Excitatory amino acid transporter 2 and excitatory amino acid transporter 1 negatively regulate calcium-dependent proliferation of hippocampal neural progenitor cells and are persistently upregulated after injury
Abstract
Using a transgenic mouse (Mus musculus) in which nestin-expressing progenitors are labeled with enhanced green fluorescent protein, we previously characterized the expression of excitatory amino acid transporter 2 (GltI) and excitatory amino acid transporter 1 (Glast) on early neural progenitors in vivo. To address their functional role in this cell population, we manipulated their expression in P7 neurospheres isolated from the dentate gyrus. We observed that knockdown of GltI or Glast was associated with decreased bromodeoxyuridine incorporation and neurosphere formation. Moreover, we determined that both glutamate transporters regulated progenitor proliferation in a calcium-dependent and metabotropic glutamate receptor-dependent manner. To address the relevance of this in vivo, we utilized models of acquired brain injury, which are known to induce hippocampal neurogenesis. We observed that GltI and Glast were specifically upregulated in progenitors following brain injury, and that this increased expression was maintained for many weeks. Additionally, we found that recurrently injured animals with increased expression of glutamate transporters within the progenitor population were resistant to subsequent injury-induced proliferation. These findings demonstrate that GltI and Glast negatively regulate calcium-dependent proliferation in vitro and that their upregulation after injury is associated with decreased proliferation after brain trauma.
Introduction
During adult neurogenesis, glial fibrillary acidic protein (GFAP)-expressing quiescent type I cells self-renew, divide, and give rise to transient amplifying type IIa cells, which will differentiate into neuronal nuclei-expressing mature granular cell neurons (Mullen et al., 1992; Gage et al., 1995, 1998; Kempermann & Gage, 2000; Blaiss et al., 2011). We previously developed and characterized a transgenic animal in which type I and IIa progenitor cells are labeled with green fluorescent protein (GFP) under the control of the nestin promoter and its second intron enhancer (Miles & Kernie, 2006, 2008; Shi et al., 2007; Koch et al., 2008; Yu et al., 2008; Gilley et al., 2011). Using this transgenic line, we created a developmental profile of the postnatal dentate gyrus that documented cell-autonomous proliferative phenotypes and identified several candidate genes that may regulate this neurogenic niche. Excitatory amino acid transporter 2 (GltI ), a glutamate transporter, was identified in this screen and found to be upregulated about 10-fold in postnatal day (P) 28 progenitors as compared with those isolated at P7. In addition, we confirmed that GltI and its closely related partner excitatory amino acid transporter 1 (Glast) (Eaat1) were expressed on type I and IIa neural progenitors in vivo (Gilley et al., 2011).
Although the function of glutamate transporters has been well documented in astrocytes, their role in early neural progenitors has not been established. In the synaptic cleft, glutamate transporters are neuroprotective and function on astrocytes to regulate extracellular concentrations of glutamate, the main neurotransmitter in the brain (Danbolt, 2001; Fonnum & Lock, 2004; Maragakis et al., 2004). In addition to its role as a neurotransmitter, glutamate has also been shown to positively regulate neural progenitor proliferation (Luk et al., 2003; Brazel et al., 2005; Suzuki et al., 2006). We therefore chose to examine how glutamate transporters regulate progenitor proliferation in vitro.
We used a combination of neurosphere cultures and models of acquired brain injury to elucidate the function of GltI and Glast in early neural progenitors. Using neurospheres derived exclusively from the hippocampal dentate gyrus, we manipulated glutamate transporter expression with small interfering RNAs (siRNAs) and overexpression plasmids, and analyzed the effect on proliferation. Our results indicate that glutamate transporters function on neural progenitors to negatively regulate calcium-dependent proliferation. By using models of brain injury to induce hippocampal neurogenesis, we were also able to demonstrate prolonged upregulation of GltI and Glast after injury. Moreover, increased transporter expression is associated with decreased proliferation after subsequent brain injury. We have therefore identified a novel functional role for GltI and Glast in regulating neural progenitor proliferation in vitro, which is closely correlated with in vivo models of induced neurogenesis.
Materials and methods
Cloning
GltI cDNA was purchased in plasmid form (pFLCI–GltI) from Geneservice (Cambridge, UK; catalog no. 6330500I22), and Glast mouse cDNA (pCMV6–Glast–Myc) was purchased from Origene (Rockville, MD, USA; catalog no. MR208665). GltI cDNA was subcloned into pCMV–IRES–DsRed2 (Clontech, Mountain View, CA, USA; catalog no. 632420) in order to create the GltI overexpression plasmid pCMV–GltI–DsRed2. The Glast cDNA construct (pCMV6–Glast–Myc) was directly used to overexpress Glast.
Transient transfections
P7 dentate gyrus-derived neurospheres were dissociated into single cells and transfected with either siRNA compounds or overexpression plasmids driven by the cytomegalovirus (CMV) promoter. Lipofectamine RNAi Max (Invitrogen, Eugene, OR, USA; catalog no. 56531) was used to reverse transfect 10 nmGltI siRNA (Sigma-Aldrich, St Louis, MO, USA; catalog no. SASI_Rn01_00062046), 10 nmGlast siRNA (Sigma-Aldrich; catalog no. SASI_Rn02_00262083) or 10 nm scrambled siRNA (Applied Biosystems, Carlsbad, CA, USA; catalog no. AM4611). Lipofectamine 2000 (Invitrogen; catalog no. 52887) was used to transiently transfect 300 ng of pCMV–GltI–DsRed2 (final concentration, 150 ng/mL), 300 ng of pCMV–Glast–Myc (final concentration, 150 ng/mL), or 300 ng of pCMV–DsRed2 (Clontech; catalog no. 632420) (final concentration, 150 ng/mL). Seven days after cells were transfected, some neurospheres were taken from each experiment and misexpression of GltI and Glast was confirmed by PCR (QPCR) analysis. Only transfections with confirmed knockdown or overexpression were used for further experiments. The siRNA sequences were as follows: GltI sense, 5′-GAU AGU GAC UGU AAG CCU U-3′; GltI antisense, 5′-AAG GCU UAC AGU CAC UAU C-3′; Glast sense, 5′-CUG UCA UUG UGG GUA CAA U-3′; and Glast antisense, 5′-AUU GUA CCC ACA AUG ACA G-3′.
QPCR
Knockdown and overexpression of GltI and Glast were confirmed with methods adapted from previous publications (Gilley et al., 2011). Seven days after transfection, RNA was isolated from neurospheres and reversed transcribed into cDNA, and relative mRNA levels were measured. mRNA levels were normalized to either GltI or Glast gene expression. The primer information is as follows: glyceraldehyde-3-phosphate dehydrogenase forward, 5′-CTC AAC TAC ATG GTC TAC ATG TTC CA-3′; glyceraldehyde-3-phosphate dehydrogenase reverse, 5′-CCA TTC TCG GCC TTG ACT GT-3′; GltI forward, 5′-GGA AGA TGG GTG AAC AGG C-3′; GltI reverse, 5′-TTC CCA CAA ATC AAG CAG G-3′; Glast forward, 5′-ACG GTC ACT GCT GTC ATT G-3′; and Glast reverse, 5′-TGT GAC GAG ACT GGA GAT GA-3′.
Neurosphere growth and assays
Neurobasal A medium lacking glutamate and aspartate was used in the preparation and growth of neurospheres (Invitrogen; catalog no. 10888-022). The methods for preparation of activated papain, neural stem cell medium and semi-solid medium were adapted from previously described protocols. All neurospheres were derived from P7 dentate gyrus and grown in neural stem cell medium for 7 days before they were used for further experiments [see Gilley et al. (2011) for prior protocols].
Misexpression neurosphere assays were performed in glutamate-free medium on neurospheres transfected with either siRNA or overexpression plasmids (see ‘Transient transfections’). Briefly, P7 neurospheres were dissociated into single cells, transiently transfected with Lipofectamine, and grown in neural stem cell medium for 7 days. Transfected neurospheres were dissociated again, counted, and plated in semi-solid medium before they were quantified 7 days later. This experiment was repeated four times, and the results were analyzed for significance with one-way anova and Bonferonni post hoc analysis.
For the glutamate neurosphere assays, neurospheres were grown in glutamate-free medium for 7 days, mechanically dissociated into a single-cell solution, and plated in semi-solid medium supplemented with either 0 μm or 5 μm glutamate. Additionally, we treated some of the cells with 10 μm (2S)-2-amino-2-[(1S,2S)-2-carboxycycloprop-1-yl]-3-(xanth-9-yl)propanoic acid (LY341495), which serves as a non-selective metabotropic glutamate receptor (mGluR) antagonist at this concentration (Kingston et al., 1998). Another group of cells were treated with 1 μm 1,2-bis(2-amino-5-fluorophenoxy)ethane-N,N,N′,N′-tetraacetic acid tetrakis ester (BAPTA-AM), which serves as an intracellular calcium chelator (Santa Cruz, Santa Cruz, CA, USA; catalog no. sc-202488). After 7 days in culture, neurospheres were quantified, and the diameters of at least 20 neurospheres from each group were measured (Gilley et al., 2011). An unpaired t-test and Bonferonni post hoc analysis were used to calculate significance. The experiment was repeated at least four times for each condition.
Bromodeoxyuridine (BrdU) incorporation
Transiently transfected neurospheres were pulsed with 10 μm BrdU for 15 min, dissociated with activated papain, and triturated into a single-cell solution before they were fixed overnight with 100% ethanol; 2 m HCl/0.5% Triton X in phosphate-buffered saline (PBS) was used to denature DNA for 30 min at room temperature. The reaction mixture was then neutralized with 0.1 m NaB4O7 before being incubated in staining solution [1.3 μL of BrdU-APC (BD Pharmingen, San Diego, CA, USA; catalog no. 552598), 5 μL of RNase and 50 μL of 0.5% Tween/1% bovine serum albumin in PBS] overnight at 4 °C. Cells were then washed and resuspended in PBS with propidium iodide (PI), and analyzed for BrdU incorporation by flow cytometry. Results are displayed as the percentage of cells that were BrdU+ and PI−. The experiment was performed in quadruplicate. Statistical significance was determined with a one-way anova followed by Bonferonni post hoc analysis.
Intracellular calcium measurement
After misexpression was confirmed 7 days after transfection, all neurospheres were dissociated and stained with Fluo4-AM calcium indicator (Invitrogen; catalog no. F14201) after cells had been exposed to a variety of compounds, including an intracellular calcium chelator and an mGluR antagonist. Cells were incubated with 1 μm BAPTA-AM according to the manufacturer’s protocols. Additionally, 10 μm LY341495, which acts as a non-selective mGluR antagonist at high concentrations, was added to a proportion of dissociated progenitors (Kingston et al., 1998). Cells were then analyzed by flow cytometry. Prior to supplementation with glutamate, baseline intracellular calcium levels were measured for 30 s. Cells were then spiked with 5 μm glutamate, and calcium measurements were made for an additional 1.5 min. The percentage of cells containing increased intracellular calcium levels (as compared with baseline) is presented. The final percentage of cells expressing Fluo4-AM was normalized to the percentage of positive cells at baseline levels (% induced cells/% baseline cells). Intracellular calcium was measured in quadruplicate for each condition, and significance was calculated with a one-way anova and Bonferonni post hoc analysis.
Mice
All animal experiments were approved by the Institutional Animal Use and Care Committee at UT Southwestern Medical Center. The Animal Resource Center within UT Southwestern, which is accredited by the Association for Assessment and Accreditation of Laboratory Animal Care, humanely housed and cared for all animals.
All experiments were performed with nestin–rtTA–enhanced GFP transgenic mice in a CD1 background, which have been well characterized (Miles & Kernie, 2006, 2008; Shi et al., 2007; Koch et al., 2008; Yu et al., 2008; Gilley et al., 2011). P28 transgenic mice were exposed to either hypoxic–ischemic injury (HI) or sham injury before being subjected to traumatic brain injury (TBI). Both groups of mice (sham + TBI or HI + TBI) were anesthetized, placed in a supine position, had their right carotid artery ligated, and were allowed to recover for 30–60 min (see below). Mice in the HI + TBI group were then exposed to 8% oxygen for 1 h in a hypoxic chamber before they were allowed to recover in room air. Mice in the sham + TBI group were not treated with hypoxia. At P60, mice from both groups were injured by controlled cortical impact (CCI) in order to induce TBI. From P64 to P67, mice were injected with a single daily dose of 100 mg/kg BrdU, and they were perfused and killed 2 h after injection on P67.
The Rice–Vanucci model was used to emulate HI, and has been described previously (Koch et al., 2008; Miles & Kernie, 2008). Briefly, mice were anesthetized and maintained on isoflurane mixed with oxygen and nitrogen. A midline incision was made, and the right carotid artery was isolated and ligated with sutures. In sham injuries, the artery was exposed but not ligated. After the incision was closed, animals were allowed to recover for up to 1 h. Only mice that fully recovered were used for further experiments. After recovery, mice were placed in a hypoxic chamber and treated with 8% oxygen for 1 h. Mice were then allowed to recover in their cage.
A CCI devise was used to induce TBI as previously described (Kernie et al., 2001). Thirty-two days after HI or sham injury (P60), mice were anesthetized and placed in a stereotactic frame, and part of the skull was removed before the brain was impacted with a steel-tipped device in order to generate the injury. The incision was closed, and the mice were allowed to recover. Daily injections of BrdU (100 mg/kg) were started 4 days after TBI at P64, and mice were killed and perfused at P67.
Immunostaining
One, 3, 7 and 45 days after HI, 50-μm vibratome sections were obtained and stained for the presence of GltI and Glast. After being blocked with normal donkey serum, sections were stained with chicken anti-GFP (1 : 500; Aves Labs, Tigard, OR, USA; catalog no. GFP-1020), mouse anti-GFAP (1 : 400; BD Biosciences, Rockville, MD, USA; catalog no. 556330), and guinea pig anti-GltI (catalog no. AB1783) or anti-Glast (catalog no. AB1782) (both 1 : 200; Millipore, Temecula, CA, USA). Fluorescent secondary antibodies were used at a concentration of 1 : 200 (Santa Cruz). See Gilley et al. (2011) for details.
Vibratome sections from HI + TBI and sham + TBI mice were blocked in normal donkey serum, stained with rat anti-BrdU (1 : 400; Abcam, Cambridge, MA, USA; catalog no. AB6326) and chicken anti-GFP 1 : 500; Aves Labs; catalog no. GFP-1020), according to previously described protocols (Gilley et al., 2011).
Only sections in which antibody and signal penetration were even were used for confocal imaging. Percentages of BrdU+/GFP+ cells were quantified (non-blinded) by colocalizing BrdU+ nuclei with GFP, which was entirely included in the section throughout the z-axis. The top and bottom of the sections were identified by the presence of the first or last nuclei that could be entirely visualized by the z-plane. Colocalization was determined by scanning serially through the z-axis of each cell with a depth of focus optical slice of 2.72 μm.
Imaging
Confocal images were acquired on a Zeiss LSM 510 (Zeiss, Jena, Gottingen, Germany) scanning confocal multicolor microscope with Ar 488-nm, He 543-nm and He 633-nm lasers (sp2, version 3.2, aim interface), as previously described (Gilley et al., 2011). A Zeiss Neofluar ×40/1.3 lens and ×63/1.3 oil DIC lens were used. A pinhole aperture of 3.46 Airy units was used.
Fluorescence-activated cell sorting
To determine the extent of GltI and Glast upregulation after injury, cells from the ipsilateral and contralateral dentate gyrus of injured transgenic mice were stained with mouse anti-polysialic acid neural cell adhesion molecule (PSA-NCAM) (1 : 200; Millipore, Billerica, MA, USA; catalog no. MAB5324) for 20 min on ice. After unbound primary antibody had been washed away, cells were stained with donkey anti-mouse Alexa Fluor 647 (1 : 200; Molecular Probes, Eugene, OR, USA; catalog no. A31571) secondary antibody for an additional 20 min on ice. Cells were then washed, and four different populations were sorted on a MoFlo (Beckman Coulter, Brea, CA, USA; catalog no. ML99030): ipsilateral GFP+/PSA-NCAM+ and GFP+/PSA-NCAM−, and contralateral GFP+/PSA-NCAM+ and GFP+/PSA-NCAM−. RNA was then extracted and normalized across samples before being analyzed by QPCR.
Results
Glutamate transporter misexpression influences neurosphere formation and progenitor proliferation in vitro
To determine whether GltI and Glast contribute to the regulation of hippocampal neurogenesis, we performed gain-of-function and loss-of-function experiments on neurosphere cultures grown in the absence of glutamate. First, we performed neurosphere assays on cells previously transfected with GltI and Glast siRNA or overexpression plasmids. Seven days after transfection, neurospheres were assayed for misexpression by QPCR (n = 4). Relative mRNA levels were normalized to GltI and Glast expression in cells transfected with scrambled siRNA as a negative control (Fig. 1A and B). Relative levels of GltI expression were 0.9 in cells transfected with DsRed2, 0.3 in cells lacking GltI, and 10.3 in cells overexpressing GltI (Fig. 1A). Glast expression levels after transfection were 1.2 for DsRed2, 0.3 for the knockdown, and 3.7 for overexpression (Fig. 1B). These data suggest that both glutamate transporters are misexpressed appropriately in culture as compared with controls (cells transfected with scrambled siRNA or DsRed2).
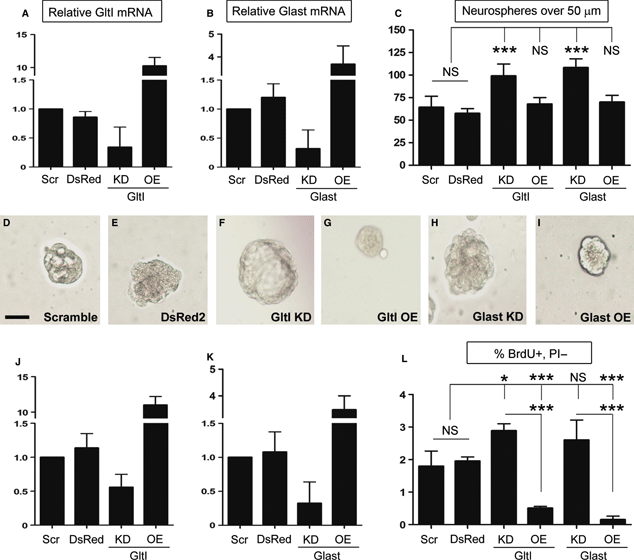
Knockdown of glutamate transporters results in increased proliferation in vitro. Seven days after neurospheres were transfected with DNA constructs or gene-specific siRNA, they were assayed for misexpression by QPCR (A, B, D and E). Relative mRNA expression levels of GltI and Glast expression were normalized to their respective expression levels in cells transfected with scrambled siRNA negative control. A, B, J and K show that both GltI and Glast were knocked down and overexpressed appropriately. After misexpression was confirmed, neurospheres were dissociated and plated in semi-solid media for 7 days before those over 50 μm were quantified (C). Knockdown of either GltI or Glast resulted in an increase in the number of neurospheres formed as compared with controls, whereas their overexpression did not have a significant effect on neurosphere formation. D–I shows representative neurospheres from each experiment, and suggest that GltI and Glast expression levels affect neurosphere size as well. Progenitors were also pulsed with BrdU and stained with an anti-BrdU-APC antibody before being analyzed for incorporation by flow cytometry. Knockdown of GltI and Glast resulted in increased BrdU incorporation, whereas their overexpression drastically decreased the percentage of BrdU/PI-double-labeled cells (L). These results suggest that glutamate transporters regulate neurosphere proliferation in vitro. Statistical analysis in C and F represents Bonferonni post hoc analysis (*P < 0.05; ***P < 0.0001). The scale bar in D represents 50 μm. DsRed, DsRed2 plasmid; KD, knockdown; NS, not significant; OE, overexpression; Scr, scrambled siRNA.
After overexpression and knockdown levels were confirmed, the remaining neurospheres were dissociated and replated in semi-solid media containing neural stem cell media (lacking glutamate) and 1.6% methylcellulose at a density of 20 cells/μL. After 7 days in culture, neurospheres over 50 μm were quantified (Fig. 1C). The average numbers of spheres per well were 64 for DsRed2, 57 for scrambled siRNA, 99 for GltI knockdown, 68 for GltI overexpression, 108 for Glast knockdown, and 69 for Glast overexpression. Analysis by one-way anova suggested that these differences were significant (P < 0.0001, F = 13.4, n = 4). Representative neurospheres are shown for each condition, and suggest that sphere size is dependent on the expression level of each glutamate transporter (Fig. 1D–I). Moreover, these results show that knockdown of glutamate transporters results in increased neurosphere formation in vitro.
To confirm this phenotype, we pulsed transfected neurospheres with BrdU and analyzed its incorporation with flow cytometry. In addition, the fidelity of misexpression of GltI and Glast was analyzed (Fig. 1J and K) (n = 4). The average relative expression of both GltI and Glast in cells transfected with DsRed2 was 1.1. Knockdown with either Glast or GltI siRNA resulted in relative mRNA levels of 0.3 and 0.6, respectively, suggesting that both genes were knocked down by at least 44%. Cells transfected with overexpression plasmids demonstrated increased GltI (11.0) and Glast (3.5) expression. From this, we can conclude that glutamate transporter knockdown and overexpression results in altered gene expression of GltI and Glast. These results are similar to those obtained previously, and suggest that we were able to consistently manipulate GltI and Glast expression levels in vitro.
Once misexpression was confirmed, the remaining neurospheres were pulsed with BrdU and stained with an anti-BrdU-APC antibody. We used flow cytometry to assess the effect of glutamate transporter misexpression on proliferation, and the results are reported as the percentage of BrdU+/PI− cells (Fig. 1L). Baseline BrdU incorporation was 1.8% for DsRed2 and 2.0% for scrambled siRNA (both negative controls); these values were not significantly different from each other. Knockdown of GltI resulted in 2.9% BrdU incorporation, whereas its overexpression decreased the percentage of BrdU+/PI− cells to 0.5% when compared with negative controls. Similarly, Glast siRNA increased the percentage of BrdU+ cells to 2.6%; however, its overexpression decreased BrdU incorporation to 0.2%. Statistical analysis with one-way anova indicated that these results were statistically different (P < 0.0001, F = 51.2, n = 4). Taken together, these results indicate that GltI and Glast negatively regulate neural progenitor proliferation in vitro.
Glutamate-induced neurosphere proliferation is dependent on calcium and mGluRs
Although previous studies have shown that glutamate stimulates neurosphere proliferation in a number of different cell types (Luk et al., 2003; Brazel et al., 2005; Suzuki et al., 2006), there are no studies that have specifically demonstrated glutamate’s effect on neurospheres derived exclusively from the dentate gyrus. To control for the presence of glutamate in most media, we prepared our neurospheres in medium lacking glutamate (Neurobasal A) before supplementing them with either 0 μm or 5 μm glutamate prior to final plating. In addition to plating neurospheres under wild-type conditions (Fig. 2A–D), we repeated the assay in the presence of 1 μm BAPTA-AM, a cell-permeable calcium chelator that neutralizes intracellular calcium (Tsien, 1980; Dieter et al., 1993) (Fig. 2E–H). Finally, to determine the role of mGluRs in proliferation, we also supplemented neurospheres with 10 μm LY341495, which non-selectively inhibits all mGluRs at this concentration (Fig. 2I–L) (Kingston et al., 1998). After 7 days in vitro, we quantified the number of neurospheres over 50 μm and their diameter as shown in Fig. 2. Under wild-type conditions, the numbers of spheres present were 27 and 28 in the presence and absence of exogenous glutamate, respectively (Fig. 2C). However, in media supplemented with glutamate, the average neurosphere size was 270 μm, as compared with 66 μm in media lacking glutamate (Fig. 2D). Although there was no difference in sphere number (unpaired t-test, P = 0.96, F = 9.0, n = 7), the difference in the size of the neurospheres was statistically significant when analyzed with an unpaired t-test (P < 0.0001, F = 24.9, n = 20). When we supplemented the media with BAPTA-AM or LY341495 in the presence and absence of glutamate, we saw a similar number of neurospheres. Among cells exposed to 1 μm BAPTA-AM, those treated with glutamate formed 44 neurospheres per well that averaged 66 μm in diameter (Fig. 2E and G). In the absence of glutamate, there were 45 spheres per well, and their average diameter was 63 μm (Fig. 2F and H). Similarly, cells treated with LY341495 and 5 μm glutamate formed 41 neurospheres, with an average diameter of 64 μm (Fig. 2I and K), whereas those grown in medium lacking glutamate formed 38 spheres, with an average diameter of 63 μm (Fig. 2J and L). According to statistical analysis with an unpaired t-test, cells treated with BAPTA-AM were not significantly different in neurosphere formation (P = 0.81, F = 1.2, n = 4) or neurosphere size (P = 0.49, F = 1.1, n = 20). Similarly, neither neurosphere number (unpaired t-test, P = 0.57, F = 3.4, n = 5) nor diameter (unpaired t-test, P = 0.72, F = 1.3, n = 20) was significantly different in wells treated with LY341495. These results suggest that intracellular calcium and mGluRs are both required for glutamate-induced neurosphere proliferation in vitro.
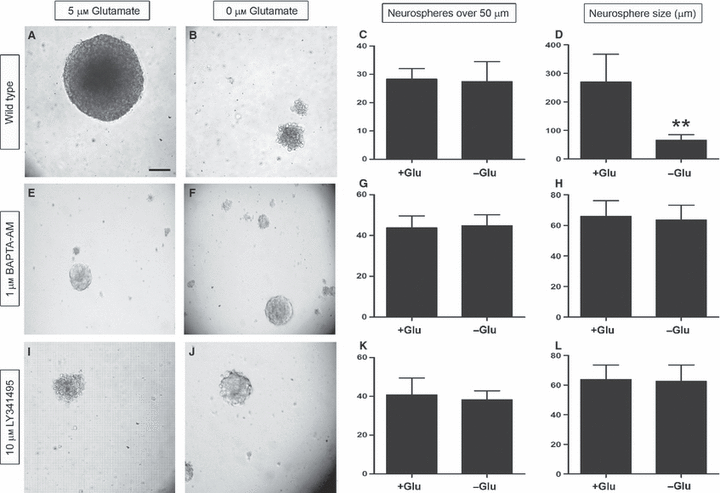
Glutamate enhances mGluR-dependent neurosphere proliferation. Neurospheres derived from P7 transgenic animals were grown in the presence (B, F and J) and absence (A, E and I) of exogenous glutamate, and were quantified and measured as shown in C, G and K, and D, H and L, respectively. Although the numbers of neurospheres in the different groups were similar under wild-type conditions (C), the difference in the overall size of the spheres was statistically significant, as shown in D, signifying that exogenous glutamate positively regulates neurosphere proliferation. In cells treated with either 1 μm BAPTA-AM or 10 μm LY341495, both the number and size of neurospheres were similar in the presence and absence of glutamate, suggesting that intracellular calcium and mGluRs are required for glutamate-induced neurosphere proliferation. Statistical analysis with a Bonferonni post hoc test is shown (**P < 0.001). The scale bar in A represents 50 μm.
Glutamate transporter misexpression affects intracellular calcium levels
Although the exact mechanisms underlying neurogenesis remain unclear, several studies have suggested that the activation of certain ionotropic (ligand-gated ion channels) and/or mGluRs (G-protein-coupled) might be required (Choi et al., 1988; Luk et al., 2003; Deisseroth et al., 2004; Brazel et al., 2005; Nacher & McEwen, 2006; Nacher et al., 2007). The evidence suggests that activation of these receptors can also be associated with increases in intracellular calcium (Deisseroth et al., 2004; Brazel et al., 2005). We therefore chose to use calcium flux to assay for pro-proliferative pathways. To determine the effect of GltI and Glast misexpression on glutamate-mediated calcium flux, we incubated transfected neurospheres with a Fluo4-AM calcium indicator and assayed for changes in intracellular calcium using flow cytometry. Prior to stimulation with exogenous glutamate, baseline levels of intracellular calcium were recorded for 30 s. Cells were then stimulated with 5 μm glutamate, and we immediately began recording the increased fluorescence indicating a proportional increase in intracellular calcium. The results are presented as the percentage of cells that were Fluo4-AM+, and are normalized to the percentage of positive cells at baseline levels prior to stimulation with glutamate (Fig. 3C).
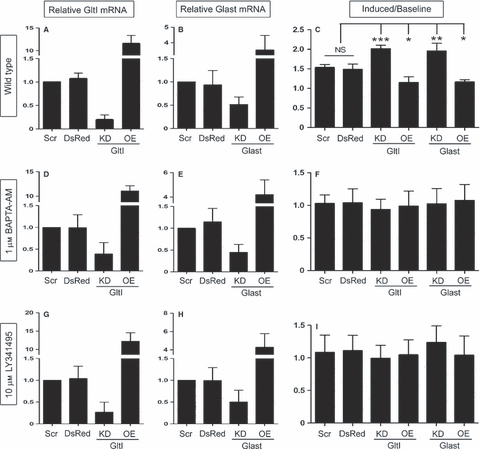
Glutamate transporters regulate intracellular calcium stores. Once again, misexpression of GltI and Glast mRNA was confirmed after transfection (A, B, D, E, G and H), and progenitors were assayed for changes in glutamate-mediated intracellular calcium (C, F and I). Under wild-type conditions, knockdown of either GltI or Glast resulted in significant increases in intracellular calcium as a result of glutamate transporter knockdown. In cells treated with BAPTA-AM, an intracellular calcium chelator, the changes in calcium flux disappeared (F). Moreover, progenitors treated with 10 μm LY341495, which serves as a non-selective mGluR inhibitor at this concentration (Kingston et al., 1998), also showed no change in intracellular calcium flux (I). These results suggest that GltI and Glast regulate calcium-dependent and mGluR-dependent proliferation of neural progenitors in vitro. In C, F and I, a Bonferonni post hoc analysis was used to determine statistical significance (*P < 0.05; **P < 0.001; ***P < 0.0001). DsRed, DsRed2 plasmid; KD, knockdown; NS, not significant; OE, overexpression; Scr, scrambled siRNA.
Before we conducted the experiment, we once again confirmed misexpression in vitro (Fig. 3A and B) (n = 4). The relative expression levels of GltI and Glast were 1.1 and 0.9, respectively, in cells transfected with DsRed2. Cells transfected with either GltI or Glast siRNA had expression levels of 0.2 and 0.5, respectively. Overexpression experiments demonstrated relative mRNA levels of 11.5 for GltI and 3.5 for Glast. Once again, these results show that GltI and Glast mRNA can be reliably manipulated in vitro. About 4% of cells above baseline levels were Fluo4-AM+ among cells transfected with either DsRed2 or scrambled siRNA prior to their stimulation with glutamate. After glutamate was added to the cells, the ratio of induced/baseline cells was 1.5 for both DsRed2 and scrambled siRNA. Whereas knockdown of GltI resulted in a ratio of 2.0, its overexpression decreased the percentage of positive cells to 1.2. Glast overexpression also demonstrated a ratio of 1.2, whereas its knockdown resulted in a ratio of 2.0. Analysis with one-way anova indicated that these results were significantly different from each other (P < 0.0001, F = 24.3, n = 4). These results suggest that glutamate transporter knockdown leads to increased intracellular calcium, presumably through activation of glutamate receptors. This therefore allows the cells to proliferate more readily than when glutamate transporters are present at baseline or increased levels.
We next wanted to determine whether this proliferative phenotype was calcium-dependent. To test this, we repeated the experiment in the presence of BAPTA-AM, a cell-permeable calcium chelator that neutralizes intracellular calcium (Tsien, 1980; Dieter et al., 1993) (Fig. 3F). Prior to incubation with BAPTA-AM, misexpression was confirmed in transfected cells (n = 4). GltI relative mRNA levels were 1.0 for scrambled siRNA, 1.0 for DsRed2, 0.4 for knockdown, and 11.1 for overexpression. Glast relative mRNA levels were 1.0 for scrambled siRNA, 1.2 for DsRed2, 0.5 for knockdown, and 4.2 for overexpression (Fig. 3D and E). After misexpression was confirmed, we incubated transfected cells with BAPTA-AM prior to staining with Fluo4-AM. This allowed us to determine whether intracellular calcium is required for the induction shown in Fig. 3C. Once again, we recorded baseline levels of calcium before we stimulated the cells with glutamate, at which point we calculated the ratio of induced/baseline cells. The results are shown in Fig. 3F. In cells transfected with scrambled siRNA, the ratio was 1.0, and cells transfected with DsRed2 also had a ratio of 1.0. Cells knocked down with either GltI or Glast siRNA had ratios of 0.9 and 1.0, respectively. Finally, cells overexpressing GltI and Glast both had ratios of 1.0. One-way anova suggested that these results were not statistically significantly different from each other (P = 0.95, F = 0.21, n = 4). This suggests that intracellular calcium is required for the phenotype observed in Fig. 3C.
To confirm that mGluRs are required for the calcium flux shown in Fig. 3C, we repeated the experiment in the presence of 10 μm LY341495 to inhibit all mGluR subtypes. Prior to experimentation, cells to be treated with LY341495 were analyzed for misexpression (n = 4). Relative GltI expression levels for scrambled siRNA, DsRed2, knockdown and overexpression were 1.0, 1.0, 0.3, and 12.2, respectively, whereas mRNA levels for Glast were 1.0, 1.0, 0.5, and 4.3, respectively (Fig. 3G and H). After misexpression was confirmed, cells previously treated with LY341495 were analyzed for glutamate-induced calcium flux. The results obtained were similar to those shown in Fig. 3F. The ratios of induced/baseline calcium flow for scrambled siRNA, DsRed2, GltI siRNA, GltI overexpression, Glast siRNA and Glast overexpression were 1.1, 1.1, 1.0, 1.0, 1.2, and 1.4, respectively (Fig. 3I). Analysis by one-way anova showed that the differences were not statistically significant (P = 0.80, F = 0.46, n = 4). Therefore, inhibiting mGluRs stifles the phenotype shown in Fig. 3C. These results suggest that glutamate transporters regulate intracellular calcium-dependent proliferation of neural progenitors in an mGluR-dependent fashion.
Glutamate transporters are persistently upregulated after HI
We previously demonstrated that GltI and Glast are expressed on type I cells in vivo (Gilley et al., 2011). Moreover, injury-induced activation of type I cells and subsequent neurogenesis have been well documented (Kernie et al., 2001; Miles & Kernie, 2006, 2008; Shi et al., 2007; Koch et al., 2008; Yu et al., 2008; Blaiss et al., 2011). We therefore hypothesized that GltI and Glast would be expressed on activated progenitors after injury. Following unilateral HI in nestin–GFP-expressing transgenic mice, we performed immunostaining for GFP, GFAP and either GltI (Fig. 4) or Glast (Fig. 5) to determine their expression at various time points after injury (n = 6). In this transgenic line, GFP is a well-established marker of slowly dividing type I cells as well as of the more rapidly dividing type IIa population (Miles & Kernie, 2006, 2008; Yu et al., 2008). GFAP in conjunction with GFP marks the type I cells only, so expression of both can be used to distinguish type I from type IIa progenitors (Shi et al., 2007; Miles & Kernie, 2008; Yu et al., 2008). On the uninjured (contralateral) side of the brain, glutamate transporter expression remained at baseline levels at all indicated time points (4, 5). However, GltI and Glast were both upregulated on type I cells on the ipsilateral (injured) side of the brain for up to 45 days after injury (4, 5).
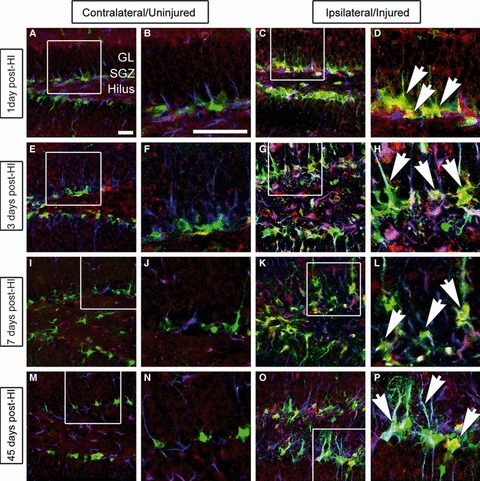
GltI is indefinitely upregulated after HI. Mice were killed 1 day (A–D), 3 days (E–H), 7 days (I–L) and 45 days (M–P) post-HI, and their brains were sectioned and stained for the presence of GFP (green), GltI (red), and GFAP (blue). The contralateral or uninjured side of the brain is shown in the left panel (A, B, E, F, I, J, M and N), and the ipsilateral (injured) side is shown on the right (C, D, G, H, K, L, O and P). White boxes represent the magnified areas shown. One day (D), 3 days (H), 7 days (L) and 45 days (P) post-HI, magnified images show activation of GltI-expressing type I cells (arrows) on the injured side of the brain. These findings indicate that GltI expression is permanently increased subsequent to HI. Scale bars in A and B represent 25 and 100 μm, respectively. GL, granular layer; SGZ, subgranular zone.
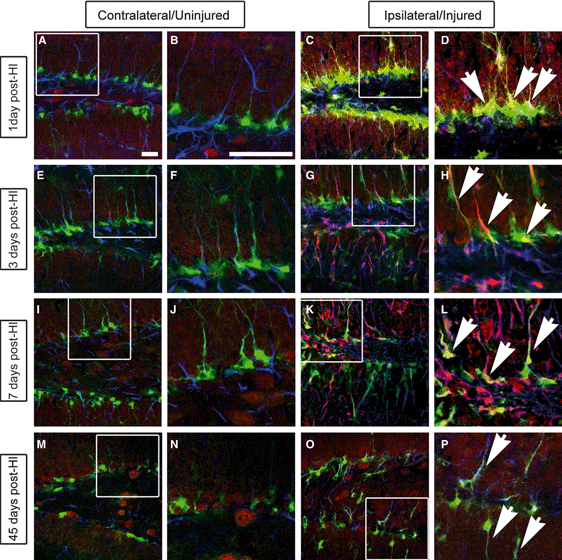
Glast is upregulated after HI. Mice were killed before their brains were sectioned and stained for GFP (green), Glast (red) and GFAP (blue) 1 day (A–D), 3 days (E–H), 7 days (I–L) and 45 days (M–P) after injury. Like GltI expression, Glast expression was increased on the ipsilateral side (D, H, L and P) of the brain as compared with the contralateral side (B, F, J and N). White boxes represent the magnified areas shown. Scale bars in A and B represent 25 and 100 μm, respectively. GL, granular layer; SGZ, subgranular zone.
Cell-specific elevation of glutamate transporter expression in injured progenitor cells
There are two possible explanations for the results shown in 4, 5. Either increased numbers of GFP+ cells on the injured side of the brain result in higher glutamate transporter expression, or the expression of GltI or Glast is elevated at the cellular level. To distinguish between these two possibilities, we used fluorescence-activated cell sorting to distinguish early and late progenitors from transgenic mice that were injured 3 days previously. We used GFP and PSA-NCAM expression to distinguish early (type I and IIa: GFP+/PSA-NCAM−) and late (type III: GFP−/PSA-NCAM+) progenitors (Chumley et al., 2007). We collected both populations from the ipsilateral (injured) and contralateral (uninjured) sides. We then isolated the RNA and normalized the concentrations across all samples before synthesizing cDNA and analyzing relative expression levels with QPCR. The expression of a particular gene was normalized to its own expression on the uninjured side of the brain. The results are shown in Fig. 6.
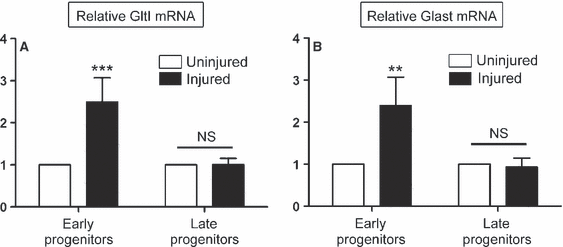
Upregulation of glutamate transporters is cell-specific following HI. Three days following HI, cells were sorted for their expression of GFP and PSA-NCAM, signifying either early (GFP+/PSA-NCAM−) or late (GFP+/PSA-NCAM+) neural progenitors. Relative expression levels of GltI (A) and Glast (B) were then measured in each population obtained from the injured and uninjured sides of the brain. Both glutamate transporters were upregulated in early progenitors from the injured side of the brain. However, their expression was not elevated in late progenitors from the injured or uninjured brain. This suggests that GltI and Glast are specifically induced in early progenitor cells isolated from the injured dentate gyrus. A Bonferonni post hoc analysis was used to calculate significance (**P < 0.001; ***P < 0.0001). NS, not significant.
We observed that the GltI mRNA level was 2.5-fold higher in early progenitor cells from the injured side of the brain than in those from the uninjured side (Fig. 6A) (two-way anova, P = 0.002, F = 25.2, n = 4). However, the relative expression level of GltI was unchanged in late progenitors from injured and uninjured dentate gyrus. The latter result is not surprising, considering that neither GltI nor Glast colocalizes with markers indicative of late type III progenitors (Gilley et al., 2011). This suggests that GltI is upregulated in early progenitors at the cellular level as a result of HI. Similar to what was found for GltI, Glast was also upregulated on early progenitors isolated from the injured side of the brain, but its expression was not significantly different in later progenitors (Fig. 6B) (two-way anova, P = 0.01, F = 14.01, n = 4). Together, these findings indicate that elevated expression of glutamate transporters on the injured side of the brain (4, 5) result from changes in gene expression at the single-cell level.
Recurrent injury impairs progenitor proliferation
As we observed long-lasting changes in the expression of GltI and Glast at the cellular level following injury, we next wanted to determine whether injured progenitors maintained their proliferative potential in the context of gene expression changes. One month after HI, a CCI model was used to induce TBI in a well-established model of increasing progenitor proliferation (Kernie et al., 2001; Shi et al., 2007; Yu et al., 2008; Blaiss et al., 2011). To analyze the subsequent effects on progenitor proliferation, we injected both groups with BrdU, performed immunostaining, and quantified the number of GFP/BrdU-double-labeled cells. In animals exposed to sham HI (ligation with no hypoxia), there were no observed differences in the percentage of proliferative cells following TBI (Fig. 7E) (unpaired t-test, P = 0.74, F = 2.2, n = 5). However, Fig. 7J shows there was a significant difference between colocalized cells within the contralateral side (15.1%) and ipsilateral (7.8%) side in mice with both HI and CCI injury (unpaired t-test, P = 0.003, F = 2.5, n = 4). These results suggest that repetitive brain injuries lead to a decreased capacity for injury-induced neurogenesis that may, in part, reflect changes in glutamate transporter expression.
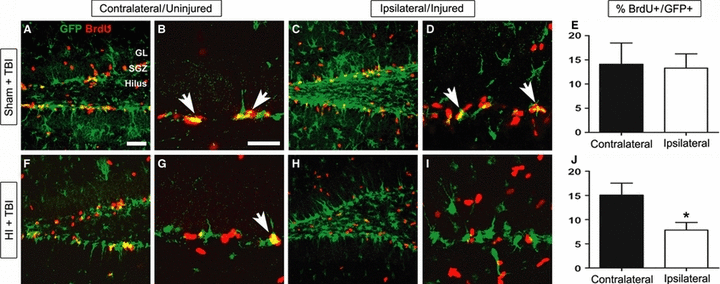
Upregulation of GltI and Glast after HI is associated with decreased proliferation following TBI. Thirty-two days prior to induction of TBI, P28 transgenic mice were exposed to either sham injury (A–E) or HI (F–J). To assess changes in proliferation, the percentages of GFP/BrdU-double-labeled cells were quantified on both sides of the brain for both groups of animals (E and J). In the sham + TBI group, there was no observed difference (E) in proliferative cells between the uninjured (A and B) and injured (C and D) sides of the brain. However, in animals exposed to HI and TBI, the percentage of double-positive cells was significantly decreased (J) on the ipsilateral side (F and G) as compared with the contralateral side (H and I). The scale bars in A and B represent 1 and 35 μm, respectively. Bonferonni post hoc analysis was used to calculate significance (*P < 0.05).
Discussion
Appropriate concentrations of extracellular glutamate are thought to regulate neurogenesis by activating glutamate receptors on the surface of the cell (Choi et al., 1988; Luk et al., 2003; Melchiorri et al., 2007). Although the exact mechanism remains unclear, there is increasing evidence suggesting that activation of ionotropic and metabotropic glutamate receptors results in elevated calcium levels, which are thought to contribute to both cellular proliferation and cell toxicity (Choi et al., 1988; Luk et al., 2003). The results from the current study suggest that glutamate transporters indirectly regulate calcium-dependent proliferation of neural progenitors via mGluRs. It appears that GltI and Glast function to regulate the availability of extracellular glutamate for various receptors expressed on the cell surface, thereby indirectly affecting calcium-induced proliferation. Moreover, when glutamate concentrations rise after injury (Andine et al., 1991; Globus et al., 1995; Bullock et al., 1998), glutamate transporters are able to transport excess glutamate into the cell, thus preventing overstimulation of glutamate receptors (Danbolt, 2001; Fonnum & Lock, 2004; Yi & Hazell, 2006). This may explain why in vitro knockdown of GltI or Glast consistently resulted in increased proliferation (1, 2).
Although some extracellular glutamate may be beneficial to the cell, excessive amounts can overstimulate glutamate receptors, leading to excitotoxicity that may ultimately exacerbate neuronal damage caused by brain injury (Choi et al., 1988; Jensen, 2002; Yi & Hazell, 2006). Glutamate transporters are therefore essential in regulating extracellular concentrations of glutamate in order to prevent excitotoxicity (Tanaka et al., 1997; Watase et al., 1998; Matsugami et al., 2006). During synaptic transmission, astrocytic GltI and Glast are neuroprotective because they transport excess glutamate, a key neurotransmitter, into the cell to prevent cytotoxic neuronal damage [see Danbolt (2001) for review]. Once glutamate is inside the astrocyte, it is converted to glutamine and then transported outside the cell. Presynaptic neurons are then able to take up glutamine and convert it back to glutamate, so that it can be reused as a neurotransmitter (Meldrum, 2000; Maragakis et al., 2004). Mice with traditional knockouts of GltI or Glast (or both) are not able to recycle excess glutamate in this manner, and this can ultimately lead to excitotoxicity, neuronal damage, and premature death (Tanaka et al., 1997; Watase et al., 1998; Matsugami et al., 2006).
In the current study, we show that manipulation of GltI and Glast mRNA in early postnatal dentate gyrus progenitors affects neurosphere formation, BrdU incorporation, and intracellular calcium signaling. Although the results from the neurosphere assay are not as striking as those from the BrdU incorporation assay, we believe that the variable sensitivities of these assays may explain this discrepancy. In the BrdU experiment, we utilized flow cytometry, which is extremely sensitive, to assess the percentage of live cells expressing BrdU. In the neurosphere assay, however, our quantification was based only on the presence of absence of neurospheres. Moreover, we used P7 neurospheres, which have high-level expression of Glast but not of GltI (Gilley et al., 2011). This may explain why overexpression of Glast in these neurospheres did not produce a significant decrease in neurosphere formation, as expected. These findings suggest that glutamate transporters function on neural progenitors to indirectly regulate calcium-dependent proliferation.
We also demonstrated that GltI and Glast overexpression correlated with decreased proliferation of neural progenitors after TBI (Fig. 7). Acquired brain injury has been shown to induce adult neurogenesis within the dentate gyrus, and has recently been shown to underlie recovery (Blaiss et al., 2011). We have previously demonstrated that this activation of progenitors is required to replace dying neuroblasts and for long-term neuronal remodeling in the hippocampus (Miles & Kernie, 2008). In addition, we have recently shown that injury-induced neurogenesis is required for cognitive recovery following TBI (Blaiss et al., 2011). Other studies have demonstrated a relationship between the severity and frequency of brain trauma and decreased capacity to recover from such injuries (Spettell et al., 1991; Slemmer & Weber, 2005). The decreased proliferation of neural progenitors associated with HI and TBI (Fig. 7) might therefore explain these phenotypes and provide insights into the mechanisms underlying recovery from multiple brain injuries. Here, we provide evidence that glutamate transporters may play a critical role in injury-induced proliferation.
Several studies have begun to characterize the role that glutamate transporters might play in neuroprotection. For instance, preconditioning with normobaric hyperoxia or middle cerebral artery occlusion increased the expression of GltI and Glast in astrocytes and was associated with marked brain protection (Bigdeli et al., 2009). Furthermore, less neuronal damage was observed in animals pretreated with 8% oxygen 1 day prior to unilateral HI. GltI was also shown to be upregulated in these animals (Cimarosti et al., 2005). When rat cortical cultures were preconditioned by glucose–oxygen deprivation, similar results were obtained. This study also concluded that GltI is a target of peroxisome proliferator-activated receptor gamma, because injection of an agonist immediately after middle cerebral artery occlusion resulted in neuroprotection and increased levels of GltI (Romera et al., 2007). Moreover, several groups have induced glutamate transporter expression with β-lactam antibiotics, in transgenic mice, and with ceftriaxone, and have also demonstrated neuroprotection in vivo (Rothstein et al., 2005; Chu et al., 2007; Weller et al., 2008). The results of the current study are consistent with these findings, and suggest that GltI and Glast are upregulated after injury and might be involved in hypoxic preconditioning and neuroprotection. However, the present study further suggests a mechanism underlying the reduction in injury-induced neurogenesis upon subsequent brain injury.
The results presented here are consistent with findings from experimental models of recurrent brain injury and clinical evidence associated with multiple brain insults. Several studies have shown increased expression of markers of central nervous system damage, including S-100β and neuron-specific enolase, in animals affected by multiple brain maladies, especially TBI (Slemmer & Weber, 2005). More relevant are findings that demonstrate a relationship between the severity and frequency of brain trauma and decreased capacity to recover from such an insult (Spettell et al., 1991; Salcido & Costich, 1992; Slemmer & Weber, 2005). The decreased proliferation of neural progenitors associated with HI and TBI (Fig. 6J) might therefore explain these phenotypes and provide insights into the mechanisms underlying recovery from multiple brain injuries. However, to elucidate the exact mechanisms involved, more focused studies on glutamate transporters and their role in injury-induced proliferation are necessary.
In summary, we provide a novel functional role for glutamate transporters in regulating neural progenitor proliferation in a calcium-dependent and mGluR-dependent manner. In addition, we demonstrate decreased proliferation associated with multiple brain injuries, which correlates with increased glutamate transporter expression. Taken together, the findings of the current study suggest that glutamate transporters contribute to the regulation of progenitor proliferation during development and may also affect induced proliferation after injury.
Acknowledgements
This work was supported by NIH grant R01 NS048192 (S. G. Kernie). The authors have no other financial interest to disclose. The authors would like to thank Gui Zhang, Kyle Lieppman and Darryl Miles for their technical support and assistance with this publication.
Abbreviations
-
- BAPTA-AM
-
- 1,2-bis(2-amino-5-fluorophenoxy)ethane-N,N,N′,N′-tetraacetic acid tetrakis ester
-
- BrdU
-
- bromodeoxyuridine
-
- CCI
-
- controlled cortical impact
-
- CMV
-
- cytomegalovirus
-
- GFAP
-
- glial fibrillary acidic protein
-
- GFP
-
- green fluorescent protein
-
- Glast
-
- excitatory amino acid transporter 1
-
- GltI
-
- excitatory amino acid transporter 2
-
- HI
-
- hypoxic–ischemic injury
-
- LY341495
-
- (2S)-2-amino-2-[(1S,2S)-2-carboxycycloprop-1-yl]-3-(xanth-9-yl)propanoic acid
-
- mGluR
-
- metabotropic glutamate receptor
-
- PBS
-
- phosphate-buffered saline
-
- PI
-
- propidium iodide
-
- PSA-NCAM
-
- polysialic acid neural cell adhesion molecule
-
- QPCR
-
- quantitative PCR
-
- siRNA
-
- small interfering RNA
-
- TBI
-
- traumatic brain injury




