Characterization of Brucella abortus lipopolysaccharide macrodomains as mega rafts
Summary
The lipopolysaccharides (LPS) of intracellular Proteobacteria such as Brucella, Chlamydia, Legionella and Rickettsia, have properties distinct from enterobacterial LPSs. These properties include deficient LPS induction of host cell activation, low endotoxicity and resistance to macrophage degradation. Together these constitute key virulence mechanisms for intracellular survival and replication. We previously demonstrated that B. abortus LPS captured by macrophages was recycled back to the plasma membrane where it was found associated with macrodomains. Furthermore, this LPS interferes with the MHC class II (MHC-II) presentation of peptides to specific T cell hybridomas. Here, we characterized the Brucella LPS macrodomains by microscopy and biochemistry approaches. We show for the first time that LPS macrodomains act as detergent resistant membranes (DRMs), segregating several lipid-raft components, LPS-binding proteins and MHC-II molecules. Brucella LPS macrodomains remain intact for several months in macrophages and are resistant to the disruptive effects of methyl β-cyclodextrin. Fluorescent anisotropy measurements show that B. abortus LPS is responsible for the formation of rigid surface membrane complexes. In addition, relocalization of MHC-II molecules is observed in these structures. The effects of B. abortus LPS on membrane properties could be responsible for pathogenic effects such as the inhibition of MHC-II-dependent antigen presentation.
Introduction
The lipopolysaccharide (LPS) of Gram negative bacteria is considered as the major bacterial antigen responsible for inducing the expression of pro-inflammatory molecules and as the principal target for the humoral antibacterial response during the infection of the host. LPS is composed of a hydrophobic lipid-A linked to a densely charged and compact core oligosaccharide associated or not to a long O-polysaccharide chain (O-chain). On the bacterial surface, the O-chain is the most exposed part of LPS while the lipid A anchors the complex in the membrane. The LPS released during division and/or death of bacteria is ingested by macrophages, which act as an early defence against bacterial pathogens by phagocytosing and degrading microorganisms. Apart from this role in innate immunity, macrophages are also essential in the adaptive immune response as they process and present bacterial antigenic peptides to T cells. After internalization, enterobacterial LPS has been shown to localize in phagosomes, endosomes, the Golgi complex, mitochondria, the nucleus, the cytoplasm and on the cell surface (Kriegsmann et al., 1993; Kitchens and Munford, 1999; Thieblemont and Wright, 1999; Gorvel and Moreno, 2002). Another study has suggested that LPS can be exocytosed in culture supernatants after bacterial processing (Duncan et al., 1986). Intracellular Proteobacteria such as Bartonella, Coxiella, Brucella, Chlamydia, Legionella, Porphyromonas and Rickettsia have unique properties, which can be attributed to distinct LPS structures (Moreno et al., 1981; Neumeister et al., 1998; Moriyon, 2004; Zahringer et al., 2004). In contrast to the LPS of enterobacteria, the modified lipid A of the intracellular Proteobacteria confers low endotoxicity, deficient host cell activation and resistance to degradation by macrophages (Erridge et al., 2002). These properties could be seen as a virulence mechanism for an intracellular parasite (Lapaque et al., 2005). We have previously shown that purified LPS from Brucella abortus accumulates in lysosomes and is then recycled to the cell surface of macrophages forming large clusters that we previously named macrodomains (Forestier et al., 1999a). LPS macrodomains remain intact in the membrane for at least 3 months in vivo. Infection of peritoneal macrophages by B. abortus leads to the formation of intracellular vesicles enriched in LPS, which are then recycled to the cell surface in association with MHC class II (MHC-II) but not MHC class I molecules to form LPS macrodomains (Forestier et al., 1999a,b). MHC-II relocalization by B. abortus LPS significantly impairs presentation of peptides to specific CD4+ T cell hybridomas (Forestier et al., 2000).
In the present study, we further characterize the B. abortus LPS macrodomains, by microscopy and biochemistry analyses, defining them, for the first time, as mega rafts, which segregate lipid-raft components and sequester most of the surface MHC-II molecules. The presence of B. abortus LPS is responsible for membrane reorganization defined by a membrane densification and the relocalization of MHC-II in these rigid structures. We propose that Brucella LPS reorganizes the cell membrane and prevents MHC-II proteins from reaching functional lipid rafts by sequestering these molecules in dense non-functional structures, which could explain the inhibition of peptide presentation to CD4+ T cells (Forestier et al., 2000).
Results
Lipopolysaccharides macrodomains form unique structures at the cell surface of peritoneal macrophages
We have previously reported that B. abortus LPS assembles into macrodomains at the surface of murine peritoneal macrophages and remains stable for at least 90 days following a single LPS injection (Forestier et al., 1999b). To further characterize the structure of LPS macrodomains, macrophages were harvested from mice injected with B. abortus 2308 LPS and analysed at the ultra-structural level. Electron microscopy revealed complex electron-dense structures at the cell surface of macrophages (Fig. 1A), which were identified as LPS aggregates (Fig. 1B). These macrodomains had diameters ranging between 1 and 2.5 µm, some of which were subdivided into LPS subunits of 0.25 µm. These macrodomains are exposed to the external environment and appear to be mounted onto peduncle-like structures. It is possible that these structures correspond to a fraction of LPS integrated into membranes serving as an anchor for LPS aggregates (Fig. 1A and C). To confirm the presence of macrodomains at the macrophage surface and interaction of B. abortus LPS with plasma membrane, we performed deep-etch electron microscopy (EM) analysis (Fig. 2). We show that intracellularly B. abortus LPS can be found in a large vacuole of 2 µm in diameter, filled with ‘amyloid-like’ fibrils (Fig. 2A, black arrows). This vacuole could be the compartment in which the LPS accumulates before reaching the cell surface. In this intracellular compartment, the LPS aggregates seem to interact with the vacuole membrane (Fig. 2A, double black arrows). At the cell surface, deep-etch EM revealed that LPS appears as a breadth of extracellular ‘mushroom-like’ cap of 1.2 µm (Fig. 2B).
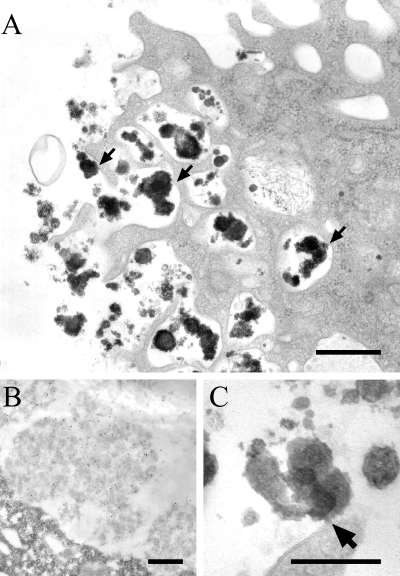
Lipopolysaccharide forms large electron-dense aggregates at the cell surface of macrophages. Peritoneal macrophages from LPS-injected C57Bl/6 mice were harvested 1 week after peritoneal injection of LPS, seeded onto culture dishes and processed for conventional electron microscopy (A, C) or immunoelectron microscopy (B). Scale bars in (A, B): 0.5 µm; (C): 0.25 µm. A. Accumulation and capping of LPS at the cell surface, with several possible sites of attachment (arrows). B. Cell thin sections were stained for LPS, showing that the large patches correspond to LPS. C. Enlarged view of a site of LPS attachment to the cell surface.
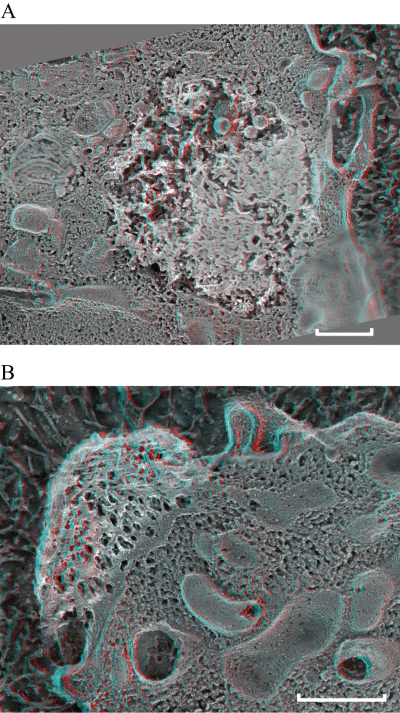
Lipopolysaccharide forms large aggregates and binds to plasma membrane at the cell surface of macrophages. Peritoneal macrophages from LPS-injected C57Bl/6 mice were harvested 1 week after peritoneal injection of LPS, seeded onto culture dishes and processed for deep-etch electron microscopy. Scale bars: 0.5 µm. A. shows a 3D anaglyph stereo view of an intracellular LPS aggregate bound to the membrane in a big vacuole (black double arrow), filled with ‘amyloid-like’ fibrils (black arrows). (× 15 000). B. shows a 3D anaglyph stereo view of LPS aggregate bound to the membrane at the cellular surface.
Lipopolysaccharides macrodomains are detergent resistant membranes that sequester MHC-II molecules
Lipid rafts have been described as functional membrane units enriched in cholesterol, gangliosides and GPI-anchored proteins. These structures have also been shown to contain MHC-II molecules (MHC-II) and to be involved in efficient MHC-II-dependent antigenic presentation to T cells. We first determined the composition of LPS macrodomains in peritoneal macrophages obtained from mice injected with B. abortus LPS. Confocal immunofluorescence data revealed LPS macrodomains at the cell surface of murine macrophages as large LPS/MHC-II clusters (Fig. 3A). In contrast to the apparent homogeneous distribution of MHC-II molecules at the surface of macrophages from γ-IFN injected mice (Fig. 3B), in the presence of B. abortus LPS, we clearly see a relocalization of most MHC-II molecules to LPS macrodomains (Fig. 3A). In order to further characterize these structures, we used filipin, a drug that labels cholesterol and we observed high levels of cholesterol surrounding the macrodomains (Fig. 3C). Similarly, using labelled cholera toxin, we found that the ganglioside GM1 accumulated around macrodomains (Fig. 3D).
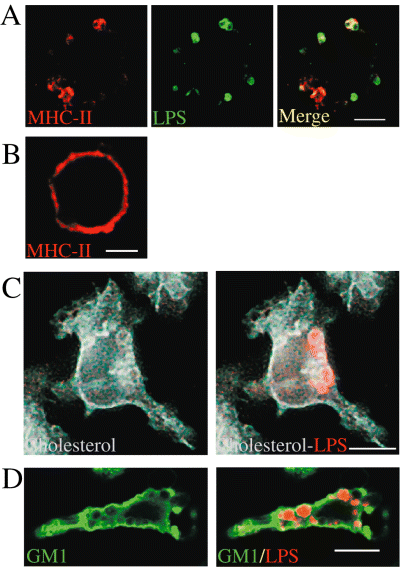
Brucella LPS macrodomains associate with MHC-II molecules, cholesterol and GM1. Peritoneal macrophages from LPS-injected (A, C and D) or γ-IFN- injected (B) C57Bl/6 mice were harvested 1 week after peritoneal injection of LPS and processed for confocal immunofluorescence. After fixation, cells were not permeabilized, thereby allowing detection of surface LPS (A), MHC-II molecules (A and B) and permeabilized for either cholesterol or GM1 detection (C and D). (A) macrophages from B. abortus 2308 LPS and (B) γ-IFN- injected mice were stained for both LPS (green) and MHC-II molecules (red). In (C), macrophages from B. abortus LPS-injected mice were stained for filipin, a cholesterol marker (blue) and for LPS (red) as shown in the right panel. In (D), macrophages from B. abortus LPS-injected mice were stained for cholera toxin, a ganglioside GM1 marker (green) and for LPS (red). LPS mega rafts were surrounded by cholesterol and by GM1. Bars: 10 µm.
In order to define if these structures are specific to smooth Brucella LPS, we used smooth Yersinia enterocolitica O:9, smooth Brucella melitensis 16 M and rough Brucella abortus 45/20 LPSs. B. melitensis LPS was able to form LPS macrodomains as shown in Fig. 4A and B, with a typical relocation of MHC-II and GM1 surrounding the macrodomains as already observed for B. abortus LPS (Fig. 3A and D). Small rough B. abortus LPS macrodomains were found at the surface of macrophages, but were not able to relocate MHC-II molecules (Fig. 4C). This suggests that the Brucella O-chain seems to be important for MHC-II interactions. Yersinia enterolitica O:9 LPS, which displays an O-polysaccharide very similar to that of B. abortus (homopolymer of N-formyl perosamine) but with core and lipid A moieties resembling those of enterobacterial LPSs (Caroff et al., 1984a,b), did not form microdomains and did not induce the relocation of MHC-II molecules at the macrophage surface (Fig. 4D). After permeabilization we were able to detect Y. enterolitica LPS in macrophages, suggesting that this LPS was endocytosed, accumulated in intracellular vesicles but was not able to reach the plasma membrane. Consequently, no perturbation of the homogeneous localization of MHC-II molecules at the cell surface was observed (Fig. 4E). This suggests that the lipid A structure seems to be important for addressing the LPS to cell surface.
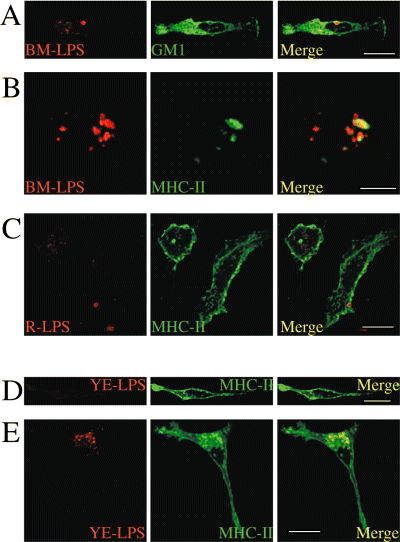
Properties of different LPSs to form macrodomains and to induce the relocation of MHC-II molecules. Peritoneal macrophages from LPS-injected C57Bl/6 mice were harvested 1 week after peritoneal injection of smooth B. melitensis 16 M, rough B. abortus 45/20 and smooth Y. enterocolitica O:9 LPS and processed for confocal immunofluorescence microscopy. In (A): macrophages from B. melitensis LPS-injected mice were fixed, permeabilized and stained for both LPS (BM-LPS, in red) and cholera toxin, a ganglioside GM1 marker (green) In (B), macrophages from B. melitensis LPS-injected mice were fixed, not permeabilized to allow surface staining only, then stained for MHC-II (green) and for LPS (BM-LPS, in red). LPS from B. melitensis form mega rafts at the cell surface accumulating GM1 and induced the relocation of MHC-II molecules within LPS macrodomains. In (C), macrophages from rough B. abortus LPS (R-LPS)-injected mice were fixed, not permeabilized and stained for both LPS (R-LPS, in red) and MHC-II molecules (green). Small R-LPS macrodomains were detected at the cell surface but were unable to relocate MHC-II molecules. In (D and E), macrophages from Y. enterocolitica LPS-injected mice were fixed and stained for both LPS (YE-LPS, in red) and MHC-II molecules (green). Macrophages were not permeabilized (D) and permeabilized in (E). Y. enterocolitica LPS was unable to form macrodomains (D) and to relocate MHC-class II molecules (E). Bars: 10 µm.
Glycosphingolipids, such as GM1, present in large amounts in lipid raft structures tend to cluster because of the hydrophobic links between lipidic fractions. This molecular clustering allows lipid rafts to be resistant to non-ionic detergents. Cholesterol, by inserting between the glycosphingolipids, enhances the compactness of these complexes. We hypothesized that LPS macrodomains could share structural homology with lipid rafts and that macrodomains are dense enough to withstand the action of non-ionic detergents. To confirm this, we purified LPS macrodomains. Peritoneal macrophages from B. abortus LPS-injected mice (Fig. 5B) and from γ-IFN-treated mice (Fig. 5A) were treated with Brij 98, a non-ionic detergent used to prepare lipid raft fractions (Drevot et al., 2002), and analysed by Western blotting after a sucrose step gradient (Fig. 5). Purified smooth B. abortus 2308 LPS distribution was also analysed after sucrose step gradient (Fig. 5C) and macrophages from γ-IFN-treated mice were used as controls (Fig. 5A). In the absence of LPS, lipid rafts from γ-IFN-treated mice were found in the light fraction (LF) of the gradient and were characterized by the presence of GM1, CD14 and CD48 GPI proteins as well as MHC-II molecules (Fig. 5A). Proteins from the endocytic and the exocytic pathways such as Lamp-1, GαS and the transferrin receptor (Tf-R) segregated into the heavy fractions (HF) (Fig. 5A). Purified LPS loaded onto the sucrose discontinuous gradient did not float up to LF and segregated in the HF (Fig. 5C). In lipid raft samples prepared from macrophages isolated from LPS-injected mice, LPS was found in intermediate fractions (IF) (Fig. 5B, surrounding by a black rectangle). Strikingly, MHC-II molecules, previously segregated in the LF (fraction 8), where microdomains-associated molecules are expected, predominantly cofractionated with LPS in IF (fraction 6). It was also noted that GM1 and GPI-proteins were also present in the IF in samples from macrophages obtained from LPS-injected mice. However, the relocation of such molecules in LPS macrodomains was partial as judged by their presence in LF (Fig. 5B).
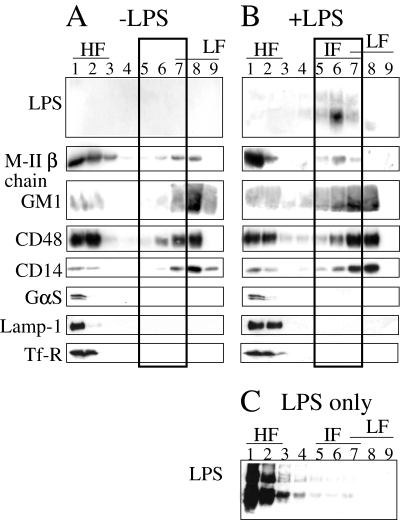
Lipopolysaccharide macrodomains have the characteristics of lipid rafts. A. Brij 98 lysates of peritoneal macrophages from γ-IFN-treated C57Bl/6 mice were used to identify lipid rafts segregating in LF of a sucrose step gradient. By Western blotting, different constituents associated with these lipid rafts were identified as GM1, CD48, CD14 and MHC-II molecules (M-IIβ chain). Markers not associated with lipid rafts such as the α-subunit of the trimeric G protein (GαS), Lamp-1 and the Tf-R migrated to HF of the gradient. B. Brij 98 lysates of peritoneal macrophages from LPS-injected C57Bl/6 mice, LPS macrodomains segregated in an IF. They were characterized by the presence of GM1, CD48, CD14 and M-II chain. C. Purified LPS was loaded onto the sucrose discontinuous gradient and segregated in the HF.
Lipopolysaccharides detergent resistant membranes (DRMs) resist cholesterol extraction
We then studied the resistance of LPS detergent resistant membranes (DRMs) to methyl β-cyclodextrin (β-CD), a chemical agent known to destabilize lipid rafts by extracting cholesterol (Christian et al., 1997). We incubated macrophages from LPS- or γ-IFN-injected mice with 20 mM of β-CD for 45 min at 37°C, and then performed the sucrose step gradient after Brij 98 solubilization (Fig. 6). In the presence of β-CD, lipid raft microdomains were disorganized (Fig. 6A), and consequently lipid raft markers such as GM1 were found in the HF of the gradient. In contrast, LPS macrodomains resisted the β-CD treatment (Fig. 6B). After β-CD treatment, LPS remained associated to MHC-II, GM1, CD14, and CD48 in IF (Fig. 6B). These results demonstrate that LPS macrodomains are unique structures, which can resist to cholesterol extraction by β-CD. This event is consistent with confocal microscopy observations of macrophages treated or not with β-CD for 45 min (Fig. 6C–E). In γ-IFN-treated macrophages, we observed an homogenous membrane labelling of GM1 (Fig. 6C), which was perturbed by β-CD treatment (Fig. 6D). In macrophages from LPS-injected mice treated with β-CD, the ganglioside GM1 labelling was also perturbed (not shown), but still accumulated around LPS macrodomains (Fig. 6E).
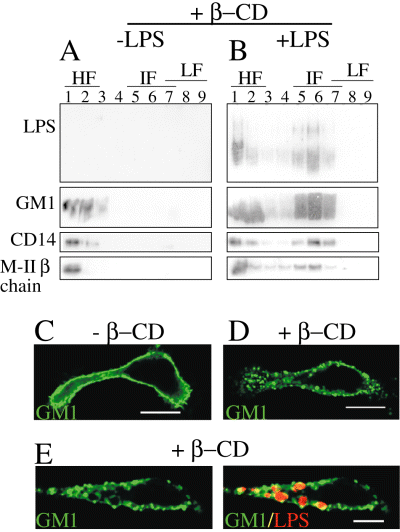
Lipopolysaccharide macrodomains are stable DRMs. Peritoneal macrophages from LPS- or γ-IFN-immunized C57Bl/6 mice were harvested 1 week and 3 days, respectively, after peritoneal injection. Macrophages were treated or not with β-CD and processed for lipid-raft purification or confocal immunofluorescence. A. In the absence of LPS, treatment with β-CD disorganized the lipid rafts and, accordingly, no raft markers were detected in LF; instead they all segregated in HF. B. LPS mega rafts resisted β-CD treatment. LPS still segregated in IF, which also contained GM1, CD48, CD14 and M-II chain. In contrast, and as expected, no raft markers were detected in LF. C. Untreated macrophages were labelled for GM1 with cholera toxin. D. After β-CD treatment, the ganglioside GM1 labelling was perturbed. In (E, GM1 in green, LPS in red), macrophages from LPS-injected mice treated with β-CD, GM1 was still accumulated around macrodomains, as observed in untreated cells (Fig. 3E). Bars: 10 µm.
Brucella abortus LPS alters plasma membrane fluidity
Fluorescence spectroscopy is a useful tool for studying the motion of fluorophores in a biological system. Steady state fluorescence anisotropy (r) measurements can provide a detailed description of the rotational motion of a fluorophore and, thus, characterizing the microviscosity of the environment surrounding the fluorophore. Here we report that the ACAS interactive laser cytometer (Okemos, MI48884 USA) can be used to obtain spatially detailed measurements of the fluorescence anisotropy within cellular membranes of macrophages from LPS- and γ-IFN-injected mice. The ACAS provides the advantage of simultaneously monitoring both the vertical (parallel) and horizontal (perpendicular) polarized fluorescence emission while automatically correlating the ratio of the emission to anisotropy. Using this technique, we compared the fluorescence anisotropy of macrophages from γ-IFN- with LPS-injected mice (Fig. 7A, left and right panel respectively). We clearly show that fluorescence anisotropy is increased in the presence of B. abortus LPS (Fig. 7A, right panel and B). Increase of anisotropy is directly related to a decreased of membrane fluidity. This leads to the hypothesis that LPS macrodomains are very rigid structures. This is consistent with the electron dense properties previously described in Fig. 1.
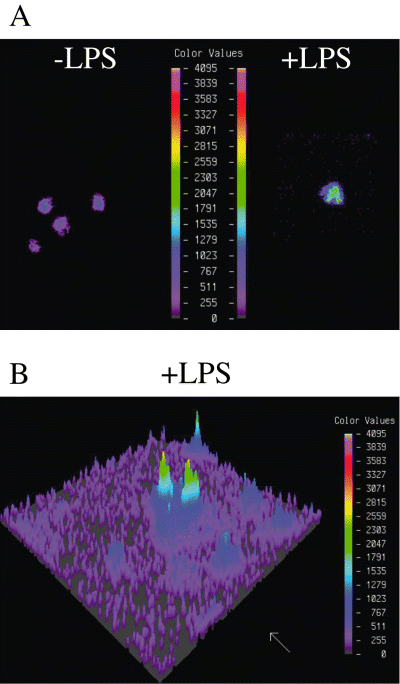
Lipopolysaccharide macrodomains are dense domains. A. Pseudocolour images of the vertical polarized fluorescence emission (detector 1) of macrophages from γ-IFN-injected mice (left) and from LPS-injected mice (right). The cells were loaded with 0.5 µM of the fluorescent probe TMA-DPH. The colour values represent the scale of fluorescent anisotropy. B. Pseudocolour image of polarized fluorescence emission of macrophages from LPS-injected mice.
Discussion
It is well described that lipid rafts are enriched in cholesterol, gangliosides and GPI-anchored proteins. These functional membrane domains are capable of transducing intracellular signals and are involved in membrane transport. Lipid rafts have been proposed to be ‘hijacked’ by various bacterial, viral and protozoan pathogens (Cherukuri et al., 2001; Manes et al., 2003; Lafont et al., 2004). For example, lipid rafts are used by different intracellular bacteria such as Mycobacterium (Gatfield and Pieters, 2000) and Shigella (Lafont et al., 2002; van der Goot et al., 2004). Brucella also uses lipid rafts for its entry and survival (Naroeni and Porte, 2002; Watarai et al., 2002) by a still unknown mechanism.
In this study, we have characterized the LPS-MHC-II molecule membrane complexes found at the surface of macrophages derived from B. abortus LPS-injected mice. These complexes are also induced by B. melitensis LPS but not by Y. enterocolitica O:9. These structures behave as mega rafts, sequestering several raft components including LPS-binding protein CD14 and the bulk of MHC-II molecules. These LPS macrodomains remain stable for several months in macrophages, are resistant to non-ionic detergent and to β-CD treatment and are also able to restrict the presentation of peptides to specific CD4+ T cells hybridoma (Forestier et al., 2000).
We show that LPS macrodomains are dense host-pathogen membrane domains with features of DRMs. Anderson et al. (2000) previously reported that the subset of functional MHC-II molecules are inserted into lipid rafts and are important for an optimal antigen presentation to CD4+ T cells. We previously used a classical antigenic presentation assay, using macrophages from γ-IFN- or LPS-injected mice as antigen presenting cells (APCs) and 3A9 T hybridoma that recognize specifically hen egg lysosyme (HEL) peptides in a MHC-II context. In the absence of LPS, γ-IFN-activated macrophages incubated with HEL were able to stimulate HEL-specific 3A9 T cells (Forestier et al., 1999b). But in the presence of LPS, specific T cell activation was inhibited (Forestier et al., 1999b). These results demonstrate that B. abortus LPS impairs antigen presentation in the context of MHC-II molecules, maybe due to the formation of macrodomains, and this inhibition is comparable with the impairment of MHC-II-dependent T cell activation by the disruption of lipid rafts after β-CD treatment (Anderson et al., 2000). Therefore, inhibition of antigen presentation may result from a relocalization of MHC-II molecules into non-functional stable and dense LPS mega rafts. This may be a consequence of the reorganization and the sequestering of essential raft components in LPS macrodomains. We propose that segregation of MHC-II molecules into dense mega rafts could be responsible for the down-regulation of antigen presentation. In addition, in contrast to lipid rafts, LPS macrodomains are resistant to β-CD. This resistance is consistent with the property of LPS macrodomains to be electron dense and to form low fluidity membrane structures. These properties may interfere with membrane dynamics necessary for the formation of synapse between T cells and antigen presenting cells.
Lipopolysaccharides macrodomains biogenesis could occur by aggregation of LPS and host molecules in intracellular compartments. Although the interaction between Brucella LPS and MHC-II remains to be characterized, it is known that Brucella O-chain strongly interacts and makes complexes with other polysaccharide and glycolipid molecules (Aragon et al., 1996). In addition, the highly hydrophobic lipid A, with its long aliphatic chains of up to 30 carbon atoms is capable of spanning the cell membrane and anchoring into the inner leaflet of the plasma membrane by the hydroxyl group close to the end of the acyl chains (Bhat et al., 1991). Poloso et al. have shown that MHC-II localize in detergent-insoluble microdomains in antigen-processing compartments (Poloso and Roche, 2004; Poloso et al., 2004). We have previously showed that B. abortus LPS accumulates intracellularly in lysosomes before reaching the membrane surface (Forestier et al., 1999a). It is tempting to postulate that the integration of LPS in MHC-II lipid rafts might occur intracellularly during LPS trafficking. As Brucella lipid A span the membrane of bacteria, we hypothesize that a subset of the Brucella LPS may be integrated into the host membrane. This idea is supported by the fact that B. melitensis LPS form macrodomains and that neither Y. enterocolitica O:9 LPS nor B. abortus R-LPS relocalizes MHC-II molecules. Because of the O-chain differences between these two Brucella LPSs and that the LPS of Y. enterocolitica 0:9 does not form macrodomains it is possible that the lipid A (and so the long acyl chains) of Brucella LPSs are required to induce macrodomain biogenesis. However, only slight differences exist between B. melitensis and B. abortus O-chains, so we cannot completely exclude a possible role of the O-chain in macrodomain biogenesis. Integration of Brucella LPS in host membranes may occur at the site of the intracellular MHC-II lipid-raft biogenesis into lysosomes.
In conclusion, we show that Brucella LPS relocated MHC-II from lipid rafts to LPS macrodomains. B. melitensis and B. abortus LPSs form macrodomains, with a characteristic relocalization of MHC-II. These structures can be considered as mega rafts as they are characterized by many components of lipid rafts and are insoluble to detergents. These results lead us to propose the hypothesis that sequestration of an important part of MHC-II into non-functional and dense structures might result in an immunomodulatory effect of Brucella by its LPS on the adaptive immune response. This relocation is specific of Brucella S-LPS and could be related to chronic evolution of brucellosis. During brucellosis, LPS released during bacterial replication and bacterial death may modify lipid-raft properties and, consequently, inhibit antigen presentation. These results are compatible with the immunosuppressive stages observed in both murine and human brucellosis (Zhang et al., 1993; Giambartolomei et al., 2002). These results led us to consider the LPS of B. abortus as a virulence factor, which may be implicated in B. abortus pathogenesis by controlling host immune response and membrane dynamics during infection.
Experimental procedures
Mice and macrophage preparation
Eight-week-old female C57Bl/6 mice were purchased from Jackson ImmunoResearch (West Grove, PA). Peritoneal murine macrophages were obtained after cervical dislocation from mice injected i.p. with 0.4 ml of B. abortus 2308, B. melitensis 16 M, B. abortus 45/20 (rough LPS) or Y. enterocoltitica O:9 LPS (1 mg ml−1) or with γ-IFN (5 µg ml−1). Peritoneal exudate macrophages were extracted by washing with 10 ml of sterile DMEM (Life Technologies, Cergy-Pontoise, France) at 4°C, sedimented and resuspended in DMEM supplemented with 10% FCS, 10 mM HEPES, 10 mM sodium pyruvate, 10 mM non-essential amino acids, 2 mM glutamine, 100 U ml−1 penicillin and 100 µg ml−1 streptomycin (all from Life Technologies). Peritoneal cells were plated on 12 mm glass coverslips in 24 well tissue culture plastic dishes or in tissue culture dish (Becton Dickinson, Franklin lakes, NJ, USA) for immunofluorescence or lipid-raft purification analysis respectively. For all experiments, peritoneal cells were plated and incubated for 2–4 h at 37°C in a 7% CO2 atmosphere. Non-adherent cells were removed from wells or dishes by aspiration, and the adherent macrophages were rinsed and incubated in fresh medium.
Lipopolysaccharides
Smooth B. abortus LPS from virulent 2308 strain, B. melitensis from B. melitensis 16 M strain, rough B. abortus 45/20 (without O-chain) and Y. enterocolitica O:9 LPS were obtained by methanol precipitation of the phenol phase from a water-phenol extract from whole bacteria. LPS purification was performed by resuspending the crude extract in 5 nM MgCl2, 0.1 M Tris-HCl (pH 7.0) and digested with nucleases [50 µg ml−1 each of DNase-II type V, and RNase-A (Sigma), 30 min at 37°C] and then three times with proteinase K (50 µg ml−1, 3 h at 55°C), sedimented by three cycles of ultracentrifugation (6 h, 100 000 g), and freeze-dried. Free lipids were then removed by a fourfold extraction with chloroform-methanol (2:1 v/v). The homogeneity of the B. abortus LPS preparation was tested by the following methods: (i) SDS-PAGE and two dimensional gel electrophoresis in combination with silver staining and Western blotting with monoclonal antibodies against LPS and outer membrane proteins), serum from B. abortus infected bovines; (ii) immuno-electrophoresis against polyclonal antiserum against B. abortus proteins and polysaccharides; (iii) high performance thin layer chromatography of O-polysaccharide and lipid A preparations and chemical analysis. For injections in mice, lyophilized LPSs were dissolved by sonication in distilled water at the appropriate concentrations and autoclaved before used.
Antibodies and reagents
The monoclonal antibody (mAb) (Baps C/Y) directed against B. abortus LPS C/Y epitope coupled to peroxidase and antiserum from infected cows were previously described (Rojas et al., 1994). The antiserum from infected cows was used to label Y. enterocolitica 0:9 LPS (same O-chain as B. abortus; for review see Moriyon, 2004). An antiserum from infected rabbit with B. melitensis 16 M was used to label B. melitensis 16 M LPS (described in Forestier et al., 1999b). An antiserum from a naturally infected sheep with rough Brucella was used to label R-LPS. 20C4 mAb (IgG 2b) (Deleuil et al., 1999), FITC-cholera toxin (Sigma) and filipin (Sigma) were used to label I-Ab MHC-II, GM1 and cholesterol respectively. The anti-MHC-IIβ cytoplasmic chain rabbit antisera, the mAb anti-Tf-R H129-121-6.8 and the mAb anti-Lamp1 were provided by Drs J. Davoust (Curie Institute, Paris), M. Pierres (CIML, Marseille) and S. Méresse (CIML, Marseille) respectively. The mAbs anti-CD48 (MCA925) anti-GαS (C18) were from Serotec, Pharmingen, Santa Cruz Biotechnology respectively. M5-114, an I-Ab-specific mAb and the anti-CD14 (rmCS-3) were purchased from BD PharMingen (San Diego, CA). Secondary Abs were: goat Alexa Fluor 594®- or Alexa 488®-conjugated anti-cow IgG, goat Alexa 594®- or Alexa 488® conjugated anti-rabbit IgG, Alexa 594®-conjugated anti-sheep IgG and goat Alexa 488®-conjugated anti-rat IgG (Molecular Probes). Rabbit IgG-peroxidase anti-mouse Ig and goat IgG-peroxidase anti-rabbit Ig conjugates and 1-(4-trimethylammoniumphenyl)-6-phenyl-1,3,5-hexatriene p-toluenesulfonate (TMA-DPH) were from Sigma. β-CD was from Sigma and was used at 20 nM for 45 min at 37°C.
Confocal and electron microscopy
Immunofluorescence studies were performed as described previously with macrophages grown on coverslips (Forestier et al., 1999a). When stipulate, cells were permeabilize with 0.1% saponin. Coverslips mounted in Mowiol (Sigma) were viewed under a Leica TCS 4D confocal microscope (Leica Lasertechnik, Heidelberg, Germany) or under a Zeiss LSM 510 laser scanning confocal microscope. For conventional electron microscopy, peritoneal macrophages were fixed and processed, as previously described (de Chastellier et al., 1993). For immunoelectron microscopy, cells were sequentially fixed with a mixture of 2% PFA and 0.1% glutaraldehyde and then with 1% PFA in cacodylate buffer and processed for embedding in LR White (Frehel et al., 2002). LR White thin sections picked up on formvar- and carbon-coated nickel grids were sequentially incubated for 60 min at room temperature with rabbit anti-Brucella LPS Abs and protein A coupled to 10 nm-wide gold particles. Dilution of antibodies and conjugates, washings between incubations and after treatment with conjugates, and staining of thin sections were as previously described (Frehel et al., 2002). As a control, thin sections were incubated with protein A-gold only; this control was negative. For freeze-fracture, cells were fixed with 2% glutaraldehyde in HpSCa buffer (Hepes 30 mM pH 7.4, 100 mM NaCl, 2 mM CaCl2) for 1 h at room temperature. Embedded cells were pelleted in warm buffer and were rapidly frozen and transferred to liquid nitrogen. Freeze-fracture, deep etching and replica preparation were carried out as described (Cover et al., 1997). Thin sections and replicas were viewed in a JEOL 100CX transmission electron microscope, operating at 100 kV and photographed in stereo with an Advanced Microscopy Techniques (Danvers, MA) charge-coupled device camera system. Micrographs (×25 000 and ×15 000) were digitized by video imaging.
Membrane preparation and isolation of lipid rafts and LPS macrodomains
The isolation technique was adapted from Drevot et al. (2002). Briefly, macrophages were gently broken through a 30 G-needle. After a 15 min solubilization at 4°C with 1% Brij 98 (Sigma Chemical), lysates were brought to 1.33 M sucrose, Brij 98 0.33% for 6 min at 37°C and then chilled down on ice. Then, lysates or 100 µg of purified LPS were placed at the bottom of a sucrose step gradient made of 0.2–0.9 M sucrose layers. Gradients were centrifuged at 38 000 rpm at 4°C for 16 h in a SW41 rotor (Beckman Instruments). Samples were run in SDS-PAGE slab gels and then proteins transferred onto Immobilon-P membranes (Millipore, Bedford, MA). Membranes were blocked in TBS/5% skim milk/0.1% Tween-20 (Sigma) for 2 h. Primary and secondary Abs were successively added in this buffer, each being left for 2 h before developing with the enhanced chemo-luminescence system (ECL; Amersham, Courtaboeuf, France).
Fluorescent anisotropy
Macrophages from γ-IFN- or LPS-injected mice were grown in DMEM culture medium. Cell cultures were washed twice with PBS, then incubated with 0.5 µM TMA-DPH in PBS for 20 min at 4°C. Following incubation, cells were washed in PBS and imaged using the ACAS spectrofluorophotometer.
The fluorescent probe was excited using the UV band (351–364 nm) of an argon laser. The vertically and horizontally polarized components of the fluorescence emission were simultaneously recorded, using a 50/50 beam splitter to direct half of the fluorescence to each of the two-photomultiplier tube (PMT) detectors. The polarized components were isolated using a vertically polarized filter placed in front of detector 1 and a horizontally polarized filter in front of detector 2. The fluorescence anisotropy (r) was calculated from the fluorescence emission as follows:
r = (Iv − Ih)/(Iv + 2Ih) = (A − 1)/(A + 2)
where Iv and Ih are the vertical and horizontal fluorescence intensities measured by detector 1 and detector 2 respectively, and A equals the ratio of the two emissions, Iv/Ih. The ACAS ratio software was used to automatically convert the ratio A to anisotropy using a standard curve.
Acknowledgements
We are grateful to Drs S. Salcedo, S. Garvis, T. Henry and S. Méresse for critically reading the manuscript. We are grateful to Drs J. Davoust (Curie Institute, Paris), M. Pierres (CIML, Marseille) and S. Méresse (CIML, Marseille) for providing antibodies. This work was supported by institutional grants from the CNRS, INSERM, and ARC (Grant N7541) and by a grant from the EU (NOVELTARGETSVACCINES).