Extrusion of misfolded and aggregated proteins – a protective strategy of aging neurons?
Abstract
Cellular senescence is the consequence of repetitive exposures to oxidative stress, perturbed energy homeostasis, accumulation of damaged proteins and lesions in their nucleic acids. Whereas mitotic cells are equipped with efficient cell replacement strategies; postmitotic neurons have - with a few exceptions - no mechanism to substitute dysfunctional cells within a complex neuronal network. Here we propose a potential strategy by which aging neurons contend against abnormal accumulation of damaged/misfolded proteins. The suggested mechanism involves the formation of ‘budding-like’ extrusions and their subsequent clearance by glia. This hypothesis emerged from our previous investigations of the aged hippocampus revealing layer-specific accumulations of Reelin, a glycoprotein with fundamental roles during brain development and adult synaptic plasticity. We showed that Reelin deposits constitute a conserved neuropathological feature of aging, which is significantly accelerated in adult wild-type mice prenatally exposed to a viral-like infection. Here, we employed two- and three-dimensional immunoelectron microscopy to elucidate their morphological properties, localization and origin in immune challenged vs. control mice. In controls, Reelin-positive deposits were dispersed in the neuropil, some being engulfed by glia. In immune challenged mice, however, significantly more Reelin-immunoreactive deposits were associated with neuritic swellings containing mitochondria, vacuoles and cellular debris, pointing to their intracellular origin and suggesting that ‘budding-like’ neuronal extrusions of misfolded proteins and glial clearance may represent a protective strategy to counteract aging-associated impairments in proteosomal/lysosomal degradation. Neurons exposed to chronic neuroinflammation with increased levels of misfolded/damaged proteins, however, may fail to combat intraneuronal protein accumulations, a process probably underlying neuronal dysfunction and degeneration during aging.
Introduction
With advancing age, neurons are confronted with numerous cellular alterations, including increased oxidative stress, impaired mitochondrial functions and cellular energy metabolism, as well as perturbations in intracellular calcium signaling and the abnormal accumulation of damaged/misfolded proteins, nucleic acids and organelles (Mattson & Magnus, 2006; Stranahan & Mattson, 2012). One of the proteins strongly affected by aging is Reelin, a conserved extracellular glycoprotein that not only plays a critical role in proper neuronal positioning during cortical development (D’Arcangelo et al., 1995; Frotscher, 1998), but also modulates synaptic function and plasticity in adulthood (Weeber et al., 2002; Beffert et al., 2005) (for recent reviews see Doehner & Knuesel, 2010; Knuesel, 2010). Interestingly, normal aging in several species is accompanied by loss of Reelin-expressing neurons and concomitant accumulation of Reelin-positive granular aggregates in the hippocampal formation (Knuesel et al., 2009; Madhusudan et al., 2009; Doehner et al., 2010; Kocherhans et al., 2010). This aging-related process is dramatically accelerated in wild-type animals that were prenatally exposed to the viral mimic PolyI:C (polyriboinosinic-polyribocytidilic acid), resulting in much earlier accumulations of Reelin along the perforant path in the adult hippocampus (Knuesel et al., 2009). Intriguingly, these Reelin-positive deposits have been shown to also contain amyloid precursor protein (APP)-derived proteolytic fragments, including amyloid-beta (Aβ) peptides both in transgenic and in wild-type mice (Doehner et al., 2010), as well as in human post-mortem Alzheimer’s disease (AD) brain tissue (IK, TN, unpublished observations).
Ultrastructurally, the cellular and subcellular localization of Reelin in the developing and adult brain has been investigated in several mammalian species, including mice (Pappas et al., 2001), rats (Pesold et al., 1998a), non-human primates (Pesold et al., 1998b; Rodriguez et al., 2000; Martinez-Cerdeno et al., 2003), ferrets (Martinez-Cerdeno et al., 2003) as well as humans (Roberts et al., 2005). These studies revealed a consistent picture of Reelin being readily detectable in the extracellular space, localised to neuronal somata, axonal processes, as well as in dendritic shafts and spines; findings that are in line with the role of Reelin in normal synaptic function, including synaptic plasticity and dendritic remodeling in the adult brain (Niu et al., 2004, 2008; Jossin & Goffinet, 2007; Chameau et al., 2009). Here, we extended these studies by investigating the ultrastructural properties of the Reelin-positive extracellular deposits in aged mice by applying two- (2D) and three-dimensional (3D) immunoelectron microscopy to gain a more precise insight into their morphology, localization and origin. In addition, to investigate putative inflammation-induced morphological differences, we compared hippocampal brain sections of aged immune challenged (PolyI:C) mice and their saline-exposed counterparts. Our findings revealed that the Reelin-positive granules in PolyI:C mice were significantly larger and more frequently contained mitochondria, vacuoles and cellular debris as compared with age-matched controls. In line with this, Reelin deposits were abundant in axonal and dendritic varicosities in immune-challenged versus saline mice. 3D serial imaging also revealed striking granular fusion events or interconnections between granules and neurites, providing the first evidence that the Reelin-positive deposits originate intracellularly. The observation of individual granules being surrounded and engulfed by glia suggests that these structures are extruded into the neuropil, potentially involving a ‘budding-like’ mechanism which might represent a defense mechanism against abnormal accumulations of neurotoxic proteins in postmitotic neurons. We discuss the significance of these findings specifically focusing on the origin and implications of Reelin protein deposition in the aging brain, followed by a more speculative interpretation of our findings in the context of sporadic AD.
Materials and methods
Animals
All procedures were approved by the local authorities of the Cantonal Veterinary Office in Zurich and were in agreement with the Principles of Laboratory Animal Care (NIH publication No. 86-23, revised 1985). All animals were housed in same-sex groups of 3–4 in an optimised in-house hygiene area (OHB, University of Zurich Irchel, Switzerland) under 12-h day–night cycle and ad libitum food and water. C57BL/6J mice were obtained from the breeding facility of the Institute of Laboratory Animal Science, University of Zurich (LTK Fuellinsdorf).
PolyI:C injections
Pregnant mouse dams of the C57Bl/6J strain were given a single intravenous injection of 5 mg/kg polyriboinosinic-polycytidilic acid (PolyI:C potassium salt, P9585–50 mg; Sigma-Aldrich, Buchs, Switzerland) dissolved in 0.9% saline with an injection volume of 5 mL/kg body weight or an equivalent volume of saline at gestation day 17. The animals were mildly restrained during the injection procedure using an acrylic mouse restrainer and immediately returned to their home cage. The offspring was weaned at 3 weeks of age and housed in groups of 4–5 siblings of the same sex. Brain tissue was collected at the age of 9 (n = 3) and 15 months (n = 3–4) per treatment group for the ultrastructural characterization of Reelin-positive plaques.
Preembedding immunoelectron microscopy
Mice were anesthetised with Nembutal (40 mg/kg body weight; i.p.) and perfused with a fixative containing 4% paraformaldehyde, 0.2% picric acid and 0.1% glutaraldehyde in phosphate buffer (PB; 0.1 m, pH 7.4). Brains were removed and post-fixed in the same fixative for 5 h at 4 °C, washed and stored in PB overnight. Coronal sections (70 μm) were cut on a Vibratome, and the hippocampi were dissected and cryoprotected in 30% sucrose in 0.1 m PB overnight at 4 °C. To enhance antibody penetration, the sections were frozen and thawed rapidly three times in the same sucrose solution by using liquid nitrogen. They were then collected and washed in Tris-buffered saline (TBS, pH 7.49) and blocked for 2 h in TBS containing 10% normal goat serum at room temperature (RT). The sections were processed for immunohistochemistry using Fluoronanogold labeling. Staining was done using the mouse anti-Reelin (clone G10, 1 : 1000, MAB5364; Millipore) primary antibody, a monoclonal antibody raised against amino acids 164–496 in the N-terminal domain of the rodent protein that recognises the full-length (∼400 kDa) form and two N-terminal products (∼300 and ∼180 kDa) on Western blots of wild-type but not Reelin-knockout (reeler) mouse brain samples (Lambert de Rouvroit et al., 1999). The antibody, previously employed in our immunohistochemical (Knuesel et al., 2009; Madhusudan et al., 2009; Kocherhans et al., 2010) and immunoelectron analyses (Doehner et al., 2010), was diluted in TBS containing 3% normal goat serum and 0.05% sodium azide, incubated for 72 h at 4 °C under constant agitation. After washing in TBS, sections were incubated in secondary immunogold-conjugated antibodies (1 : 200; Alexa Fluor-488-Fluoronanogold-Fab, Nanoprobes, Yaphank, NY, USA) diluted in TBS containing 3% normal goat serum overnight at 4 °C. The latter immunoprobe consisted of a 1.4-nm gold particle conjugated with goat anti-mouse Fab-fragment and fluorescein. For optimal visualization of the 1.4-nm gold particles at the electron microscopic level, specimens were processed for gold enhancement using the GOLDENHANCETM kit following the manufacturer’s protocol (Nanoprobes). After the enhancement, samples were kept in Na-cacodylate buffer (0.1 m, pH 7.4) overnight. Finally, sections were post-fixed for 15 min in 0.5% OsO4 (on ice), counterstained with 1% uranyl acetate for 30 min at RT, dehydrated in graded acetone and flat embedded in Epon812 (Science Services, Munich, Germany). The Epon812 polymerization was achieved by incubation for at least 48 h at 60 °C. Ultrathin sections (50 nm) were cut on an ultramicrotome (EM UC6; Leica Microsystems) and collected on adhesive-coated nickel grids (300 mesh). As controls, some brain sections were processed without immunolabeling. After cutting and dissecting the hippocampi, these sections were directly collected in Na-cacodylate buffer (0.1 m, pH 7.4) and processed without antibodies as described above. Before imaging, ultrathin sections were contrasted in 2% lead citrate solution for 10 min at RT. Ultrathin sections were examined using a Philips CM100 transmission electron microscope equipped with a side-mounted CCD camera (4k × 3k; Gatan Bioscan GmbH, Munich, Germany) and images were acquired with the software program digital micrograph (Gatan Inc., Pleasanton, CA, USA). Cropping of images and adjustments of brightness and contrast were achieved using adobe photoshop (Adobe Systems, San Jose, CA, USA).
Tissue preparation for serial block-face scanning electron microscopy
For 3D reconstruction of the granular structures, the electron microscopy protocol was adjusted slightly. Prenatal PolyI:C-exposed mice were perfused at 15 months of age with a fixative containing 4% paraformaldehyde, 0.2% picric acid and 2% glutaraldehyde in PB (0.1 m, pH 7.4). Brains were removed and post-fixed in the same fixative for 3 h at 4 °C, washed and stored in PB overnight. Coronal sections (70 μm) were cut on a Vibratome, and the hippocampi were dissected and washed in Na-cacodylate buffer (0.1 m, pH 7.4). The sections were then postfixed in reduced osmium (1.5% KFeCN, 1% OsO4 in 0.1 m Na-cacodylate buffer) for 30 min at RT, followed by post fixation in 1% osmium diluted in 0.1 m Na-cacodylate buffer for 30 min at RT. Subsequently, the sections were subjected to counterstaining in 1% uranyl acetate solution for 30 min at RT, dehydrated in increasing acetone concentration and flat embedded in Durcupan ACM (Science Services). The Durcupan polymerization was achieved by incubation for at least 24 h at 60 °C. The ultrastructure of the granules was investigated using serial block-face scanning electron microscopy (SBF-SEM), which is suited for fast data collection from larger samples. The region of interest was mapped by light microscopy, cut out of the resin and mounted on a Plexiglas stub. The block was trimmed to obtain a trapezoid block of maximally 500 × 500 μm and placed in the SBF-SEM system, consistent of a microtome (3VIEW; Gatan) mounted in a scanning electron microscope (Quanta200VP-FEI; Endhoven, the Netherlands). The surface of the block was imaged and cut using a diamond knife in alternation to obtain a stack of images. Once the surface was scanned, 80 nm of tissue was removed and the fresh surface imaged. In the present work, imaging was performed at an accelerating voltage of 4.5 kV (spot size 3) in low-vacuum mode (0.25 T water pressure) and images were taken as a mosaic of 3 × 3 fields of view (2048 × 2048 pixels) with an overlap of 10%. As control, ultrathin sections (50 nm) were collected on adhesive-coated nickel grids (300 mesh) and examined using a Philips CM100 transmission electron microscope equipped with a side-mounted CCD camera (4k × 3k; Gatan Bioscan). All images were acquired and equalised for contrast in digital micrograph (Gatan). Cropping of images and adjustments of brightness and contrast were achieved using imagej software and adobe photoshop.
Tissue preparation for immunohistochemistry
Wild-type mice exposed prenatally either to PolyI:C or NaCl were deeply anesthetised at the age of 15 months (Nembutal; 40 mg/kg body weight; i.p.) and transcardially perfused through the ascending aorta with 4% paraformaldehyde and 15% saturated picric acid in phosphate-buffered saline (PBS). After postfixation in the same fixative for 4 h at 4 °C, the brains were cryoprotected in 30% sucrose. Free-floating sections (40 μm) were cut coronally on a sliding microtome and stored at –20 °C in cryoprotectant solution until further processing. A 30-min pepsin pretreatment (0.15 mg/mL in 0.2 m HCl at 37 °C) was applied to all free-floating sections and subsequently employed for double-immunofluorescence staining as described previously (Kocherhans et al., 2010) using the following antibodies: mouse anti-rodent Reelin (clone G10; MAB5364; Millipore; 1 : 1000) and rabbit anti-myelin-basic protein (MBP; AB980, Chemicon, , 1 : 200). Brain sections were incubated overnight at 4 °C in the primary antibody solution diluted in PBS containing 2% normal goat serum and 0.2% Triton X-100. After three washes in PBS, tissue sections were incubated for 30 min at RT in the corresponding secondary antibodies conjugated to Alexa488 (1 : 1000; Molecular Probes, Eugene, OR, USA) and Cy3 (1 : 500) and processed as described (Kocherhans et al., 2010). Brain sections were then mounted and air-dried in the dark and cover slipped with aqueous permanent mounting medium (Dako). Double immunofluorescence labelings were visualised by confocal microscopy (LSM-710; Zeiss, Jena, Germany) using a 40× (NA 1.3) and 63× (NA 1.4) objective and sequential acquisition of separate channels. Z-stacks of consecutive optical sections (1024 × 1024 pixels, spaced 0.5–1 μm in z) were acquired. For visual display, Z-sections of all channels were summed and projected in the z-dimension (maximal intensity) and merged using the image analysis software imaris (Bitplane, Zurich, Switzerland). Cropping of images and adjustments of brightness and contrast were identical for each labeling and were done using adobe photoshop.
Silver staining
For the detection of dystrophic neurites, and extracellular and intracellular proteinaceous aggregates, the FD NeuroSilver Kit II (FD NeuroTechnologies Inc., Ellicott City, MD, USA) was used. Free floating perfusion-fixed brain slices (40 μm) prepared for normal immunohistochemistry were processed according to the manufacturer’s instruction with slight modifications. Here, mounted instead of free-floating sections were employed, and washing was carried out in PBS instead of ddH2O to prevent shriveling and damage of the tissue sections.
Quantification of size and mitochondria in granular structures
Quantitative analyses of the area size of the Reelin-positive granules and the amount of granules associated with mitochondria were done on randomly selected granules from three to six electron micrographs of six NaCl- and seven PolyI:C-exposed animals (including 9- and 15-month-old animals). A total of 62 (NaCl group) and 69 granules (PolyI:C group) were outlined and the area was measured using ImageJ software. In addition to the size, the granules were also evaluated regarding the presence of mitochondria. Histograms and pie charts were composed using Microsoft Office Excel.
Statistical analysis
Statistical analysis was performed using the software statview version 5.0 (Abacus Concepts, Inc., Berkeley, CA, USA). The nonparametric Kolmogorov–Smirnov (KS) test was used to statistically compare the size distribution of the Reelin-positive granules between the two treatment groups. anova with Age and Treatment as main between-subject factors was performed to statistically compare the mean size and frequency of granules containing mitochondria in saline vs. PolyI:C mice. Statistical significance was set at P < 0.05.
Results
We have previously reported that Reelin accumulates in granular-like deposits in the hippocampal formation during aging in several species (Knuesel et al., 2009; Madhusudan et al., 2009; Doehner et al., 2010; Kocherhans et al., 2010). Our ultrastructural analysis confirmed the presence of Reelin in extracellular spherical deposits of 1–3 μm in size, containing distinct fibrillary material in their center, and being largely devoid of any organelles (Doehner et al., 2010). Besides Reelin, these globular structures were also immunoreactive for several APP-derived proteolytic fragments, including N-terminal sAPP and Aβ species (Doehner et al., 2010), indicating the accumulation of multiple, aggregation-prone proteins and peptides. Based on our findings that a prenatal immune challenge accelerates the formation of Reelin-positive deposits (Knuesel et al., 2009), we explored here their origin and localization and investigated whether the long-term effects of the systemic infection involve alterations in their morphology. We employed 9- and 15-month-old wild-type mice that were exposed to a single viral-like infection using PolyI:C in utero (gestation day 17). Prenatal exposure of wild-type mice to the same volume of saline (NaCl) served as control. Immunoelectron microscopy using anti-Reelin antibodies (clone G10) confirmed the selective accumulation of Reelin in the hippocampal CA1 area, most prominently in the stratum radiatum and lacunosum-moleculare (Fig. 1). In PolyI:C-exposed mice, the Reelin-positive granules were significantly larger and were more frequently associated with mitochondria as compared with control mice (Fig. 1). Statistical analysis of the size distribution yielded a significant treatment effect (KS test: Dmax = 0.3, χ2 = 13.0, P = 0.003), with the mean granular area shifted towards larger values in PolyI:C compared with NaCl mice (Fig. 1C). anova of the numerical density of granules containing mitochondria confirmed the long-term consequences of a prenatal PolyI:C exposure and yielded a significant main effect of Treatment (F1,9 = 65.3, P < 0.001). In addition, it revealed a significant effect of Age (F1,9 = 5.4, P = 0.04) and an Age × Treatment interaction (F1,9 = 24.3, P < 0.001), indicating that the phenotype significantly aggravated during aging in the PolyI:C compared with the NaCl mice.
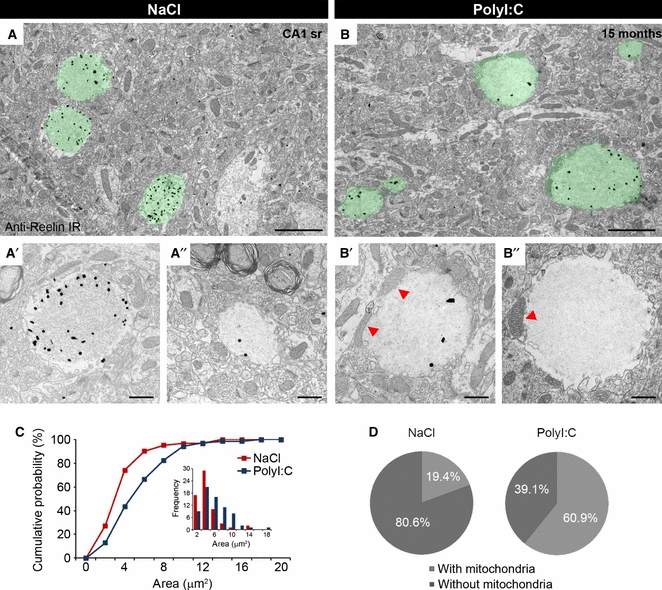
Enrichment of mitochondria in Reelin-positive granules in aged PolyI:C mice. Immuno-electron microscopy of hippocampal brain tissue obtained from prenatal NaCl- (A) and PolyI:C-exposed mice (B) at 15 months of age, processed for Fluoronanogold labeling. The long-term effect of prenatal PolyI:C administration on granule morphology was evident by an increase in size and the more frequent presence of mitochondria within the granules (B, red arrowheads). (C) This morphological evaluation was confirmed by statistical analysis, which yielded a significant treatment effect (KS test: χ2 = 13.0, P = 0.003). (D) The increase in size was accompanied by a significantly higher number of mitochondria detected within the granules in PolyI:C compared with NaCl subjects (anovaTreatment: F1,9 = 65.4, P < 0.001). In NaCl subjects (n = 6), the proportion of individual granules containing mitochondria reached 19.4%, while in PolyI:C mice (n = 7), almost two-thirds of the granules (60.9%) were associated with mitochondria. Scale bars: A, B = 2 μm; A′, A″, B′, B″ = 500 nm.
To explore the origin and precise localization of the mitochondria, we applied 3D SBF-SEM (Leighton, 1981; Denk & Horstmann, 2004), a technique allowing better visualization and reconstruction of the source of cellular organelles. Using this approach, we detected a distinct association of Reelin-positive granules with neurites, pointing to ‘budding-like’ extrusions in aged PolyI:C mice (Fig. 2A and C). Using a higher glutaraldehyde concentration than employed for regular immunoelectron microscopy, we achieved a much better structural preservation, which allowed the detection of continuous membranes and distinct fibrillary structures that were denser compared with their appearance at lower fixation condition (compare Fig. 1B and 2B). Importantly, the optimised tissue processing allowed the reconstruction of individual granular structures in three dimensions. The sequence of 24 serial sections obtained by SBF-SEM of a 15-month-old PolyI:C mouse revealed that granules were interconnected (Fig. 3B, image C10) or connected to dendritic structures (Fig. 3C, image C18), confirming that the granular Reelin structures represent extrusions of neuritic compartments, a process that in PolyI:C subjects appears to be strongly exacerbated compared with saline controls (Knuesel et al., 2009), thereby increasing the likelihood of detecting ‘budding-like’ events.
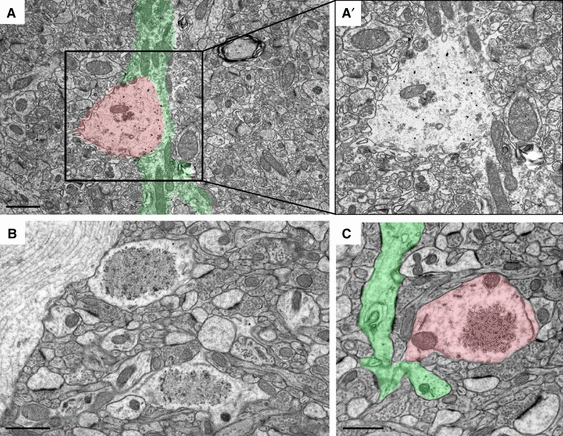
Reelin granules are found within neuritic compartments. Representative immunoelectron micrographs taken from Fluoronanogold-labeled hippocampal brain sections of 10- (A) and 15-month-old (B and C) PolyI:C subjects using anti-Reelin antibody (G10). (A) Distinct granular deposits (red overlay) along an unmyelinated axonal process green overlay) were detected in the CA1 stratum lacunosum-moleculare, indicative of axonal swellings or putative ‘budding’ events. (A′) Higher magnification of the boxed area outlined in A. (B) Increased glutaraldehyde concentration (2% vs. 0.1%) and Durcupan embedding resulted in optimal tissue preservation and detection of granular structures. The optimised protocol allowed the detection of continuous membranes surrounding the fibrillary material in the center of the granules (compare with Fig. 1). (C) Higher magnification image revealing the interconnection between a dendrite (green) and a granular structure (red), pointing to potential extrusion of fibrillary material from neuritic compartments. Scale bars: A–C = 1 μm.
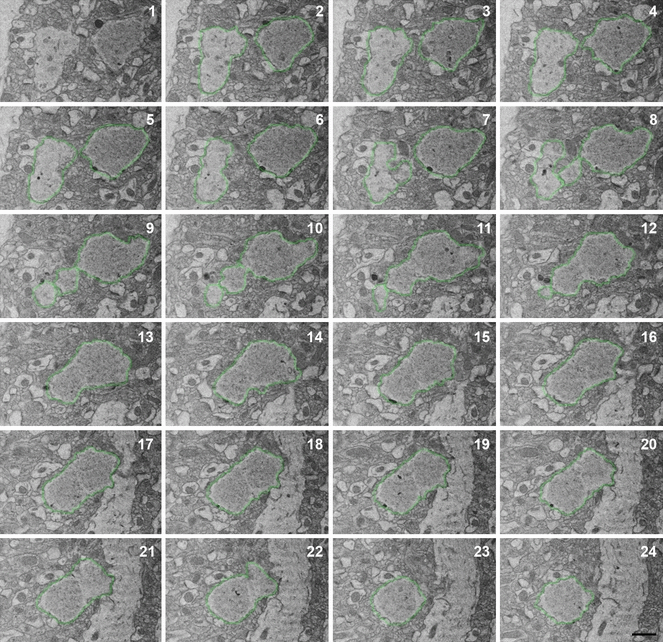
Visualization and reconstruction of the 3D structure of individual granules. Twenty-four consecutive sections taken from the CA1 stratum radiatum in the dorsal hippocampus obtained by SBF-SEM showing the appearance of interconnected granular structures (outlined in green). Scale bar = 1 μm.
To test this assumption, we also performed a light microscopical analysis of hippocampal brain sections obtained from 15-month-old PolyI:C- and NaCl-exposed mice. Based on the selective enrichment of the Reelin deposits in the terminal zone of perforant path afferents we focused on CA1 stratum lacunosum moleculare (Knuesel et al., 2009; Doehner et al., 2010) and performed double-immunofluorescence staining using anti-MBP and anti-Reelin antibodies. Reelin-positive granular structures were closely associated but not co-localised with myelinated axons in NaCl subjects (Fig. 4A). In contrast, a strong enrichment of Reelin immunoreactivity in myelinated axons was detected in the PolyI:C subject (Fig. 4B), accompanied by the accumulation of Reelin within axonal compartments (Fig. 4C). Hence, we hypothesised that the intraneuronal accumulation of proteins and organelles, probably linked to impaired proteasomal and autophagosomal clearance (Mattson & Magnus, 2006), induces also a failure in transport processes. The protein extrusion of misfolded and aggregated proteins might therefore represent a protective mechanism.
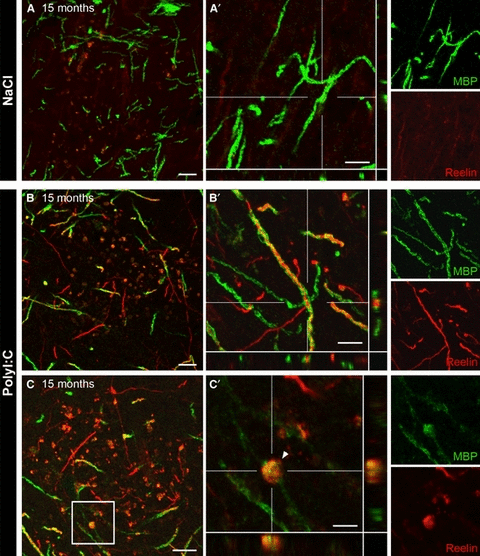
Reelin accumulates in myelinated axons in aged PolyI:C mice. (A–C) Representative confocal images of double-immunofluorescence staining of brain sections obtained from 15-month-old NaCl and PolyI:C subjects using mouse anti-Reelin (red) and rabbit anti-MBP (myelin basic protein, green) antibodies. (A) No Reelin immunoractivity was observed in axonal projections in saline (NaCl)-treated mice. (B) In contrast, Reelin immunoreactivity was strongly enriched in myelinated axons in aged immune challenged PolyI:C animals. Note the appearance of granular structures at the terminals of the axonal projections (C), pointing to their intracellular accumulations. (C′) Higher magnification of the boxed area outlined in C containing also the xz- and yz-views shown at the bottom and right side (also included in A′ and B′). Scale bars: A–C = 10 μm, A′, B′ = 5 μm, C′ = 3 μm.
To investigate this possibility, we examined the extent of neurodegenerative processes in aged PolyI:C-exposed subjects compared with controls. Evidence for abnormal protein accumulation was provided by the observation that Reelin immunoreactivity was frequently associated with granules containing significant amounts of cell debris, swollen vacuoles, dispersed neurofilaments, disintegrated mitochondria and other undefined structures indicative of neuronal degeneration (Fig. 5A) in PolyI:C subjects. In line with the ultrastructural findings, silver staining of hippocampal brain sections of 15-month-old PolyI:C-exposed animals selectively and very prominently labeled the granular deposits (Fig. 5B–C), whereas no or only very weak silver precipitates were found in age-matched controls (data not shown). Also in agreement with the EM data, the silver staining was restricted to the granular structures and associated with neuritic compartments, indicating that degenerative processes did not involve the whole cell or neighboring neurons at this stage.
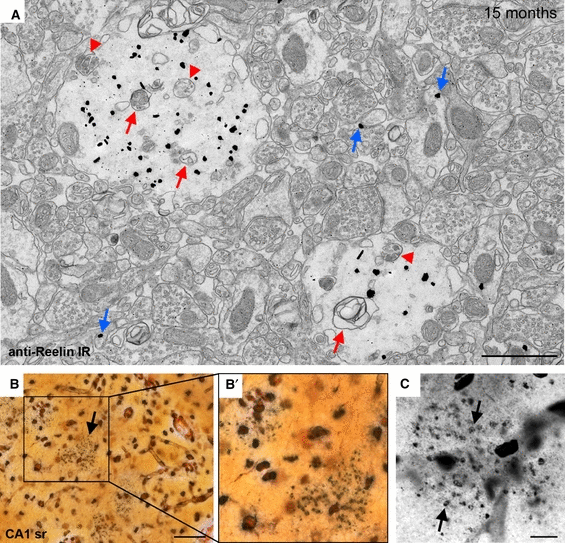
Reelin-positive granules show characteristics of degenerative processes. (A) Representative immunoelectron micrograph taken from Fluoronanogold-labeled hippocampal brain sections of a 15-month-old NaCl subject using the anti-Reelin antibody (G10). Signs of neurodegeneration were evident by the presence of cell debris, including disintegrated mitochondria (red arrows) and multivesicular bodies (red arrowheads) within the Reelin-positive granules. Reelin immunoreactivity was also detected in extracellular space and/or associated with spines (blue arrows) in line with previous EM studies. (B and C) Degenerative features were also clearly evident at the light microscopic level in aged PolyI:C-exposed subjects. Brain sections obtained from a 15-month-old PolyI:C-exposed animal processed for silver staining revealed distinct silver precipitates within the granules and associated neuritic compartments (black arrows), indicative of degeneration. Scale bars: A = 1 μm, B = 50 μm, C = 10 μm.
Based on the preferential neuropil localization of Reelin-positive granules in NaCl mice and our previous findings showing a close association of Reelin-positive granules with glia and perivascular astrocytic end-feet in aged wild-type mice (Knuesel et al., 2009; Doehner et al., 2010), we analysed the specificity of this association using immunoelectron microscopy. Qualitative examinations provided first evidence for the presence of fine gliofilaments in the periphery of some granules and bundles of glial filament processes (astrocytic processes) passing around individual granules (Fig. 6A, gf). Moreover, Reelin-positive granules were observed in glial somata/cytoplasm or in close association with microglia and astrocytes (Fig. 6B and C), indicating that completely extruded protein deposits were cleared by glia cells through phagocytosis, thereby potentially preventing progressive neurodegeneration induced by intraneuronal accumulations of Reelin and other misfolded/damaged proteins.
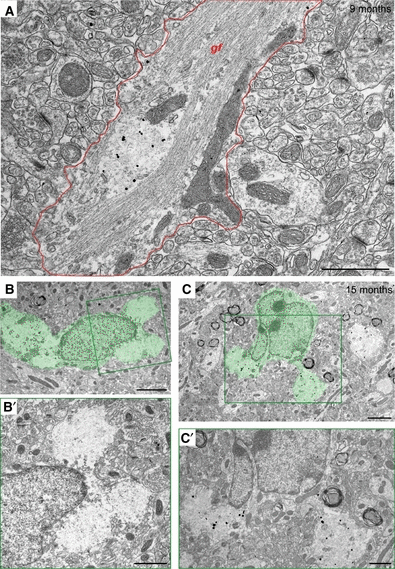
Fibrillary granules are associated with glial cells. Ultrastructural investigation of the association of Reelin-positive granules with astrocytes and microglia revealed the presence of fine gliofilaments (gf) in astrocytic processes (outlined in red) passing around an individual Reelin-positive granule in a NaCl-exposed mouse (9 months) as shown by Fluoronanogold labeling (A). Granules were also observed in the somata/cytoplasm of glial cells as seen in 15-month-old NaCl (B) and PolyI:C (C) subjects (green), indicative of clearance of the extruded fibrillary material by glia. (B′ and C′) Enlarged views of the boxed areas (green) in B and C, respectively, showing two fibrillary granules in the cytoplasm of glia cells. Scale bars: A, B′, C′ = 1 μm, B, C = 2 μm.
Discussion
Here, we complement and extend our previous studies, showing that a prenatal viral-like infection using the Toll-like receptor 3 agonist PolyI:C has a significant long-term effect on adult neuronal health by accelerating and aggravating aging-associated accumulations of misfolded proteins in the hippocampus. Our EM analyses revealed that the PolyI:C-exposed mice showed significantly larger deposits as well as a significantly higher frequency of mitochondria, vacuoles and cell debris located within Reelin-positive granules as compared with saline controls. Their close association with neuritic swellings and varicosities provides support for inflammation-induced neurodegenerative processes and point to the – rather unexpected – intracellular origin of Reelin-positive granular structures. In line with this, our 3D serial EM imaging revealed interconnected granular structures and distinct conjunctions between granules and neurites, suggesting fusion and/or incomplete extrusion events. It is tempting to speculate that the ‘budding-like’ exclusions might represent a protective reaction of postmitotic neurons to counteract aging-associated impairments in proteosomal/lysosomal protein degradation and the accumulation of neurotoxic protein aggregates. Chronic inflammatory conditions paired with aging-associated impairments in lipid and cholesterol metabolism, however, are expected to result in a complete failure of protein clearance, disruption of transport and signaling, degeneration of the affected neurite, and ultimately death of the neuron.
Below we discuss our findings with a specific focus on the relevance of Reelin-positive granular deposits in the aging brain, followed by a more speculative interpretation of the generality of our findings in the context of sporadic AD.
Ultrastructural analysis of Reelin deposits in aged wild-type versus immune challenged mice
Reelin-positive granules are of intracellular origin
Here we provide further ultrastructural evidence that corroborate our previous observations (Knuesel et al., 2009; Madhusudan et al., 2009; Doehner et al., 2010; Kocherhans et al., 2010) showing that in aged NaCl-exposed wild-type mice, most of the fibrillary Reelin-positive granules detected in the CA1 lacunosum-moleculare or radiatum are dispersed in the neuropil, sharing several ultrastructural features with polyglycan bodies (PGs), which have been described in senescence accelerated mice (SAMP8) as well as aged C57Bl/6 mice (Lamar et al., 1976; Akiyama et al., 1986; Mandybur et al., 1989; Jucker et al., 1992, 1994a; Jucker & Ingram, 1994; Mitsuno et al., 1999). Their morphological and biochemical features including the positive reaction using Periodic Acid Schiff staining (Kuo et al., 1996), as well as the presence of several extracellular matrix proteins and proteoglycans [e.g. heparan sulfate proteoglycan, (HSPG)] (Jucker et al., 1994a) have led to the conclusions that the PGs are of extracellular origin. Newer studies involving SAMP8 mice, however, have described the localization of a multitude of intracellular proteins, including mitochondrial components in the PGs (Nakamura et al., 1995; Del Valle et al., 2010; Kern et al., 2011; Manich et al., 2011), which is in line with the data presented here, pointing to their intracellular origin and putative extrusion into the extracellular matrix. Interestingly, HSPGs coexist with Aβ in the cores of many senile plaques found in AD brains (Snow et al., 1988; Snow & Wight, 1989; Narindrasorasak et al., 1991; Su et al., 1992; Armstrong, 2006). This is very similar to the localization of Reelin-positive granules in the core of Aβ plaques in transgenic AD mice (Doehner et al., 2010), supporting the idea that (i) Reelin is a major component of the PGs, and (ii) these structures may serve as seed for aggregation-prone peptides and facilitate the development of amyloid plaques along axonal varicosities in aged individuals.
Chronic inflammation accelerates the formation of Reelin deposits
In line with this assumption, our light as well as 2- and 3D electron microscopical investigations revealed the accumulation of Reelin in varicosities in dendritic and axonal compartments, particularly prominent in aged immune challenged mice. The morphological features of ‘budding’ and fusion events that probably contribute to the significant increase in size, as well as the significant increase in mitochondria, vacuoles and cellular debris within the Reelin-positive granules in aged PolyI:C compared with NaCl mice not only confirm their intracellular origin, but also indicate that these varicosities form at a much higher rate under chronic inflammatory conditions. This observation is in agreement with our previous study in which we provided first evidence that a prenatal immune challenge during late gestation results in significant acceleration of aging-associated neuropathological alterations in non-transgenic wild-type mice (Knuesel et al., 2009). The hypothesis also fits with the immunohistochemical data reported here showing that Reelin accumulates in myelinated axons in aged PolyI:C mice. It is conceivable that the chronic inflammatory state in these mice (Krstic et al., 2012) induces demyelination and thereby further facilitates the accumulation of misfolded/damaged proteins and organelles in axonal varicosities. This interpretation also fits with post-mortem analyses in AD patients showing that focal swellings, which develop along the axons and dendrites (Nixon et al., 2005; Yu et al., 2005; Nixon, 2007; Shacka et al., 2008), typically include significant amounts of accumulated organelles, likely related to impaired autophagic–lysosomal clearance mechanisms (Morfini et al., 2009). Our ultrastructural findings, highlighting the abundant presence of disintegrated mitochondria and enlarged multivesicular bodies in Reelin-positive granules, suggest that chronic inflammation may impair the autophagic–lysosomal pathway, thereby inducing an increase in misfolded and aggregated proteins.
Reelin deposits are associated with glial cells
We previously reported a close association between Reelin-positive granules and astrocytes and microglia cells (Knuesel et al., 2009; Madhusudan et al., 2009; Doehner et al., 2010), in line with reports assessing the relationship between PGs and glia (Akiyama et al., 1986; Jucker et al., 1992, 1994b; Kuo et al., 1996; Mitsuno et al., 1999; Knuesel et al., 2009; Del Valle et al., 2010; Doehner et al., 2010; Manich et al., 2011). Here we extend these findings using immunoelectron microscopy and show that individual Reelin deposits are being engulfed by glia cells, indicating that the aggregated, extruded fibrillary material is cleared by surrounding glia. However, we have also observed that glia seem incapable of phagocytosing larger deposits (Madhusudan et al., 2009), suggesting that the increase in number and size of Reelin-positive granules in PolyI:C compared with NaCl mice may not only be due to overproduction of protein deposits but also related to impaired clearance through glia cells. Altogether, this scenario is expected to further aggravate the neuropathology by favoring the accumulation of other aggregation-prone proteins.
Damaged/misfolded protein extrusion – an adaptation of a developmental process?
During brain development, the large neuronal glycoprotein Reelin is constitutively secreted from the ramified axonal plexus of Cajal Retzius (CR) cells in the marginal zone to coordinate the lamination of the developing cortex (D’Arcangelo et al., 1995; Frotscher, 1998; Derer et al., 2001). The prominent localization of Reelin in cisterns of the rough endoplasmic reticulum (RER) in the axonal cones of CRs, named ‘axonal reservoirs’ (Derer et al., 2001), is believed to promote efficient delivery and diffusion of Reelin, as well as induction of the signal transduction via the very low-density lipoprotein receptor (VLDLR) and apolipoprotein E receptor 2 (ApoER2) located on the apices of cortical cells. Interestingly, in reeler orleans (RelnOrl) mice, which produce a C-terminally truncated form of Reelin that aggregates intracellularly and therefore is not secreted (de Bergeyck et al., 1997), the RER cisterns are heavily distorted and hugely dilated. They are accumulating in numerous axonal varicosities each containing spheroidal cisterns filled with Reelin-positive fibrillary material and covering a size of up to 3 μm in diameter (Derer et al., 2001). This distinct axonal phenotype in RelnOrl mice, which also includes a significant increase in the numerical density of the axonal beads compared with wild-type mice, is not seen in full reeler knockout mice (Derer et al., 2001), indicating that the neuropathology is not due to a loss of Reelin function but directly related to the accumulation of the truncated protein. Based on the striking similarity between the morphological features of the Reelin-positive granular structures reported here and the ultrastructural descriptions of the axonal phenotype of the RelnOrl mice (Derer et al., 2001), we suggest that the mechanism of bulk transport and secretion of RER-derived cisterns along axons or dendrites of adult neurons may represent an adaptation of the process utilised by the developing brain. It is conceivable that via this mechanism, adult neurons might have a way to extrude damaged/misfolded protein, constituting a strategy to counteract impairments in autophagosomal/lysosomal clearance and prevent abnormal accumulations of neurotoxic proteins in postmitotic neurons.
Relevance of chronic inflammation and accumulation of misfolded-protein extrusions – implications for sporadic AD
The impact of systemic infection on the developing brain
Neuroinflammation is not only a critical player in acute central nervous system conditions, such as stroke and traumatic injury, but represents also a central factor in chronic and neurodegenerative conditions, such as AD or Parkinson’s disease. However, despite the growing number of studies linking inflammatory responses to AD pathogenesis (Akiyama et al., 2000; Hoozemans et al., 2006; Wyss-Coray, 2006; Parachikova et al., 2007; Lucin & Wyss-Coray, 2009; Salminen et al., 2009), very little information is available on early neuroinflammatory processes which might possess stronger disease-modifying potential than the Aβ plaque- and neurofibrillary tangle-associated inflammatory responses. Our recent experimental approach provides the first evidence that a prenatal immune challenge during late gestation using the viral mimic and cytokine releaser PolyI:C results in long-term changes in several inflammatory modulators accompanied by a significant acceleration of aging-associated neuropathological alterations in non-transgenic wild-type mice (Knuesel et al., 2009; Krstic et al., 2012), indicating that neuroinflammatory changes can trigger and drive AD-relevant pathophysiological processes. In line with the suggestion that synaptic and cellular dysfunctions occur decades before the manifestation of overt histopathological changes in AD patients (Thal et al., 2004), our data complement this view by pointing to a detrimental long-term effect of inflammatory cytokines and other immune signaling molecules during fundamental developmental processes that appear to reduce restorative capabilities of neurons during aging. It is conceivable that the acute exposure of several inflammatory cytokines during late gestation (Meyer et al., 2006) significantly impairs synaptogenesis and interferes with the recently identified function of immune molecules in synaptic pruning and refinement (Paolicelli et al., 2011), in line with previous data showing that the balance between excitatory and inhibitory synaptic activity can be disturbed for extended periods of time following treatment with cytokines during synaptogenesis (Biber et al., 2002; Brask et al., 2004; Hellstrom et al., 2005; Serantes et al., 2006). In addition, it has been demonstrated that several inflammatory mediators can cause hypomyelination (Corbin et al., 1996; LaFerla et al., 2000; Chen et al., 2002; Pang et al., 2003), thereby significantly increase the brain’s susceptibility to axonal degeneration.
The adverse environment induced through a prenatal immune challenge is also expected to involve a detrimental effect on the developing microglia, the macrophage-derived immune cells of the brain that start to colonise the brain during late gestation (Herbomel et al., 2001; Ginhoux et al., 2010). Besides interfering with their fundamental role in immune surveillance and phagocytosing apoptotic neurons (Peri & Nusslein-Volhard, 2008), the prenatal infection-induced expression of inflammatory cytokines and other mediators of the innate immunity may also reduce the proliferative capacity of microglia. In addition, this environmental manipulation may prime microglia, thereby creating an innate memory allowing a faster and exaggerated response upon further immune challenges and exposure to adverse stimuli, changes very similar to mechanisms of the adaptive immune system. This combination is expected to have a devastating effect on neurons during aging by promoting cytokine-mediated demyelination and release of reactive oxygen species through primed microglia on the one hand, and reduced phagocytic activity due to precocious degeneration of microglia on the other. Although offering a relevant theory regarding AD pathogenesis, the molecular mechanisms underlying aging-associated microglia dysfunction are largely unknown. Besides a decline in proteasome activity and other aging-associated impairments in protein homeostasis (Stolzing & Grune, 2003), limited information is available regarding the lifespan of microglia (Streit, 2006). Nevertheless, it is conceivable that repetitive exposures to brain injury and neuroinflammation induce replicative senescence, eventual loss of mitotic activity and degeneration of microglia during aging. Evidence for this hypothesis has been provided by in vitro findings showing that microglia stimulation by mitogens induces telomere shortening (Flanary & Streit, 2004). Recent data also showed that interferon-γ exposure is sufficient to trigger activation-induced microglia cell death (Yun et al., 2011), in line with post-mortem investigations of AD patients showing caspase activity in plaque-associated microglia in AD (Yang et al., 1998).
The long-term consequences of a prenatal infection on brain aging
A primed stage of microglia and the elevated levels of inflammatory cytokines during adulthood and aging are also expected to increase the translational activity of neurons. This hypothesis is based on the findings that interferon-γ and numerous other inflammatory stimuli induce, via the mTor signaling pathway, an increase in protein synthesis (Ma et al., 2006; Reits et al., 2006; Seifert et al., 2010). However, a large fraction of newly generated proteins are defective in folding, translation and/or assembly, probably linked to the effect of oxygen radicals that are the result of the concomitant activation of the inducible nitric oxide synthase (iNOS) system through the inflammatory stimulus (van Deventer & Neefjes, 2010). Seifert et al. (2010) have provided the first evidence that this inflammation-induced pool of damaged proteins is selectively degraded through the immunoproteasome, a fast acting variant proteasome that – in addition to promoting an adaptive immune response through increased antigen presentation – also protects cells from the damaging side effects of an innate inflammatory response (van Deventer & Neefjes, 2010). It is conceivable that chronic elevation of inflammatory cytokines as seen following a prenatal immune challenge (Krstic et al., 2012) impairs the activity of the immunoproteasome in aging neurons, resulting in the accumulation of damaged/aggregated proteins intracellularly. Support for this hypothesis is provided by our present immunohistochemical and ultrastructural findings demonstrating that a prenatal viral-like immune challenge induces the intraneuronal accumulation of Reelin, a large glycoprotein that is normally secreted from perforant path afferents as well as a subset of GABAergic interneurons in the hippocampus (Knuesel et al., 2009). The earlier appearance, higher density, as well as the frequent presence of mitochondria, vacuoles and other degenerative features in Reelin-positive granular structures in aged PolyI:C- compared with NaCl-exposed mice supports a detrimental effect of aggregated proteins on neuronal integrity and function in aging neurons. The hypothesis of an aging-associated impairment in immunoproteasome function is in line with previous findings demonstrating that proteosomal degradation decelerates during aging (Mattson & Magnus, 2006). It will be interesting to investigate whether subsets of neurons show differential expression and activity of the immunoproteasome, potentially providing an explanation of the selective neuronal vulnerability characteristic of neurodegenerative disorders. Moreover, it will be essential to demonstrate a functional decline of this fundamental machinery in different types of neurons during aging.
Extrusion of misfolded proteins as a neuroprotective strategy?
Is it possible that neurons can – as a neuroprotective strategy – extrude toxic protein aggregates? Support for a ‘budding-like’ extrusion mechanism is provided by our 3D EM analysis of Reelin-containing protein aggregates, showing interconnected granules and distinct connections between neurites and granules. Based on these and previous findings from other groups investigating PG bodies, it is tempting to speculate that the postmitotic neurons have adapted a neuroprotective strategy to extrude misfolded proteins, which are then cleared through phagocytic glia cells. Hence, young neurons would be able to counteract the inflammation-induced increase in misfolded/damaged proteins by efficient recruitment of immunoproteosomal/lysosomal pathways, thereby preventing the harmful intraneuronal accumulation of aggregated proteins. During adulthood, neurons exposed to inflammatory stimuli might additionally remove misfolded proteins via an extrusion mechanism, a strategy to counteract the reduced capabilities in proteosomal and lysosomal clearance processes described in aging neurons (Mattson & Magnus, 2006). However, prolonged exposure to inflammatory modulators and impairments in lipid and cholesterol homeostasis during aging may lead to aberrant ‘budding-like’ extrusions and thereby result in incomplete clearance of abnormal protein accumulations. It is therefore tempting to speculate that a dysregulated protein extrusion mechanism may create a potent seeding condition for the accumulation of several aggregation-prone proteins and formation of amyloid plaques in the surrounding neuropil.
Extrusion of misfolded proteins – implications for AD
This speculative hypothesis also suggests that chronic inflammation and concomitant augmentation of misfolded/damaged proteins constitute a crucial initiating event in AD neuropathology, including the formation of extracellular Aβ deposits (senile plaques) and intracellular aggregates of hyperphosphorylated Tau (neurofibrilary tangles). Our view is in agreement with several lines of evidence pointing to the crucial role of the brain’s innate immune system in late-onset AD: (i) several pro-inflammatory markers of neuroinflammation are selectively enriched in brain areas affected by AD pathology (McGeer & McGeer, 2002); (ii) positron emission tomography imaging studies revealed that the cognitive status inversely correlates with microglial activation in AD subjects (Edison et al., 2008); and (iii) recent genome-wide association studies have identified significant correlations between components of the innate immunity and incidence of sporadic AD (Lambert et al., 2009). It also supports a crucial relationship between alterations in the immune system and AD pathophysiology, as suggested by previous retrospective epidemiological studies in humans (Schmidt et al., 2002; Engelhart et al., 2004). Finally, ADAPT (the Alzheimer’s Disease Anti-inflammatory Prevention Trial) hypothesis was recently revised based on new data that strongly support a beneficial role of anti-inflammatory drugs during early, asymptotic, phases of the disease (McGeer et al., 1996; Breitner et al., 2011), pointing again to a potentially causative role of neuroinflammation in the etiology of this age-related disease.
In light of these findings and the protein extrusion mechanism proposed here, we hypothesise that young neurons might be able to counteract the inflammation-induced increase in misfolded/damaged proteins via efficient clearance and extrusion mechanisms. However, prolonged exposure to inflammatory modulators, impairments in proteosomal/lysosomal degradation, and failure or incomplete protein extrusion during aging may induce abnormal varicosities along axons and dendrites. These swellings would represent a potent seeding site for several aggregation-prone proteins and formation of amyloid plaques in the surrounding neuropil. Moreover, the accumulation of degenerated mitochondria and other disintegrated organelles, e.g. lysosomes, in the proximity of these protein accumulations might also allow proteolytic events and generation of aggregation-prone peptides that would not occur under normal physiological conditions. This scenario would fit with many previous observations showing that inhibition of the lysosomal proteolysis, a process affected by aging (Keller et al., 2004) and neuroinflammation (Alirezaei et al., 2011), and being altered in AD brains (Nixon & Cataldo, 2006), leads to Alzheimer’s-like axonal dystrophy involving also axonal swellings and accumulation of proteolytic APP fragments (Lee et al., 2011). Besides confirming a proximal role of chronic neuroinflammation in amyloid plaque deposition, this hypothesis may also implicate that the presence of dystrophic neurites closely accompanying amyloid plaques (Adalbert et al., 2009) may not be the consequence of extracellular Aβ accumulation, but, conversely, represent a putativecause for amyloid plaque deposition.
Acknowledgements
We are grateful to Corinne Sidler and Cornelia Schwerdel for their excellent technical support. We also thank Prof. Jean-Marc Fritschy and Dr Patrizia Panzanelli for their invaluable help in establishing and optimizing the EM protocol. The present study was supported by SNF Grants 310000-117806 and 310030-132629 (IK).
Abbreviations
-
- AD
-
- Alzheimer’s disease
-
- APP
-
- amyloid precursor protein
-
- Aβ
-
- amyloid-β
-
- EM
-
- electron microscopy
-
- GFAP
-
- glial fibrillary acidic protein
-
- HSPG
-
- heparan sulfate proteoglycan
-
- MBP
-
- myelin-basic protein
-
- PB
-
- phosphate buffer
-
- PBS
-
- phosphate-buffered saline
-
- PGs
-
- polyglycan bodies
-
- PolyI:C
-
- polyriboinosinic-polyribocytidilic acid
-
- RER
-
- rough endoplasmic reticulum
-
- RT
-
- room temperature
-
- SBF-SEM
-
- serial block-face scanning electron microscopy
-
- TBS
-
- Tris-buffered saline




