Presynaptic nitric oxide/cGMP facilitates glutamate release via hyperpolarization-activated cyclic nucleotide-gated channels in the hippocampus
Abstract
In hippocampal neurons, synaptic transmission is affected by a variety of modulators, including nitric oxide (NO), which was proposed as a retrograde messenger as long as two decades ago. NO signals via two NO-sensitive guanylyl cyclases (NO-GCs) (NO-GC1 and NO-GC2) and the subsequent increase in cGMP. Lack of long-term potentiation in mice deficient in either one of the two NO-GCs demonstrates the involvement of both NO-GCs in synaptic transmission. However, the physiological consequences of NO/cGMP and the cellular mechanisms involved are unknown. Here, we analyzed glutamatergic synaptic transmission, most likely reflecting glutamate release, in the hippocampal CA1 region of NO-GC knockout mice by single-cell recording, and found glutamate release to be reduced under basal and stimulated conditions in the NO-GC1 knockout mice, but restorable to wild-type-like levels with a cGMP analog. Conversely, an inhibitor of NO/cGMP signaling, ODQ, reduced glutamate release in wild-type mice to knockout-like levels; thus, we conclude that presynaptic cGMP formed by NO-GC1 facilitates glutamate release. In this pathway, NO is supplied by endothelial NO synthase. In search of a cGMP target, we found that two mechanistically distinct blockers of hyperpolarization-activated cyclic nucleotide-gated (HCN) channels (ZD7288 and DK-AH269) abolished the cGMP-induced increase in glutamate release, suggesting that cGMP either directly or indirectly signals via HCN channels. In summary, we unravel a presynaptic role of NO/cGMP most likely in glutamate release and propose that HCN channels act as effectors for cGMP.
Introduction
As a rapidly diffusible messenger molecule, nitric oxide (NO) has been implicated as a neuromodulator in synaptic transmission for two decades (Snyder & Bredt, 1991; Boehning & Snyder, 2003; Garthwaite, 2008). NO is generated by NO synthases (NOSs), two isoforms of which, endothelial NOS (eNOS) and neuronal NOS (nNOS), are responsible for the formation of NO as a signaling molecule under physiological conditions. Functionally, various effects of NO on neuronal excitability and/or synaptic transmission have been described, and can be generally classified as excitatory or inhibitory. NO formation in response to NMDA receptor activation is the archetype of neurotransmitter-induced NO formation, and is explained by the physical association of nNOS and NMDA receptor subunit NR2B via postsynaptic density protein 95 (PSD95) (Christopherson et al., 1999).
Many of the effects of NO as a signaling molecule are mediated by NO-sensitive guanylyl cyclases (NO-GCs), which act as receptors for NO (Garthwaite, 2008; Mergia et al., 2009). The enzymes contain a prosthetic heme group as the NO receptor site, and respond to NO binding with an up to 200-fold increase of cGMP-forming activity (Koesling et al., 2004). The resulting cGMP increases are transduced by the cGMP effector molecules – cGMP-dependent protein kinase type I and cGMP-dependent protein kinase type II (Hofmann et al., 2006), cGMP-activated phosphodiesterases (Bender & Beavo, 2006) and cGMP-gated ion channels (Biel et al., 2009).
Two isoforms of NO-GC exist, the more widely expressed NO-GC1, and NO-GC2, which mainly occurs in the central nervous system (Mergia et al., 2003). Whereas no differences have been detected between the isoforms in catalytic or regulatory properties (Russwurm et al., 1998), NO-GC2 was shown to be able to interact with a PDZ domain of PSD95 (Russwurm et al., 2001), indicating that it is located in close proximity to nNOS, which also interacts with PSD95 (see above). Knockout (KO) mice deficient in either one of the NO-GCs have been generated (Mergia et al., 2006), and allow study of the contribution of each NO-GC to synaptic transmission. Analysis of long-term potentiation (LTP) in the visual cortex and hippocampus has shown that both NO-GCs are required for LTP, as LTP was abolished in mice lacking either NO-GC1 or NO-GC2 (Haghikia et al., 2007; Taqatqeh et al., 2009).
Here, we analyzed excitatory synaptic transmission, most likely reflecting glutamate release, in the hippocampi of the NO-GC1 and NO-GC2 KO mice by single-cell recordings, and found reductions in glutamate release in the NO-GC1 KO mice under basal and stimulated conditions, but no changes in NO-GC2 KO mice. The NO-GC1 KO phenotype with reduced glutamate release was mimicked in wild-type (WT) mice upon pharmacological inhibition of NO/cGMP signaling, underlining a role of cGMP, most likely in glutamate release. Endothelial NOS-derived, and not nNOS-derived, NO was shown to be responsible for glutamate release under our experimental conditions, that is, in the presence of an NMDA receptor blocker. Finally, blockers of hyperpolarization-activated cyclic nucleotide-gated (HCN) channels [4-ethylphenylamino-1,2-dimethyl-6-methylaminopyrimidinium chloride (ZD7288) and DK-AH269] reduced glutamate release in WT mice to KO-like levels, and did not affect glutamate release in NO-GC1 KO mice, suggesting that HCN channels are involved in the execution of cGMP effects.
Materials and methods
KO animals
The generation and genotyping of NO-GC1 and NO-GC2 KO mice has been described previously (Mergia et al., 2006). Briefly, exon 4 of the α1-subunit and exon 4 of the α2-subunit floxed by loxP sites (α1+/flox and α2+/flox) were removed by crossing with the general deleter mouse EIIa-Cre. The resulting heterozygous KO mice were crossed with WT mice (C57BL/6) to remove the Cre recombinase gene. Finally, the generated heterozygotes were intercrossed to yield homozygous mice (NO-GC1 KO and NO-GC2 KO). All experiments were carried out with postnatal day 21–28 KO mice and the respective WT littermates of either sex with a C57BL/6 background. As no differences were detected between the respective WT mice of either KO strain, the WT data were pooled regardless whether of they were derived from NO-GC1 KO or NO-GC2 KO littermates.
Electrophysiology
The present study was performed in full accordance with the guidelines of the local animal ethics commission and German law. Mice were deeply anesthetized with ether and decapitated. Slice preparation and whole-cell patch-clamp recordings of CA1 pyramidal cells were performed as previously described in Taqatqeh et al. (2009). Neurons were recorded at 32 ± 2 °C and visually identified with differential interference contrast optics and a × 40 objective (Olympus, Hamburg, Germany). Patch pipettes (4–6 MΩ) pulled from borosilicate glass capillaries (GB 150F-8P; Science Products, Frankfurt, Germany) contained either 135 mm potassium gluconate, 20 mm KCl, 2 mm MgCl2, 10 mm Hepes and 10 mm EGTA for characterization of the intrinsic cell membrane properties, or 130 mm CsGlu, 8 mm KCl, 10 mm EGTA, 10 mm Hepes, 2 mm MgCl2, 2 mm CaCl2, 2 mm Mg-ATP and 0.3 mm GTP for all other experiments.
Passive membrane properties and firing behavior of neurons were studied in current-clamp mode by use of an Axopatch 200 B amplifier (Axon Instruments, Union City, CA, USA) with depolarizing and hyperpolarizing current steps through the recording electrode. Access resistance ranged from 12 to 15 MΩ, and the seal resistance was > 1 GΩ. Data were discarded if one of the parameters changed by more than 20%. Voltage-clamp recordings were not corrected for liquid junction potentials and series resistance.
Excitatory postsynaptic current (EPSC) recordings were performed in cell somata at a holding potential of −80 mV, as described in detail by Abidin et al. (2006). In brief, miniature EPSCs (mEPSCs) and evoked EPSCs were evaluated through recordings of AMPA receptor (AMPAR)-mediated EPSCs isolated by bath application of 25 μm d-(–)-2-amino-5-phosphonopentanoic acid (D-AP5) and 100 or 50 μm picrotoxin (PTX). For mEPSC recordings, 0.5 μm tetrodotoxin (TTX) was also applied. mEPSCs were recorded for at least 10 min, and the events were analyzed offline. Only those with a clear signal-to-noise ratio, a defined baseline and smooth rising phase were used.
Minimal electrical stimulation was applied through a glass electrode (4–6 MΩ) stimulating the afferent Schaffer collateral–commissural fibers projecting onto the CA1 neurons. The position of the stimulation electrode was carefully adjusted to fulfill three criteria: (i) minimal evoked EPSC (meEPSC) signals should be evoked to in an all or non manner; (ii) the responses should be similar in amplitude to mEPSC recordings; and (iii) within each recording, the latencies of meEPSCs should always be in a range of 5–10 ms following the stimulation artefact. Generally, signals with amplitudes > 5–6 pA were used for further analysis. In addition, all selected signals showed a clear sharp-rising phase.
Evoked EPSCs were measured in the presence of 5 mm QX-314 in the intracellular solution. Prior to high-frequency stimulation (HFS), the maximal EPSC amplitude was calculated for each cell by determining an input–output relationship. A stimulation intensity inducing 70% of the maximal amplitude was always chosen for application of HFS. Although the maximally evoked EPSC amplitude was lower in the NO-GC1 KO mice than in the WT mice, the strength of the stimulation intensity needed to evoke 70% of the maximum signal amplitude did not differ between the groups. HFS was performed by application of 40 pulses at 20 Hz. Paired-pulse ratios (PPRs) were derived from two consecutively evoked EPSCs at a holding potential of −80 mV and with interstimulus intervals (ISIs) of 30, 50 and 100 ms at different extracellular calcium concentrations (0.5, 2.5 and 4 mm). The PPR was calculated from the mean amplitudes of six to eight paired stimulations.
All recorded data were filtered at 2 kHz and digitized at 5 kHz with a Digidata-1200 system equipped with PClamp10 software (Molecular Devices, Sunnyvale, CA, USA). Data were analyzed offline with the same software.
Drugs
PTX (100 or 50 μm), D-AP5 (25 μm), 6,7-dinitroquinoxaline-2,3-dione (DNQX) (20 μm), l-NG-nitroarginine (L-NNA) (100 μm) and ZD7288 (50 and 10 μm) were obtained from Tocris Biozol (Eching, Germany); TTX (0.5 μm) was from ICS (Munich, Germany); 1H-[1,2,4]oxadiazole[4,3-a]quinoxalin-1-one (ODQ) (10 μm), QX-314 (5 mm) and DK-AH269 (10 μm) were from Sigma-Aldrich (Munich, Germany); vinyl-l-N5-(1-imino-3-butenyl)-l-ornithine (L-VNIO) (0.1 μm) was from Alexis (Lausen, Switzerland); and 8-Br-PET-cGMP (100 μm) was from BioLog (Bremen, Germany).
Preparation of synaptosomal fraction and western blot analysis
Preparation of synaptosomes was perforfmed as in Dunkley et al. (2008). In brief, hippocampi (20 mice) were removed and homogenized (9 mL/g tissue) with 10 strokes of a glass/glass homogenizer (500–800 r.p.m.) in an isotonic sucrose solution (0.32 m sucrose, 1 mm EDTA, 0.25 mm dithiothreitol, 20 mm Tris, pH 7.4). After centrifugation (1000 g, 10 min, 4 °C), a small portion of the supernatant was used as hippocampal homogenate for western blots (see below). The remaining supernatant was adjusted to a protein concentration of 4–5 mg/mL, and applied to the Percoll gradient (3, 10, 15 and 23% Percoll). Centrifugation and collection of synaptosomes was performed as previously described (Dunkley et al., 2008). Synaptic proteins such as NR2B, PSD95 and synaptophysin were shown to be enriched in the synaptosomal fraction. Western blot analysis and antibodies against the NO-GCs were as described in Mergia et al. (2006). Antibodies against eNOS and nNOS were from BD Transduction Laboratories (No. 610297; Franklin Lakes, New Jersey, USA) and Alexis, respectively. Antibodies against the HCN channels (HCN1, HCN2, HCN3 and HCN4) were from Alomone Labs (Jerusalem, Israel).
Immunohistochemistry
Mice were anesthetized with pentobarbital (300 mg/kg body weight, i.p.; Narcoren; Rhone Merieux GmbH, Laubheim, Germany) and intracardially perfused with heparinized saline, followed by 50 mL of cold 4% paraformaldehyde in 0.1 m phosphate-buffered saline (PBS) (pH 7.4). The brains were removed and postfixed overnight at 4 °C in the same fixative. Thereafter, brains were embedded in paraffin and cut into 5-μm horizontal sections.
Randomly chosen sections containing the ventro-caudal region of the hippocampus were mounted on slides, dewaxed in xylene, and dehydrated in a descending ethanol series. After 90 min of preincubation in blocking solution (10% normal goat serum diluted in PBS containing 0.2% Triton X-100), sections were exposed overnight to the primary peptide antibody raised against the β1-subunit of NO-GC (1 : 500; rabbit; see below) diluted in PBS containing 1% normal serum and 0.2% Triton X-100. After extensive washing in PBS, sections were incubated with the biotinylated secondary anti-rabbit FAB (1 : 200; goat polyclonal; Dianova, Hamburg, Germany) diluted in PBS with 1% normal serum and 0.2% Triton X-100 for 90 min. After washing, slices were incubated with Cy5-conjugated streptavidin (1 : 3000; Jackson ImmunoResearch, West Grove, PA, USA) for another 90 min. For further double and triple labeling, the second and third primary antibodies were applied sequentially to exclude cross-reactivity. Additionally, a preincubation step in blocking solution (90 min) containing PBS with 10% normal serum, 20% avidin and 0.2% Triton X-100 was used each time. The second primary antibody, vesicular glutamate transporter 1 (vGLUT1) (1 : 8000; guinea pig; catalog no. 5905; Millipore GmbH, Schwalbach, Germany), diluted in PBS containing 1% normal serum, 20% biotin and 0.2% Triton X-100 was applied overnight and visualized with an appropriate biotinylated secondary anti-guinea pig antibody (1 : 500; goat; Dianova) followed by Cy2-conjugated streptavidin (1 : 250; Jackson ImmunoResearch). For triple labeling, the third primary antibody, PSD95 (1 : 200; rabbit; catalog no. 2507; Cell Signalling Technology, Danvers, MA, USA) diluted as described above and applied overnight, was detected by a secondary antibody conjugated to Cy3 (1 : 1000; Jackson ImmunoResearch). Possible cross-reactivity between antibodies was excluded, as omission of a primary antibody resulted in a lack of staining in the corresponding channels. Furthermore, the same results were observed when the sequential order of primary antibodies or fluorophores was changed. Possible autofluorescence or unspecific background labeling of the secondary antibodies or Cy-conjugated streptavidin were excluded by staining sections without the primary antibodies.
Sections were examined with a TCS SP5 confocal laser-scanning system (Leica Microsystems, Wetzlar, Germany) equipped with a Leica DM6000 CFS microscope and Ar/HeNe continuous wave lasers and a × 40 water immersion objective (NA 0.8). The Leica software (LAS AF; Leica Microsystems) was used to obtain z-stacks of six optical sections (0.5 μm apart), with application of an additional × 6 zoom and a pixel size of 26 or 51 nm. A small pinhole and a sequential scan of each channel optimized the detection of the correct signal corresponding to the fluorescent dyes used. Images recorded from independent experiments (n = 4 animals) were subjected to colocalization analysis with ImageJ (Rasband, W.S., ImageJ; US National Institutes of Health, Bethesda, MD, USA; http://rsb.info.nih.gov/ij/, 1997–2011). After background subtraction in each image, the NO-GC1 channel was hidden to prevent bias, and 54 ± 12 regions of interest (ROIs) (0.5–1.5 μm in diameter) were selected from the vGLUT1 and PSD95 channels. ROIs were defined as containing vGLUT1 apposed to PSD95 signals, indicating the presence of synaptic contact. Analysis of ROIs was performed with the colocalization test plug-in (Tony J. Collins, http://www.macbiophotonics.ca), according to the method of Fay et al. (1997), to evaluate the significance of the Pearson coefficient obtained. P-values ≥ 0.95 were considered to be significant and to be indicative of colocalization. Furthermore, images were analyzed without selection of ROIs by the same method.
The antibody against the β1-subunit of NO-GC was generated by immunization of rabbits with two antigenic peptides (RIIYDDSKTYD and GTEETNEEDEN). As purification of the antibody did not improve the signal in western blot analysis, serum was used in the respective experiments. The serum was extensively characterized by western blot analysis, and the specificity was controlled by using lung homogenate of the NO-GC1 animal, in which the β1-subunit is reduced by about 80%.
Statistical analysis
All data are presented as means ± standard errors of the mean. Quantitative differences between the three genotypes (wild type, NO-GC1 KO and NO-GC2 KO) were statistically evaluated by one-way anova followed by a post hoc Fischer LSD test. Effects upon treatment (inhibitor, cGMP analog) were analyzed by Student’s t-test or paired Student’s t-test on a per cell basis, unless stated otherwise. Data from the PPR experiment were analyzed with the Mann–Whitney U-test performed on a per animal basis. All statistical tests were performed with the spss software package (version 15.0.0; SPSS, Chicago, IL, USA). A P-value < 0.05 was considered to be significant.
Results
In the KO mice deficient for either NO-GC1 or NO-GC2, the physiological function of each of the NO-GCs can be investigated separately (Mergia et al., 2006). Here, we studied presynaptic changes in the hippocampal CA1 region by single-cell recordings. Both NO-GCs are expressed in the hippocampus in comparable amounts, as shown by western blot analysis (Supporting Information Fig. S1A). Moreover, detection in hippocampal synaptosomes is compatible with a synaptic localization of both NO-GCs.
Analysis of intrinsic membrane properties of hippocampal slices did not reveal any alterations between WT and NO-GC1 KO mice (Supporting Information Fig. S1B–D).
Miniature EPSC frequency is greatly reduced in NO-GC1 KO mice and depends on cGMP formed in response to eNOS-derived NO
To study the effects of NO-GC1 and NO-GC2 deficiency on glutamatergic neurotransmission, mEPSCs were studied in hippocampal slices of WT, NO-GC1 KO and NO-GC2 KO mice, respectively, by recording pharmacologically isolated AMPAR-mediated mEPSCs in whole-cell voltage-clamp configuration. AMPAR dependency of mEPSCs was ensured by treating the slices with DNQX (20 μm; Fig. 1A). Analysis of mEPSCs revealed a reduced frequency in the NO-GC1 KO slices (0.37 ± 0.02 Hz in NO-GC1 KO slices as compared with 0.96 ± 0.07 Hz in WT slices; F = 121, P < 0.0001), suggesting a role of NO-GC1 in neurotransmitter release (Fig. 1A and B). The mean mEPSC amplitude, however, was not affected (8.8 ± 0.5 pA in NO-GC1 KO slices as compared with 8.1 ± 0.2 pA in WT slices; P = 0.84), indicating that the amount of neurotransmitter released per vesicle and the postsynaptic responsiveness in the NO-GC1 KO mice were not altered (Fig. 1C). In contrast to the mEPSC frequency in NO-GC1 KO slices, the mEPSC frequency in NO-GC2 KO slices (0.99 ± 0.1 Hz, P = 0.74) was indistinguishable from that in WT slices. These results are compatible with reduced presynaptic glutamate release in the NO-GC1 KO slices. To determine whether the reduced mEPSC frequency was attributable to a lack of cGMP produced by NO-GC1, we used ODQ, an NO-GC inhibitor. The mean frequency of mEPSCs in ODQ-treated WT slices (0.93 ± 0.06 Hz in WT slices; 0.45 ± 0.03 Hz in WT + ODQ slices; t = 5.651, P < 0.0001) was reduced to that observed in NO-GC1 KO slices, in which ODQ did not cause any reduction (0.34 ± 0.03 Hz in NO-GC1 KO slices; 0.33 ± 0.03 Hz in NO-GC1 KO + ODQ slices; t = 1.317, P = 0.22) (Fig. 1D). Conversely, a cGMP analog, 8-Br-PET-cGMP, reconstituted WT-like frequency in the NO-GC1-deficient slices (0.4 ± 0.02 Hz in NO-GC1 KO slices vs. 0.94 ± 0.03 Hz in NO-GC1 KO + cGMP slices; t = −8.9, P < 0.0001) (Fig. 1D). In all of these and the following experiments, the mean mEPSC amplitudes were unaltered. Together, the results show that the NO-GC1 KO phenotype is caused by an acute cGMP shortage, most likely in the presynaptic nerve terminal, and is not a result of long-term changes caused by gene deficiency. Moreover, the results obtained with ODQ in WT slices point to the continuous production of NO-induced cGMP, with cGMP most likely exerting a stimulatory effect on presynaptic glutamate release in this experimental setting.
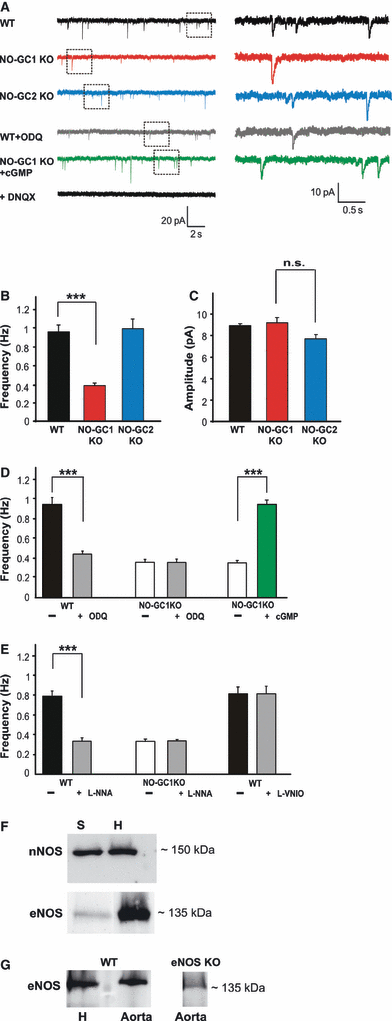
Miniature EPSC frequency is greatly reduced in NO-GC1 KO mice, and depends on cGMP synthesized in response to eNOS-derived NO. (A) Representative mEPSC traces of WT, NO-GC1 KO, NO-GC2 KO, ODQ-treated WT and 8-Br-PET-cGMP-treated NO-GC1 KO slices recorded as described in Materials and methods. DNQX-treated WT slices are shown as a control. (B) Miniature EPSC frequency and (C) mEPSC amplitudes of WT, NO-GC1 and NO-GC2 KO slices (WT slices, 15 cells of four mice; NO-GC1 KO slices, 19 cells of 11 mice; NO-GC2 KO slices, 13 cells of three mice; ***P < 0.001; n.s., not significant; anova followed by post hoc Fisher LSD test performed on a per animal basis). (D) Miniature EPSC frequencies of WT and NO-GC1 KO slices before and after incubation with the NO-GC inhibitor ODQ (10 μm, 10 min) and of NO-GC1 KO slices before and after incubation with the cGMP analog (8-Br-PET-cGMP 100 μm, 10 min) (WT ± ODQ slices, 17 cells of four mice; NO-GC1 KO ± ODQ slices, eight cells of four mice; NO-GC1 KO ± ’cGMP’ slices, 13 cells of four mice; ***P < 0.001; Student’s paired t-test). (E) Miniature EPSC frequencies of WT and NO-GC1 KO slices before and after incubation with L-NNA (100 μm, 30 min) and of WT slices before and after incubation with L-VNIO (0.1 μm, 30 min) (WT ± L-NNA slices, 11 cells of four mice; NO-GC1 KO ± L-NNA slices, eight cells of three mice; WT ± L-VNIO slices, eight cells of four mice; ***P < 0.0001; Student’s paired t-test). (F) Detection of nNOS and eNOS with specific antibodies in western blots of the hippocampal homogenate (H, 10 μg protein per lane) and synaptosomal fraction of hippocampi (S, 10 μg protein per lane). (G) Comparison of the amount of eNOS detected in hippocampal and aortic homogenates (10 μg protein per lane) and lack of the signal in eNOS-deficient aortic homogenate (10 μg).
To identify the NOS isoform that produces the NO-GC1-stimulating NO, we used the nNOS-specific inhibitors L-VNIO and L-NNA, inhibiting both nNOS and eNOS, in the mEPSC measurements in WT slices. In WT slices, L-NNA caused a reduction in mEPSC frequency similar to that observed in NO-GC1 KO slices (0.79 ± 0.05 Hz in WT slices vs. 0.33 ± 0.01 Hz in WT + L-NNA slices; t = 7.8, P < 0.0001), whereas in the NO-GC1 KO slices, L-NNA did not cause any reduction. L-VNIO had no effect in WT slices (0.82 ± 0.07 Hz in WT slices vs. 0.81 ± 0.07 Hz in WT + L-VNIO slices; t = 0.225, P = 0.829) (Fig. 1E). For critical evaluation of this finding, however, it should be kept in mind that these results were obtained in the presence of an NMDA receptor blocker. The effectiveness of L-VNIO was controlled by measuring HFS (20 Hz, 40 pulses) of cells clamped at a membrane potential of −30 mV without the NMDA receptor antagonist, to allow NMDA receptor-dependent NOS activation. Under these conditions, the nNOS inhibitor L-VNIO caused a reduction in evoked EPSCs (data not shown). Thus, we conclude that eNOS-derived and not nNOS-derived NO is responsible for the NO/cGMP-induced effect, most likely on glutamate release, under our experimental conditions. Both NOS isoforms were shown to be expressed in the hippocampus in western blots (Fig. 1F and G); in contrast to eNOS, nNOS was enriched in the synaptosomal fraction.
Minimal evoked EPSC recordings also yielded results compatible with a reduced probability of glutamate release in the NO-GC1 KO slices (Supporting Information Fig. S2). Analogously to the decrease in mEPSC frequency, these experiments revealed a reduction of the success rate to evoke these signals in NO-GC1 KO slices. Also here, the success rate to evoke minimally evoked EPSCs was reduced by ODQ in WT slices (0.59 ± 0.02 in WT slices; 0.35 ± 0.01 in NO-GC1 KO slices; 0.33 ± 0.02 in WT + ODQ slices; F = 36.5, P < 0.0001; Supporting Information Fig. S2B).
In summary, an increase in glutamate release caused by NO/cGMP appears to be the most likely explanation for the results obtained with mEPSC and meEPSC measurements of WT and NO-GC1 KO slices, although other factors, for example postsynaptic changes in silent synapses, cannot completely be ruled out. However, for reasons of clarity, we decided to refer to the observed NO/cGMP effects as increases in glutamate release in the following.
Evoked glutamate release is reduced in NO-GC1 KO mice and greatly depends on cGMP
To test whether the evoked neurotransmitter release was also altered in NO-GC1 KO mice, we performed HFS (20 Hz, 40 pulses). The stimulus intensity used in the HFS experiment was adjusted to induce 70% of the maximal signal amplitude (see Materials and methods). Whereas the stimulus intensity inducing 70% of the maximal amplitude did not differ between the groups, maximal signal amplitudes of NO-GC1 KO cells (187 ± 9 pA) were lower than the WT ones (298 ± 17 pA), indicating reduced excitability. HFS-induced evoked EPSC signals recorded in the whole-cell configuration are shown in Fig. 2A. In the NO-GC1 KO slices, the first amplitude was significantly reduced (131 ± 11.8 pA in NO-GC1 KO slices; 207.8 ± 15.4 pA in WT slices; F = 3.35, P < 0.006) (Fig. 2B), whereas the second amplitude was significantly higher and comparable to the WT one (second amplitude – 229.3 ± 21.4 pA in NO-GC1 KO slices; 232.6 ± 17.8 pA in WT slices) (Fig. 2C), as were all the subsequently induced amplitudes. Together, these results are compatible with an initially low probability of release in the NO-GC1 KO slices, and indicate that the cGMP produced by NO-GC1 increases excitability. The results obtained in the NO-GC2 KO slices were indistinguishable from those obtained in the WT slices.
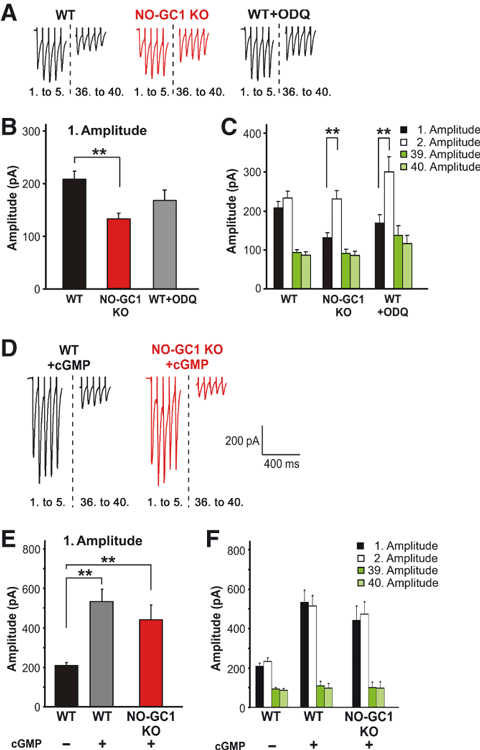
Evoked glutamate release is reduced in NO-GC1 KO mice and greatly depends on cGMP. (A) Representative current traces (1st. to 5th. eEPSC; 36th. to 40th. eEPSC ) of WT, NO-GC1 KO and ODQ-pretreated WT slices (10 μm, 10 min) in response to HFS (20 Hz, 40 pulses) recorded as described in Materials and methods. (B) First HFS-induced amplitude of WT, NO-GC1 KO and ODQ-pretreated WT slices (WT slices, 46 cells of 23 mice; NO-GC1 KO slices, 32 cells of 18 mice; WT + ODQ slices, seven cells of six mice; **P < 0.006; anova followed by post hoc Fischer LSD test performed on a per animal basis). (C) Amplitudes (1, 2, 39, 40) of the slices described in B (**P < 0.005; anova followed by post hoc Fischer LSD test performed on a per animal basis). (D) Representative current traces (1–5; 36–40) of WT and NO-GC1 KO slices pretreated (10 min) with the cGMP analog (8-Br-PET-cGMP 100 μm) in response to HFS (20 Hz, 60 pulses) recorded as in A. (E) First HFS-induced amplitude of untreated WT and WT and NO-GC1 KO slices pretreated with the cGMP analog (WT ± ’cGMP’ slices, four cells of three mice; NO-GC1 KO ± ’cGMP’ slices, 11 cells of three mice; **P < 0.007; anova followed by post hoc Fischer LSD test performed on a per animal basis). (F) Amplitudes (1, 2, 39, 40) of the slices described in E.
To exclude any alteration in the NO-GC1 KO slices other than NO-GC1 deficiency, we studied ODQ-treated WT slices, and found that the first amplitude tended to be reduced (first amplitude – 168.8 ± 21.9 pA), corresponding to what was found in the NO-GC1 KO slices (Fig. 2A–C). However, the reduction was not statistically significant, as the number of measured cells was low. The cGMP analog 8-Br-PET-cGMP not only increased the first amplitude in the NO-GC1 KO slices (NO-GC1 KO slices – 131 ± 11.8 pA without cGMP vs. 440.4 ± 132 pA with cGMP), but also had a stimulatory effect in WT slices, with an almost 2.5-fold increase in the first amplitude (WT slices – 207.8 ± 15.4 pA without cGMP vs. 535.8 ± 110 pA with cGMP; Fig. 2D–F). These results demonstrate the cGMP dependency of the evoked glutamate release. We conclude that endogenously produced or exogenously added cGMP has a stimulatory impact, most likely on the evoked glutamate release.
The results obtained with ODQ in the WT slices indicate the relevance of continuously produced cGMP for the evoked glutamate release. Again, we investigated the source of the generated NO, and performed HFS in the presence of the NOS inhibitors. The non-specific NOS inhibitor L-NNA, but not the nNOS-specific inhibitor L-VNIO, significantly reduced the response to the first stimulus in WT slices (285.1 ± 37.8 pA before L-NNA in WT slices, and 167.4 ± 19.3 pA in WT + L-NNA slices, t = 2.58, P = 0.033; 155.3 ± 23.05 pA before L-VNIO in WT slices, and 172.9 ± 18.8 pA in WT + L-VNIO slices, t = –0.117, P = 0.910; Fig. 3A–C). As in the NO-GC1 KO slices, the second amplitude was significantly higher in the presence of L-NNA (Fig. 3D) and comparable to that in the WT slices. We conclude that eNOS-derived NO is responsible for cGMP facilitating the evoked glutamate release in the presence of an NMDA receptor antagonist. As can be seen in Fig. 3, the signal amplitudes in WT slices differed considerably. We have to point out that absolute amplitudes are shown throughout this article, and these are known to vary from cell to cell, owing to the variability in the strength of synaptic connections. In the experiments with the NOS inhibitors, however, the differences in amplitudes are not critical, as each cell is its own control, the cells being first measured without and then with the drug.
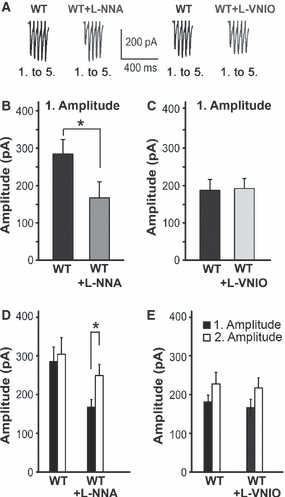
Evoked glutamate release is reduced by the eNOS and nNOS inhibitor L-NNA but not by the nNOS-specific inhibitor L-VNIO. (A) Representative current traces (1st. to 5th. eEPSC) of WT slices before and after pretreatment with L-NNA or L-VNIO (100 and 0.1 μm, respectively, 30 min) in response to HFS (20 Hz, 60 pulses) recorded as described in Materials and methods. (B and C) The first amplitude of WT slices before and after L-NNA or L-VNIO pretreatment (WT ± L-NNA slices, nine cells of thre mice; WT ± L-VNIO slices, nine cells of three mice; *P < 0.03; Student’s paired t-test). (D and E) Amplitudes (1, 2) before and after L-NNA or L-VNIO pretreatment of the slices measured above (*P < 0.045; Student’s t-test performed on a per cell basis).
Finally, to support the notion of a presynaptic localization of NO-GC1, we addressed the question in immunohistochemical experiments with antibodies against the β1-subunit of NO-GC1 and antibodies against the presynaptic marker protein vGLUT1. Antibodies against PDS95 were used to visualize the postsynaptic compartment. The β1 antibodies are not specific for one of the NO-GC isoforms, as the β1-subunit is a component of both NO-GC1 and NO-GC2, but the experiments were carried out in NO-GC2 KO slices, in which only NO-GC1 is present. As can be seen in Fig. 4, multiple puncta immunoreactive for NO-GC1, vGLUT1 and PSD95 were detected in the stratum radiatum of the CA1 region. For quantitative analysis of colocalization, ROIs containing vGLUT1 apposed to PDS95 signals, indicating synaptic contacts, were selected in images derived from four animals. Analysis of ROIs (54 ± 12 per image) as described in Materials and methods revealed that, in 65% ± 10% of the ROIs, NO-GC1 colocalized with vGLUT1, and only in 5% ± 3% of the ROIs with PDD95. Furthermore, analysis of images without selection of ROIs by the same method also indicated colocalization of only NO-GC1 and vGLUT1 (P-values > 0.95). Thus, NO-GC1 colocalizes more with vGLUT1 than with PDS95, in agreement with our proposed presynaptic localization of NO-GC1.
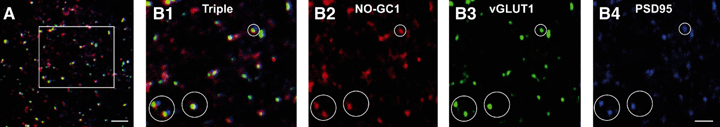
NO-GC1 is colocalized with the presynaptic protein vGLUT1 in the stratum radiatum of the CA1 region. (A) Triple immunofluorescence labeling with antibodies against the β1-subunit of NO-GC1 (red), the presynaptic marker vGLUT1 (green) and the postsynaptic marker PSD95 (blue) shown in a section of the stratum radiatum of CA1 in NO-GC2 KO mice. (B1–4) Higher magnification of the area indicated in A revealed puncta that were double-stained for NO-GC1 and vGLUT1, adjacent to PSD95-labeled puncta (an example of a puncta is indicated by white circle). Scale bars – A, 5 μm; B1–4, 2.5 μm. The quantification is given in Results.
HCN channels – possible targets for presynaptic cGMP
Next, we investigated how cGMP could increase glutamate release, and considered the HCN channels as candidates. To test the possible involvement of presynaptic HCN channels, we monitored mEPSCs in the presence of the HCN blocker ZD7288 (50 μm). A wide range of ZD7288 concentrations (10–100 μm) have been used in slices by others (Meuth et al., 2006; Tsay et al., 2007). The mEPSC frequency of WT slices was reduced by the HCN channel blocker to that determined in the NO-GC1 KO slices (0.9 ± 0.03 Hz in WT slices; 0.4 ± 0.02 Hz in WT + ZD7288 slices; 0.38 ± 0.4 Hz in NO-GC1 KO slices; F = 107.197, P < 0.0001), whereas the mEPSC frequency of NO-GC1 KO slices was not further reduced (0.35 ± 0.04 Hz; Fig. 5A and B). Comparable results were obtained with a lower concentration of ZD7288 (10 μm vs. 50 μm), supporting the involvement of HCN channels in presynaptic glutamate release.
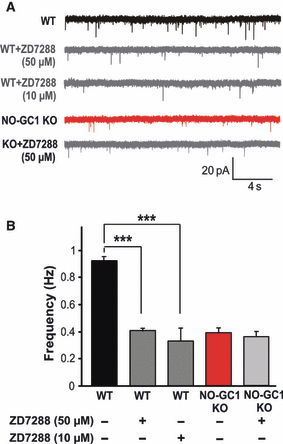
Miniature EPSC frequency in hippocampal slices is greatly reduced by the HCN channel blocker ZD7288. (A) Representative mEPSC traces of WT and NO-GC1 KO slices before and after pretreatment with ZD7288 (50 μm for 10 min, or 10 μm for 20 min, respectively) recorded as described in Materials and methods. (B) Miniature EPSC frequencies of untreated and ZD7288-pretreated WT and NO-GC1 KO slices [WT ± ZD7288 (50 μm) slices, 12 cells of four mice; WT ± ZD7288 (10 μm) slices, six cells of three mice; NO-GC1 KO ± ZD7288 (50 μm) slices, 13 cells of three mice; ***P < 0.001; anova followed by post hoc Fischer LSD test performed on a per cell basis].
Next, the HCN channel blocker was studied with regard to evoked glutamate release, and caused a reduction of the first WT amplitude to that measured in the NO-GC1 KO slices, whereas the first NO-GC1 KO amplitude was not further altered [WT slices, 203.6 ± 41 pA; WT + ZD7288 (50 μm) slices, 124.9 ± 12.5 pA; NO-GC1 KO slices, 124.8 ± 14.3 pA; NO-GC1 KO + ZD7288 slices, 130.7 ± 14.9 pA; F = 4.82, P < 0.019; Fig. 6A–C]. Also here, comparable results were obtained with the lower ZD7288 concentration. We repeated the experiment with DK-AH269, another HCN channel blocker with a different mechanism of action (Fig. 6D–F). This blocker also caused a reduction in the first WT amplitude to that measured in the NO-GC1 KO slices, and had no effect in the NO-GC1 KO slices (WT slices, 205 ± 43.9 pA, and WT + DK-AH269 slices, 118.5 ± 17.8 pA, t = 2.653, P = 0.045; NO-GC1 KO slices, 109.2 ± 9.5 pA, and NO-GC1 KO + DK-AH269 slices, 113.9 ± 12.3 pA). These results suggest that cGMP either directly or indirectly exerts its effect by modulating gating of HCN channels, thereby providing a depolarizing current that enhances the spontaneous and evoked glutamate release. The lack of an HCN channel blocker effect in the NO-GC1 KO slices suggests that, in the absence of cGMP, the HCN channel-mediated current (Ih) does not contribute to the glutamate release. With antibodies specific for each of the HCN isoforms, we detected HCN1, HCN2, HCN3 and HCN4 channels in the hippocampus (Fig. 6G). In the presence of the respective antigenic peptide, the signals detected by the antibodies were abolished (data not shown). All channels were also detected in synaptosomal fractions, compatible with a synaptic localization of the channels.
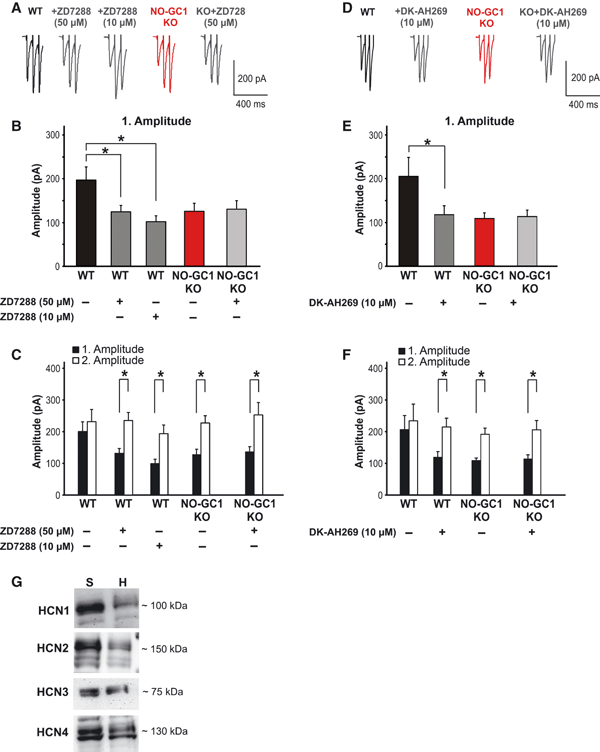
Evoked glutamate release is reduced by the HCN channel blocker ZD7288. (A) Representative current traces (1st. to 3rd. eEPSC) of WT and NO-GC1 KO slices before and after pretreatment with ZD7288 (50 μm for 10 min, or 10 μm for 20 min, respectively) in response to HFS (20 Hz, 60 pulses) recorded as described in Materials and methods. (B) The first amplitude of untreated and ZD7288-pretreated WT and NO-GC1 KO slices [WT slices, 13 cells of six mice; WT ± ZD7288 (50 μm) slices, seven cells of three mice; WT ± ZD7288 (10 μm) slices, six cells of three mice; NO-GC1 KO ± ZD7288 (50 μm) slices, eight cells of three mice; *P < 0.019; anova followed by post hoc Fischer LSD test performed on a per animal basis]. (C) First and second amplitudes of the slices described in B (*P < 0.045; Student’s t-test performed on a per animal basis). (D) Representative current traces (1st. to 3rd. eEPSC) of WT and NO-GC1 KO slices before and after pretreatment with DK-AH269 (10 μm, 20 min) in response to HFS (20 Hz, 60 pulses) recorded as described in Materials and methods. (E) The first amplitude of untreated and DK-AH269-pretreated WT and NO-GC1 KO slices (WT ± DK-AH269 slices, six cells of three mice; NO-GC1 KO ± DK-AH269 slices, six cells of three mice; *P < 0.045; Student’s paired t-test). (F) First and second amplitudes of the slices described in E (*P < 0.035; Student’s t-test performed on a per cell basis). (G) Detection of HCN1, HCN2, HCN3 and HCN4 with specific antibodies in western blots of hippocampal homogenates (H, 10 μg protein per lane) and the synaptosomal fraction of hippocampi (S, 10 μg protein per lane).
If cGMP increases the open-probability of HCN channels via a shift in the voltage activation curve, the formed cGMP should allow an inward depolarizing current that facilitates the opening of voltage-dependent calcium channels and the resulting glutamate release. If the proposed mechanism of action is right, then high concentrations of extracellular calcium should be able to overcome the reduced glutamate release in the NO-GC1 KO slices, at least partially. The glutamate release probability was determined through the level of PPR (ISI – 30, 50 and 100 ms) at different calcium concentrations (0.5, 2.5 and 4 mm). PPR in WT slices decreased at higher extracellular calcium concentrations (Fig. 7). In NO-GC1 KO slices, PPR was generally higher than in WT slices and was calcium-dependent, with the difference from WT slices becoming smaller at higher calcium concentrations. In accordance with the proposed mechanism of cGMP either directly or indirectly increasing the probability of HCN channels being open, the difference in glutamate release between KO and WT slices was almost completely overcome by high extracellular calcium concentrations.
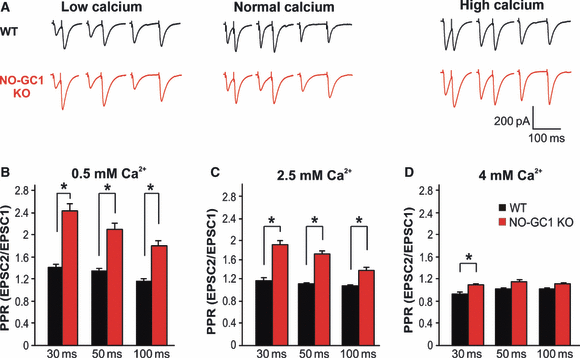
(A) Increased PPR in NO-GC1 KO mice is overcome by high extracellular calcium concentrations. PPR in WT (black) and NO-GC1 KO (red) slices monitored as described in Materials and methods determined at different ISIs (30 ms, 50 ms, 100 ms) at the indicated calcium concentrations. (B, C and D) Original traces summarized in bar plots (WT slices, nine cells of three mice; NO-GC1 KO slices, 11 cells of three mice; *P < 0.05; Mann–Whitney U-test performed on a per animal basis).
In summary, we found a profound influence of continuously produced NO/cGMP, most likely on presynaptic glutamate release, via direct or indirect modulation of HCN channels under spontaneous and stimulated conditions.
Discussion
As indicated by the finding that both NO-GCs are required for hippocampal LTP, both NO-GC1 and NO-GC2 may play a role in synaptic transmission, possibly at either side of the synaptic cleft. In favor of this hypothesis, postsynaptic changes are observed in the NO-GC2 KO slices (manuscript in preparation), and a presynaptic localization of NO-GC1 is indicated by immunofluorescence analysis performed in this study, which is in accordance with results published by others (Burette et al., 2002).
Moreover, in the current study, we found that cGMP formed in response to eNOS-derived NO increases synaptic transmission, and identified HCN channel blocker-sensitive channels as possible targets for cGMP. All of our results obtained with single-cell recordings under spontanous (mEPSCs) and evoked (meEPSCs and evoked EPSC) conditions in WT and NO-GC1 KO slices are compatible with the assumption that NO/cGMP increases presynaptic glutamate release. The possible involvement of presynaptic GABAb receptors and metabotropic glutamate receptors can be ruled out, as the corresponding antagonists did not exert any effect on the mEPSC and evoked EPSC measurements. As glutamate release was not measured directly, we admit that the involvement of other factors that alter postsynaptic responsiveness and/or postsynaptic changes in silent synapses cannot be completely ruled out (Kerchner & Nicoll, 2008). On the other hand, we take the interchangeability of the NO-GC1 KO-derived and WT-derived results with ODQ and cGMP, respectively, as an indicator of the comparability of the WT and KO strains, and therefore exclude functionally relevant alterations of the NO-GC1 KO despite NO-GC1 deficiency. We propose increased presynaptic glutamate release as the most likely explanation for the NO/cGMP-induced effects, and discuss the results in the light of this concept in the following.
Spontaneous and evoked glutamate release is reduced in NO-GC1 KO mice and depends greatly on cGMP
The spontaneous glutamate release in the KO mice deficient in either of the NO-GCs was studied by mEPSC measurement. Whereas the NO-GC2 KO mice exhibit WT-like properties, glutamate release in the NO-GC1 KO mice is greatly reduced, as shown by the decrease in mEPSC frequency. The reduction of mEPSC frequency is attributable to the lack of presynaptic cGMP in the KO mice, because, with the cGMP analog 8-Br-PET-cGMP, WT-like properties are found in the NO-GC1 KO mice. It should be noted that the lipophilicity of this cGMP analog is about 45-fold higher than that of 8-Br-cGMP, which was used in former LTP experiments (Selig et al., 1996). In accordance with cGMP enhancing glutamate release, inhibition of NO-induced cGMP formation with the NO-GC inhibitor ODQ in WT mice results in an NO-GC1 KO-like reduction of mEPSC frequency. To our knowledge, this is the first report on ODQ and L-NNA (see below) affecting baseline synaptic transmission mediated by AMPARs in acute slices. Several previous papers reported that these compounds did not influence baseline synaptic transmission; this may be partially attributable to the applied method, as field potentials were measured (Boulton et al., 1995; Bon & Garthwaite, 2001; Taqatqeh et al., 2009). Also, application of ODQ in whole-cell recordings did not reveal an effect of ODQ on baseline synaptic transmission (Boulton et al., 1995; Serulle et al., 2007). However, Boulton et al. measured isolated NMDA and not AMPA receptor currents, and Serulle et al. determined the frequency and amplitude of mEPSCs in another experimental setting, namely cultured hippocampal neurons instead of acute slices.
In addition to the reduced spontaneous glutamate release, the NO-GC1 KO mice showed a decreased eEPSC amplitude in response to the first stimulus, indicative of a low glutamate release probability. Hence, the amplitude in response to the second stimulation was significantly higher than that in response to the first, and even as high as the WT one. According to our working hypothesis, the depolarizing current through the cGMP-gated HCN channel indirectly contributes to the height of the first amplitude in WT mice most likely by increasing calcium influx. Obviously, the calcium remaining from the first stimulus in the NO-GC1 KO mice is able to overcome the missing contribution of I(h) and reveals additive effects to the second response, as outlined in the paradigm of paired pulse facilitation, which also explains the pronounced facilitation of the second stimulus. Again, the first amplitude of WT mice was reduced to KO-like levels by ODQ treatment, whereas the first amplitude of the NO-GC1 KO mice was increased to WT levels with the cGMP analog, confirming that the observed effects were solely attributable to the absence or presence of cGMP.
The cGMP dependency of the glutamate release is demonstrated by the almost 2.5-fold increase in WT EPSC amplitude caused by the cGMP analog. The increase in amplitude can be explained by cGMP enhancing the open-probability of the HCN channels, allowing an additional depolarizing current that increases calcium influx. The lack of a further increase in the second amplitude indicates that enhanced cGMP induced already maximal glutamate release in response to the first stimulus and is not limited by the efficiency of the calcium influx.
The effect of the NO-GC inhibitor ODQ on spontaneous and evoked glutamate release in WT mice implies that NO-GC1 is permanently active. Taking our findings together, we conclude that cGMP: (i) is continuously formed presynaptically; and (ii) increases the glutamate release probability.
eNOS-derived NO is responsible for presynaptic cGMP increases
The continuous cGMP formation has to be a consequence of permanent NO production. Experiments with NOS inhibitors suggest that eNOS-derived, and not nNOS-derived, NO is responsible for the continuous effect on glutamate release under the conditions tested, that is, the presence of a NMDA receptor antagonist. This is unexpected, but an involvement of eNOS in synaptic plasticity has been shown in eNOS-deficient KO mice, and eNOS-derived NO has previously been postulated to participate in neuronal transmission (O’Dell et al., 1994; Wilson et al., 1997; Garthwaite et al., 2006). Here, we found eNOS to be permanently activated, as shown by the reduction in glutamate release in WT mice upon inhibition of the NO-GCs. Although eNOS was originally characterized as a calcium/calmodulin-stimulated enzyme, it later became clear that eNOS activity can be regulated by post-translational control mechanisms, such as fatty acid modification and phosphorylation, and by protein–protein interactions, for example with caveolin (Sessa, 2005). The expression of eNOS in brain, other than in blood vessels, has been a matter of debate (Dinerman et al., 1994; Hopper & Garthwaite, 2006). Our finding that eNOS is not enriched in synaptosomal fractions points to a non-synaptic localization of the enzyme. The involvement of eNOS in glutamate release described here does not argue against an additional role of nNOS in synaptic transmission. Rather, one has to consider that, in our experimental setting, an NMDA receptor antagonist was present, and, according to the dogma of nNOS activation, calcium influx through NMDA receptors is required to activate nNOS (Christopherson et al., 1999). Therefore, under our measurement conditions, postsynaptic nNOS activation is not expected to occur. Conceivably, NMDA receptor-mediated nNOS activation not only causes cGMP increases in the postsynaptic nerve terminal, but also supplies NO to act as a retrograde messenger, activating presynaptically localized NO-GC1, and thereby further increasing glutamate release (Taqatqeh et al., 2009). This concept of tonic and phasic NO produced by eNOS and nNOS, respectively, has already been proposed (Garthwaite et al., 2006), and our data substantiate the tonic NO production part of this.
HCN channels as possible effectors for presynaptic cGMP enhance the glutamate release probability
In our search for a cGMP target, we hypothesize that the cGMP effects are mediated by HCN channels that are known to be modulated by direct binding of cGMP or cAMP. Four HCN channels (HCN1–4) have been identified (Ludwig et al., 1998; Biel et al., 2009); these occur mainly in heart and brain, are activated at hyperpolarized membrane potentials, and produce an inward (depolarizing) current (Ih). The activation curve of HCN2 and HCN4 are shifted to the right by 5–25 mV by cAMP and cGMP; generally, cGMP is not considered to be an endogenous HCN ligand, as cAMP binds with 10-fold higher affinity than cGMP (1 μm vs. 10 μm) (Ludwig et al., 1998; Craven & Zagotta, 2006). The distribution of HCN channels has been studied extensively at the mRNA and protein levels, and in KO mice. Messenger RNA for HCN1, HCN2 and HCN4 has been shown to occur in the hippocampus (Moosmang et al., 1999). With the antibodies, we found all four HCN channels in the hippocampus. As all signals obtained with the antibodies could be competed with the respective antigenic peptide they appeared to be specific and it is unclear at present why our results do not match those of others. On the other hand, one has to take into account that the intensity of the signal does not reflect the amount of HCN channel protein present in the hippocampus, but the sensitivity of the antibody.
HCN channels have been proposed as possible cGMP targets before. In the rat optic nerve, NO derived from endothelial cells persistently depolarized axons through cGMP acting on HCN channels (Garthwaite et al., 2006). During the preparation of this article, HCN channels were shown to be potential downstream mediators of NO signaling in deep cerebellar nuclei neurons (Wilson & Garthwaite, 2010).
In our study, two HCN blockers (ZD7288 and DK-AH269) with different mechanisms of action (Raes et al., 1998) caused a pronounced reduction in glutamate release in WT mice, suggesting that the inward current supplied by HCN channels enhances the probability of release. Conversely, the cGMP analog caused a massive, almost 2.5-fold, increase in the evoked glutamate release in WT mice. The notion of cGMP allowing an inward (depolarizing) current leading to depolarized resting membrane potentials is supported by experiments on the calcium dependency of glutamate release, in which the difference in the NO-GC1 KO mice is overcome by extracellular calcium concentrations. The question remains of how the HCN channel, which is possibly involved in presynaptic glutamate release, is activated by cGMP. Because of the rather poor affinity for cGMP, a direct interaction of the HCN channels with cGMP is considered to be unlikely; however, it should be kept in mind that NO-GC is activated up to 200-fold by NO, so very high local cGMP concentrations cannot be ruled out. On the other hand, mainly recombinantly expressed channels were studied, and in their physiological environment, HCN channels may have increased sensitivity towards cGMP, owing to post-translational modifications (Pian et al., 2006) or interactions with additional proteins (Santoro et al., 2009). Cyclic GMP-dependent phosphorylation of the HCN channels could theoretically account for the observed effects; however, lack of LTP impairment in cGMP-dependent protein kinase type I or cGMP-dependent protein kinase type II KO mice argues against the involvement of these kinases (Kleppisch et al., 1999). Nevertheless, our identification of an HCN channel was indirect, being achieved with the use of HCN channel blockers, and the existence of other cGMP-regulated HCN channel blocker-sensitive channels cannot be completely ruled out. In the absence of cGMP, the HCN channel blocker-sensitive channels do not participate in glutamate release, as shown by the lack of an effect of the HCN channel blocker in NO-GC1 KO mice.
Taken together, our findings show that NO/cGMP enhances glutamate release by an effect on HCN channel blocker-sensitive channels, and identify eNOS and NO-GC1 as the enzymes responsible for NO and cGMP formation, respectively. By unraveling a physiological function, we provide the molecular basis from which to study the involvement of NO/cGMP in diseased states and to develop pharmacological interventions (phosphodiesterase inhibitors; HCN channel activators) to enhance the signaling cascade.
Acknowledgements
We thank Medah Özcan, Ursula Krabbe, Ute Neubacher and Petra Küsener for excellent technical assistance. We are grateful to Andreas Daiber for providing the aorta of the eNOS KO mice. This work is supported by the DFG.
Abbreviations
-
- AMPAR
-
- AMPA receptor
-
- D-AP5
-
- d-(–)-2-amino-5-phosphonopentanoic acid
-
- DNQX
-
- 6,7-dinitroquinoxaline-2,3-dione
-
- eNOS
-
- endothelial nitric oxide synthase
-
- EPSC
-
- excitatory postsynaptic current
-
- HCN
-
- hyperpolarization-activated cyclic nucleotide-gated
-
- HFS
-
- high-frequency stimulation
-
- ISI
-
- interstimulus interval
-
- KO
-
- knockout
-
- L-NNA
-
- l-NG-nitroarginine
-
- LTP
-
- long-term potentiation
-
- L-VNIO
-
- vinyl-l-N5-(1-imino-3-butenyl)-l-ornithine
-
- nNOS
-
- neuronal nitric oxide synthase
-
- NO
-
- nitric oxide
-
- NO-GC
-
- NO-sensitive guanylyl cyclases
-
- NOS
-
- nitric oxide synthase
-
- meEPSC
-
- minimal evoked excitatory postsynaptic current
-
- mEPSC
-
- miniature excitatory postsynaptic current
-
- ODQ
-
- 1H-[1,2,4]oxadiazole[4,3-a]quinoxalin-1-one
-
- PBS
-
- phosphate-buffered saline
-
- PPR
-
- paired-pulse ratio
-
- PSD95
-
- postsynaptic density protein 95
-
- ROI
-
- region of interest
-
- PTX
-
- picrotoxin
-
- TTX
-
- tetrodotoxin
-
- vGLUT1
-
- vesicular glutamate transporter 1
-
- WT
-
- wild-type
-
- ZD7288
-
- 4-ethylphenylamino-1,2-dimethyl-6-methylaminopyrimidinium chloride




