1994).
Immunohistochemical studies revealed that there are both common and distinct features exhibited by the human and mouse pulmonic granulomatous tissues with respect to spatial organization of the various types of infiltrating immunocytes. Specifically, macrophages and T lymphocytes (4, 5 versus 7-9) infiltrating the tuberculous lungs of mice and humans exhibit distinct patterns of distribution. A most conspicuous common feature of the granulomatous tissues obtained from the tuberculous lungs of the two species is the B cell aggregates. B lymphocyte clusters have been described in granulomatous tissues of the lungs of tuberculous mice and humans (Gonzalez-Juarrero et al., 2001; Ulrichs et al., 2004). The spatial relationship of the B aggregates with other immune cells in the inflammatory site and the kinetics of influx during infection were, however, not defined. The present study revealed differences in the cell types with which the B cell clusters in the two species associate. In the mouse, macrophages encircle the B cell clusters (4, 5). In humans, abundant T cells are seen at the periphery of a subset of B cell aggregates while other clusters contain evenly distributed T lymphocytes within (Fig. 7). The different cell types that the B aggregates in human and mouse associate with suggest that B cells in the granulomatous response of these two species may serve distinct functions. Alternatively, a function of B cells common to both human and mouse may be achieved through interaction with different inflammatory leucocytes in the two species. The presence of B cells in mouse lungs without necrosis suggests that the aggregates do not necessarily form in response to necrotic tissues. Lesional aggregates of B cells, generally considered to be the product of lymphoid neogenesis, have been described in a variety of chronic inflammatory diseases such as rheumatoid arthritis (Takemura et al., 2001). It has been hypothesized that B cell aggregates present in the inflamed synovium may play a role in the pathogenesis of rheumatoid arthritis (Goronzy and Weyand, 2003). However, prominent B cell aggregates are not necessarily a hallmark of chronic inflammation: studies of lung tissues of individuals with sarcoidosis, a granulomatous disease of unknown aetiology, revealed the conspicuous absence of B cell aggregates (Fig. 12). This datum suggests that the formation of B cell aggregates in inflammatory tissues is disease-specific. Notwithstanding the controversy regarding the role of humoral immunity in tuberculous infection (Vordermeier et al., 1996; Johnson et al., 1997; Bosio et al., 2000; Turner et al., 2001), the function of the granulomatous B cell clusters deserves further investigation.
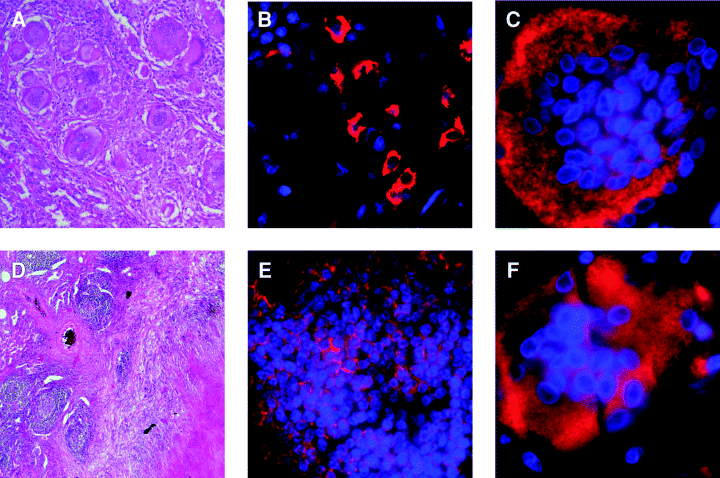
The human tuberculous (D,E,F) and sarcoidosis (A,B,C) granulomatous response. A and D represent H&E-stained 6 to 8 µm sections of formalin-fixed, paraffin-embedded human sarcoidosis and tuberculous lung tissues respectively. Magnification: 10×. B and E are immunofluorescence analyses of human sarcoidosis and tuberculous lung tissues, respectively, using anti-CD20 antibodies to detect B cells (CD20+ B cells: red; DAPI-stained nuclei: blue). C and F used anti-CD68 antibodies to immunofluorescently detect multinucleated giant cells (CD68: red; DAPI-stained nuclei: blue). Magnification of immunofluorescence studies (B, C, E, F): 60×.
Finally, this study has provided evidence strongly suggesting that granulomatous tissues in the lungs of mice with chronic tuberculosis are not anaerobic. A caveat to the interpretation of the EF5 data is that the method employed does not allow direct probing of the oxygen tension within a mycobacteria-containing subcellular compartment. Nevertheless, the EF5 observation should raise issues with the validity of the use of chronic murine experimental tuberculosis models in evaluating anti-tuberculous agents that target anaerobicity-related mycobacterial components. Indeed, despite the demonstration of the hypoxia-induced susceptibility of the tubercle bacillus to metronidazole (Wayne and Sramek, 1994), this drug has not been found to be effective in the treatment of chronic persistent tuberculosis in the mouse (Brooks et al., 1999). The utility of the mouse model in the study of the tubercle bacillus has been well established, and shortcomings of this system have also been well documented (Flynn and Chan, 2001). The lack of evidence of hypoxia in granulomatous lung tissues in mice with chronic tuberculosis suggests that the murine experimental tuberculosis model may not be suitable for evaluation of the hypoxic response of M. tuberculosis. These results should also underscore the importance of the use of human tissues to study the host granulomatous response to M. tuberculosis.
Experimental procedures
Animals
Eight- to 10-week-old C57BL/6 female mice (Charles River, Rockland, MA) were used in all studies. Animal protocols employed in this study have been approved by the Institutional Animal Care and Use Committee.
Mycobacteria and mouse infection
The maintenance and cultures of M. tuberculosis strain Erdman (Trudeau institute, Saranac, NY), and aerogenic infection of mice (∼100 cfu) and assessment of tissue bacterial burden were carried out as described (Mohan et al., 2001; Scanga et al., 2001). At appropriate times post infection, tissues procured from infected mice were subjected to histopathological, immunohistochemical and flow-cytometric studies (see below).
Human tissues
Lung tissues from individuals with tuberculosis and sarcoidosis, embedded in paraffin blocks, were obtained from the archive of the Department of Pathology of the Albert Einstein College of Medicine. A total of two tuberculous lung and one lymph node specimens were examined. One lung sample obtained from an individual with sarcoidosis was used as control. The study of these human tissues has been approved by the Institutional Review Board.
Histopathological and immunohistochemical studies of mouse tissues
Six to eight micrometre sections of formalin-fixed, paraffin-embedded tissue samples were H&E-stained for histopathological examination (Mohan et al., 2001; Scanga et al., 2001). For immunohistochemical detection of different leucocyte subsets and endothelial cells, lungs were insufflated with 30% OCT compound (Tissue-Tek, Sakura) in phosphate-buffered saline (PBS) via catheterization of the trachea using a 20 guage angiocatheter (Infusion, therapy System, UT), snap-frozen in OCT, and stored at −80°C. Six micrometre sections were cut, air-dried on poly- l-lysine-coated slides, fixed in cold acetone for 5 min, and fixed for 4 min with 80% ethanol. Sections were treated with 0.3% hydrogen peroxide for 20 min. Endogenous biotin was blocked using an avidin/biotin blocking kit (Vector Laboratories, Burlingame, CA). Five per cent goat serum was used to block non-specific binding before incubation with primary antibodies. The antibodies used were (i) rat anti-mouse CD4 (CD4+ T cells), CD8 (CD8+ T cells), CD19 (B cells), TCR β and TCR γδ, LY6G (neutrophils) (Pharmingen); (ii) rat anti-mouse DEC205 (dendritic cells) and F4/80 (macrophages) (Serotec, USA); rabbit anti-human CD3 (T lymphocytes) (Dako) that cross-react with murine CD3; rabbit anti-mouse CD34 (endothelial cells). Sections were washed with PBS prior to reaction with the appropriate biotinylated secondary antibodies (Vector, CA). The avidin-biotin complex-based signal amplification was achieved using Vectastain Elite ABC Kit (Vector, CA). Hydrogen peroxide and diaminobenzidine were used for signal development. For double-staining studies designed to colocalize B lymphocytes and macrophages, using as substrates diaminobenzidine and VIP respectively (Vector, CA).
Histopathological and immunohistochemical studies of human tissues
Six to eight micrometre formalin-fixed, paraffin-embedded sections of human tuberculous lung tissues were H&E-stained for histological examination. For immunohistochemical detection of different leucocyte subsets, 6 to 8 µm sections were subjected to antigen retrieval by microwave warming using Antigen Unmasking Solution (Vector Laboratories, Burlingame, CA). Endogenous peroxidase activity and biotin sites, and non-specific antibody binding were blocked as described above for mouse tissues before incubation with primary antibodies specific to cell-surface markers: rabbit anti-human CD3 (T cells), mouse anti-human CD8 (CD8+ T cells), mouse anti-human CD20 (B cells), mouse anti-human CD68 (macrophages), mouse anti-human CD31 (endothelial cells) (DakoCytomation Denmark A/S). The appropriate biotinylated Ig's (Dako) were used as secondary antibodies (Dako). Colorization of tissue sections was carried out using the method for analysis of OCT frozen mouse sections (see above). For immunofluorescence studies of human tissues, goat anti-mouse secondary antibodies conjugated to Alexa Fluor® 555 or 594 (Molecular Probes, Eugene, OR) were used. Upon completing incubation with the secondary antibodies, sections were stained with 0.3 µM DAPI (Molecular Probes), and immediately mounted with ProLong® Antifade reagent (Molecular Probes) and coverslipped.
Positive depletion of CD45+ cells from lung homogenates
To obtain cells from lungs of M. tuberculosis-infected mice for flow-cytometric analysis, pulmonary intravascular blood was flushed via the right ventricle with 10 ml of PBS containing 2 mM of ethylenediaminetetraacetic acid (EDTA; Sigma). Lung tissues were minced and treated for 1.5 h (37°C; 5% CO2) with collagenase type IA [10 mg ml−1 (Sigma)] and DNase [800–1400 IU ml−1 (Sigma)] in DMEM supplemented with 10% fetal bovine serum (FBS) and 2 mM EDTA. Single-cell suspension was obtained by passing the digested materials through increasingly smaller calibre needles (18, 23 and 26 gauge), and erythrocytes lysed with NH4Cl (0.17 M, pH: 7.2). Cells were then washed once with MACS buffer (PBS supplemented with 2 mM EDTA and 0.5% BSA). Treatment with rat anti-mouse CD16/CD32 (FcγIII/FcγII) Fc blocker (Pharmingen, CA) was carried out to prevent non-specific binding prior to the magnetic-labelling of CD45+ cells (Miltenyi Biotec). Procurement of magnetically labelled CD45+ cells was achieved using the MACS separation column (MS separation columns, Miltenyi Biotec). Both the CD45-negative and CD45-positive cells were collected and resuspended separately into 1 ml of FACS buffer (PBS containing 5% mouse serum and 10% FBS; pH 7.35). For each time interval studied, cells obtained from the lungs of five infected animals were pooled for further analysis. The CD45-positive cells obtained from the lungs of five uninfected mice were similarly prepared and pooled.
Flow-cytometric analysis
The CD45+ cells obtained from the lungs of M. tuberculosis-infected mice were subjected to four-colour flow-cytometric analysis. Dead cells were identified and excluded from analysis by means of staining with the Live/Dead Reduced Biohazard Cell Viability Kit (LDRBCVK) ♯2 (green) or ♯4 (blue) (Molecular Probes, OR). The following fluorochrome-conjugated antibodies were used for the study: anti-CD4-PE, anti-CD8-APC, anti-CD3-PerCP, anti-CD45-PE, anti-CD45-APC, anti-CD45-Cy, anti-LY6G-APC, anti-CD19-PE, anti-γδ TCR-PE and anti-β TCR-APC (the above obtained from Pharmingen), F4/80-PE (Serotec), and anti-DEC205-FITC (RDI, Research and Diagnosis, NJ). The CD45-negative fraction was stained with anti-CD45-PE/LDRBCVK-Green. Antibody-staining was carried out as follow: positive and negative controls for each fluorochromes were included in all analyses. A total of 2 × 106 cells were pelleted, washed once with FACS buffer, and resuspended in the same containing the standardized dilutions of the appropriate fluorescence-conjugated antibodies. After a 40 min incubation at 4°C, cells were washed once with FACS buffer and resuspended in 1 ml of MACS buffer containing the predetermined dilutions (Green dye: 1:20 000; Blue dye: 1:500) of the appropriate LDRBCVK dye (Molecular Probes, OR, USA). The live/dead staining was carried out for 10 min at 4°C. Cells were then washed once in MACS buffer and fixed for 45 min at 4°C in 200 µl of cold 4% paraformaldehyde in PBS (pH: 7.35). One ml of PBS was added to the stained cells, which were then subjected to four-colour flow-cytometric analysis using the FACSCalibur cytometer and the CellQuest software (Becton Dickinson, San Jose, CA).
EF5 [2-(2-nitro-1H-imidazol-1-yl)-N-(2,2,3,3,3-pentafluoropropyl) acetamide]: detection of tissue hypoxia
EF5 is a 2-nitroimidazole that undergoes reduction and binding to cellular macromolecules in anaerobic environment (Lord et al., 1993; Laughlin et al., 1996; Koch, 2002). In vivo assessment of tissue hypoxia was carried out as previously described (Graeber et al., 1996; Bialik et al., 1997). Briefly, at 1 month, 3 months, and 6 months post infection, mice were injected with 200 µl of EF5 (10 mM) via the tail vein. At appropriate time intervals after EF5 treatment, lung tissues were formalin-fixed and paraffin-embedded. The anti-EF5-adduct antibody ELK3-51 was used for the immunohistochemical detection of hypoxic tissues using the avidin-biotin complex-based Vectastain Elite Pk-6100 kit (Vector, CA) for signal amplification. EMT6 tumours with hypoxic areas were used as positive controls (Laughlin et al., 1996).
Acknowledgements
This work was supported by the NIH HL71241, HL68526, and the Albert Einstein College of Medicine CFAR P30 AI051519. We thank members of the Chan and Flynn lab for helpful discussion.