Postnatal refinement of proprioceptive afferents in the cat cervical spinal cord
Abstract
Proprioceptive afferent (PA) information is integrated with signals from descending pathways, including the corticospinal tract (CST), by spinal interneurons in the dorsal horn and intermediate zone for controlling movements. PA spinal projections, and the reflexes that they evoke, develop prenatally. The CST projects to the spinal cord postnatally, and its connections are subsequently refined. Consequently, the tract becomes effective in transmitting control signals from motor cortex to muscle. This suggests sequential development of PAs and the CST rather than co-development. In this study we determined if there was also late postnatal refinement of PA spinal connections, which would support PA–CST co-development. We examined changes in PA spinal connections at 4 weeks of age, when CST terminations are immature, at 8 weeks, after CST refinement, and at 11 weeks, when CST terminations are mature. We electrically stimulated PA afferents in the deep radial nerve. Evoked PA responses were small and not localized at 4 weeks. By 8 and 11 weeks, responses were substantially larger and maximal in laminae VI and dorsal VII. We used intramuscular injection of cholera toxin β subunit to determine the distribution of PAs from the extensor carpii radialis muscle in the cervical enlargement at the same ages as in the electrophysiological studies. We found a reduction of the distribution of PAs with age that paralleled the physiological changes. This age-related sharpening of PA spinal connections also paralleled CST development, suggesting coordinated PA–CST co-development rather than sequential development. This is likely to be important for the development of adaptive motor control.
Introduction
Development of the motor systems and motor control is protracted. During prenatal and early postnatal development, higher brain centers, responsible for movement planning and execution, connect with spinal interneurons and motoneurons, responsible for translating control signals into muscle contraction. Proprioceptive afferents (PAs), which provide limb state information for feedforward planning (Ghez & Sainburg, 1995) and feedback regulation (Windhorst, 2007), develop spinal connections prenatally (Skoglund, 1960; Mears & Frank, 1997; Arber et al., 2000).
After establishing early connections, spinal circuits are refined (Vinay & Jean-Xavier, 2008; Mentis et al., 2010). Activity-dependent processes, during an early postnatal critical period, refine connections of the corticospinal tract (CST), the only descending path that has been thoroughly studied (Ohno et al., 2004; Martin et al., 2009; Yoshioka et al., 2009). Activity-dependent processes, also involving the CST, promote increases in the numbers of interneurons expressing a choline acetyltransferase (ChAT) phenotype within the territories receiving dense CST terminations (Chakrabarty et al., 2009). This likely contributes to the development of the CST signal transmission from motor cortex (M1) to muscle (Meng et al., 2004; Chakrabarty & Martin, 2010). After spinal circuit maturation, the M1 motor map develops (Bruce & Tatton, 1980; Chakrabarty & Martin, 2000) and animals begin to express a broad behavioral repertoire (Martin & Bateson, 1985).
We are beginning to understand the development of descending, particularly CST (Martin et al., 2009), control. However, less is understood about the development of PA control. What we do know is largely the development of the monosynaptic reflex and group Ia motoneuronal terminations in the motor pools (Gibson & Clowry, 1999; Mentis et al., 2010). Little is known about the development of the majority of PA spinal connections within the dorsal horn and intermediate zone. Importantly, muscle spindle primaries make substantially more synapses on interneurons than motoneurons (Burke et al., 1979; Brown, 1981). Many of these interneurons also receive descending inputs, allowing for motor output regulation by PA or descending signals. In this study, we examined the postnatal development of PA terminations in this region of the cat spinal cord. Building on our knowledge that important spinal circuit development occurs between postnatal weeks 5 and 7 in cats (Chakrabarty et al., 2009), we determined if this was also a time of refinement of PA connections with dorsal horn and intermediate zone interneurons. Prenatal development of PAs and postnatal CST development imply sequential development of the two systems. By contrast, late PA refinement implies PA–CST co-development. In cats at different ages, we activated deep radial nerve (DRN) PAs and recorded evoked spinal monosynaptic field potentials. We marked PAs from the extensor carpii radialis muscle, which is innervated by DRN, with cholera toxin beta subunit (CTB).
We found refinement of PA connections with dorsal horn and intermediate zone interneurons during the same period when CST refines its spinal connections. PA refinement produced substantial strengthening of connections in the same spinal cord region where there is concurrent cholinergic interneuron development (Chakrabarty et al., 2009). Our findings indicate a coordinated spatial and temporal PA–CST co-development, rather than sequential development. This is likely to be important for the development of adaptive motor control.
Materials and methods
Animals (n = 10) were obtained from an Association for Assessment and Accreditation of Laboratory Animal Care-accredited supplier. Kittens were delivered in litters of four or five along with a lactating mother at postnatal week 2. The City College of the City University of New York, Columbia University and New York State Psychiatric Institute Institutional Animal Care and Use Committees approved all experimental procedures.
General surgical procedures
For all surgical procedures, animals were administered atropine (0.04 mg/kg, i.m.). A mixture of acepromazine (0.03 mg/kg, i.m.) and ketamine hydrochloride (32 mg/kg, i.m.) was given to induce anesthesia. Cats were intubated after anesthesia was induced and maintained in an areflexive condition during surgery using 1–2% isoflurane. Animals were given a broad-spectrum antibiotic at the time of surgery (cefazolin, 25 mg/kg, i.m.) and an analgesic after surgery (buprenorphine, 0.03 mg/kg, i.m.). Animals resumed nursing after recovery from anesthesia, and were given supplemental milk [Feline Milk Replacement (KMR); PetAg] as needed to ensure adequate weight gain.
For spinal electrophysiological experiments, animals were anesthetized (ketamine, 30 mg/kg, i.m.; xylazine, 0.6–0.8 mg/kg, i.m.; maintained using ketamine infusion at 10–30 mg/kg/h, i.v.), the head was placed in a stereotaxic frame and a cervical (C) laminectomy was made, as in our previous studies (Chakrabarty & Martin, 2010). Animals were administered atropine (0.04 mg/kg, i.m.) during the experiment. The first thoracic spinous process was clamped to stabilize the vertebral column. The DRN was isolated and placed in a stimulating cuff.
Muscle afferent tracing
To label myelinated muscle afferents, we injected cholera toxin β subunit (CTB) (lot#10428A2, List Biological Laboratories; unconjugated, low salt, reconstituted with sterile purified water to a 1% concentration in 0.01 m sodium phosphate buffer) into the extensor carpii radialis muscle. A blue dye (Evans Blue) was added to the CTB solution to help to verify that no injected CTB leaked from the muscle. The skin and fascia over the muscle were slit. A volume of 50 μL was slowly injected into multiple locations. The needle was held within the muscle for several minutes after each injection to prevent leakage. Care was taken to avoid injury to peripheral nerves, which may result in the uptake of CTB by smaller diameter afferent fibers (Shehab et al., 2003). We used a survival time of 10 days to allow transganglionic transport of CTB from the muscle to the terminations of the primary muscle afferents within the spinal cord. CTB also retrogradely labels motoneurons. This allowed us to verify the efficacy of transport by counting the numbers of retrogradely labeled motoneurons across animals of different ages.
Histology and tracer histochemistry
At the termination of experiments, cats were deeply anesthetized (sodium pentobarbital, 30 mg/kg, i.v.) and perfused transcardially with warm saline, followed by a solution of 4% paraformaldehyde, pH 7.4. Heparin was injected (200–500 U, i.v.) at the onset of perfusion. For perfusion, a peristaltic pump was used at a predetermined flow rate that depended on the animal’s weight. The total perfusion time was 20–30 min. The brain and spinal cord were removed, postfixed in the same fixative for 2–3 h, and then transferred to 20% sucrose in 0.1 m phosphate buffer overnight at 4 °C. Frozen transverse sections (40 μm) through the cervical spinal cord (C6–C8) were cut and processed for CTB immunohistochemistry to determine the distribution of labeled CTB terminals.
To visualize axons labeled with CTB, sections were incubated at 4 °C overnight in phosphate-buffered saline containing goat anti-CTB toxin B subunit (1 : 1000, Lot#7032A4, List Biological Laboratories) in blocking buffer (3% donkey serum in 1 × phosphate-buffered saline with 0.2% Tween, pH 7.4). After rinsing, sections were incubated for 2 h at room temperature in blocking buffer containing 0.2% anti-rabbit secondary antibody conjugated to peroxidase (1 : 200; pH 7.4), and then incubated with the chromogen. We commonly used fluorescence to mark CTB terminations. We used a secondary antibody either conjugated to Cy3 (1 : 800; incubated at room temperature; 1 h; donkey anti-rabbit, anti-goat or anti-mouse) or to fluorescin isothiocyanate (1 : 500; room temperature; 2 h; donkey anti-rabbit, anti-goat or anti-mouse). We also used diaminobenzidine, incubated for 5–30 min, in selected experiments to verify the sensitivity of the labeling.
Topography of cholera toxin beta subunit terminations and motoneuron labeling
Cholera toxin beta subunit produces a punctate pattern of intra-axonal labeling, similar to that of horseradish peroxidase using the tetramethylbenzidine reaction. Whereas some puncta are larger than others, it is difficult to distinguish axonal varicosities that might correspond to putative presynaptic sites (i.e. boutons) from non-varicose axonal regions. For this reason we restricted our anatomical analyses to a topographic distribution. See Fig. 3 for representative high-magnification micrographs of transganglionic dorsal horn CTB labeling. We first captured multiple images at 20 × using a fluorescence microscope, all at the same exposure. We created a montage using MosaicJ (Thevenaz & Unser, 2007). This created a high-resolution, low-magnification view of the sections (see Fig. 2, left and center columns). The images were then thresholded and the labeling intensity was measured in pixels using ImageJ (version 1.44k; available at http://rsb.info.nih.gov/ij/; developed by Wayne Rasband, National Institutes of Health, Bethesda, MD, USA) along predefined axes that depended on the particular spinal gray matter region examined. The three distinct areas measured were: (i) dorsoventrally from lamina III to the dorsal portion of lamina VII (see Fig. 4) centrally within the gray matter, deep to the large-diameter myelinated fiber entry zone; (ii) mediolaterally within laminae III–IV (see Fig. 5); and (iii) mediolaterally, within lamina VI (see Fig. 6) (Rexed, 1954). The regional distribution of axonal labeling was measured as a percentage area of the region of interest. This generated a plot of pixel density along the axis measured. The values were then smoothed (Kaleidagraph, Synergy Software, Reading, PA, USA; version 3.6; using the ‘lowess’ function), normalized to the peak (Prism, Graphpad, La Jolla, CA, USA; version 5.0) and graphs were plotted for each animal (average of five sections per animal). These plots were filled and the percentage labeled was obtained (measure function in ImageJ). The mean ± SEM for each group was then calculated (see 4-6).
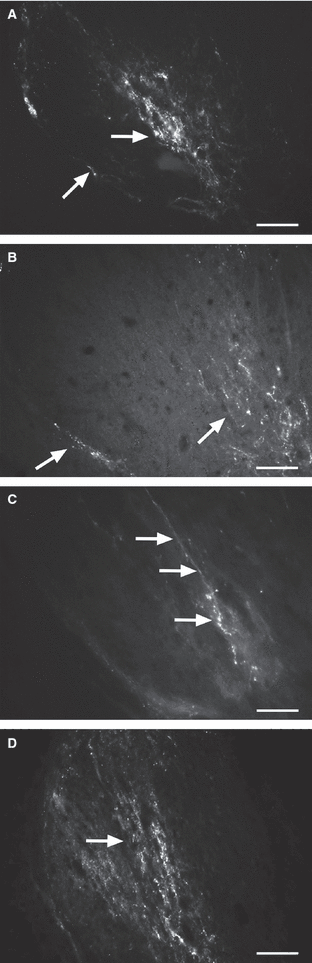
Representative high-magnification micrographs of transganglionic dorsal horn CTB labeling. (A) 4-week-old, (B and C) 8-week-old and (D) 11-week-old animals. Calibration: 100 μm.
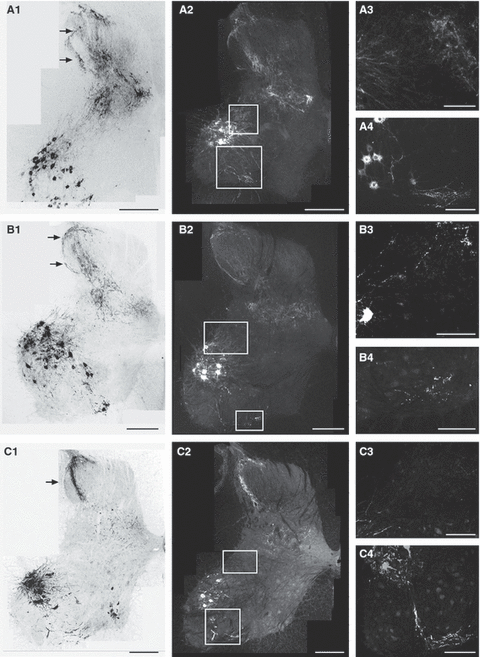
Typical patterns of CTB-labeled terminations in the cervical enlargement of the spinal cord in a 4-week-old animal (A), 8-week-old animal (B) and 11-week-old animal (C). Left panels (A1, B1 and C1) show four overlaid sections, as montages, obtained by epifluorescence microscopy. Images were converted to gray scale and inverted. CTB labeling is black. Middle panels (A2, B2 and C2) show single sections from the same animals as in the left panel. CTB labeling is white. Right panels (A3, A4, B3, B4, C3 and C4) show selected regions at higher magnification. Boxes in the middle panel show the approximate location of micrographs in the right panel. Note that the higher magnification views are not necessarily from the same section or animal. In A3, B3 and C3, the dorsolateral lamina VII is shown. This is where previous studies have shown PA fiber elimination (Gibson & Clowry, 1999; Clowry et al., 2004). In A4, B4 and C4, CTB-labeled recurrent terminations in the ventral lamina VII, where Renshaw interneurons are located, are shown. Calibration: A1, A2, B1, B2, C1 and C2, 500 μm; A3, A4, B3, B4, C3 and C4, 100 μm.
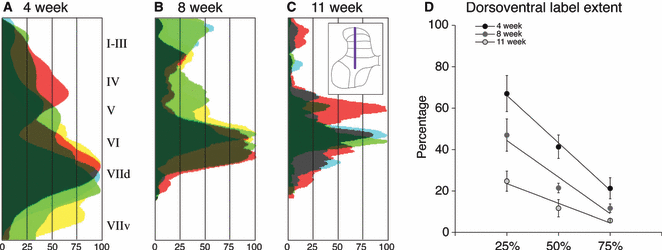
Age-dependent changes in distribution of CTB labeling along the dorsoventral axis. The inset in C shows the location where these densities were measured. (A–C) For each animal, CTB label pixel density from five sections was averaged and the data were normalized to peak label density for each animal (maximal label 100%). Data from animals of the same age are superimposed, with the amount of overlap between animals represented by the darkness. At each age there was a peak in labeling density. The X-axis indicates the density of labeling (0–100%) and the Y-axis shows the data as measured in each lamina indicated in inset in C. At 4 weeks (A), the peak was broad and large, superimposed on diffuse labeling throughout the dorsal and dorsal intermediate zone. At 8 weeks (B) and 11 weeks (C) of age, there is narrowing of the dorsoventral spread and a progressive reduction in the ventral extent of labeling. (D) Percentage of the dorsoventral extent of the gray matter with CTB labeling. The amount of label at three density levels (25, 50 and 75%) is plotted. Across the three criterion percentages, the highest percentage of labeled dorsal horn and upper intermediate zone was at 4 weeks, with the least at 11 weeks and an intermediate amount at 8 weeks (see Results for details).
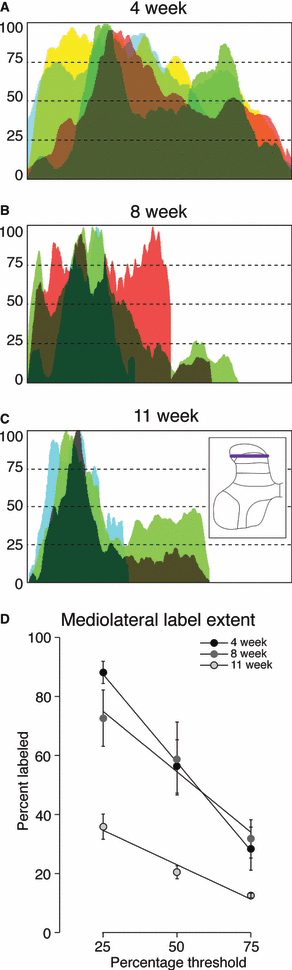
Age-dependent changes in distribution of CTB labeling along mediolateral axis of dorsal horn. This is in the same format as Fig. 4 but oriented vertically. (A) 4 weeks, (B) 8 weeks and (C) 11 weeks. At all ages, the lateral dorsal horn had more labeling than medial dorsal horn. In C, 11 weeks, labeling was absent most medially. (D) Percent of gray matter labeled, across the mediolateral extent. There were age-related differences at only the 25 and 50% criterion levels.
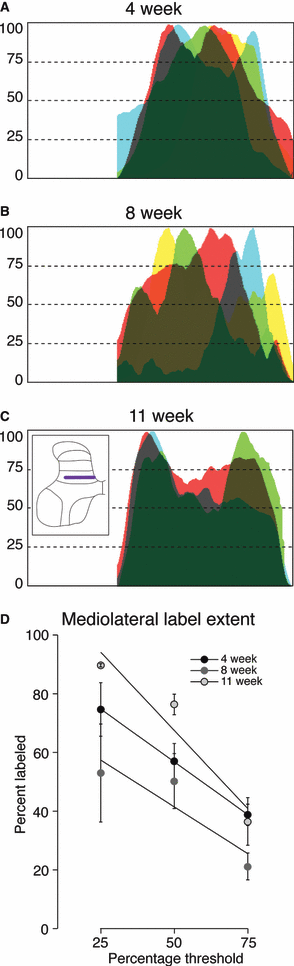
Age-dependent changes in distribution of CTB labeling along mediolateral axis lamina 6. Same format as 4, 5. (A) 4 weeks, (B) 8 weeks and (C) 11 weeks. There is a striking reduction in interanimal variability in the locations of peak labeling by 11 weeks of age; labeling was denser medially and laterally. (D) Percentage of gray matter labeled. There was no significant difference in percentage of gray matter labeled.
To verify that age-related differences in the distribution of CTB-labeled terminations were not due to tracer efficacy, we counted all motoneurons labeled on randomly selected sections in each animal, using the program Neurolucida (MicroBrightfield Inc., Williston, VT, USA; version 8.26.1).
Image acquisition
Bright-field and epifluorescence images were acquired on an Olympus BX-60 microscope and a Microfire-CCD camera (version 2.3A; Optronics, Goleta, CA, USA) mounted on the microscope. For epifluorescence, a 460–500 lambda excitation filter and a 510–560 lambda emission filter were used for fluorescin isothiocyanate, and a 535–550 lambda excitation filter and a 610–675 lambda emission filter were used for Cy3. To adjust contrast and brightness, either ImageJ or Adobe Photoshop CS3 (Adobe Systems Inc., San Jose, CA, USA) was used. When comparing images, all image capture and adjustment parameters were kept identical.
Nerve stimulation, recording and data acquisition
To activate PAs, the DRN was stimulated with a 0.2 ms monophasic square pulse generated using a constant-voltage stimulation (model 2100; A-M Systems). In all experiments, the threshold (T) was defined as the minimum stimulus strength required to evoke a group I response. The nerve was electrically stimulated at 2.5T to evoke maximal group I effects while minimizing stimulation of group II and other afferents (Jack & Roberts, 1974; Edgley & Jankowska, 1987). The cord dorsum potential was recorded on the surface at the dorsal root entry zone at C6 using a silver ball electrode. Depth recordings were made with a glass micropipette (2 m potassium citrate, tip diameter 1.5–2 μm, resistance 2–5 MΩ) using conventional amplification and filtration (< 100 Hz and > 10 kHz), and digitized. The small pipette tip was required to record focal responses, affording recording unit activity. We recorded at caudal C6, from the dorsal horn, intermediate zone and medial aspect of the lateral motor pools. This is the rostrocaudal level of maximal DRN evoked responses. We recorded nominally at 400 μm depth intervals in 4-week-old animals and 500 μm depth intervals in older animals. The records all show positive up. Data were collected at a sampling rate of 100 kHz, in 25 ms epochs. We examined responses to single stimulus pulses. Spinal potentials were acquired using an analog-to-digital converter (Digidata; Molecular Devices) at 25 kHz per channel. We used the program Axograph X (Axograph Scientific, Sydney, Australia) for the Apple Macintosh computer to measure the latency and amplitude of spinal responses. For measurements and presenting potentials (see Fig. 1), we constructed ensemble averages synchronized on the stimulus artifact.
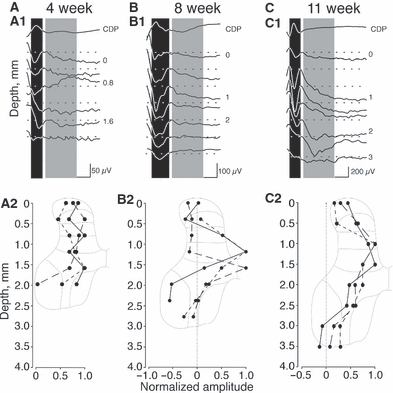
DRN stimulation-evoked field potentials recorded along the dorsoventral axis of the caudal C6 spinal segment. (A1, B1 and C1) The cord dorsum potential (CDP) (top traces) and associated field recordings at the depths indicated (in mm). The dark bands indicate the evoked monosynaptic response and the gray bands indicate the period of oligosynaptic responses. Dotted lines in A–C indicate baseline. (A2, B2 and C2) Plot of mean changes in evoked field potential amplitude, normalized as a function of the peak value for individual animals. Scaled schematics of the spinal cord are shown to help localize the responses to their corresponding laminae. All field potentials were evoked with stimulation of the DRN at 2.5T. (A1 and A2) 4 weeks; in all animals (3/3) the evoked field amplitude was unchanged. (B1 and B2) 8 weeks; in 2/3 animals there was a clear phasic increase in amplitude at a depth of 1 mm in one and at 1.5 mm in the other. The depths correspond with lamina VI in 8-week-old animals. (C1 and C2) 11 weeks; in 2/3 animals a clear phasic increase in amplitude was seen at a depth of 1 mm in one and at 1.5 mm in the other. The depths correspond with lamina VI in 11-week-old and mature animals. The difference in amplitude between peak and baseline values (indicated by the dotted line) was significant in both 8- and 11-week-old animals (see Results for details).
Criteria for focal synaptic potentials
As we have previously discussed (for details, see Chakrabarty & Martin, 2010), focal synaptic potentials were defined as a deflection (negative or positive) in the extracellular record evoked by the stimulus that changed over distances of > 250 μm and began to return or returned to baseline by the end of the recording epoch (typically within 5–10 ms). As described for sensory afferents and descending projections to the cord, focal synaptic potentials occurring within approximately 1 ms after the volley are considered monosynaptic (Edgley & Jankowska, 1987; Noga et al., 1995).
Analysis of focal synaptic potentials
We measured the onset latency, latency to peak negative response and amplitudes of the responses relative to the onset of the response ensemble averages of focal synaptic potentials.
Statistical analyses
Standard statistical tests were conducted (Prism, Graphpad) including: Student’s t-test, one-way anova and repeated-measures anova using Bonferroni posthoc testing at a significance level of P < 0.05.
Results
Development of the monosynaptic proprioceptive afferent response within the dorsal horn and intermediate zone
We activated PAs using electrical stimulation of the DRN at 2.5T (Fu & Schomburg, 1974; Fu et al., 1974). This current maximally activates group I afferents while minimizing activation of group II and other smaller diameter afferents (Jack & Roberts, 1974; Edgley & Jankowska, 1987). We examined the monosynaptic PA response from stimulation of the DRN in kittens at three key developmental ages: 4, 8 and 11 weeks. At 4 weeks of age the group I monosynaptic reflex has developed (Wilson, 1962), but this is before ChAT interneuron development in the spinal cord (Chakrabarty et al., 2009) and CST axon terminal refinement (Li & Martin, 2001, 2002). At 8 weeks, the spinal interneurons have developed their mature neurotransmitter phenotype and CST topographic refinement has occurred, but this is before late-stage development of CST axon termination branching (Li & Martin, 2001) and development of the M1 motor map (Chakrabarty & Martin, 2000). Finally, at 11 weeks there has been further development of CST terminations and the M1 motor map is essentially mature.
Field recordings in response to 2.5 times threshold DRN stimulation are shown in Fig. 1 (top row) for representative animals at 4, 8 and 11 weeks (Fig. 1A1–C1). The dotted line in each trace shows the baseline and the top trace is the cord dorsum potential. The spinal cord inset (Fig. 1A2) shows the approximate mediolateral location of all penetrations shown in Fig. 1. Recordings in 4-week-old animals revealed clear phasic negative (downward directed) focal synaptic potentials, as others have reported. This is the first, monosynaptic, response. Other later responses were recorded, but not further studied because it is not known if they were dominated by disynaptic group I responses or group II (i.e. secondary spindle afferents) responses. As shown in most of the traces in the 4-week-old animals (Fig. 1A1), response amplitude did not change substantially from the pial surface to the ventral recording point. The response pattern of each of the three animals examined at this age was similar, with a broad dorsoventral region in which we recorded the response. This was not due to use of a non-selective extracellular recording electrode because the recorded potentials differed in subtle ways between adjacent recording sites. Importantly, the amplitude of the monosynaptic component did not differ systematically within the dorsal horn or intermediate zone. There was no significant amplitude difference with depth [Fig. 1A2; F = 1.453; P = 0.2870, df = (5,12)]. It should be noted that, as we approached the motor pools, we began to record a combination of the antidromic motoneuron activation and the orthodromic field; it was difficult to clearly distinguish the two. For this reason we did not quantitatively examine these deeper responses.
The amplitude of the evoked responses was substantially larger by 8 weeks. Note that the amplitude calibration in Fig. 1B1 is double that of Fig. 1A1 and Fig. 1B1 is approximately one-half that of Fig. 1C1. In contrast to the 4-week-old animal, there was a clear depth localization of the monosynaptic response at 8 and 11 weeks, as shown in the field recordings (Fig. 1B1 and C1). In all animals, there was a single peak within the dorsal horn, at approximately lamina 6. At 8 weeks, the locations of the peak responses varied between animals (Fig. 1B2), whereas at 11 weeks, the depth response profiles were better aligned (Fig. 1C2). The difference in response amplitude with depth was significant for the 11-week-old animals [Fig. 1C2; F = 10.22; P = 0.0001, df = (7,16)], but not for the 8-week-old group (Fig. 1B2). However, by aligning the dorsoventral distributions at the peak response, the differences in the 8-week-old response amplitude profiles were significant [Fig. 1B2; F = 9.658; P = 0.0014, df = (7,14)]. The depth profile of the monosynaptic response at 11 weeks was essentially the same as that of the adult (Abrahams & Swett, 1986; Hishinuma & Yamaguchi, 1990). Our results show that, by the age CST axon terminations achieve a mature topographic refinement, PAs produced more focused monosynaptic responses in the dorsal horn and intermediate zone. We also noted an overall increase in amplitude in the responses as animals grew older. This is similar to what we observed for the developing CST (Meng & Martin, 2003).
It should be noted that we found clear late evoked responses within lamina 7 when the DRN was stimulated at 2.5T in the 11-week-old, but not in the 4- and 8-week-old animals. The late response in the 11-week-old cat (Fig. 1C1) was probably a group I disynaptic field, as the stimulus amplitude range used was not optimal for recruiting group II afferents in the adult (Fu & Schomburg, 1974; Fu et al., 1974). However, we cannot rule out a contribution from group II afferents (Edgley & Jankowska, 1987; Jankowska & Edgley, 2010). In two 4-week-old animals we stimulated the DRN at 5T and recorded evoked fields within the same region as with 2.5T stimulation. Despite the larger stimulus, one that recruits group II afferents in maturity, consistent late fields were not seen at this young age. When late fields were present, distinct group I or group II components could not be distinguished.
Development of proprioceptive afferents within the dorsal horn and intermediate zone
To better understand the development of functional proprioceptive signals from muscle to spinal segmental circuits we injected CTB into the extensor carpii radialis muscle in kittens at the same three postnatal ages that we studied electrophysiologically. The extensor carpii radialis muscle is innervated by the DRN. Our starting hypothesis, based on the electrophysiological findings, was that PAs would have a broad zone of termination within the dorsal horn and intermediate zone at 4 weeks of age, without clear localization. Further, this broad termination pattern would be sculpted later in development, so that a laminar localization would be present by 8 weeks and further specified at 11 weeks. We obtained strong evidence for refinement and localization of the distribution of PAs within the gray matter.
Figure 2 presents montages of the distribution of CTB labeling within the cervical enlargement at 4 (Fig. 2A1 and 2), 8 (Fig. 2B1 and 2) and 11 (Fig. 2C1 and 2) weeks of age. Retrograde motor pool labeling was strong at all ages. The small differences in the numbers of labeled motoneurons in this figure at the three ages do not reflect systematic changes in tracer efficacy, but rather section-to-section variability. To validate tracer efficiency at all ages, we counted motoneurons in each animal. There were no significant differences in the numbers of motoneurons labeled in animals of different ages [4 weeks: 8.417 ± 0.556 motoneurons labeled; 8 weeks: 6.567 ± 0.342; 11 weeks: 7.275 ± 0.5651; anova, P = 0.1832, F = 2.673, df = (2,6)].
The left column presents overlaid images of four nearby cervical sections for each age. The 4-week-old animal (Fig. 2A) showed dense labeling within the dorsal horn, including the superficial laminae (top horizontal arrow), as well as labeling throughout most of the remaining dorsal horn and intermediate zone (lamina 7). From section to section, the exact location of the dorsal horn labeling varied, so that over the limited rostrocaudal range shown most of the dorsal horn was labeled. Dorsal horn and intermediate zone labeling is quantified below. The middle column shows individual sections at the three ages. These images are shown in darkfield because sparse labeling from single sections (e.g. Fig. 2B2) is best shown this way. Note that the single section in the 4-week-old animal labels a small subregion of the dorsal horn. Figure 3A shows dorsal horn labeling at higher magnification. Labeling in the dorsolateral dorsal horn shows a mixture of non-varicose and varicose axons. Examples of wider, bouton-like swellings are to the right of the horizontal arrow. However, most axon varicosities were not a substantial multiple of the width of non-varicose axons (i.e. 3 × non-varicose width) (Chakrabarty et al., 2009), precluding quantification as presynaptic sites. Dense CTB labeling was consistently observed within and immediately dorsal to the motor pools (Fig. 2A2, upper box; Fig. 2A3). We show this labeling because this area has been extensively examined in the rat during development (Gibson & Clowry, 1999; Clowry et al., 2004). Although this labeling looked like anterograde afferent fiber labeling, we cannot eliminate the possibility that this is a combination of afferent and motoneuronal labeling in the distal dendrites. We also observed CTB-labeled axons at the ventral horn border (see Fig. 2A2, lower box; Fig. 2A4). This was distinctly different from the dorsal labeling, with thicker labeled axons and large bouton-like swellings. Often we could follow bundles of labeled axons from within the motor pool into this ventral site (better shown in the mature animal; see Fig. 2C4). As this labeling was in the vicinity of Renshaw cells (Jankowska & Lindstrom, 1971), we presume that this labeling is contained within the motor axon recurrent collaterals. It should also be noted that labeling in laminae VI–IX, at all ages, appeared somewhat different from labeling within the dorsolateral region (lateral laminae 3 and 4). Ventral label, as others have noted (Gibson & Clowry, 1999), is probably terminal on the basis of its location and that most CTB puncta are scattered randomly without line-like continuity. In the lateral laminae III–IV, although most labeling was not ordered, suggestive of terminal label, there was also some continuous labeling, suggestive of axons (Fig. 2; lower arrows in Fig. 2A1 and B1; single arrow in C1). Further, this labeling could sometimes be followed ventrally into laminae V and VI. We also noted consistent labeling in the superficial dorsal horn (Fig. 2; upper arrows in Fig. 2A1, B1 and C1). It generally appeared denser in 4-week-old animals. The origin of this labeling is considered in the Discussion.
At 8 weeks (Fig. 2B1), despite overlaying four nearby sections, dorsal horn labeling was much more restricted, both superficially and in the deeper laminae. As with the 4-week-old animal, there were varicose portions of axons in the lateral dorsal horn (Fig. 3B, left arrow, lamina 3) and more medially (Fig. 3B, right arrow, lamina 3). Figure 3C shows a particular clear, albeit rare, example of non-varicose (top two arrows) and varicose (bottom arrow) axonal segments in lateral lamina 3. Labeling in the dorsolateral region of the motor pools (lamina IX) was much less than at 4 weeks (Fig. 2B2), as reported for rats at older postnatal ages (Gibson & Clowry, 1999). Labeling in the ventral gray matter (Fig. 2B3) was similar to 4-week-old animals.
By 11 weeks of age (Fig. 2C1), labeling in the dorsal horn was further restricted. Typically, only a thin band of labeling was present (arrow). In addition to this restriction, the location of labeling was highly stereotypic from section to section, as shown for the four overlaid sections. Moreover, it was clear that the lateral position of the labeled bundle was similar across animals. Figure 3D shows an example of lateral dorsal horn labeling in an 11-week-old animal at high magnification. The bundle of labeled axons in the superficial laminae had the characteristics of both varicose and non-varicose axons (arrow), the latter suggesting synaptic contacts with dorsal horn neurons. Labeling in the dorsolateral motor pool region was very sparse (Fig. 2C3); labeling of motor axon collaterals in the region of the Renshaw cells was similar to that of younger ages (lower box; Fig. 2C4). Our findings indicate that PA labeling at 4 weeks of age has a distinctive pattern of densely labeled regions. It is not uniform as suggested by the electrophysiological data. By 8 weeks of age, there is an overall substantial elimination of terminations within the densely labeled regions. There appeared to be a further elimination by 11 weeks and stereotypy in the locations of labeling.
Development of proprioceptive afferent dorsoventral regional topography
We next determined whether the age-dependent reduction in PA labeling paralleled the changes in the monosynaptic depth profiles. We measured the distribution of CTB labeling dorsoventrally (from laminae I to VII) at three different ages (Fig. 4A, 4 weeks; 4B, 8 weeks; C, 11 weeks). For each animal, we averaged CTB label pixel density from five sections. Data are normalized to peak label density for each animal (maximal label, 100%). Data from animals of the same age are superimposed, with the amount of overlap between animals represented by the darkness. Although there was a peak in labeling density at each age, at 4 weeks, more than at 8 and 11 weeks, the peak was broad and superimposed on diffuse labeling throughout the dorsal horn and upper intermediate zone. Thus, as animals grew older there was a narrowing of the dorsoventral spread of terminations. Importantly, there was also a progressive reduction in the ventral extent of labeling.
Figure 4D graphs the percent of the dorsoventral extent of the gray matter in which CTB labeling was present. We plot the amount of label at three criterion density levels: 25, 50 and 75%. These percentages correspond to the gray vertical lines shown in Fig. 4A–C. For each criterion percentage, the amount of label was inversely ordered according to age. The highest percentage of labeling within the dorsal horn and upper intermediate zone was at 4 weeks, with the least at 11 weeks, and an intermediate amount at 8 weeks. At each criterion percentage, there was a clear trend of change with age [one-way anova; 25%: F = 6.923, P = 0.0180; 50%: F = 11.8, P = 0.0041; 75%: F = 4.764, P = 0.0434; df = (2,8)]. However, amongst the three ages the only differences that were significant were at the 25% criterion (4 weeks vs. 11 weeks, mean difference 42.35, t = 3.715; 4 weeks vs. 8 weeks, mean difference 19.91, 3.379; 4 weeks vs. 11 weeks, mean difference 29.65, 4.658).
We also determined the localization of age-related changes in the dorsoventral extent of CTB labeling within particular dorsal and intermediate laminae (see Fig. 7A). For this analysis, we computed the percent area occupied by the CTB label within three regions of interest that together combine to form the total shown in Fig. 4: (i) laminae I–IV, (ii) laminae V–VI and (iii) dorsal portion of lamina VII. For the dorsal laminae examined, there was significantly less labeling at 11 weeks of age than at 8 weeks. For laminae V and VI, there was no significant difference with age. Finally, for the dorsal portion of lamina VII, the amount of labeling was significantly different between 4 weeks and the two older ages (see Fig. 4 legend for statistics).
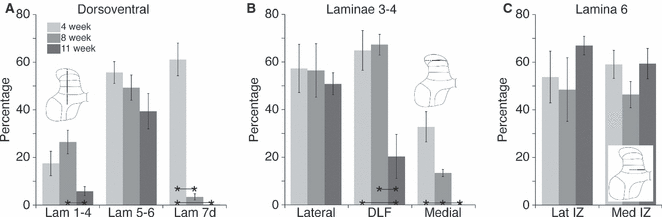
Age-dependent localization of CTB labeling within specific laminae. Measurements were made along three different axes. (A) Localization of age-related changes in CTB labeling along dorsoventral axis. CTB labeling was measured in three locations: dorsal (lam 1–4) and intermediate laminae (lam 5–6 and lam 7d). In dorsal horn, labeling at 11 weeks of age was less than that at 8 weeks. In intermediate laminae (lam 5 and 6), there was little difference with age. In the deeper intermediate zone (lam 7d), the difference in labeling between 4 weeks and the two older ages was significantly different. Laminar comparison: one-way anova measures, lam 1–4: F = 4.616, P = 0.0465; lam 5–6: F = 1.990, P = 0.1988; lam 7d: F = 60.93, P < 0.0001, df = (2,8). Bonferroni’s multiple comparison, significance P < 0.05; lam1 −4: 8 vs. 11, mean difference 20.61, t = 3.038; lam 7d: 4 vs. 8, mean difference 57.68, t = 13.49; 4 vs. 11, mean difference 60.98, t = 13.21. (B) Localization of age-related changes in CTB labeling along mediolateral axis of laminae 3–4. The area was subdivided into three parts: lateral, DLF and medial. There was no significant change in distribution of labeling in the lateral region. In the DLF region, there was a reduction in labeling at 11 weeks. In the medial region, virtually all labeling was eliminated in 11-week-old animals. Laminar comparison: one-way anova measures; lateral: F = 0.1574, P = 0.8573; DLF: F = 15.44, P = 0.8152; medial: F = 24.93, P = 0.0007, df = (2,8). Bonferroni’s multiple comparison, significance P < 0.05; DLF: 8 vs. 11, mean difference 46.94, t = 4.693; 4 vs. 11, mean difference 47, t = 5.023; medial: 4 vs. 8, mean difference 25.52, t = 4.49; 4 vs. 11, mean difference 38.8, t = 6.839. (C) Localization of age-related changes in CTB labeling within laminae 6. There was no significant difference at any age or within either region examined. Laminar comparison: one-way anova measures; lateral intermediate zone (Lat IZ): F = 0.6640, P = 0.5410, df = (2,8); medial intermediate zone (Med IZ): F = 1.655, P = 0.2503, df = (2,8). * with the connecting lines, indicate the columns that are significantly different.
Development of proprioceptive afferent regional topography in the dorsal horn and intermediate zone
Figure 5 presents data for age-dependent mediolateral changes in dorsal horn labeling (see inset, Fig. 5C), in the same format as Fig. 4. At all ages, more of the lateral dorsal horn was labeled, than medial dorsal horn (i.e. clustering of labeling to the left). In fact, by 11 weeks, labeling was absent most medially. Graphs of percent of gray matter labeled, across the mediolateral extent, are shown in Fig. 5D. There were age-related differences at only the 25 and 50% criterion levels [25%: F = 20.23, P = 0.0012; 50%: F = 5.236, P = 0.0407; 75%: F = 2.525, P = 0.1494; df = (2,6)]. Posthoc testing revealed that the only significant differences were between 11 and both 4 and 8 weeks, at 25% criterion (4 weeks vs. 11 weeks, mean difference 52.26, t = 6.294; 11 weeks vs. 8 weeks, mean difference −36.78, t = 4.143) and 50% criterion (8 weeks vs. 11 weeks, mean difference −38.53, t = 3.333).
We also determined the localization of age-related changes in CTB labeling within three separate mediolateral portions of laminae III–IV (Fig. 7B). This analysis is similar to that described above for Fig. 7A. For the lateral region, there were no significant changes in distribution of labeling. For the dorsolateral funiculus (DLF) region, the distribution of labeling became restricted at 11 weeks of age. Labeling at 11 weeks was significantly less than that in 4- and 8-week-old animals. For the medial region, virtually all labeling was eliminated. As a consequence, there was a significant reduction from each age to the next. Again, labeling at 11 weeks was significantly less than that in 4- and 8-week-old animals.
Figure 6 plots mediolateral data for lamina VI, in the same format as 4, 5. In contrast to the dorsoventral and lamina 4 distributions, there were no significant changes in the percentage of gray matter labeled (Fig. 6D). However, there was a striking reduction in interanimal variability in the locations of peak labeling by 11 weeks of age; labeling was denser medially and laterally. As with the other regions examined, we also determined the localization of age-related changes in CTB labeling within lamina 6 (Fig. 7C). The most lateral location was not examined as there was no labeling in this region of the cord at all ages. There were no significant differences at any age or within either region examined.
Discussion
Development of the motor system is commonly considered to be a bottom-up process. Spinal circuits, which comprise the lower levels of the hierarchy, develop prenatally or early postnatally. The simplest motor circuit is the group Ia monosynaptic reflex, which is functional before birth (Naka, 1964; Mears & Frank, 1997). However, it undergoes substantial refinement postnatally, losing connections with heteronymous motoneurons and maintaining homonymous connections (Seebach & Ziskind-Conhaim, 1994). These changes occur before the M1 motor representations mature (Chakrabarty & Martin, 2000) and diverse behavioral repertoires are expressed (Martin & Bateson, 1985). The CST refines its terminations between these early and late time-points (Martin et al., 2009). Our finding that PAs within the dorsal horn and intermediate zone undergo refinement during the same period that the CST refines its spinal connections shows that this simple hierarchical developmental plan is not valid. To our knowledge, this is the first analysis of the topographic and functional postnatal development of PA terminations outside the motor pools. This is an important PA projection; it is the first relay for information about limb joint angle, muscle length and other limb conditions to other spinal and supraspinal targets (Jankowska, 1992). This information is used for diverse functions, including motor planning and feedback regulation (Ghez & Sainburg, 1995).
Segmental connections of proprioceptive afferents undergo late postnatal refinement
We used a 2.5T stimulus to activate PAs. The evoked responses had short segmental latencies (< 1 ms), consistent with monosynaptic activation of spinal neurons. This stimulus optimizes for maximal group I activation and minimizes group II activation (Fu & Schomburg, 1974; Fu et al., 1974). We preferentially labeled myelinated PAs – probably myelinated – using CTB. The pattern of labeling at different ages matched that of the fields, suggesting that an important component of the labeling was associated with group I afferents. Further, we observed motor pool afferent labeling consistent with an important contribution of group Ia terminations (Brown, 1981) (see Materials and methods). Group Ia single axon fills in mature cats also show a similar deep dorsal horn, intermediate zone and ventral horn labeling pattern (Brown, 1981). In contrast to the studies of Brown and colleagues, however, we observed more terminal labeling within the dorsal horn than suggested by either group Ia or Ib single axon fills. There is some evidence for group Ia afferents contacting interneurons in lamina IV in mature animals (Jankowska & Edgley, 2010). Alternatively, this labeling could be due to group II afferents, which terminate in the lateral dorsal horn (Brown, 1981), or the use of a more sensitive tracing method than acute axon fills and short survival. As discussed in the Results, the absence of consistent late fields in the young animal at 2.5T suggests that we preferentially studied group I development, but contributions from group II fibers cannot be excluded. In mature animals, group Ia afferents traverse lateral laminae II to VI in the same segment before projecting to lamina IX to contact motoneurons (Brown & Fyffe, 1978). En route, they arborize in three locations: lamina VI, lamina VII (principal location of Ia inhibitory interneurons) (Jankowska & Lindstrom, 1972) and within the motor pools in lamina IX. Our findings in 11-week-old animals match this distribution, indicating that the PA termination pattern is mature by this age. We found partial elimination of terminations in lamina VII with age (2, 4).
At 4 weeks, we found substantial labeling within the dorsal horn. This labeling was largely eliminated by 8 weeks. In the rat, there is a loss of labeling in the region of the dorsolateral motor pools, and this elimination showed a dependence on the CST (Gibson & Clowry, 1999). CTB labeling within lamina VI persisted throughout development, but connections became substantially stronger with age. Labeling in dorsal lamina VII was partially reduced and that in ventral lamina VII was largely eliminated. Interestingly, CTB persistently labeled a small region in the superficial dorsal horn. Recently, it was shown using spinal cord slices that large-diameter fibers contact GABAergic neurons in laminae I–III of the mouse (Daniele & MacDermott, 2009). This could reflect a spinal mechanism by which PAs suppress pain systems as they co-activate more ventral mechanosensory and motor circuits.
There was persistence of labeling within laminae VI and dorsal VII (2, 7). This is the location of potentially important motor control interneurons. Interneurons that are active during fictive locomotion with mono- or disynaptic inputs from the DRN (Hishinuma & Yamaguchi, 1990) are present here. The medial lamina VI contains spinocerebellar neurons in the cervical segment (Verburgh et al., 1989; Krutki & Mrowczynski, 2002) and, somewhat more laterally and ventrally, premotor interneurons in cats (Alstermark & Kümmel, 1990; Hishinuma & Yamaguchi, 1990) and mice (Asante & Martin, 2010; Stepien et al., 2010). The evoked field potentials in this region of the cord increased in amplitude by 8 weeks of age, indicating improved efficacy of activation of the local interneurons and improved throughput from PAs to muscle. As discussed below, this is the location of the increase in ChAT-positive interneurons, which also occurs by 8 weeks of age (Chakrabarty et al., 2009). Most PA projections to more dorsal and ventral laminae are eliminated later in development. PAs may develop functional connections throughout the dorsal horn and intermediate zone but are subsequently eliminated within certain laminae, as reported for Renshaw cells (Mentis et al., 2006). Maintenance of terminations within lamina VII may reflect development of a more targeted projection to particular interneuron classes, as reported for V1-derived 1a inhibitory interneurons (Siembab et al., 2010).
Coordinated spatial and temporal co-development of the corticospinal tract and proprioceptive afferents
The increase in the amplitude of the monosynaptic PA response occurred as the CST is refined; there is a loss of the most dorsal and ventral axons (Li & Martin, 2000), as well as most of the ipsilateral axons (Theriault & Tatton, 1989; Alisky et al., 1992; Martin et al., 1999). Further, the peak responses were localized to the laminae where we demonstrated an activity-dependent increase in the number of interneurons expressing ChAT.
A shift from an inhibitory to a facilitatory interneuronal network ‘bias’ may occur at this time. At 4 weeks of age, putative inhibitory interneurons, marked by the presence of immunostaining for the calcium-binding protein parvalbumin (Antal et al., 1991), are present in the spinal cord, whereas cholinergic interneurons (i.e. expressing ChAT) are sparse, limited solely (Chakrabarty et al., 2009) to the Pitx2 group in medial lamina VII (Miles et al., 2007; Zagoraiou et al., 2009; Enjin et al., 2010). Whereas the putative inhibitory interneurons remain stable by 8 and 11 weeks of age, cholinergic interneurons become plentiful throughout laminae VI and VII. Unilateral activity blockade of M1/CST activity largely blocked the increase in cholinergic neurotransmitter phenotype (Chakrabarty et al., 2009). Thus, with putative inhibitory interneurons stable and a substantial increase in excitatory (cholinergic) interneurons, the overall balance of excitation and inhibition within the cord shifts toward excitation.
This change in the postsynaptic target phenotype probably plays a significant role in defining how the afferent terminations are refined. We propose the following PA–CST co-development model to help explain the changes in distribution and strength of PA responses in the dorsal horn and intermediate zone. At 4 weeks of age, PAs terminate within the territory of abundant putative inhibitory interneurons – in both the dorsal laminae I–III (Spike & Todd, 1992; Todd & Spike, 1993) and deeper, in laminae V–VII (Antal et al., 1991; Anelli & Heckman, 2005; Chakrabarty et al., 2009) – as well as the future territory of the cholinergic interneurons (Barber et al., 1984; Phelps et al., 1984; Chakrabarty et al., 2009). Their postsynaptic actions, weak by mature standards, were not different across laminae at this age. PAs could activate a mixture of these abundant putative inhibitory neurons, as well as a small population of excitatory interneurons; small because most cholinergic interneurons develop later. The net postsynaptic effect of PA actions would be muted. By 8 and 11 weeks of age, excitatory actions are greater because there are more cholinergic interneurons (Chakrabarty et al., 2009). The net result would be larger PA-evoked responses because of the excitatory bias at these ages. We previously reported that the increase in cholinergic interneurons in the spinal gray matter occurs as there is enhancement of CST throughput (Chakrabarty & Martin, 2010). This may have a complementary effect on the postsynaptic PA response by further increasing the excitability of interneuronal circuits that are the convergent targets of both PAs and the CST. These findings show that there is a coordinated spatial and temporal co-development of the CST and PAs that produces strong and well-localized postsynaptic responses in the dorsal horn and intermediate zone. This functional development is likely to play an important role in the development of a more flexible and adaptive motor control, characteristic of kittens from postnatal week 7 onward (Martin & Bateson, 1985).
Acknowledgements
We thank Xiuli Wu for histochemistry and histology. Supported by NIH NS36835 (J.H.M.).
Abbreviations
-
- C
-
- cervical
-
- ChAT
-
- choline acetyltransferase
-
- CST
-
- corticospinal tract
-
- CTB
-
- cholera toxin β subunit
-
- DLF
-
- dorsolateral funiculus
-
- DRN
-
- deep radial nerve
-
- M1
-
- motor cortex
-
- PA
-
- proprioceptive afferent
-
- T
-
- threshold




