Effects of agrin on the expression and distribution of the water channel protein aquaporin-4 and volume regulation in cultured astrocytes
Abstract
Agrin is a heparan sulfate proteoglycan of the extracellular matrix and is known for organizing the postsynaptic differentiation of the neuromuscular junction. Increasing evidence also suggests roles for agrin in the developing CNS, including the formation and maintenance of the blood–brain barrier. Here we describe effects of agrin on the expression and distribution of the water channel protein aquaporin-4 (AQP4) and on the swelling capacity of cultured astrocytes of newborn mice. If astrocytes were cultured on a substrate containing poly dl-ornithine, anti-AQP4 immunoreactivity was evenly and diffusely distributed. If, however, astrocytes were cultured in the presence of agrin-conditioned medium, we observed an increase in the intensity of AQP4-specific membrane-associated staining. Freeze-fracture studies revealed a clustering of orthogonal arrays of particles, representing a structural equivalent of AQP4, when exogenous agrin was present in the astrocyte cultures. Neuronal and non-neuronal agrin isoforms (agrin A0B0 and agrin A4B8, respectively) were able to induce membrane-associated AQP4 staining. Water transport capacity as well as the density of orthogonal arrays of intramembranous particles was increased in astrocytes cultured with the neuronal agrin isoform A4B8, but not with the endothelial and meningeal isoform A0B0. RT-PCR demonstrated that agrin A4B8 increased the level of the M23 splice variant of AQP4 and decreased the level of the M1 splice variant of AQP4. Implications for the regulation and maintenance of the blood–brain barrier including oedema formation under pathological conditions are discussed.
Introduction
It is generally accepted now that astrocytes play a decisive role in the maintenance of the barrier properties of the brain microcapillary endothelial cells. The astroglial differentiation can be morphologically recognized as the polarization of astrocytes which arises concomitantly with the maturation of the blood–brain barrier (BBB; Wolburg, 1995a; Nico et al., 2001; Brillault et al., 2002). Polarization of astrocytes implies a molecular and structural heterogeneity of specific membrane domains of the astroglial surface. Where the endfeet of glial cells contact the superficial or perivascular basal lamina (glia limitans superficialis et perivascularis), the glial membrane is studded with numerous orthogonal arrays of intramembranous particles (OAPs) which can only be visualized by means of the freeze-fracture technique (Wolburg, 1995b). Apart from the area of direct contact of the glial cell membrane with the basal lamina the density of OAPs is dramatically reduced, suggesting that the establishment of the glial endfoot specializations depends on basal lamina-associated extracellular matrix molecules.
Interestingly, BBB endothelial cells are strictly surrounded by astrocytes highly polarized in terms of OAP distribution (Wolburg, 1995b; Nico et al., 2001). In brain tumours where the BBB is known to be leaky, the OAP-related polarity of glial cells as well as the OAP density were decreased (Neuhaus, 1990). As several lines of evidence suggest that OAPs consist of, or at least contain, the water channel protein aquaporin-4 (AQP4), it was unexptected that the AQP4 content as detected by immunocytochemistry was increased rather than decreased in brain tumours (Saadoun et al., 2002; Warth et al., 2004). This apparent contradiction suggests that, at least under glioma conditions, AQP4 exists in a non-OAP-associated form in the glial membrane and that AQP4 is not restricted to the glial endfeet membranes. AQP4 exists in two alternatively spliced variants: M1, with 323 amino acids, and M23, with 301 amino acids (Jung et al., 1994). The isoform M23 seems to assemble in large OAPs whereas the isoform M1 only forms small arrays. If isoform M23 is transfected together with isoform M1, arrays are formed wich resemble those found in astrocytic endfeet in vivo (Furman et al., 2003). These endfeet are adjacent to the perivascular basal lamina containing many extracellular matrix components such as laminin, collagen and the heparansulfate proteoglycan agrin.
Agrin is an extracellular heparan sulfate proteoglycan (Tsen et al., 1995) and was originally identified as an essential molecule for clustering acetylcholine receptors at the motor endplate (McMahan, 1990; Bezakova & Ruegg, 2003), but it has also been described as being important within the CNS and for the integrity of the BBB (Barber & Lieth, 1997; Berzin et al., 2000; Smith & Hilgenberg, 2002). The agrin splicing variant A0B0 is reported to be specifically present in the subendothelial basal lamina of CNS capillaries (Stone & Nikolics, 1995). If agrin is absent from the basal lamina, AQP4 immunoreactivity is randomly distributed across the whole surface of the cell (Warth et al., 2004). This suggests that agrin might play a role in the restriction or stabilization of AQP4 molecules in the membrane of glial endfeet.
Materials and methods
Culture of murine brain astrocytes
All animal care and experimental protocols conformed to the University of Tübingen Animal Ethics Committee guidelines and the German legislation regulating the use of animals in research. The use of animals in this study was minimized to the necessary numbers for quantitative and qualitative analysis. Murine brain astrocytes were isolated from BALB/c mice (age between 2 and 5 days) according to the method of Beyer & Raab (1998) and Ivanova et al. (2001, 2002). Briefly, mice were killed by cervical dislocation after anaesthesia (25% chlorale hydrate, 1 mL per 100 g body weight). To establish primary cortical astroglial cultures, brains were removed and the cortices were prepared and collected according to Beyer et al. (1994). After removal of meninges, the tissues were digested with 0.1% trypsin and 0.02% EDTA (Cambrex, Verviers, Belgium) in PBS for 15 min at room temperature. The cell suspension was then transferred into culture medium, filtered through a 40-µm nylon mesh, and centrifuged (400 g, 5 min). Cell pellets were resuspended in the culture medium and seeded on poly dl-ornithine (Sigma, Deisenhofen, Germany)-coated plastic dishes. Cells were cultured in Dulbecco's modified Eagle's medium (DMEM; Cambrex) supplemented with 20% fetal calf serum (Cambrex), penicillin (10 000 units/mL; Cambrex), streptomycin (10 000 µg/mL; Cambrex) and Fungizone® (1%; Invitrogen, Karlsruhe, Germany) under standard conditions up to passage 2. Cells were split twice after having reached confluency. Finally, astrocytes were cultured with agrin-conditioned medium for 1–2 weeks and processed for immunocytochemistry, freeze fracture, volume measurements and RT-PCR.
Agrin-conditioned culture medium
Transfected HEK 293 cells producing agrin A4B8 or agrin A0B0 (as continuously proved by means of RT-PCR) were seeded in culture flasks and cultured in DMEM and 10% fetal calf serum to confluency. The presence of agrin in the media was frequently tested by means of ELISA. Every third day the medium was collected, centrifuged to remove cell debris and stored at 4 °C for further use. The medium contained on average 1 unit of active agrin per 10 µL (Kröger, 1997).
Culture of murine brain meningeal cells
After enzymatic digestion of collected meninges with trypsin in EDTA, cells were seeded in poly dl-coated plastic flasks and cultured in endothelial growth medium (EGM bullet kit; Cambrex) under standard conditions up to passage 2. The meningeal cells were then seeded for coculture experiments and further processed for immunocytochemistry or freeze-fracturing.
Immunocytochemistry
Cultured astrocytes were fixed in 4% paraformaldehyde for 15 min. Anti-AQP4 antibodies (rabbit polyclonal 1 : 100; Chemicon, Hofheim/TS, Germany) were used to localize AQP4 in the cells and detected with secondary Cy3-labelled antibodies (Dianova, Hamburg, Germany). To control for nonspecific staining or autofluorescence, the primary antibodies were omitted. Nonspecific binding was blocked by incubation for 30 min in 4% normal goat serum and 1% BSA in PBS. Antibodies were diluted in 0.25% TritonX and 1% DMSO in PBS and the samples incubated at 4 °C overnight. Afterwards, the cells were rinsed three times in PBS and secondary antibody was applied for 60 min at room temperature. Following washes in PBS, specimens were mounted in Mowiol (Calbiochem, Merck, Germany). Fluorescence was visualized with a confocal laser scanning microscope (Zeiss LSM510 META, Oberkochen, Germany) using a HeNe laser for excitation at 543 nm with appropriate filter sets and a 40× oil-immersion objective (N.A. 1.3).
Freeze-fracture experiments
Monolayers of cultured cells were fixed with 2.5% glutaraldehyde in 0.1 m cacodylate buffer (pH 7.4) for 2 h at room temperature. The cells were then cryoprotected for freeze-fracturing in 30% glycerol and quick-frozen in nitrogen slush (−210 °C). Subsequently, the specimens were fractured in a Balzer's freeze-fracture device (BAF400D; Balzers, Liechtenstein) at 5 × 10−6 mbar and −150 °C. The fracture faces were shadowed with platinum–carbon (2 nm, 45°) for contrast and carbon (20 nm, 90°) for stabilization of the replica. After removing the cell material in 12% sodium hypochlorite, the replicas were cleaned several times in double-distilled water and mounted on Pioloform-coated copper grids. The replicas were observed using a Zeiss EM10 electron microscope (Oberkochen, Germany). OAP densities were determined as OAPs/µm2 at a magnification of 100 000 : 1 in 30 replicas of three different assays in each experiment. The statistical evaluation was performed using the Kruskal–Wallis one-way anova on ranks followed by Dunn's method for comparisons (SigmaPlot Software; Scientific Solutions, Lausanne, Switzerland).
Cell volume measurements
For volume measurements, cells were grown on round coverslips fitting into the incubation chamber. Cells were incubated in 5 µm calcein (Invitrogen) in culture medium at 37 °C for 30 min. This dye is taken up by the cells but does not leave the cell upon esterization, and can be used for cytoplasmic volume measurements (Sonnentag et al., 2000; Zelenin et al., 2000). Afterwards the cells were kept in Hanks's balanced salt solution (HBSS) in HEPES buffer (pH 7.3) for at least 15 min. The coverslips were then mounted in a closed, flow-through incubation chamber (LaCon, Staig, Germany) surrounded by a heating frame that kept the temperature at 37 °C. Cells were imaged on a confocal microscope (Zeiss LSM 510 META) using a 63× long-distance objective. The 488 nm argon laser line was used for excitation with appropriate filter sets and the laser intensity was kept low to avoid cell damage and bleaching during the experiment.
To test for cellular volume changes the following paradigm was used: to establish a baseline, the chamber was perfused with isotonic HBSS (300 mosmol) at a flow rate of 300 µL/min. After 180 s, the solution was switched to hypotonic (200 mosmol) HBSS for an additional 180 s and finally back to isotonic buffer for at least another 200 s. During the entire experimental time, confocal images (pinhole settings at one Airy unit) of selected cells were acquired in an automated time series with an interval of 10 s, with each confocal scan set to take 4–5 s. Cells were recorded in a line scan through the z-axis of the selected cells. In some experiments, images were acquired in the x–y plane through the centre of the cells; this provided essentially the same results. Prior to and after the experiment, a higher quality image was acquired to control for the cellular integrity. Only cells without structural damage were further analysed. For cell volume changes, increase or decrease in fluorescence intensity were taken as a measure for cell shrinkage or swelling, respectively. For each cell, the fluorescence intensity was measured in three regions of interest in every recorded image from the time series applying the Zeiss LSM 510 software. Subsequent calculations were performed with the computer program Micrososft Excel. The first measurements (during the isotonic phase) were used to determine a bleaching or leakage curve for which all data in the series were corrected. The corrected fluorescence measurements were then plotted as dF/F0, where F0 is the averaged initial fluorescence intensity and dF the difference between the measured fluorescence and the initial fluorescence. The initial fluorescence values were compared to fluorescence minima (highest volume) and tested for statistical significance applying the nonparametric Wilcoxon rank test (n > 50 cells were measured in each experimental group).
Reverse transcriptase (RT)-PCR
RT-PCR analysis for AQP4 isoform M23, M1 and hypoxanthine guanine phosphoribosyl transferase (HPRT) mRNAs was performed as described previously (Ivanova et al., 2002). Total RNA was isolated using the peq Gold RNApure extraction kit (Peqlab, Germany) according to the manufacture's protocol. The cDNA was synthesized from 1 µg of each RNA, 1 µL dNTP (20 mm), 1 µL RT (MMLV), 5 µL 5x-Puffer and 1.5 µL hexanucleotide (10 pmol/µL; all reagents from Gibco) for 1 h at 37 °C followed by enzyme inactivation for 5 min at 95 °C. PCR was conducted with 1.5 µL probe of RT reaction, 0.5 µL sense and antisense primers, 0.4 mm dNTP, 1.5 mm MgCl2 and 1.5 U Taq polymerase (all from Gibco, Karlsruhe, Germany). PCR conditions were: 32 cycles (M23 and M1 primers) or 29 cycles (HPRT primer) of denaturation for 1 min at 95 °C, annealing for 1 min at 62 °C (M23 and M1 primers) or at 61 °C (HPRT primer), extension for 1 min at 72 °C, followed by a final elongation step at 72 °C for 3 min. Concurrent RT-PCR amplification of HPRT was carried out as an internal control for variations in the efficiencies of RNA isolation and RT. The primer sequences are shown in Table 1.
| Target mRNA (mouse) | Primer-Sequence (5′→3′) | Product size (bp) | Annealing (°C) |
|---|---|---|---|
| HPRT | |||
| s | GCT GGT GAA AAG GAC CTC T | 250 | 61 |
| as | CAC AGG ACT AGA ACA CCT GC | ||
| AQP4 m23 | |||
| s | GGA AGG CTA GGT TGG TGA CTT C | 460 | 62 |
| as | TGG TGA CTC CCA ATC CTC CAA C | ||
| AQP4 m1 | |||
| s | CTC CCA GTG TAC TGG AGC CCG | 510 | 62 |
| as | TGG TGA CTC CCA ATC CTC CAA C | ||
- HPRT, housekeeping gene hypoxanthine guanine phosphoribosyl transferase; s, sense sequence; as, antisense sequence.
The PCR products (each 10 µL) were separated by electrophoresis on a 1.5% agarose gel and stained with ethidium bromide. For semiquantitative product analysis, gels were analysed with a UV transilluminator and densitometrically quantified using ImageJ software (available at http://rsb.info.nih.gov/ij). Absolute optical densities (OD) were normalized to the ODs of the corresponding bands of the housekeeping gene HPRT and expressed as relative abundance in arbitrary units. Each experiment was performed at least nine times; RT-PCRs were confirmed in replicates. Prior to the semiquantitative analysis, we determined that 32 PCR cycles were well within the linear detection range. The statistical analysis for comparison of the experimental groups was carried out by Kruskal–Wallis one-way anova on ranks followed by the Tukey test for multiple comparisons (SigmaPlot Software).
Results
We studied physiological and morphological properties of astroglial cultures grown in medium containing two different agrin isoforms, the neuronal agrin A4B8 and the non-neuronal agrin A0B0. We compared these cultures to control cultures without agrin regarding the AQP4 protein level (immunocytochemistry), molecular membrane morphology (freeze-fracturing) and water transport capacity, and on the AQP4 mRNA level (RT-PCR).
Immunocytochemistry
Previously, we have established that most cells in the cultures from brains of BALB/c mice (age between 2 and 5 days) stain positively for GFAP and can therefore be identified as astrocytes (Beyer & Raab, 1998; Ivanova et al., 2001, 2002). Cell cultures of similar growth and density were stained with an antibody against AQP4 recognizing the C-terminal segment of the molecule and, thus, not differentiating between the M1 and the M23 isoforms of AQP4. We observed that the immunoreactivity was diffusely distributed within the control cells; the membranes were not particularly labelled (Fig. 1A). In contrast, when astrocytes from the same source were cultured for 14 days in the presence of medium conditioned with either agrin A0B0 or A4B8 and were processed for anti-AQP4 immunocytochemistry we observed an increased immunoreactivity. Especially, in astrocytes cultured in the presence of agrin A4B8 the immunoreactivity was more pronounced in membrane regions than in cells cultured with agrin A0B0 (Fig. 1B and C). These results suggested that agrin influences the distribution or expression of AQP4.
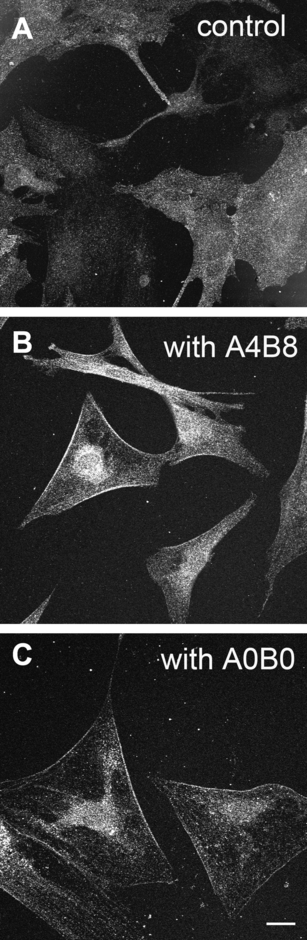
Immunocytochemical staining of AQP4 in astrocytes cultured for 2 weeks under different conditions. (A) Control cells without agrin-condtioned medium. AQP4 immunoreactivity was found diffusely within the cells. (B) Astrocytes cultured with agrin A4B8 revealed immunoreactivity associated predominantly with membranes and less immunoreactivity in the cytoplasm. (C) Agrin A0B0 also caused strong immunoreactivity. Scale bar, 20 µm.
Freeze-fracturing
Astrocyte cultures which were identical in growth and density to those tested by immunocytochemistry were scraped off the culture dish and freeze-fractured. OAPs, known to contain AQP4, were associated strictly with the protoplasmic (inner) fracture face (P-face) leaving pits in the external fracture face (E-face). An OAP was identified as a rectangular array of 4–30 intramembranous particles. These particles measured 7 nm each in diameter. In control cultures without agrin, the OAPs were randomly distributed across the cellular surface (Fig. 2A). In cultures treated with the agrin isoform A0B0, there was no obvious difference in the OAP frequency compared to the control cultures, except that they were often arranged in small accumulations of several OAPs rather than occurring as single arrays (2, 3). These accumulating effects were even strengthened by coculturing astrocytes and menigeal (pial) cells, which are known to produce agrin A0B0 (Stone & Nikolics, 1995). Indeed, these cocultures revealed focal ‘hot spots’ of OAP clusters (Fig. 2E–G). The density of these clusters locally reached that of astroglial endfeet in vivo. With the agrin isoform A4B8, the density significantly increased (2, 3, Table 2). In addition, some raft-like lattices consisting of > 100 subunits occurred (Fig. 2D). These lattices were exclusively present in membranes of astrocytes treated with agrin A4B8 and not found in control cells or agrin A0B0-treated cells.
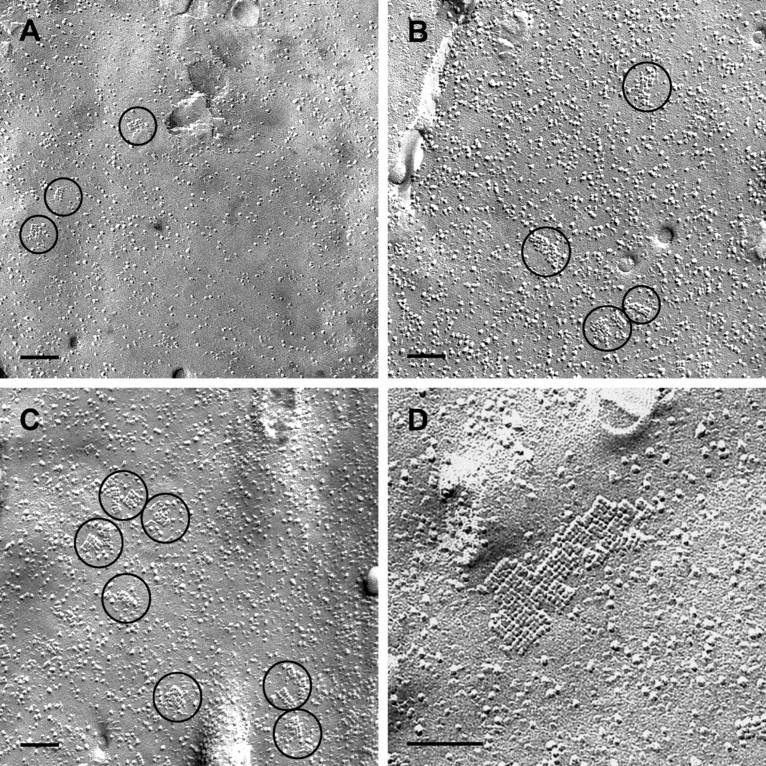
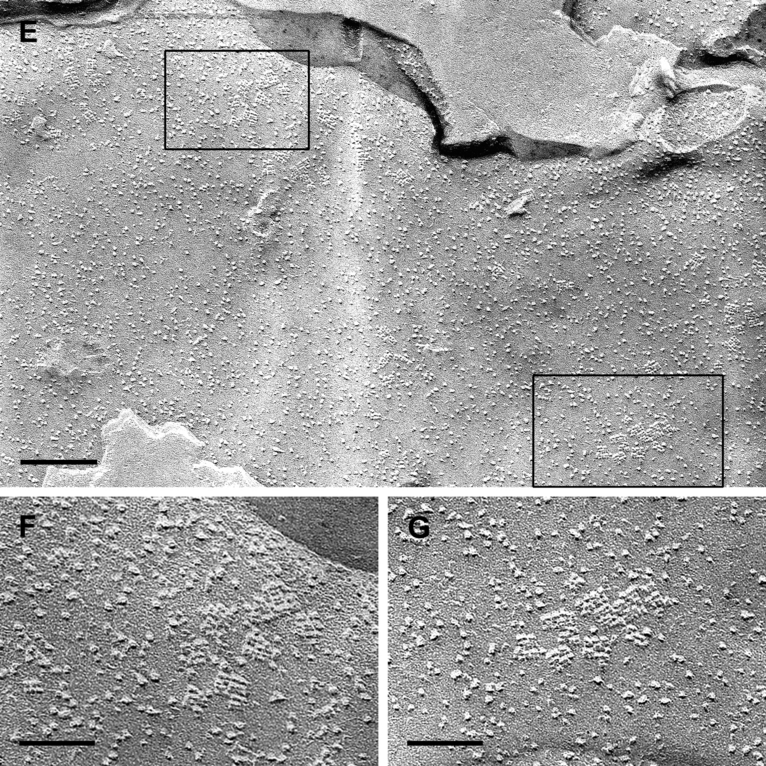
Freeze-fracturing of cultured astrocytes. (A) Control cell cultured without agrin revealing only few and small OAPs which were randomly distributed all over the cell membrane. (B) Astrocytes cultured with agrin A0B0. OAPs occurred in small accumulations but not more frequently than in control cultures. (C and D) Astrocytes cultured with agrin A4B8. Membranes showed more OAPs, often in groups. (D) Some OAPs formed large lattices with many subunits. (E–G) Astrocytes cocultured with meningeal cells. (E) Overview of an astrocytic membrane revealing different areas containing clusters of OAP. (F and G) Enlarged areas of OAPs indicated by the frames in E. Scale bars, 0.2 µm (A–C and E), 0.1 µm (D), 0.04 µm (F and G).
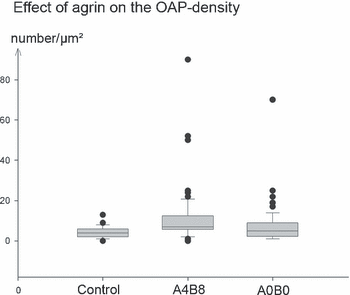
Box blot diagram showing the quantitative morphometric evaluation of OAP densities (OAPs/µm2) in cultured astrocytes treated with agrin A4B8 and agrin A0B0. The untreated control group offered no significant difference from the A0B0-treated astrocytes. In contrast, agrin A4B8-treated cells revealed a significant increase in OAPs (P < 0.001) in comparison to the other groups.
| Immunocyto-chemistry AQP4 | Freeze-fracture OAPs | Volume regulation | PCR | ||
|---|---|---|---|---|---|
| M23 | M1 | ||||
| Agrin A4B8 | ↑ | ↑ | ↑ | ↑ | ↓ |
| Agrin A0B0 | ↑ | ↔ | ↔ | ↔ | ↔ |
- For quantification of the OAP densities, see Fig. 3.
Cell volume regulation
Cultured astrocytes were brought into the flow-through chamber and allowed to equilibrate for a few minutes at constant flow in isotonic buffer solution. After the beginning of acquisition of confocal images (the first 180 s), the solution was switched to hypotonic buffer (∼ 200 mosmol) for another 180 s and finally returned to isotonic buffer again. Under normal growth conditions (controls), the calcein fluorescence intensity decreased by ∼ 8% dF/F0 (Fig. 4C), indicating a volume increase in the imaged cell, and then returned to the initial intensity in isotonic condition. Agrin-treated astrocytes were subjected to the same experimental paradigm as control cultures. In the presence of the A4B8 isoform we observed a decrease in calcein-fluorescence (volume increase) from 8% dF/F0 under agrin-negative conditions up to 16% under agrin-positive conditions (Fig. 4A–D). The agrin A0B0 isoform did not show any effect on the swelling capacity of astrocytes in comparison to astrocytes not treated with agrin (Fig. 4C and D). In addition to the volume changes observed, the change rate, i.e. the water uptake, was faster in astrocytes treated with the A4B8 isoform than in controls or the A0B0 isoform (3.2% in 100 s compared to 1.7 and 1.6%, respectively). This can be seen by the steeper slope of the swelling curve for cells in A4B8 agrin-conditioned medium. These results showed that agrin A4B8 was capable of influencing the rate of water transport across the membrane of cultured astrocytes.
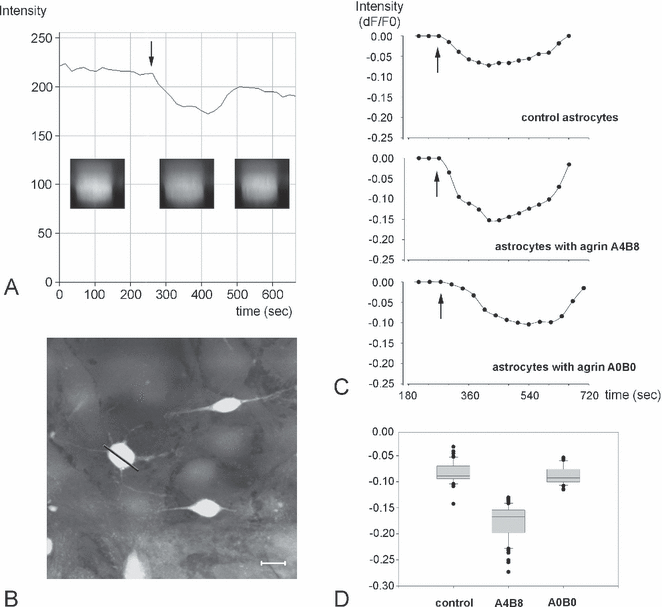
Cell volume measurements of cultured astrocytes. (A) Original trace of a recording from a time series of calcein-loaded cells. The buffer solution was changed from isotonic to hypotonic (arrow) and back to isotonic 180 s later. The arrow indicates the actual solution change in the incubation chamber. The insets show fluroescence intensity changes in acquired images from the time series in z-scans of an astrocyte indicated by the bar in B. (B) Calcein-loaded astrocytes in the x-y plane recorded after the experiment. (C) Averaged and corrected recording traces from control and agrin-treated cultures (see Materials and methods). After exposure to a hypotonic solution (arrows), astrocytes treated with agrin A4B8 showed a larger fluorescence intensity decrease than did control or agrin A0B0-treated cells. This indicates a higher cell volume change. The fluorescence intensity returned almost to baseline in isotonic solution. (D) The lowest corrected fluorescence intensity values were compared between the three groups and showed a significant difference between the agrin A4B8-treated cells and the other two groups (Wilcoxon rank test, P < 0.001). Scale bar in B, 10 µm.
Rt-pcr
The expression of the AQP4 isoforms M23 and M1 was analysed in astrocyte cultures which were treated as described in the experiments above. The expression of the two AQP4 isoforms did not differ in untreated astrocytes. The agrin A0B0-treated cultures displayed the same expression of the AQP4 isoforms as the control group (Fig. 5A–C). This can be seen in similar M23/M1 ratios of ∼ 1.0 for the control and A0B0 groups (Fig. 5D). In contrast, in cultures treated with the agrin A4B8 isoform the mRNA level of the M23 isoform was increasead and mRNA level of the M1 isoform was decreased (P < 0.001). There was a highly significant difference (P < 0.001) in the M23/M1 ratio of the agrin A4B8-treated astrocytes compared to the other groups (Fig. 5D). These results demonstrate that agrin A4B8, but not agrin A0B0, increases the expression of the M23 isoform mRNA of AQP4 and decreases the M1 isoform mRNA of AQP4 in cultured astrocytes.
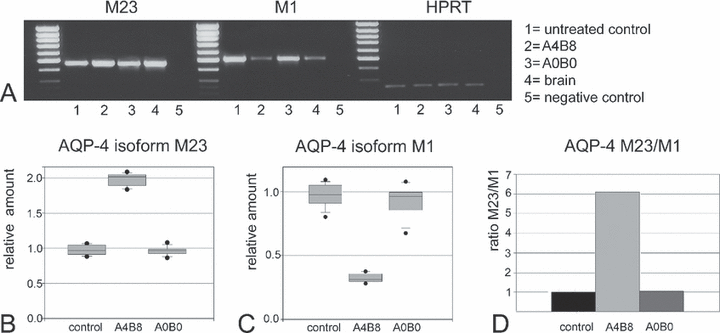
RT-PCR analysis of the AQP4 isoforms M1 and M23. (A) Representative RT-PCR on an agarose gel showing bands corresponding to the AQP4 isoforms M23 and M1 mRNA, and the internal standard HPRT. The M23 isoform showed an increase in mRNA in astrocytes treated with agrin A4B8 (lane 2) compared to the expression in untreated (lane 1) and A0B0 agrin-treated (lane 3) astrocytes. In contrast to the M23 isoform, the M1 isoform was reduced in agrin A4B8-treated cells (lane 2), whereas the two other groups showed similar expression levels (lanes 1 and 3). Whole mouse brain samples (lane 4) were used as positive control tissues for both isoforms. Lane 5 shows the negative H2O control. (B) Semiquantitative results of the M23 isoform expression (each group n = 9): the M23 isoform was significantly more abundant in A4B8-treated cultures (P < 0.001), and there was no significant difference between the other groups. (C) Semiquantitative analysis of the M1 isoform (each group n = 9); again, control astrocytes and the agrin A0B0-treated cells displayed similar expression levels in the range of the relative amounts found for M23 (in B). In contrast, the band for agrin A4B8-treated astrocytes indicated a significantly (P < 0.001) lower expression (relative amount < 0.5) of the M1 isoform. (D) The M23/M1 ratio of expression levels showed a highly significant difference (P < 0.001) between the agrin A4B8-treated cells and the ratio of agrin A0B0-treated and control groups.
Discussion
The present study has provided four main novel findings: first, the presence of agrin increased the membrane-associated immunoreactivity of AQP4 in cultured astrocytes. Second, freeze-fracturing revealed alterations in the morphological properties of the membranes of astrocytes grown in agrin-containing medium. Third, agrin A4B8, but not agrin A0B0, increased the swelling capacity of cultured astrocytes under hypotonic conditions. Fourth, semiquantitative RT-PCR demonstrated that agrin A4B8 increased the level of the M23 isoform of AQP4 and decreased the level of the M1 isoform of AQP4. These results are summarized in Table 2.
The role of the extracellular heparan sulfate proteoglycan agrin for the differentiation of the motor endplate as well as for the regulation of synaptic processes in the CNS has been extensively demonstrated (McMahan, 1990; Bezakova & Ruegg, 2003). In addition, a significant function of agrin for the BBB has been postulated (Barber & Lieth, 1997; Berzin et al., 2000; Smith & Hilgenberg, 2002). Indeed, agrin has been reported as disappearing under pathological conditions and at places where agrin was absent from the perivascular basal lamina in human glioblastoma, tight junctions of the adjacent capillary endothelial cells were deficient of one or several tight junctional molecules (Rascher et al., 2002).
The astrocyte in situ is first of all characterized by a typical polarization. The endfoot membrane adjacent to the perivascular basal lamina is enriched in ion and water channels and important for water homeostasis of the extracellular space. For example, the uneven distribution of the potassium channel Kir4.1 in the astrocytic membrane is fundamental for the spatial buffering capacity of astrocytes (Kofuji & Newman, 2004; Simard & Nedergaard, 2004). As the water flux is osmotically driven by ion fluxes, it is plausible that Kir4.1 is codistributed with AQP4 (Nagelhus et al., 2004). The molecular complex which might be responsible for this codistribution has been assumed to be the dystrophin–dystroglycan complex (DDC) which is connected to the extracellular matrix. Guadagno & Moukhles (2004) reported on a laminin-induced aggregation of Kir4.1 and AQP4 via the DDC. Furthermore, in human glioblastoma the water channel protein AQP4 was redistributed and no longer restricted to the astroglial endfoot membrane where agrin could not be detected in the perivascular basal lamina (Warth et al., 2004). Thus, these observations already suggested that agrin may be important for the polarization of astrocytes. In this context, it is remarkable that in the presence of either of the two agrin isoforms the immunoreactivity against AQP4 accumulated in the membrane of cultured glial cells (Fig. 1). This suggests that both agrin isoforms (A0B0 and A4B8) induced an aggregation of AQP4 in the astrocytic membrane. Agrin A0B0 influences the insertion and/or aggregation of preexisting AQP4 into or within the membrane, respectively, rather than the expression strength of AQP4. Quantification of OAPs showed no significant difference between the agrin A0B0-treated astrocytes and the controls. However, as was clearly visualized in the replicas, these membranes carried considerably more focal accumulations of OAPs. This feature of OAP distribution represents a qualitative difference between A0B0-treated and -untreated astrocytes. In any case, the effect of AQP4 aggregation (Fig. 1) and of increased OAP number/µm2 (Fig. 3) was more pronounced in agrin A4B8-treated astrocytes. Moreover, if cocultured with meningeal (pial) cells which produce agrin (Stone & Nikolics 1995; Fig. 2E–G), astrocytes developed focal ‘hot spots’ of OAP clusters which were not observed in pure astrocyte cultures. In the absence of agrin, AQP4 immunoreactivity was detectable only diffusely within the cytoplasm of the glial cells.
It is well known now that AQP4 is the only aquaporin that can be visualized by freeze-fracture electron microscopy. The OAPs have been known among morphologists since 1969 (for review, see Wolburg, 1995b), but only ∼ 10 years ago did it become clear that they contain AQP4. This was demonstrated by the absence of OAPs in astrocytes of AQP4-deficient mice (Verbavatz et al., 1997) as well as by the formation of OAPs in stably transfected CHO cells with AQP4 cDNA (Yang et al., 1996). Most directly, Rash et al. (1998, 2004) demonstrated by means of the immunogold fracture-labelling technique that AQP4 is a component of the arrays. Moreover, Nielsen et al. (1997) were able to demonstrate by immunogold immunocytochemistry that the distribution of the AQP4-related immunoreactivity was identical to that of the OAPs. Nicchia et al. (2000) presented evidence suggesting that AQP4 is mainly responsible for rapid water movement in the brain. AQP4 knockdown in cultured astrocytes significantly contributed to protection against water influx during hypoxia, suggesting dominant roles of AQP4 in oedema formation (Fu et al., 2007). AQP4 exists in different alternative splice variants, M1 and M23 (Jung et al., 1994). Transfection of AQP4-M23 but not of AQP4-M1 into CHO cells induced the formation of OAPs but these OAPs displayed large lattices (Furman et al., 2003). If, however, both AQP4 isoforms were cotransfected the OAPs formed closely resembled those found on astrocytic endfeet in vivo.
When astrocytes were grown in the presence of agrin-A4B8, we observed a stronger and faster volume increase after hypotonic stimulation. Interestingly, Jones et al. (1997) have shown in the muscle system that the insertion of eight amino acids at the B splicing site of agrin was able to influence the expression of motor endplate-related proteins. In addition, the expression of AQP4-M23 was up-regulated and that for AQP4-M1 down-regulated (Fig. 5). Thus, the M23/M1 ratio was dramatically increased. These results are consistent with the report of Silberstein et al. (2004) that AQP4-M23-mediated water transport was more efficient than AQP4-M1-mediated water transport. Thus, our data speak in favour of the assumption that both the increase in M23 expression and the increase in the M23/M1 ratio may be responsible for the increased water transport capacity. In addition, lattices known to be formed after AQP4-M23 transfection (Furman et al., 2003) were observed in cultured astrocytes treated with agrin A4B8 (Fig. 2D). These differential effects of agrin isoforms on AQP4 isoforms would not be detected by Western blotting because antibodies used in recognizing AQP4 can not differentiate between the isoforms (data not shown; Fu et al., 2007).
The agrin splicing variants A0B0 and A4B8 have been reported to be specifically expressed by endothelial cells and neurons, respectively (Stone & Nikolics, 1995). The endothelial agrin isoform A0B0 did not increase the water influx into astrocytes after the switch to hypotonic solution (Fig. 4). From the well-known distribution of AQP4 in vivo it could be expected that, where endothelial agrin A0B0 contacts the astroglial membrane, AQP4-based water transport should be enhanced but, obviously, this enhancement is below the detection level of the volume measurement performed. The A0B0 isoform seems to fulfil the limited task of mediating insertion of AQP4 from the cytoplasmic pool into the membrane of cultured agrin-treated astrocytes, as shown in Fig. 1.
In the parenchymal compartment, the astroglial cell is in close contact with neurons and synapses. Here, the extracellular K+ concentration strongly increases during synaptic activity and is counter-regulated by the spatial buffering system of the astrocytes. K+ uptake is followed by water entry. This parenchymal water transport is performed by AQP4 channels as well (for review see Amiry-Moghaddam et al., 2004), but these channels are only sparsely present at this domain of the glial membrane as demonstrated by anti-AQP4 immunocytochemistry (Nielsen et al., 1997; Nagelhus et al., 1999, 2004; Amiry-Moghaddam et al., 2003, 2004) and by the low OAP density in freeze-fracture replicas (Neuhaus, 1990; Wolburg, 1995a). It is all the more essential to equip this domain with fast water channels. Indeed, what we have shown in the present study is the strong increase in the AQP4-M23-isoform expression and the accumulation and insertion of AQP4 as a response to the neuronal agrin isoform A4B8. In vivo, the insertion and activation of AQP4-M23 by means of the neuronal agrin isoform A4B8 is restricted to the parenchymal membranes in synaptic regions of the brain. However, in the situation of our in vitro experiments, the entire surface of the cell represents a target for A4B8 activity. In other words, the membrane of the entire cell in culture, if under the influence of neuronal agrin, reacts as the perisynaptic membrane domain in vivo. Concerning the endothelial agrin isoform A0B0 in vivo, it is important that AQP4 is inserted into the membrane domain directly adjacent to blood vessels. As in vivo this glial membrane domain represents a small proportion of the entire astrocytic surface membrane, it is all the more necessary to accumulate the water channels at the perivascular domain.
As under pathological conditions agrin and astrocytic polarity are lost, the directionality of water flux is disturbed leading to cytotoxic oedema followed by disruption of the BBB and subsequently by vasogenic oedema. Both forms of oedema are pivotal in the development of high brain pressure with all known clinical detrimental consequences. We therefore propose that therapy for intracranial pressure should implement the recovery of astrocyte polarity via substitution of agrin or a similar compound with polarity-inducing properties.
Acknowledgements
Yeliz Donat-Krasnici and Ria Knittel are thanked for the skilful technical assistance in cell culturing, freeze-fracturing and microscopy. With support by the Deutsche Krebshilfe–Mildred Scheel-Stiftung to H.W. (grant number 107686).
Abbreviations
-
- AQP4
-
- aquaporin-4
-
- BBB
-
- blood–brain barrier
-
- HPRT
-
- hypoxanthine guanine phosphoribosyl transferase
-
- OAP
-
- orthogonal array of intramembranous particles.