Gene switching and odor induced activity shape expression of the OR37 family of olfactory receptor genes
Abstract
Olfactory sensory neurons (OSNs) which express distinct odorant receptor (OR) genes are spatially arranged within the mouse olfactory epithelium. Towards an understanding of the mechanisms which determine these patterns, representative OR genes which are typically expressed in the unique central patch of the epithelium were investigated. Inside the patch, numerous OSNs which initially selected a representative gene from this OR group finally expressed another gene from the group, indicating that OSNs inside the patch ‘switch’ between these genes. If an OSN successively chose genes from the same OR gene cluster, these originated from the same parental chromosome. A deletion of the olfactory cyclic nucleotide-gated ion channel altered the distribution pattern of distinct OSN populations; they were no longer located exclusively inside the patch. Together, the results indicate that OSNs inside the patch initially sample several OR genes for expression; for their correct patterning in the OE, odor-induced activity appears to play a critical role.
Introduction
The capability of the mammalian olfactory system to detect thousands of odor molecules is based on the existence of a large and diverse repertoire of odorant receptor (OR) proteins (Buck & Axel, 1991) which are encoded by the largest gene family in vertebrate genomes (Zhang & Firestein, 2002; Young et al., 2003; Mombaerts, 2004; Zhang et al., 2004; Godfrey et al., 2004; Zhang et al., 2007a). Evidence from a series of different experimental approaches have led to the conclusion that each of the several million olfactory sensory neurons (OSNs) in the olfactory epithelium (OE) specifically expresses just one of the numerous OR genes (Chess et al., 1994; Vassar et al., 1994; Ressler et al., 1994; Mombaerts et al., 1996; Malnic et al., 1999; Ishii et al., 2001). This singularity of OR gene expression is believed to be the basis for the discriminatory power of the system. Interestingly, the cell populations which express the same OR genes are not homogenously dispersed throughout the OE but are spatially organized. Most OR-specific populations are arranged in so-called zones (Vassar et al., 1993; Ressler et al., 1993; Strotmann et al., 1994; Iwema et al., 2004; Miyamichi et al., 2005); however, there are prominent exceptions to this rule – OSNs which express a member from the so-called OR37 gene family are restricted to a small patch in the centre of the OE (Strotmann et al., 1992, 1994, 2000).
The molecular mechanisms that enable an individual OSN to selectively express one particular OR gene, one which is, moreover, appropriate for its position in the OE, are largely unknown. It is thought that multiple steps establish the regional and monogenic patterns (for recent reviews see Fuss & Ray, 2009; Imai & Sakano, 2009; McClintock, 2010). The spatial restriction is believed to be determined by regulatory sequences present in the OR gene promotors (Sosinsky et al., 2000; Lane et al., 2001; Vassalli et al., 2002; Hoppe et al., 2003; Rothman et al., 2005; Hoppe et al., 2006). The interaction of a gene cluster-specific locus control region with one defined OR gene promotor is thought to then result in the selection of that gene for expression (Lomvardas et al., 2006; Fuss et al., 2007; Nishizumi et al., 2007). Finally, the emergence of a functional OR protein within an individual OSN is believed to elicit a (still unknown) signal which stabilizes the expression of that gene; OSNs which fail to produce a suitable receptor can ‘switch’ to the expression of another OR (Serizawa et al., 2000; Ishii et al., 2001; Serizawa et al., 2003; Lewcock & Reed, 2004; Feinstein et al., 2004; Shykind et al., 2004; Bozza et al., 2009).
Although initial insights have emerged concerning the mechanisms governing the expression patterns of OR genes, further studies are required to elucidate in more detail the process of OR gene choice and to discover in particular the checkpoints which allow an individual OSN to express a gene appropriate for its position. Towards this goal, the patch region of the OE offers an ideal system as it represents a well circumscribed area with a defined and small set of OR genes. We therefore extended our analyses on the expression control mechanisms of genes which are confined to this region.
Materials and methods
Experimental animals
The present study was performed using adult (3–12 months old) mice. Two Cre-reporter strains were employed: in one, EYFP (Srinivas et al., 2001) and in the other, tandemdimerRFP (Luche et al., 2007) is turned on in cells expressing Cre recombinase. Three strains carrying targeted mutations of IRES-taulacZ or IRES-tauGFP at the mOR37A−, mOR37B− or mOR37C locus, respectively (Strotmann et al., 2000), were used. Homozygous females [olfactory cyclic nucleotide-gated channel (OCNC)1−/−] and hemizygous males (OCNC1−/○) were employed from the strain which carries a knockout (ko) mutation of the OCNC1 subunit [also known as cyclic nucleotide-gated (CNG)A2] of the olfactory CNG channel (Zheng et al., 2000). Animals were housed at the Central Unit for Animal Research at the University of Hohenheim. For tissue preparations animals were killed by cervical dislocation and subsequent decapitation as approved by the regional administrative authority (Regierungspräsidium Stuttgart # S136/02 Phy).
Tissue preparation and cryosectioning
After decapitation all bones of the head surrounding the olfactory bulb and the nasal turbinates were excised. For in situ hybridization experiments the freshly prepared tissue was embedded in Tissue Freezing Medium (Leica Microsystems, Bensheim, Germany) and frozen on dry ice. For immunohistochemistry the tissue was fixed by immersion in 4% paraformaldehyde (in 150 mm phosphate buffer, pH 7.4) on ice. To remove the air from the nasal cavity, the specimens were first immersed in fixative and a light vacuum was applied for a few minutes. Fixation was performed for between 15 min and 2 h. Subsequently the tissue was cryoprotected by incubation in 25% sucrose overnight at 4°C. Finally, the tissue was embedded in Tissue Freezing Medium and frozen on dry ice. Cryosections (12 μm) were generated using a CM3050S cryostat (Leica Microsystems) and mounted onto microscope slides (Superfrost or Polysine slides; Menzel, Braunschweig, Germany).
In situ hybridization
Digoxigenin-labeled antisense riboprobes were generated from partial cDNA clones in pGem-T plasmids encoding mOR37A-CDS (NCBI accession number NM_019473.1, position 4-997), mOR37A-3′UTR (Strotmann et al., 1999), mOR37C-3′UTR (NM_019475.3, position 960-1696) and mOR118-1 (NM_009605, position 25-939) by using the T7/SP6 RNA transcription system (Roche Diagnostics, Mannheim, Germany) as recommended by the manufacturer. The specificity of the signals was confirmed by control experiments in which the corresponding sense probe did not label cells in the main olfactory epithelium.
Cryosections (12 μm) were fixed with 4% paraformaldehyde in 0.1 m NaHCO3, pH 9.5 for 45 min at 4°C. Slices were washed in 1 × phosphate-buffered saline (PBS; NaCl, 0.85%; KH2PO4, 1.4 mm; and Na2HPO4, 8 mm; pH 7.4) for 1 min, incubated in 0.2 m HCl for 10 min and in 1% Triton X-100 for 2 min, and again washed twice in 1 × PBS for 30 s, all at room temperature. Subsequently, sections were incubated in 50% formamide in 5 × standard sodium citrate (SSC; 1 × SSC – NaCl, 150 mm and Na-citrate, 15 mm; pH 7.0) for 10 min. The tissue was then covered with hybridization buffer (formamide; with 2 × SSC, 50%; dextran sulfate, 10%; yeast t-RNA, 0.2 mg/mL; and sonicated herring sperm DNA, 0.2 mg/mL) containing the probe and incubated in a humid chamber (50% formamide) at 65°C overnight. For posthybridization, slides were washed twice for 30 min in 0.1 × SSC at 65°C and treated with 1% blocking reagent (Roche) in Tris-buffered saline (TBS; Tris, pH 7.5, 100 mm; and NaCl, 150 mm) with 0.3% Triton X-100 for 30 min at room temperature. Afterwards the slices were incubated with an antidigoxigenin alkaline phosphatase-conjugated antibody (Roche) diluted 1 : 750 in 1% blocking reagent (Roche) in TBS with 0.3% Triton X-100 for 30 min at 37°C. After two washes in TBS for 15 min, slides were rinsed in alkaline phosphatase (AP) buffer (TRIS, pH 9.5, 100 mm; NaCl, 100 mm; and MgCl2; 50 mm). Hybridization signals were visualized by using NBT (nitroblue tetrazolium) and BCIP (5-bromo-4-chloro-3-indolyl phosphate) in AP buffer as substrates. Finally, sections were dried and mounted in Vectamount mounting medium (Vector Laboratories, Burlingame, CA, USA).
Immunohistochemistry
Cryosections (12 μm) were air-dried and rinsed in 1 × PBS for 10 min at room temperature. For single and double labeling experiments, primary antibodies were diluted in 0.3% Triton X-100 in 1 × PBS containing either 10% normal goat serum (NGS; Dianova, Hamburg, Germany) or 10% normal donkey serum (NDS; Dianova). Antibodies were used in the following dilutions: rabbit anti-GFP (Invitrogen, Karlsruhe, Germany) 1 : 700 (for the detection of EYFP) or 1 : 800 (for the detection of GFP); mouse anti-beta-galactosidase (Promega, Mannheim, Germany) 1 : 800 and goat anti-DsRed (Santa Cruz Biotechnology, Santa Cruz, CA, USA) 1 : 700. Sections were incubated with the diluted primary antibodies overnight at 4°C. After three rinses for 5 min in 1 × PBS, the bound primary antibodies were visualized using appropriate secondary antibodies conjugated to Alexa 488 or Alexa 568 (Invitrogen, Karlsruhe, Germany) diluted in 1 × PBS with 0.3% Triton X-100 containing either 10% NGS or 10% NDS for 2 h at room temperature. After washing three times for 5 min the sections were counterstained with 4,6- diamidino-2-phenylindole (DAPI; 1 μg/mL, Sigma Aldrich, Schnelldorf, Germany) for 3 min at room temperature, rinsed with H2O, and finally mounted in MOWIOL [10% polyvinylalcohol 4-88 (Sigma), 20% glycerol in 1 × PBS]. When the staining procedure was performed on nontransgenic mice as controls, no immunoreactivity could be observed.
Microscopy and photography
Sections were analyzed using a Zeiss Axiophot microscope (Carl Zeiss MicroImaging, Jena, Germany). Images were captured using a Zeiss Axiocam for transmitted light and a ‘Sensi-Cam’ CCD-camera (PCO-imaging, Kelheim, Germany) for fluorescent images. Brightness and contrast were adjusted for the whole images.
Quantitative analysis
Labelled cells inside the patch region were counted at high magnification on every coronal section (12 μm thick) along the rostrocaudal axis of the main olfactory epithelium. Values were represented as means and SD of absolute cell numbers.
For the quantification of glomeruli in the olfactory bulb, two individuals were investigated. The densely innervated glomeruli in the ventral part of the olfactory bulb were counted. The achieved value is the mean ± SD number of glomeruli per bulb.
Results
Employing a transgenic approach in mice by which all OSNs that have ever turned on the mOR37C (Olfr157) gene were permanently labeled with histological markers, we have recently demonstrated that this OR gene is selected for expression not only by cells residing in the OR37-typical central patch, but also by many cells outside this patch; however, all ectopically positioned OSNs had subsequently shut down their expression (Strotmann et al., 2009). These results demonstrated that OR37 genes are transiently chosen for expression by widely distributed OSNs, and their long-term expression exclusively in the central patch results from a rapid down-regulation in the non-appropriate regions, thereby ultimately restricting them to the patch.
This concept implies that all OSNs located inside the patch which have chosen a particular OR37 gene continue to express this gene; however, it is unclear whether this paradigm is in fact fulfilled. To approach this question, mOR37C as one representative of the subfamily was analyzed. We exploited the fact that each OSN expresses a particular OR gene in a monoallelic manner, i.e. a given cell randomly expresses either the maternal or the paternal allele (Chess et al., 1994; Ishii et al., 2001; Vassalli et al., 2002); this has also been shown for the OR37 genes (Strotmann et al., 2000). Thus, the fraction of OSNs which express each allele has been shown to be always very close to 50%. Based on this knowledge we determined the number of labeled OSNs in animals which carried two different targeted mutations at the mOR37C locus [Fig. 1A; one allele, mOR37C-IRES-Cre, short 37CCre* (Strotmann et al., 2009); the other allele, mOR37C-IRES-tauGFP, short 37C-ITGFP (Strotmann et al., 2000)]; in addition, all mice contained a Cre-reporter at the ROSA26-locus (R-tdRFP or R-EYFP; see Fig. 1A; Srinivas et al., 2001; Luche et al., 2007). Figure 1B shows a segment from a cross-section through the middle part of the nasal cavity of such a mouse. The frame exemplifies the characteristic extension of the patch region which includes parts of endoturbinates II and III and of ectoturbinate 3. At higher magnification, cells expressing the 37C-ITGFP allele are clearly visible by their green fluorescence (Fig. 1C). In the area many red fluorescent cells can also be seen (Fig. 1D); these are cells which had turned on the 37CCre* allele and subsequently expressed the red fluorescent protein from the ROSA26 locus. In the overlay (Fig. 1E) it becomes clear that the labeled cells are either green or red, supporting the idea that they express exclusively one or the other allele. From the inspection of this region it appeared, however, that the number of cells with the different labels was not identical; the 37CCre* cells (red) were more abundant. To obtain a quantitative measure, the green fluorescent cells which defined the patch, and the red fluorescent cells within the corresponding region, were counted. On serial sections through the entire patch region, 2407 ± 382 (n = 3) green fluorescent OSNs (Fig. 1F), thus expressing the 37C-ITGFP allele, were determined. The number of cells which had chosen the 37CCre* allele was almost four times (approximately 3.8 times) higher (9377 ± 1057; Fig. 1F). The notion that more cells inside the patch region were labeled from the 37CCre* allele was thus confirmed. These results suggested that, although they were accurately located inside the patch region, numerous (approximately 7000) OSNs had turned off the once-chosen mOR37C gene. It is interesting to note that actually more OSNs inside the patch had turned it off than had maintained its expression.
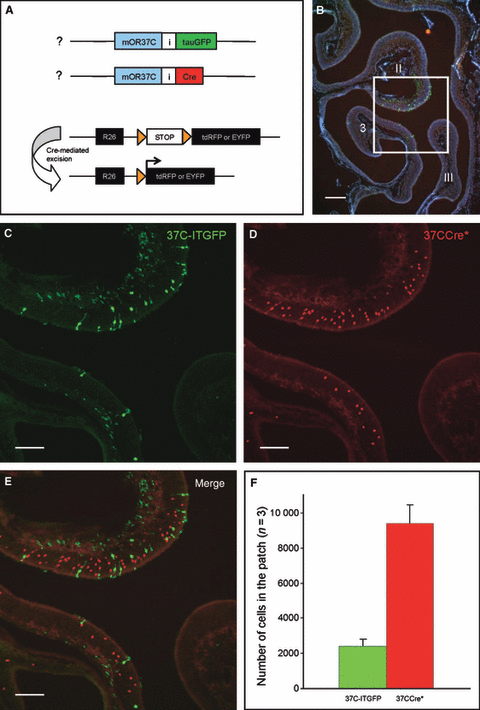
(A) Schematic representation of the gene locus in 37CCre*/R-tdRFP/37C-ITGFP triple-mutant animals; both parental alleles of mOR37C carry a targeted mutation. The maternal allele of mOR37C is coexpressed with the Cre-recombinase (red); OSNs expressing the paternal allele are labeled by the marker tauGFP (green). Cre directs loxP (orange triangles)-mediated recombination and subsequent marker expression (i.e. tdRFP or EYFP). As this Cre-mediated recombination is irreversible, expression of the marker will persist even if the expression of 37CCre* is extinguished. tauGFP, tau-green fluorescent protein; Cre, Cre-recombinase; R26, ROSA26 promotor; I, internal ribosomal entry site; tdRFP, tandemdimer red fluorescent protein; EYFP, enhanced yellow fluorescent protein. (B) Cross-section through one nasal cavity of a 37CCre*/R-tdRFP/37C-ITGFP mouse. The section is stained with DAPI to show the structure of the turbinates (endoturbinates II and III; ectoturbinate 3). The boxed area represents the typical OR37 patch and is shown at a higher magnification in C–E. (C) The patch region contains numerous cells which express the 37C-ITGFP allele. (D) Many 37CCre*-positive cells are visible in the patch. (E) Merging the pictures from C and D reveals that OSNs in the patch do express either the 37C-ITGFP allele (green) or the 37CCre* allele (red). (F) Quantification of 37CCre*-positive (red bar) and 37C-ITGFP-expressing cells (green bar) inside the patch region. Data are means of cell counts from three adult animals (± SD).The number of 37CCre*-positive cells in the patch region is approximately four times higher than the number of 37C-ITGFP-expressing cells. Scale bars, 200 μm (B), 100 μm (C–E) .
These data suggested that cells inside the typical patch may also have ‘switched’ to another OR gene, as was recently observed for the ectopically located OSNs (Strotmann et al., 2009). This of course raised the question of which OR gene was subsequently chosen by these cells. As all members of the OR37 subfamily are expressed inside the patch, it seemed likely that a fraction of the 37CCre* cells had ‘switched’ to one of the other OR37 subtypes. To address this question we crossed 37CCre*/R-tdRFP mice with our previously generated transgenic lines in which OSNs expressing mOR37A (Olfr155) or mOR37B (Olfr156), respectively, co-express the marker tau-GFP or tau-lacZ (37A-ITGFP or 37B-ITlacZ; Fig. 2A; Strotmann et al., 2000). If OSNs ‘switch’ from mOR37C to one of these receptors, there might be double-labeled cells detectable. Figure 2B shows a typical result from a 37CCre*/R-tdRFP/37A-ITGFP animal. On this representative section, numerous red and green fluorescent OSNs are visible but none of them is double-labeled. The same results were obtained for 37CCre*/R-tdRFP/37B-ITlacZ animals (data not shown). A quantification of cells from the entire patch region revealed that among roughly 2000 cells expressing 37A-ITGFP, only a very few were double-labeled (37A-ITGFP, 36 ± 5 or 1.83%; 37B-ITlacZ, 47 ± 12 or 2.39%).
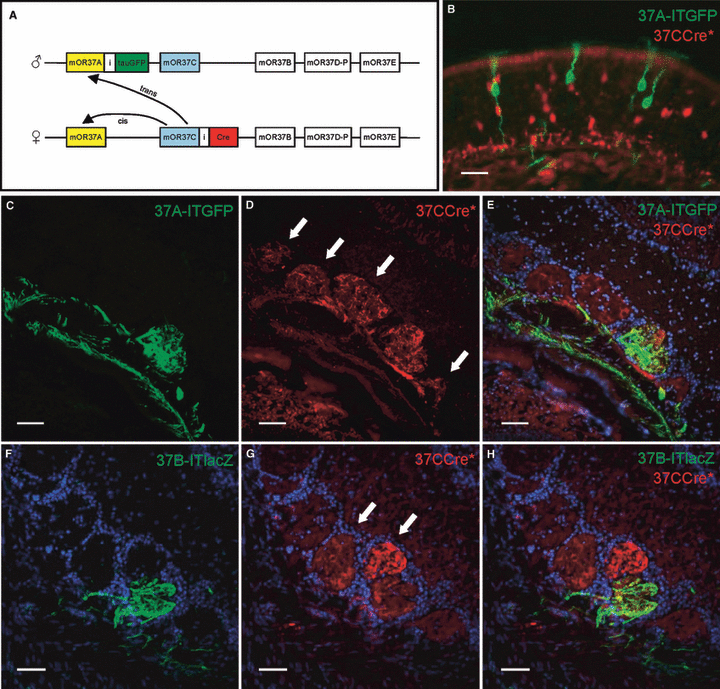
(A) Scheme of the genomic segment on chomosome 4 with five members of the OR37 subfamily; both parental chromosomes are shown. 37CCre*/R-tdRFP/37A-ITGFP animals carry two targeted mutations in this cluster of OR genes. The mOR37C gene located on the maternal chromosome is coexpressed with the Cre-recombinase, whereas the mOR37A gene located on the paternal chromosome is coexpressed with tauGFP. A switch from mOR37C to another gene from the cluster could occur in cis, i.e. to a gene also present on the maternal chromosome, or in trans, i.e. to a gene on the paternal chromosome. (B) In a high magnification of the olfactory epithelium representative for a 37CCre*/R-tdRFP/37A-ITGFP mouse, no double-labeled cells can be seen. (C) High magnification of the ventral olfactory bulb from a 37CCre*/R-tdRFP/37A-ITGFP mouse. Fibers of OSNs expressing the 37A-ITGFP allele converge in a single glomerulus. (D) On the same section numerous glomeruli are visible which are innervated by 37CCre*-positive fibers. Arrows mark glomeruli which are neighbors of the mOR37A-glomerulus. (E) Merging the pictures from C and D reveals that 37CCre*-positive fibers (red) do enter the glomerulus formed by mOR37A-expressing OSNs (green). (F) High magnification of the ventral domain of the olfactory bulb from a 37CCre*/R-tdRFP/37B-ITlacZ mouse. The ventral domain of the olfactory bulb is shown. Fibers of OSNs expressing the 37B-ITlacZ allele converge in a single glomerulus. (G) On the same section numerous glomeruli are innervated by 37CCre*-positive fibers. Arrows mark glomeruli which are neighbors of the mOR37B-glomerulus. (H) Merging the pictures from F and G reveals that 37CCre*-positive fibers (red) do enter the glomerulus formed by mOR37B-expressing OSNs (green). Scale bars, 20 μm (B), 50 μm (C–H).
These results seemed to indicate that basically no ‘switching’ from mOR37C to mOR37A or to mOR37B occurred. However, at this point it could not be excluded that the lack of double-labeled cells was due to the fact that a ‘switch’ was restricted to a cis-located gene, i.e. to a gene on the same chromosome, whereas the other labeled mOR37 gene in our transgenic animals was located in trans, thus on the other chromosome (see Fig. 2A). In order to visualize whether a ‘switch’ to another OR37 gene in cis might have occurred, we decided to analyze the projection pattern of the respective OSN populations. For example, if a switch from 37CCre* to mOR37A had taken place, the fibers from these 37CCre* cells should be detectable in the glomerulus formed by OSNs expressing mOR37A. As shown in Fig. 2C–E, the mOR37A glomerulus indeed contained numerous 37CCre* fibers. Similar results were obtained when the glomerulus formed by OSNs expressing 37B-ITlacZ was examined (Fig. 2F–H). Together, these results strongly support the idea that in fact many 37CCre* cells inside the patch had ‘switched’ to another member from the OR37 gene family. On the other hand, a considerable fraction of the 37CCre* cells should have continued to express mOR37C after their initial choice. To make sure that this was the case, we examined whether the glomerulus formed by the 37C-ITGFP-expressing cells contained 37CCre* fibers. As shown in Fig. 3, this could indeed be confirmed; numerous 37CCre*-positive fibers terminated in the same glomerulus as the axons of 37C-ITGFP-expressing OSNs.
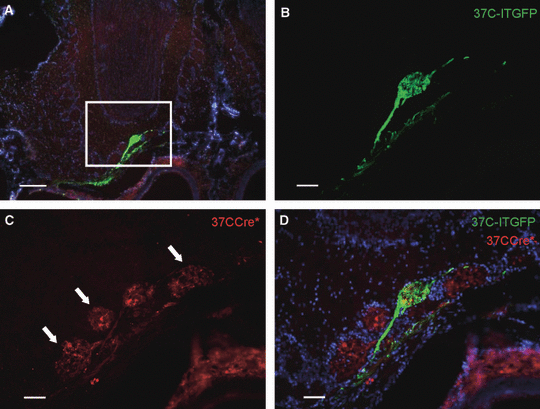
(A) Cross-section through the olfactory bulb of a 37CCre*/R-tdRFP/37C-ITGFP mouse. The nuclei are stained with DAPI. The boxed area is shown in B-D. (B–D) 37CCre*-expressing OSNs (red fibers) project to the glomerulus that is innervated by the axons of 37C-ITGFP-expressing OSNs (green). The mOR37C glomerulus (green) is surrounded by glomeruli that are also densely innervated by 37CCre*-positive fibers (red; arrows). Scale bars, 200 μm (A), 50 μm (B–D).
A close examination of the glomeruli which received input from OSNs expressing the respective mOR37 subtypes revealed that they were always surrounded by several glomeruli which also contained a dense innervation by 37CCre* fibers (see arrows in 2, 3). In a previous study we showed that OSNs expressing a certain member of the OR37-subfamily project to neighboring glomeruli in the ventral domain of the olfactory bulb (OB; Strotmann et al., 2000). Therefore, our data indicated that 37CCre* cells ‘switched’ to several related OR genes, raising the question of which OR genes are actually available for a ‘switch’ in the 37CCre* cells. It seemed conceivable that it were simply the other mOR37 genes from the cluster which contains mOR37C, thus also including mOR37D and mOR37E (Strotmann et al., 1999). Unfortunately, there are no transgenic mouse lines available which allowed us to directly visualize the OSNs expressing these genes or their target glomeruli; therefore we also addressed this question by analyzing the projection pattern of the 37CCre* cells. We reasoned that the number of glomeruli which contained a high number of 37CCre* fibers should be indicative of the approximate number of OR genes available for the ‘switching’ process. A close examination of the OB in 37CCre*/R-EYFP mice revealed a rather large group of glomeruli in the ventral domain that received robust input from 37CCre* fibers (Fig. 4A–E). This set of glomeruli was easily distinguishable from glomeruli in their neighborhood which contained only a few labeled fibers (Fig. 4F); these latter ones have been described previously and represent glomeruli which receive input from ectopically positioned 37CCre* cells (Strotmann et al., 2009). Interestingly, the densely innervated glomeruli were exclusively detectable in the ventral domain of the OB, positioned in the immediate neighborhood to each other (Fig. 4A–E). A thorough examination revealed that 22 ± 1 (n = 4 bulbs) glomeruli formed this ensemble. Based on this number of characteristically labeled glomeruli it seems unlikely that only the genes from the cluster containing mOR37C are involved in the ‘switching’ process. Actually, it correlates quite well with the currently known 18 OR genes which are expressed exclusively in the patch (Strotmann et al., 1999; Hoppe et al., 2003, 2006); therefore, it seems likely that those OR genes which are selectively expressed inside the patch are available for a ‘switch’ in the 37CCre* cells.
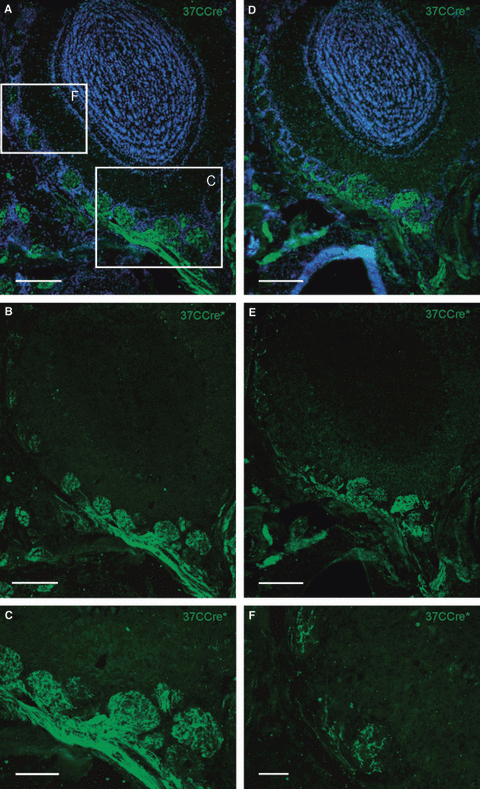
(A) Cross-section through the olfactory bulb of a 37CCre*/R-EYFP mouse. Cell nuclei are stained with DAPI. Boxed areas are shown in B and F. (B) In the ventral part of the olfactory bulb multiple, neighboring glomeruli are densely innervated by 37CCre*-positive fibers. (C) At higher magnification the dense innervation of glomeruli in the ventral part of the olfactory bulb becomes more obvious. (D and E) Cross-section through a more anterior region (approximately 100 μm) of the olfactory bulb. Also, glomeruli in this neighboring region are densely innervated by 37CCre*-positive fibers. (F) In more dorsal regions of the olfactory bulb, glomeruli are only sparsely innervated by 37CCre*-positive fibers. Scale bars, 200 μm (A, B, D and E), 100 μm (C), 50 μm (F).
Our data so far imply that, if OSNs inside the patch ‘switch’, for example from mOR37C to mOR37A, this occurs between the genes from that locus on the same chromosome (see Fig. 2A). To further corroborate this notion, we quantified the co-labeled OSNs from animals in which the 37CCre*-allele and the 37A-ITGFP- or 37B-ITlacZ-allele, respectively, were labeled in trans. As shown in Fig. 5A and B, these numbers were very low (37A-ITGFP, 36 ± 5; 37B-ITlacZ, 47 ± 12), supporting the notion that the ‘switching’ process is restricted to OR37 genes from the cluster on the same chromosome. These results suggest that in each given cell probably only one of the two homologous gene clusters is transcriptionally competent. To provide additional evidence for this hypothesis, the number of double-labeled cells in animals which have both alleles of OR37C targeted, one with Cre and the other one with ITGFP, was determined. The number of such cells was again very low (51 ± 21; n = 3). Taken together, these results could be an indication that one of the two allelic clusters is made competent for expression before the choice process starts; consequently, all selections can be made exclusively from this competent cluster. To test this hypothesis we compared 37CCre* homozygous vs. heterozygous individuals. As shown in Fig. 5C–E, the number of labeled OSNs inside the patch in homozygous animals (17 360 ± 2153; n = 3) was about twice as high (1.8 times) as in heterozygous animals (9377 ± 1056; n = 3), consistent with the notion that one cluster is pre-determined for selection.

(A) Quantification of 37CCre*-positive (red bar), 37A-ITGFP-expressing (green striped bar) and double-labeled cells (yellow bar) inside the patch region. Data are means ± SD of counts from three adult 37CCre*/R-tdRFP/37A-ITGFP animals. The number of double-labeled cells is very low. (B) Number of 37CCre*-positive (red bar), 37B-ITlacZ-expressing (green dotted bar) and double labeled cells (yellow bar) inside the patch region. Data are means of counts from three adult 37CCre*/R-tdRFP/37B-ITlacZ animals (± SD). For mOR37B also, the number of double-labeled cells is extremely low. (C) Cross-section through the patch region of a 37CCre*/R-EYFP mouse heterozygous for the targeted mutation at the mOR37C locus. Numerous labeled cells are visible. (D) In animals homozygous for the mutation, more 37CCre*-positive cells in the patch are visible. (E) Quantification of 37CCre*-positive cells inside the patch region in heterozygous (white bar) and homozygous (dashed bar) animals. Data are means ± SD of counts from three adult 37CCre*/R-EYFP animals. The number of 37CCre*-positive cells inside the patch in homozygous animals is approximately 1.8 times higher than in heterozygous animals. Scale bars, 50 μm.
Altogether, our data indicate that mechanisms must operate within a particular OSN that assess the suitability of the OR gene choice. It is currently not known whether initially only one OR gene is selected for expression or whether a few OR genes are expressed simultaneously at first. However, the procedure that finally stabilizes the expression of one defined OR gene in an individual OSN and prevents a continuation of the choice process is still an enigma. Results from previous studies suggest that a functional OR protein and possibly the transduction process is necessary to generate a feedback reaction which blocks further selections (Serizawa et al., 2003; Lewcock & Reed, 2004). To follow these possibilities we set out to investigate the expression of the OR37 genes in mice which lack a key component in the signal transduction cascade in OSNs, the OCNC (Zheng et al., 2000). As a first approach, the distribution of OSNs which express OR37 genes was examined by in situ hybridization experiments on cross-sections through the nose of OCNC1-knockout (ko) mice. For this purpose, a digoxigenin-labeled antisense riboprobe against the coding sequence of mOR37A was used; due to the high sequence similarity of the OR37 coding regions it is capable of visualizing several OR37 subtypes. As shown in Fig. 6A and B, numerous OR37-expressing OSNs could be detected in the OE of OCNC1-ko mice. Interestingly, many labeled cells were ectopically positioned (arrows in Fig. 6A and B), in contrast to the wildtype situation where almost exclusively OSNs inside the typical patch were stained (Fig. 6C and D). A close inspection revealed that the ectopic cells were dispersed over all turbinates and the septum, except for the dorsal region of the nasal epithelium. Thus, the lack of a functional CNG channel obviously altered the expression pattern of the mOR37 genes. At this point it was not clear, however, whether only one particular subtype was affected; therefore, in situ hybridizations were performed with specific probes for two representative members (mOR37A and -C ). With these probes similar distribution patterns emerged although, as expected, the number of labeled OSNs was lower than with the pan-OR37 probe (data not shown). Together, these results demonstrated that the expression pattern of distinct mOR37 genes was altered in the OE of OCNC1-ko mice. This is an interesting finding as the expression pattern of several OR genes which are organized in zones was reported to be unchanged in OCNC1-ko mice (Brunet et al., 1996; Zheng et al., 2000). Experiments using probes for M71/M72 (Olfr151/Olfr160) and mOR233-4 (Olfr1217) in fact provided no evidence for cells outside their typical expression zone in the OE of OCNC1-ko mice (data not shown). These results raised the question of whether the change in expression pattern was specific for the mOR37 subfamily or common to all OR genes expressed inside the patch. As two representatives, mOR120-2 (Olfr460) and mOR118-1 (Olfr49; Hoppe et al., 2006) were analyzed. With both probes, cells outside the patch were stained, most prominently with the mOR118-1 probe (Fig. 6E and F). Altogether, these results demonstrated that OSN populations which are normally confined to the patch were more broadly dispersed throughout the OE in OCNC1-ko mice.

(A) Representative cross-sections through the nose of an OCNC1-ko mouse hybridized with a pan-mOR37 antisense RNA probe. Arrows highlight regions of the olfactory epithelium where ectopically located cells are detectable. The boxed area (as in C and E) shows a typical OR37 patch. (B) Dorsolateral region of the section in A shown at higher magnification. Numerous ectopic cells are visible that are labeled by the pan-mOR37 probe (arrows). (C) Cross-sections through the nose of a wildtype (wt) mouse. Cells labelled by the pan-mOR37 probe are only detectable in the typical patch region. (D) A higher magnification of the dorsolateral region of the section shown in C reveals that in wt mice no labeled cells are detectable on turbinates outside the patch. (E) Cross-sections through the nose of an OCNC1-ko mouse probed with a mOR118-1 antisense RNA. For this particular OR, cells are also labeled on turbinates distinct from the patch. (F) At higher magnification, it is obvious that numerous mOR118-1-expressing cells are visible for example in the dorsolateral part of the OE. Scale bars, 200 μm.
The observation that OSN populations which are typically restricted to the patch were no longer confined to this special region of the OE in OCNC1-ko mice suggested that the down-regulation of the respective genes outside that region might not operate correctly. This hypothesis implies that ectopic cells which do not employ this particular CNG channel should manage the shutdown process properly in the OCNC1-ko mice. To test this concept we exploited the fact that many vomeronasal sensory neurons also select mOR37C, but all of them down-regulate it (Strotmann et al., 2009). As vomeronasal organ (VNO) neurons do not employ the respective CNG channel in their transduction cascade (Berghard & Buck, 1996), we expected that the shutdown process should work. In fact, no cells expressing mOR37C could be detected on cross-sections through the VNO of OCNC1-ko mice (data not shown).
Our findings suggested that in the OE of OCNC1-ko mice, ectopic OSNs which had selected OR37-related genes could not down-regulate their expression. Due to the continued expression of OR37-related genes in OSNs positioned at remote locations in the OE, the question arose as to whether the axons of these cells still find the receptor-specific glomeruli in the bulb or rather form extra glomeruli in the OB. To investigate this aspect we explored the projection pattern of OSNs expressing 37A-ITGFP in OCNC1-ko mice. On series of cross-sections through the OB of mutant mice, the typical glomerulus formed by mOR37A-expressing OSNs was found to be established in the ventral domain of the OB (Fig. 7A and B; n = 8 bulbs); in two bulbs, two similar glomeruli were visible close to each other (data not shown), a feature that has been observed before for OSNs expressing 37A-ITGFP (Strotmann et al., 2000). However, in addition, at two to five extra positions in the bulb, aggregated fibers were detected. In all cases, the fibers were positioned in the glomerular layer, but differently organized. In one type, they were visible within a glomerulus which contained mainly unlabeled fibers (Fig. 8A and B). In the other cases, the green axons converged in separate small glomerulus-like structures (Fig. 8C and D). Furthermore, each bulb contained a small population of individual green fibers (Fig. 8E–G); it was not possible to determine their site of termination. Together, these results indicated that a fraction of OSNs which expressed 37A-ITFGP targeted novel glomeruli in the OB. In all cases, these extra glomeruli were positioned quite distantly from the typical large glomerulus which was formed by the majority of axons from 37A-ITGFP-expressing cells.
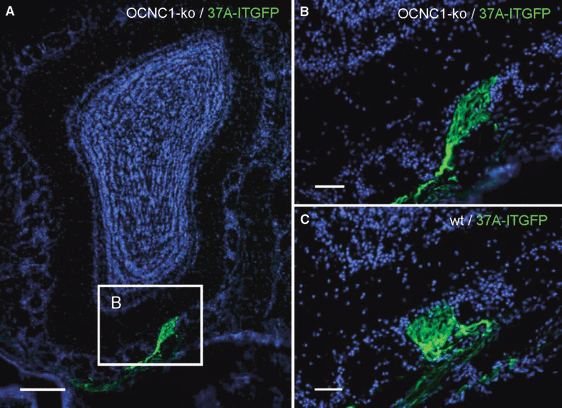
(A) Cross-section through the OB of an OCNC1-ko/37A-ITGFP mouse. The glomerulus formed by 37A-ITGFP-expressing neurons is located in the ventral part of the OB. Cell nuclei are stained with DAPI. Boxed area is shown in B. (B) Higher magnification shows that axons from 37A-ITGFP-expressing neurons project precisely to one well-defined glomerulus. (C) The glomerulus formed by 37A-ITGFP-expressing neurons in a wt background is similar to the ventrally located glomerulus observed in OCNC1-ko mice. Scale bars, 200 μm (A), 50 μm (B and C).
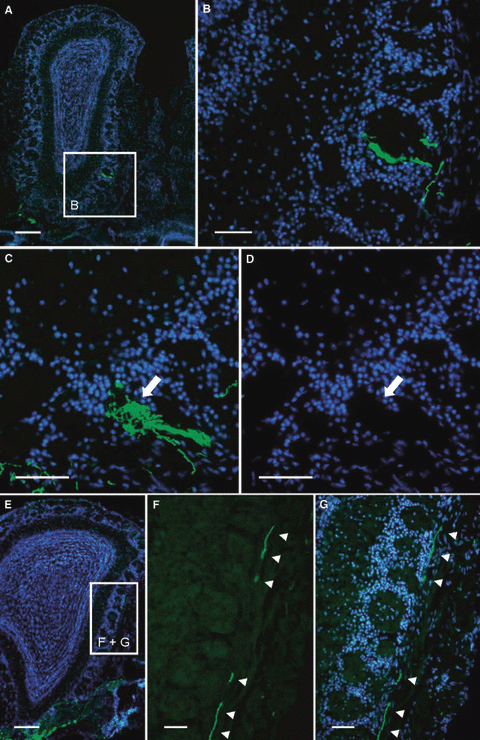
(A) Cross-section through the OB of an OCNC1-ko/37A-ITGFP mouse. Cell nuclei are stained with DAPI. Boxed area is shown in B. (B) Higher magnification of the glomerular layer shows fibers from 37A-ITGFP-expressing OSNs in a glomerulus that is mainly filled by unlabeled fibers. (C) Some small glomerular structures (arrow) are densely filled with labeled fibers in OCNC1-ko/37A-ITGFP mice. (D) The DAPI staining outlines the shape of the structure shown in C (arrow) which is targeted by fibres from 37A-ITGFP-expressing OSNs; it appears like a small glomerulus which is free of cell nuclei. (E) Cross-section through the OB of an OCNC1-ko/37A-ITGFP mouse. Boxed area is shown in F and G. (F) Higher magnification reveals that fibers of 37A-ITGFP-expressing OSNs (arrowheads) travel far more dorsal than the typical OR37 glomerulus is positioned. (G) Counterstaining with DAPI shows that these fibers (arrowheads) navigate exclusively within the olfactory nerve layer. Scale bars, 200 μm (A and E), 50 μm (B–D and F and G).
Discussion
In the present study we have shown that numerous OSNs, which were located inside the OR37-typical patch of the OE and which had selected a suitable OR37 subtype (mOR37C) for expression, down-regulated this gene and instead expressed another OR. The fact that these OSNs did not continue to express mOR37C, although they were appropriately positioned inside the patch region, indicates that positional information in the OE does not unequivocally predefine the selection of only one gene from this subfamily. This result is consistent with a previous study which has shown that OSNs which selected a zone-compatible gene (mOR28) subsequently stopped expressing this gene and ‘switched’ to another OR (Shykind et al., 2004). However, a significant difference was observed – only 10% of the OSNs performed the ‘switch’ from mOR28 to another OR, whereas almost 75% of the OSNs that had chosen mOR37C turned it off again. The reason for this significant difference is unclear; it might be determined by the region of the OE where the respective OSNs are located. This view is supported by the finding that OSNs in the dorsal zone which selected M72 did not show a ‘switch’ at all (Li et al., 2004). The finding by Li et al. (2004) that these OSNs never ‘switch’, moreover, could be an indication that the dorsal zone employs mechanisms for OR gene choice that are distinct from those in other regions of the OE. Our previous finding that mOR37C was also selected by OSNs outside the typical patch area, but not by those in the dorsal zone (Strotmann et al., 2009), is in line with this view. However, as only a few OR genes have been analyzed, it is too early for a final conclusion.
Regarding the question of which set of OR genes can be recruited by OSNs inside the patch that have first selected mOR37C, it seems conceivable that the organisation of OR genes in clusters is the crucial determinant. Thus only OR genes organized in the same gene cluster would be accessible for switching. However, we provide evidence that OR genes located in different gene clusters can be chosen. Our data suggest that switching includes the patch-like expressed genes which are located in different gene clusters. The view is based on the projection pattern of 37CCre* cells, which innervated approximately 22 glomeruli in the ventral domain of the OB. This number correlates quite well with the 18 OR genes which are selectively expressed in the patch area, and the projection pattern confirms and extends the results of previous studies, demonstrating for four OSN populations inside the patch region that their axons converge on a single glomerulus in this particular ventral domain of the OB (Strotmann et al., 2000; Pyrski et al., 2001). The notion that the gene choice of OSNs inside the patch is not random but restricted to particular OR genes implies that those OR genes share relevant features for gene regulation. Indeed, we have previously discovered similar DNA motifs upstream of their transcription start sites, and this argues in favour of common expression control mechanisms (Hoppe et al., 2006).
The fact that each OSN eventually expresses just a single OR gene and the transcription is selectively made from the maternal or the paternal allele (Chess et al., 1994; Ishii et al., 2001) has raised the question of whether these features arise from a common mechanism or represent independent processes (Capello et al., 2009; for recent reviews, see Fuss & Ray, 2009; Imai & Sakano, 2009; McClintock, 2010). Our results concerning the ‘switch’ process between the OR37 genes that are located within the same gene cluster clearly demonstrated that the ‘switch’ was always to genes on the same chromosome. These results suggest that the initial choice is already restricted to either the maternal or the paternal locus. It seems very likely, at least for the OR37 cluster, that only one copy is selectively prepared for expression, and choices are therefore only possible from this cluster. Thus, for the OR37 genes, the choice of the cluster obviously precedes that of one particular gene, implying that these are distinct processes. The data are consistent with previous studies for M71 and MOR28 which reported that basically no ‘switch’ occurred between the two alleles of these genes (Li et al., 2004; Shykind et al., 2004). However, whether it is a general principle for all OR genes cannot yet be answered. Interestingly, the recent study of a cluster of vomeronasal receptor genes, which are also expressed in a monogenic and monoallelic fashion (Rodriguez et al., 1999), revealed that vomeronasal sensory neurons which selected a V1R pseudogene from one cluster made their second choice exclusively from the respective cluster on the other chromosome (Roppolo et al., 2007); these results suggest that for V1R genes the choice mechanisms might be different.
The signals that finally stabilize the expression of one particular OR37 gene per OSN are still unknown. In the present study we have demonstrated that a deletion of the CNG channel, which is a key component in the signal transduction cascade in OSNs, resulted in numerous ectopic OSNs containing the mRNA for OR37 genes, suggesting that the turn-off mechanisms for these genes are not active. Interestingly, such a phenomenon was not observed for OR genes which are expressed in zones (Baker et al., 1999; Zheng et al., 2000; Zhao & Reed, 2001). This difference could be due to the fact that the patterning of the patched OR genes is established stepwise, via an initial selection for expression in widely distributed OSNs followed by a down-regulation in the nonappropriate regions (Strotmann et al., 2009). No similar mode of pattern formation has been observed for any zonal OR gene. In this respect, the genes expressed in the patch are thus likely to require unique control mechanisms.
The observation that, in OCNC1-ko mice, OSNs at ectopic positions contain mRNA for OR37 receptors raised the question of whether these cells may in addition produce a position-related OR type. Analyses of the projection pattern of OR37 neurons in the OCNC1-ko mice revealed that they project either to one glomerulus located in the ventral domain of the OB, which is typical of the wildtype (see Fig. 7), or to several small glomerulus-like structures in more dorsomedial and dorsolateral positions in the OB (see Fig. 8). The results are consistent with the idea that all OSNs with mRNA for OR37, those inside and outside, express only the OR37 receptor. In this concept, the cells inside the patch project to their typical glomerulus whereas those outside the patch would generate the extra glomeruli. This notion is in line with previous studies which have demonstrated that OSNs which express a particular OR in an ectopic position form extra glomeruli in the OB (Vassalli et al., 2002; Nakatani et al., 2003; Zhang et al., 2007b). These results have led to the concept that the projection pattern of OSNs is determined by a combination of molecular cues in OSNs which depend both on their position in the OE and on the OR subtype. Previous studies have demonstrated that for the maintenance of a glomerulur structure a certain number of OSNs are required (Ebrahimi & Chess, 2000; Nakatani et al., 2003); in the case of the ectopically positioned cells which express an OR37 gene, it seems likely that enough OSNs with a common molecular phenotype exist to form the small extra glomeruli.
Acknowledgements
The authors thank Hans Jörg Fehling (University Clinics Ulm) for providing the ROSA26-tdRFP Cre-reporter mice and Peter Mombaerts (MPI Frankfurt) for the OCNC1-ko mice. This work was supported by the Deutsche Forschungsgemeinschaft.
Abbreviations
-
- CNG
-
- cyclic nucleotide-gated
-
- DAPI
-
- 4,6-diamidino-2-phenylindole
-
- ko
-
- knockout
-
- OB
-
- olfactory bulb
-
- OCNC
-
- olfactory cyclic nucleotide-gated channel
-
- OE
-
- olfactory epithelium
-
- OR
-
- odorant receptor
-
- OSN
-
- olfactory sensory neuron
-
- PBS
-
- phosphate-buffered saline
-
- SSC
-
- standard sodium citrate
-
- TBS
-
- TRIS-buffered saline




