Neuroligin-3 is a neuronal adhesion protein at GABAergic and glutamatergic synapses
Abstract
Synaptic adhesion molecules are thought to play a critical role in the formation, function and plasticity of neuronal networks. Neuroligins (NL1–4) are a family of presumptive postsynaptic cell adhesion molecules. NL1 and NL2 isoforms are concentrated at glutamatergic and GABAergic synapses, respectively, but the cellular expression and synaptic localization of the endogenous NL3 and NL4 isoforms are unknown. We generated a panel of NL isoform-specific antibodies and examined the expression, developmental regulation and synaptic specificity of NL3. We found that NL3 was enriched in brain, where NL3 protein levels increased during postnatal development, coinciding with the peak of synaptogenesis. Subcellular fractionation revealed a concentration of NL3 in synaptic plasma membranes and postsynaptic densities. In cultured hippocampal neurons, endogenous NL3 was highly expressed and was localized at both glutamatergic and GABAergic synapses. Clustering of NL3 in hippocampal neurons by neurexin-expressing cells resulted in coaggregation of NL3 with glutamatergic and GABAergic scaffolding proteins. Finally, individual synapses contained colocalized NL2 and NL3 proteins, and coimmunoprecipitation studies revealed the presence of NL1–NL3 and NL2–NL3 complexes in brain extracts. These findings suggest that rodent NL3 is a synaptic adhesion molecule that is a shared component of glutamatergic and GABAergic synapses.
Introduction
The function of the nervous system critically depends on the pattern of synaptic connectivity established during development. Assembly of neuronal circuits requires specific recognition between afferents and targets and subsequent formation and stabilization of the appropriate number of synapses. The organization of individual synapses is mediated, in part, by bi-directional signalling between the pre- and postsynaptic neurons (Scheiffele, 2003; Waites et al., 2005). Target-derived signals direct the recruitment of synaptic vesicles and the assembly of presynaptic active zones, whereas axon-derived signals promote the nucleation of postsynaptic machinery, including neurotransmitter receptors and scaffolding molecules. Specific trans-synaptic signals are believed to ensure the accurate matching of presynaptic neurotransmitter phenotype with the appropriate postsynaptic neurotransmitter receptors, but the specific molecules mediating these interactions are not known.
Synaptic adhesion molecules are attractive candidates for governing recognition events between pre- and postsynaptic cells, as well as for orchestrating the structural organization of synaptic junctions (Yamagata et al., 2003). Neuroligins (NLs) are one family of postsynaptic adhesion molecules that includes isoforms localizing at glutamatergic and GABAergic synapses (Ichtchenko et al., 1995, 1996; Song et al., 1999; Varoqueaux et al., 2004). NLs form trans-synaptic complexes with presynaptic neurexins (NRXs), a second family of neuronal cell surface proteins (Ushkaryov et al., 1992), and bi-directional signalling via the NL–NRX complex can organize both pre- and postsynaptic compartments (Levinson & El-Husseini, 2005; Dean & Dresbach, 2006). For example, studies in cultured neurons have demonstrated that postsynaptic NLs are sufficient to induce presynaptic clustering of synaptic vesicles and active zone components, and presynaptic NRXs can recruit postsynaptic NLs, scaffolding molecules and neurotransmitter receptors to synaptic sites (Scheiffele et al., 2000; Dean et al., 2003; Graf et al., 2004; Prange et al., 2004; Chih et al., 2005).
In mice, there are four highly homologous neuroligin variants (NL1–4) that share 50–60% amino acid sequence identity (Ichtchenko et al., 1996; Bolliger et al., 2001). In vivo, NLs are essential for nervous system function, as NL1,2,3 triple knockout mice die at birth due to defects in respiration (Varoqueaux et al., 2006). Brain stem circuits in these mice exhibit a 20% reduction in synapse density and an impairment of synaptic transmission that might result from a loss of neurotransmitter receptors from postsynaptic sites. However, the specific roles of each NL isoform or how individual NL isoforms may act in concert are still not well understood. On the cellular level, NL1 and NL2 are the best characterized of the NL isoforms. Alternative splicing at two sites (A and B) in the extracellular domain generates multiple variants that differ in interactions with presynaptic NRXs and association with glutamatergic and GABAergic synapses (Boucard et al., 2005; Chih et al., 2006). NL1B, the most abundant splice variant of NL1, is concentrated at glutamatergic synapses, whereas NL2A is localized preferentially at GABAergic synapses (Song et al., 1999; Graf et al., 2004; Varoqueaux et al., 2004; Chih et al., 2006). This selective distribution may contribute to the stabilization of specific synapse types in an activity-dependent manner (Chubykin et al., 2007). In contrast to NL1 and NL2, the NL3 and NL4 isoforms have not been well characterized. In the mouse central nervous system, NL4 appears to be expressed only in the adult, where it represents less than 5% of total NLs (Varoqueaux et al., 2006). NL3 has been proposed to be primarily expressed in glia (Gilbert et al., 2001); however, in situ hybridization studies and in vitro experiments utilizing gain and loss of function approaches in hippocampal neurons indirectly support a neuronal role for NL3 (Chih et al., 2005; Varoqueaux et al., 2006). Resolving these discrepancies is of great importance, not only with respect to the function of the NL–NRX complex, but also because inactivating mutations in NL3 have been identified in individuals with autism spectrum disorders (Jamain et al., 2003; Chih et al., 2004; Comoletti et al., 2004). Therefore, any insight into the localization and function of NL3 in the nervous system should be valuable for understanding the basic cellular defects associated with these disorders.
A major obstacle in the analysis of NL3 has been the lack of immunological reagents suitable for immunohistochemistry. In this study, we generated a panel of NL isoform-specific antibodies and examined the expression, biochemical interactions and subcellular localization of endogenous NL3. We show that NL3 protein is highly expressed in neurons and is localized at both glutamatergic and GABAergic synapses. Furthermore, our results demonstrate that single synapses can contain multiple NL isoforms, and that NL3 can associate in complexes with other NL isoforms in vivo.
Materials and methods
All animal care and use was in accordance with the institutional guidelines and approved by the Institutional Animal Care and Use Committee of Columbia University.
Antibody production
Antibodies to NL isoforms were raised in rabbit and guinea pig using synthetic peptide antigens (Global Peptide) including terminal cysteines for coupling. Peptides that elicited a strong immune response were as follows (single-letter amino acid code; additional amino acids introduced as spacer or for coupling are in parentheses): NL3: N-(CG)-QAPAPTVNTHFGKLRGAR-C, corresponding to amino acids 35–52 in the extracellular domain, and N-(C)-EAGPPHDTLRLTALPDYT-C, corresponding to amino acids 753–770 in the cytoplasmic domain of mouse NL3 (splice variant A2). NL2: N-(C)-RGGGVGADPAEALRPACP-C, corresponding to amino acids 750–767 in the cytoplasmic domain of mouse NL2 (splice variant A). NL1: N-QKLDDVDPLVTTNFGKIR-(C)-C, corresponding to amino acids 46–63 in the extracellular domain of mouse NL1.
Peptides were conjugated to Imject KLH Maleimide (Pierce) and injected into two rabbits and one guinea pig each (Covance). Peptide columns for affinity purification were generated by cysteine coupling using SulfoLink Coupling Gel (Pierce). Guinea pig and rabbit antisera were affinity purified on separate peptide columns. All purified antibodies and sera were tested for NL isoform specificity by detection of overexpressed epitope-tagged NL isoforms in HEK293 cells.
Western blotting
Animals were killed by CO2 inhalation followed by cervical dislocation. Rodent tissues were homogenized in 10% (tissue weight/buffer volume) lysis buffer [100 mm NaCl, 50 mM Tris, pH 8.0, 1% (w/v) Triton X-100, 0.1% (w/v) sodium dodecylsulphate, 2 mm dithiothreitol, complete protease inhibitor cocktail (Roche)]; the protein concentration was measured with a bicinchoninic acid protein assay (Pierce), and the homogenized tissue was prepared in 4× Laemmli buffer for western blotting. Thirty micrograms of total protein was loaded in each lane. Primary antibodies used were: mouse anti-PSD95 (Upstate), mouse anti-N-methyl-d-aspartate receptor (NR) subunit 1 (NR1) (Synaptic Systems), rabbit anti-NR2B (Molecular Probes), rabbit anti-glutamate receptor (GluR) subunit 2/3 (GluR2/3) (Chemicon), rabbit anti-synaptophysin (Synaptic Systems), mouse anti-actin (Sigma), and mouse anti-Tuj1 (Covance). The rabbit panNL antibody has been described previously (Taniguchi et al., 2007).
Subcellular fractionation and coimmunoprecipitation
Subcellular fractionation from adult rat brain was performed as previously described (Carlin et al., 1980), with minor modifications. Briefly, brains from three adult male rats were homogenized in 0.32 m sucrose and 4 mm HEPES (pH 7.4) (lys) and centrifuged for 10 min at 1000 g to generate the P1 nuclear pelleted fraction (P1). The supernatant was centrifuged for 15 min at 10 000 g to yield the crude synaptosomal pellet (P2). The synaptosomal pellet was resuspended in 10 mL of HEPES-buffered sucrose and recentrifuged at 10 000 g for 15 min to generate the washed crude synaptosomal fraction (P2′). The pellet was lysed by hypo-osmostic shock in 9 mL of cold H2O with complete protease inhibitors (Roche), 40 µL of 1 m HEPES (pH 7.4) was added, and the sample was mixed at 4 °C for 30 min. Centrifugation at 25 000 g for 20 min yielded the light membrane fraction containing plasma membrane fragments and synaptic vesicles (S3) as the supernatant and the pelleted heavy synaptosomal membrane fraction (P3). The pellet was resuspended in 2 mL of HEPES-buffered sucrose, layered on top of a sucrose gradient, and centrifuged for 2 h at 150 000 g. The synaptic plasma membrane fraction was collected from the 1.2/1.0 m sucrose interface, diluted by adding 2.5 volumes of 4 mm HEPES (pH 7.4), and centrifuged for 30 min at 150 000 g to yield the pelleted synaptic plasma membrane fraction (SPM). The pellet was resuspended in 50 mm HEPES (pH 7.4), 2 mm EDTA and complete protease inhibitors (Roche). The fraction was solubilized with 0.5% Triton X-100, rotated at 4 °C for 15 min, and centrifuged at 200 000 g for 20 min to yield the insoluble postsynaptic density (PSD) fraction and the solubilized supernatant (sol). Aliquots from each fraction were saved, the protein concentration was determined with a bicinchoninic acid protein assay (Pierce), and 15 µg of total protein were loaded per well for western blot analysis.
In vivo coimmunoprecipitation was performed using juvenile rodent total brain lysates. Postnatal day 20 (P20) mice were killed with CO2 inhalation and brains were dissected and homogenized in NP-40 lysis buffer [150 mm NaCl, 50 mm Tris-HCl, pH 7.4, 1% (w/v) NP-40, 0.1% (w/v) sodium deoxycholate, 0.5 mm CaCl2, 1 mm dithiothreitol, complete protease inhibitors (Roche)]. Lysates were incubated for 12 h at 4 °C with guinea pig NL isoform-specific antisera or preimmune guinea pig sera, and this was followed by 2 h of incubation with 30 µL of protein A sepharose beads (Amersham Pharmacia) at 4 °C. Lysate was also incubated with beads alone as a negative control. Samples were washed three times with NP-40 lysis buffer without sodium deoxycholate. Laemmli buffer was added, samples were boiled, and 25% was loaded per lane. Purified rabbit NL isoform-specific antibodies were used in western blot analysis.
Neuronal culture
Dissociated hippocampal cultures were prepared from embryonic day 18 (E18) rats. Briefly, pregnant mothers were killed with CO2 inhalation, embryos were removed, and hippocampi were dissected. Neurons were plated on poly(d-lysine)-coated glass coverslips at a density of 25 000/cm2 and maintained in neurobasal medium (Invitrogen) with B27 supplement (Invitrogen) and Glutamax (Invitrogen). Transfections were carried out at 10 days in vitro (DIV 10) with Lipofectamine 2000 (Invitrogen) as previously described (Chih et al., 2005). At DIV 16, neurons were fixed in 4% (w/v) paraformaldehyde in phosphate-buffered saline (PBS) containing 4% (w/v) sucrose at room temperature for 5 min, followed by 100% methanol at −20 °C for 10 min, and prepared for immunocytochemistry as described for detection of endogenous proteins.
Lentiviruses
Previously described lentiviral vector constructs Lenlox3.7-U6-shRNA NL3-BA-EGFP (NL3 shRNA) or Lenlox3.7-U6-shRNA dsRed-BA-EGFP (control shRNA) (Chih et al., 2005) were used to generate lentiviruses for knockdown of endogenous NL3 in hippocampal cultures. HEK293T cells were triple-transfected with small hairpin (sh)RNA DNA, delta 8.9 and VSVg using the calcium phosphate method. Seventy-two hours later, the supernatant containing viral particles was harvested. The culture medium was passed through a 0.45 µm filter unit and the virus was concentrated by ultracentrifugation (80 000 g for 1.5 h). The resulting pellet was incubated in 100 µL of PBS for 12 h at 4 °C and resuspended, and aliquots were frozen in liquid nitrogen.
Viruses expressing shRNA directed against NL3 or dsRed as a negative control were added to hippocampal cultures at DIV 10. At DIV 16, culture lysates were collected in PBS, 1.0% Triton X-100, 0.1% sodium dodecylsulphate, 5 mm EDTA, 2 mm dithiothreitol and complete protease inhibitor (Roche), and analysed by western blotting.
Immunocytochemistry and antibodies used
Neurons stained for endogenous proteins were fixed in 4% (w/v) paraformaldehyde in PBS containing 4% (w/v) sucrose at room temperature for 5 min, followed by 100% methanol at −20 °C for 10 min, except when using the GABA receptor (GABAR) antibody, when fixation was performed for 10 min with 4% PFA on ice. Coverslips were blocked in 10% (v/v) normal donkey serum and 0.125% (w/v) Triton X-100 for 1 h at room temperature. Coverslips were stained with primary antibodies overnight at 4 °C. Commercially available antibodies used included: mouse anti-PSD95 1 : 200 (ABR), rat anti-homer 1 : 400 (Chemicon), mouse anti-gephyrin 1 : 400 (Cedar Lane), mouse anti-GABARβ2/3 1 : 100 (Chemicon), mouse anti-GluR2 1 : 600 (Chemicon), guinea pig anti-VGlut1 1 : 20 000 (Chemicon), mouse anti-VGAT 1 : 2000 (Synaptic Systems), guinea pig anti-VGAT 1 : 2000 (Calbiochem), and chicken anti-green fluorescent protein 1 : 1000 (Upstate). Affinity-purified rabbit anti-NL3 was used at a dilution of 1 : 1000 and guinea pig anti-NL2 was used at a dilution of 1 : 200. All secondary antibodies were Cy2-, Cy3- or Cy5-conjugated donkey anti-IgGs cross-absorbed against all other species used (Jackson).
Cos cell drop assay
Cos7 cells were transfected with haemagglutinin (HA)-tagged NRX1β constructs or EGFP as a negative control using Fugene6 (Roche) transfection reagent. After 36 h, cells were trypsinized and pelleted by centrifugation at 1.2 g for 3 min, and 20 000 cells were added per well (24-well plate) of hippocampal neurons at DIV 11. Forty-eight hours later, neurons were fixed in 4% (w/v) paraformaldehyde in PBS containing 4% (w/v) sucrose at room temperature for 5 min, followed by 100% methanol at −20 °C for 10 min, and prepared for immunostaining as for staining for endogenous proteins.
Image acquisition and analysis
Images were acquired using a 63× oil objective (numerical aperture, 1.40) on an LSM510 meta confocal microscope (Zeiss) with 2048 × 2048 pixel resolution. Each channel was scanned individually with identical laser power and photomultiplier settings for different conditions within each experiment. With such settings, no crosstalk or bleedthrough between different channels occurred. Five to 10 optical sections acquired at 0.5 µm intervals were assembled as a Z-stack projection and analysed with Metamorph software as previously described (Chih et al., 2005). Briefly, sections of dendrites 40 µm in length, which typically contained around 100 synaptic puncta, were selected for analysis of endogenous NL3 localization. For colocalization studies, it was established that Z-stack projections produced similar results as analysis of single optical sections. Synaptic puncta were isolated by thresholding of images such that background staining was excluded. Puncta smaller than 0.04 µm2 in area were excluded from analysis. Masks of thresholded signal were created in one channel and were overlaid onto the other two thresholded channels, and the amount and percentage overlap of different channels was recorded. Postsynaptic proteins were considered to be colocalized if greater than a 0.04 µm2 overlap was observed, and apposition of pre- and postsynaptic proteins was defined as at least 0.016 µm2 overlap of the thresholded signals.
Results
Expression of NL3 in rodent brain
We raised polyclonal antipeptide antibodies in rabbits and guinea pigs using NL isoform-specific peptides as antigens. For NL1 and NL2, we selected one single epitope from each isoform as antigens. For detection of NL3, we chose two epitopes, one derived from the extracellular domain (NL3ex) and one from the intracellular domain (NL3in). Specificity of the affinity-purified sera for individual NL isoforms was confirmed by probing lysates of HEK293 cells overexpressing HA-tagged NL1–4 cDNAs in western blots (Fig. 1). NL1, NL2 and NL3ex antibodies recognized the respective NL isoform without noticeable cross-reactivity with other isoforms. The NL3in antibody showed a low level of cross-reactivity with overexpressed NL4 (Fig. 1A). This cross-reactivity is not a major concern, as NL4 is expressed at levels 5–10 times lower than NL3 in rodent brain (Varoqueaux et al., 2006) and our experiments suggest that the majority of immunoreactivity from endogenous proteins is derived from NL3 (see below). Western blot specificity profiles for the sera obtained from rabbit and guinea pig were very similar, and specificity was further confirmed by immunostaining of NL isoform-expressing HEK293 cells (data not shown). In total brain lysates from adult mice, NL1, NL2 and NL3in antibodies revealed single immunoreactive bands with an apparent molecular mass of approximately 100 kDa. The NL3ex antibody recognized an additional nonspecific band of 45 kDa (Fig. 1B). Therefore, this latter antibody was used exclusively in western blotting where the interpretation of the data was not compromised by the cross-reacting protein.
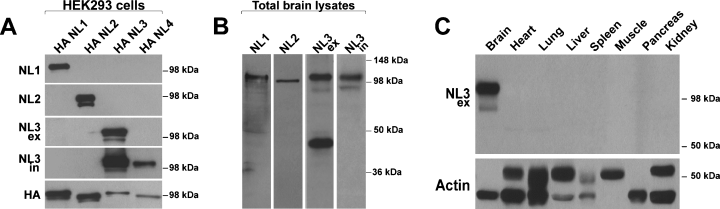
NL3 protein is highly enriched in brain tissue. (A) Generation of NL isoform-specific antibodies. Full-length HA-tagged NL isoforms (NL1–4) were overexpressed in HEK293 cells, and cell lysates were probed with rabbit anti-NL1, anti-NL2 and anti-NL 3 specific antibodies. The NL3ex antibody was raised against an antigen in the extracellular domain of NL3, and the NL3in antibody was raised against an antigen in the intracellular domain of NL3. The same lysates were probed with an antibody to the HA epitope tag. (B) Lysates from adult mouse brain probed with NL antibodies. Rabbit anti-NL1, anti-NL2 and anti-NL3in each detect a single band of around 100 kDa corresponding to the endogenous NL isoforms. NL3ex also detects an additional band of a lower molecular mass. Knockdown of endogenous NL3 in cultured neurons by RNAi did not change the level of this protein (data not shown). Therefore, we concluded that it represents a nonspecific cross-reacting band. Purified antibodies raised in guinea pig produced very similar results (data not shown). (C) NL3 is enriched in rodent brain. Tissues from adult mouse were harvested and lysates containing equal amounts of protein were probed with the NL3ex antibody or actin as a loading control.
NL3 was originally identified in brain (Ichtchenko et al., 1996), but expression of NL3 mRNA in other tissues has been detected by northern blot analysis (Philibert et al., 2000). To determine whether significant NL3 protein is produced in these tissues, tissue lysates from adult mouse were probed with the NL3ex antibody (Fig. 1C). We found that NL3 protein was highly enriched in brain and was not observed at detectable levels in adult heart, lung or muscle tissue (Fig. 1C). The same result was also obtained with the NL3in antibody and with a panNL antibody (Taniguchi et al., 2007) that recognizes all NL isoforms (data not shown). Although we cannot exclude the possibility that an uncharacterized variant of NL3 lacking all three epitopes detected by our antibodies is present in non-neuronal tissues, our data strongly suggest selective expression of full-length NL3 protein in the nervous system.
Next, we examined the developmental pattern of NL3 expression in rodent brain (Fig. 2A). NL3 was detectable as early as E12. Expression increased throughout development, peaked around P14, and was maintained in the adult (>P90). This expression profile is similar to what has been previously reported for NL1 and NL2 (Song et al., 1999; Varoqueaux et al., 2004) and coincides with the developmental peak in synaptogenesis during the second and third postnatal week in rodents. To test whether NL3 is associated with synapses, subcellular fractionation was performed from adult rat brain tissue. Synaptosome isolation and detergent extraction revealed that NL3 was enriched in synaptic plasma membranes. A significant fraction of NL3 was recovered in the Triton X-100-insoluble postsynaptic density fraction containing PSD95 and NMDA (NR1) and AMPA (GluR2/3) glutamate receptors (Fig. 2B). NL3 was also detected in other subcellular fractions, which might reflect the existence of nonsynaptic and intracellular pools of NL3 (see below). Such nonsynaptic pools are routinely observed for synaptic proteins, including neurotransmitter receptors (Perez-Otano et al., 2006). Taken together, these data demonstrate that endogenous NL3 is highly expressed in the developing and adult brain and is enriched in synaptic fractions.
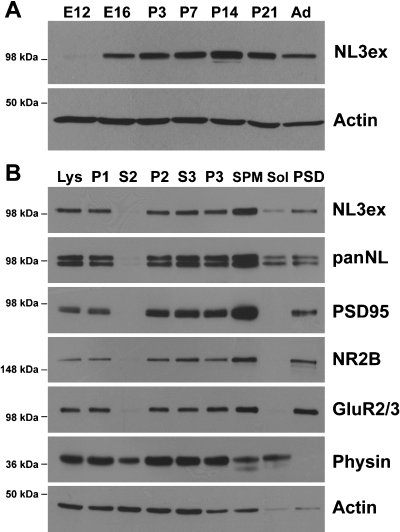
NL3 in rodent brain is enriched in synaptic membrane fractions. (A) Developmental time course of NL3 expression in rodent brain. Mouse total brain lysates from E12 and E16 and P3, P7, P14 and P21 and adult (>90 days) (Ad) were harvested and 30 µg of protein from each sample was probed with the NL3ex antibody and actin as a loading control. (B) NL3 is enriched in synaptic fractions. Subcellular fractions of rat brain were probed with the NL3ex and panNL antibodies and a panel of synaptic markers. For each fraction, 30 µg of protein was loaded. lys, brain homogenate; P1, nuclear pellet; S2, myelin/microsome/small soluble protein supernatant; P2, crude synaptosomal pellet; P3, heavy synaptosomal membrane pellet; S3, light synaptic membrane fraction; SPM, synaptosome fraction; Sol, Triton X-100-soluble fraction; PSD, postsynaptic density/Triton X-100-insoluble fraction. See Materials and methods for more details on the fractionation procedure.
NL3 is synaptically localized in hippocampal neurons
To examine the cellular and subcellular localization of NL3 in more detail, we immunostained dissociated hippocampal neurons with NL3 antibodies. We observed punctate staining along neuronal processes costained with the dendritic marker MAP2 (data not shown). Double-labelling with a presynaptic vesicle marker, synaptobrevin/VAMP2, indicated that a significant proportion of the NL3-positive structures are apposed to presynaptic terminals (Fig. 3A). Although somewhat more diffuse, a similar pattern of synaptic localization was observed with the NL3ex antibody (see Supplementary Material, Fig. S1). However, due to the cross-reacting protein detected by this antibody in western blotting, the NL3in antibody was used for all of the following immunostaining experiments. To further confirm specificity of the antibody detection for NL3, we took advantage of a previously characterized shRNA that suppresses endogenous NL3 (Chih et al., 2005). Compared to a control shRNA, transfection of hippocampal neurons with the NL3 shRNA dramatically reduced the cell body labelling and punctate staining of dendritic processes observed with the NL3 antibody (Fig. 3B). This decrease is not an indirect consequence of the reduction of synaptic contacts resulting from knockdown of NL3 (Chih et al., 2005), as NL1- and NL2-positive puncta were maintained in NL3 knockdown neurons (supplementary Fig. S2). Therefore, our NL3in antibody specifically detects endogenous NL3 in cultured hippocampal neurons.
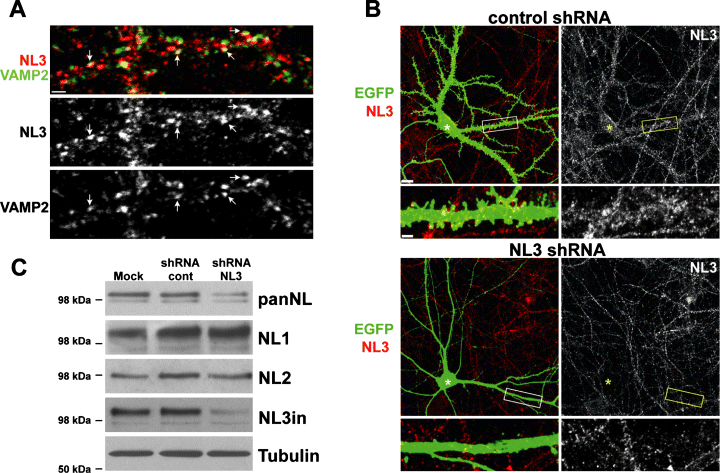
NL3 is highly expressed in hippocampal neurons. (A) NL3 is localized at mature synapses in vitro. Dendritic segments of DIV 21 hippocampal neurons were costained with the NL3in antibody (red) and an antibody to the presynaptic vesicle marker, synaptobrevin/VAMP2 (green). Arrows indicate examples of apposed puncta. Scale bar, 2 µm. (B) Punctate staining of hippocampal neurons with NL3 is diminished upon shRNA-mediated knockdown of endogenous NL3. At DIV 10, hippocampal cultures were transfected with a lentiviral shRNA expression construct targeting NL3 (NL3shRNA, bottom panel) or a control sequence (control shRNA, top panel) and analysed 6 days later. Cells were immunostained with antibodies to EGFP (green), which is expressed in all transfected cells, and with the NL3in antibody (red). White and yellow asterisks mark the location of the cell body of the transfected neuron in each frame. Scale bar, 10 µm in large frame and 2 µm in enlarged view of dendrite. (C) NL3 represents a significant fraction of total NL proteins in hippocampal cultures. Lentiviral infection at 10 DIV was used to deliver NL3 shRNA into cultured hippocampal neurons (nearly 95% of total cells are infected), and infection with viruses carrying an unrelated shRNA construct was used as a control. In the mock-treated condition, culture medium alone was added to cultures. Cell lysates were collected 6 days later and processed for western blotting. Blots were probed with panNL and NL1, NL2 and NL3in antibodies. Tubulin β3 levels were probed as a loading control.
To estimate the relative abundance of endogenous NL3 as compared to other NL isoforms in hippocampal cultures, we suppressed NL3 expression with lentiviral delivery of NL3 shRNA at DIV 10. This protocol results in infection and knockdown of endogenous protein in more than 90% of cultured neurons. At DIV 16, cell lysates were collected and probed with the panNL antibody and isoform-specific antibodies for NL1, NL2 and NL3. Knockdown of NL3 resulted in a 40–50% reduction of total NL protein levels (Fig. 3C). Whereas detection of NL3 was almost completely abolished, levels of NL1 and NL2 were unchanged. In combination with the immunohistochemical findings described above, these results suggest that our NL3in antibody specifically detects endogenous NL3 protein at synapses and demonstrate that NL3 comprises a substantial fraction of total NL protein in cultured hippocampal neurons.
Localization of NL3 at both glutamatergic and GABAergic synapses in hippocampal neurons
The expression of NL1 and NL2 isoforms is largely segregated between glutamatergic and GABAergic synapses, respectively (Song et al., 1999; Varoqueaux et al., 2004; Chih et al., 2006). To investigate whether NL3 specifically localizes at either synapse type in vitro, DIV 14 hippocampal neurons were triple-stained with antibodies to NL3 and pre- and postsynaptic markers of glutamatergic and GABAergic synapses (Fig. 4). Glutamatergic puncta were detected by colabelling with the presynaptic marker VGlut1 and postsynaptic markers PSD95 or the AMPA-type glutamate receptor subunit GluR2; GABAergic synapses were identified by colocalization with VGAT and gephyrin or GABAA receptor beta subunits. Using these markers, we found that endogenous NL3 is associated with both glutamatergic and GABAergic synapses during the period of synaptogenesis in hippocampal neurons (Fig. 4A and B). In addition to the synaptically localized NL3, we occasionally observed colocalization of NL3 with postsynaptic scaffolds or neurotransmitter receptors in the absence of an apposed presynaptic terminal, and a substantial proportion of NL3 puncta were not colocalized with any of the synaptic markers tested. Finally, the association of NL3 with glutamatergic and GABAergic synapses was also seen in more mature cultures (DIV 21 and DIV 30), demonstrating that the association of NL3 with both synapse types persists upon synapse maturation (data not shown and supplementary Fig. S3).
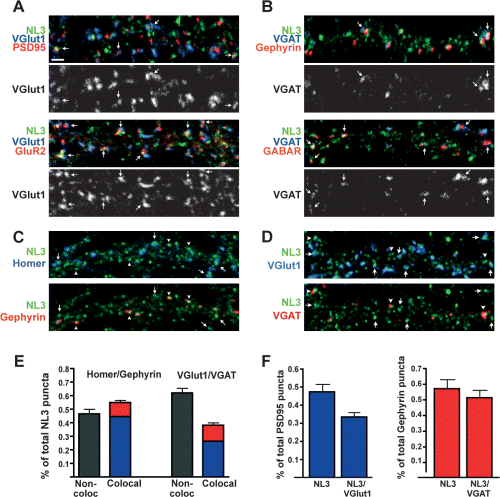
NL3 is localized at both glutamatergic and GABAergic synapses in hippocampal neurons. At DIV 14, cultured hippocampal neurons were triple labelled to determine colocalization of endogenous NL with glutamatergic and GABAergic synaptic proteins. Each image shows puncta on one dendritic segment. (A) Glutamatergic synapses were stained with antibodies against NL3 (NL3in, green), the presynaptic vesicle marker VGlut1 (blue) and the postsynaptic scaffold PSD95 (red) or AMPA receptor subunit GluR2 (red). Scale bar, 2 µm. (B) GABAergic synapses were stained with antibodies against NL3 (NL3in, green), the presynaptic vesicle marker VGAT (blue) and the postsynaptic scaffold molecule gephyrin (red) or GABARβ subunits (red). Single-channel images are shown for the presynaptic vesicle markers. Arrows indicate examples of colocalization of NL3 with both pre- and postsynaptic markers. (C and D) To determine the relative distribution of NL3 at glutamatergic and GABAergic synapses, triple staining was performed with the NL3in antibody (green) and postsynaptic (C) or presynaptic (D) markers of glutamatergic (blue) and GABAergic (red) synapses. Postsynaptic scaffolds homer (blue) and gephyrin (red) and the presynaptic vesicle markers VGlut1 (blue) and VGAT (red) were used. Arrows indicate colocalization/apposition of NL3 with glutamatergic markers, and arrowheads indicate colocalization/apposition of NL3 with GABAergic markers. (E) The distribution of total NL3 puncta at glutamatergic or GABAergic synapses is quantified for both postsynaptic and presynaptic markers. Quantification was performed as described in Materials and methods (n = 10 neurons and >800 NL3 puncta per staining condition). (F) To estimate the relative contribution of NL3 at glutamatergic and GABAergic synapses, the percentage of total postsynaptic scaffold puncta that colocalize with NL3 and the percentage of total postsynaptic scaffolds that both colocalize with NL3 and are apposed to presynaptic terminals were quantified. Quantification shows data for n = 10 neurons and >800 NL3 puncta per staining condition. Multiple independent quantifications from different experiments yielded similar results.
To quantify the percentage of NL3 associated with glutamatergic and GABAergic sites, we triple stained cultured hippocampal neurons for NL3 and homer and gephyrin, postsynaptic scaffolding molecules at glutamatergic and GABAergic synapses, respectively (Fig. 4C). In parallel, triple staining was performed for NL3 and the presynaptic markers VGlut1 and VGAT (Fig. 4D). Of all NL3 puncta, approximately 54 ± 3% were associated with a synaptic marker. Of this synaptic pool, 70–80% colocalized with glutamatergic markers and 30% colocalized with GABAergic markers (Fig. 4E, n = 10 neurons, > 800 NL3 puncta). Whereas a greater percentage of total synaptic NL3 puncta are localized at glutamatergic synapses, in hippocampal cultures glutamatergic synapses are far more numerous than GABAergic synapses. Therefore, in order to obtain an estimate of the contribution of NL3 at each synapse type, we analysed the percentage of glutamatergic and GABAergic synapses that also contained detectable levels of NL3 (Fig. 4F). Of all PSD95 puncta, 48 ± 3% contained detectable levels of NL3, whereas 57 ± 5% of all gephyrin-positive puncta contained NL3. The majority of these postsynaptic sites were apposed to presynaptic terminals, as 71 ± 3% of PSD95 puncta colocalizing with NL3 were apposed to VGlut1 puncta and 90 ± 2% of gephyrin puncta containing NL3 were apposed to VGAT (Fig. 4F).
In summary, these data demonstrate that endogenous NL3 is a synaptic protein in cultured hippocampal neurons. In contrast to NL1 and NL2, which are largely restricted to glutamatergic and GABAergic synapses, respectively, NL3 is a common component of both synapse types.
Recruitment of NL3 to NRX-induced postsynaptic structures
Previous work has demonstrated that NRX expressed in non-neuronal cells can promote the assembly of postsynaptic structures through association with NLs (Graf et al., 2004). In this assay, NRX splice variants that differ in interactions with NLs exhibit preferential induction of glutamatergic and GABAergic sites (Boucard et al., 2005; Chih et al., 2006; Graf et al., 2006). For example, NRX1β4(+), which interacts with NL splice variants NL1A and NL2A but not with NL1B, induces the recruitment of GABAergic postsynaptic structures containing gephyrin and GABAA receptors but fewer glutamatergic structures containing PSD95 (Chih et al., 2006; Graf et al., 2006). Based on the ability of NL3 to bind both NRX1β4(–) and NRX1β4(+) (Boucard et al., 2005), we predicted that both isoforms would cluster NL3, and we investigated the coclustering of postsynaptic components along with NL3. We found that both NRX1β4(–)- and NRX1β4(+)-expressing cells promote clustering of endogenous NL3 and that both the glutamatergic scaffolding molecule PSD95 and the GABAergic scaffolding molecule gephyrin can be recruited to NL3 postsynaptic sites (Fig. 5). These data further support the notion that NL3 is not restricted to glutamatergic sites but can also be incorporated into GABAergic postsynaptic structures.
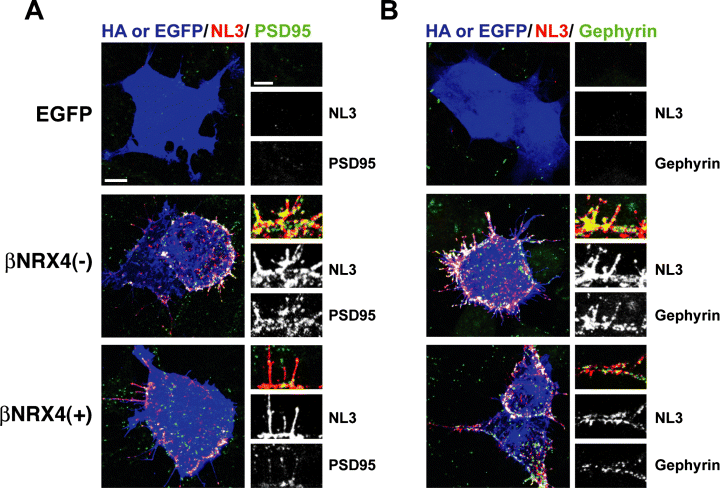
Neurexin promotes coaggregation of NL3 with glutamatergic and GABAergic postsynaptic scaffolds. COS cells expressing HA-NRX1β4(+), HA-NRX1β4(–) or EGFP were dropped onto hippocampal neurons at DIV 11 and analysed 2 days later. Triple labelling was performed for the NRX epitope tag HA or EGFP (blue), NL3in (red) and the postsynaptic scaffold molecules PSD95 (A) or gephyrin (B) (green). Both NRX1β isoforms strongly recruited endogenous NL3. Additionally, both glutamatergic (PSD95; A) and GABAergic (gephyrin; B) scaffolds were recruited in clusters overlapping NL3, as shown in the magnified insert panels. Note that due to the strong concentration of NL3 at contacts with the NRX-expressing cells (NL3 labelling intensity at Cos cell contacts is >3-fold higher than labelling at neuron–+neuron contacts) the endogenous NL3 puncta are not clearly visible in the adjacent neuronal processes. However, the overall distribution of NL3 in neighbouring processes was unchanged. Scale bar, 10 µm for large panel, 5 µm for inset.
Association of NL3 with NL1 and NL2
The association of multiple NL isoforms with glutamatergic and GABAergic synapses in hippocampal neurons raises the question of whether synapses might contain only one NL isoform or whether multiple different NL isoforms are coexpressed at single synapses. To test whether NL2 and NL3 colocalize at single GABAergic synapses, we performed triple immunostaining of hippocampal neurons using antibodies to NL2, NL3 and gephyrin. As shown previously (Varoqueaux et al., 2004), the majority of NL2-positive puncta contained the GABAergic scaffolding protein gephyrin. Of all gephyrin/NL2-positive sites, close to 60% also contained significant levels of NL3 (Fig. 6A), demonstrating the coexistence of multiple NL isoforms at individual GABAergic synapses. In light of this colocalization data, we performed coimmunoprecipitation experiments from brain lysates to test whether NL isoforms might associate together in complexes in vivo. NL2 antibodies coprecipitated NL3 but not NL1 (Fig. 6B). NL3-specific antibodies immunoprecipitated both NL1 and NL2, whereas NL1-specific antibodies coimmunoprecipitated NL3 but not NL2. Preimmune sera did not precipitate any of the NL isoforms, and none of the NL antibodies coimmunoprecipitated β-catenin (data not shown), suggesting that the interactions detected in these experiments represent specific associations of NL protein complexes in vivo.
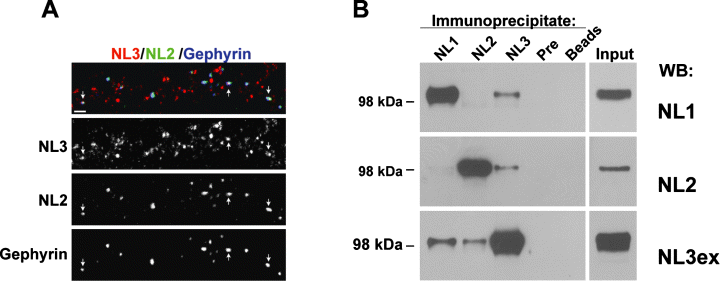
NL3 is colocalized with NL2 at single synapses in vitro and is associated in separate complexes with NL1 and NL2 in vivo. (A) DIV 14 hippocampal neurons were triple stained with antibodies to NL3 (rabbit anti-NL3in, red), NL2 (guinea pig anti-NL2, green) and gephyrin (blue). Arrows indicate puncta with colocalized NL3, NL2 and gephyrin. Scale bar, 2 µm. (B) Western blot analysis of NL isoform coimmunoprecipitations from mouse brain. Total brain lysates were subjected to immunoprecipitation with guinea pig sera specific for NL1, NL2 or NL3 (NL3in), preimmune guinea pig sera, or protein A sepharose beads alone. Immunoprecipitates were probed with rabbit NL isoform-specific antibodies (NL3ex was used for detection of NL3 in western blotting). Input lysate was 2% of the total lysate used for the precipitation.
Discussion
In this study we performed a detailed analysis of expression, localization and biochemical interactions of rodent NL3. Our data strongly suggest that endogenous NL3 is a neuronal postsynaptic adhesion molecule that represents a common component of glutamatergic and GABAergic synapses in hippocampal neurons.
Whereas NL1 and NL2 have been previously characterized in detail, the expression and localization of NL3 have been controversial. NL3 has been proposed to be expressed primarily in glia and to represent an orthologue of Drosophila gliotactin, a component of septate junctions (Gilbert et al., 2001). Our study supports a predominantly neuronal role for NL3 at synapses. At this time, we cannot exclude the possibility that a small pool of NL3 might be expressed in non-neuronal cells. However, by immunohistochemistry we did find that the majority of NL3 protein is present in neurons, where it is synaptically localized. This is further supported by the enrichment of NL3 in synaptosomal and postsynaptic density fractions. Although it has been proposed that epitope-tagged NL3 might be selectively associated with glutamatergic synapses (Graf et al., 2004), several lines of evidence support localization of endogenous NL3 at both glutamatergic and GABAergic synapses. We found that the majority of GABAergic synapses contain NL3 and demonstrated a biochemical association of NL3 with NL2, which is primarily found at GABAergic synapses. Moreover, NRX1β-mediated aggregation induced coclustering of postsynaptic NL3 along with the GABAergic scaffolding molecule gephyrin. Taken together, our findings strongly support an association of endogenous NL3 with GABAergic proteins and synapses in hippocampal neurons.
Our studies will require confirmation of this localization pattern of NL3 by in vivo analysis. Examination of NL3 localization in tissue sections was not possible in the present study, as suitable conditions for staining have not yet been established. However, the high neuronal expression of NL3 protein observed in vitro is consistent with the distribution of NL3 as detected by in situ hybridization studies on brain tissue (Varoqueaux et al., 2006; Lein et al., 2007). Additionally, future studies will be required to clarify the significance of the nonsynaptic NL3 observed here. As the epitope for the antibody (NL3in) is located in the cytoplasmic domain, it was not possible to test whether the extrasynaptic staining for NL3 was present at the cell surface or whether it represents an intracellular pool.
Currently, it is not known what determines the synapse specificity of NL3. Previous studies on NL1 and NL2 suggest two potential mechanisms mediating NL synaptic localization, both of which could also contribute to the localization of NL3. First, levels of the cytoplasmic scaffolding molecule PSD95 can modulate association of NLs with glutamatergic vs. GABAergic synapses (Prange et al., 2004; Levinson & El-Husseini, 2005; Levinson et al., 2005). Second, the synapse specificity of NLs can be regulated by the inclusion of alternatively spliced regions in the extracellular domain (Chih et al., 2006). NL3 undergoes alternative splicing at one site analogous to NL1 and NL2 (site A) (Ichtchenko et al., 1996), and at least three distinct splice variants of NL3 are expressed in hippocampal cultures (our unpublished observations). As the antibodies used in this study detect all variants of NL3, we were unable to distinguish whether distinct splice isoforms of NL3 preferentially localize at one particular synapse type. Further work will be required to investigate the potential role of alternative splicing in the regulation of NL3 localization and function. An alternative possibility is that NL3 might specifically associate with a component common to both types of synapse that directs the synaptic localization of NL3. Future studies examining the distinction among synapses containing or lacking NL3 should provide insights into possible candidates for such a role.
Another interesting observation in our studies is the association of NL3 with NL1 and NL2 in vitro and in vivo. Immunostaining revealed colocalization of NL2 and NL3 at GABAergic synapses, and NL3 was found to associate in separate complexes with NL1 and NL2 as detected by immunoprecipitation. NL3 antibodies coimmunoprecipitated both NL1 and NL2 from rodent brain, whereas NL2 antibodies precipitated only NL3 but not NL1 and vice versa. This may be significant, as NL1 has been demonstrated to form oligomers in vitro and oligomerization is required for the ability of NLs to induce clustering of synaptic vesicles in cultured neurons (Comoletti et al., 2003; Dean et al., 2003). Furthermore, recent structural models of NLs based on X-ray scattering techniques demonstrate preferential formation of dimers of NL1–4 and indicate that similar dimerization domains are present in all NL isoforms (Comoletti et al., 2007). Additionally, examination of an NL–NRX complex revealed the association of an NL dimer with two NRX monomers, suggesting that the functional three-dimensional orientation of NLs is in fact based on NL dimerization (Comoletti et al., 2007). Whether the NL1–NL3 and NL2–NL3 complexes identified in our study represent hetero-oligomers or larger protein assemblies remains to be shown. By either mechanism, the association of NL3 with NL2, which is primarily localized at GABAergic synapses, further supports our finding that NL3 is not restricted to glutamatergic synapses.
The presence of multiple NLs at the same synapse raises interesting questions regarding the contribution of each NL isoform. It remains unclear whether NL3 functions redundantly or in a distinct manner as compared to NL1 and NL2. All NL variants share the ability to interact with NRXs and to induce the assembly of presynaptic structures (Ichtchenko et al., 1996; Chih et al., 2004, 2005). However, differences in isoform identity and alternative splicing of NL1 and NL2 lead to selectivity in the binding to specific NRX isoforms and to differential effects on glutamatergic and GABAergic synapses (Boucard et al., 2005; Chih et al., 2006; Comoletti et al., 2006; Graf et al., 2006). Furthermore, isoform identity leads to selectivity in regulating functional postsynaptic electrophysiological properties (Chubykin et al., 2007). However, the mechanisms underlying this selectivity are not well understood.
With respect to the proposed role for functional defects of NLs in autism spectrum disorders, our finding that NL3 is associated with neuronal synapses further strengthens the hypothesis that such disorders result from aberrations in synaptic wiring and synaptic function (Zoghbi, 2003). Further characterization of NL3-specific function should contribute to a better understanding of cellular defects associated with these disorders.
Acknowledgements
We thank members of the Scheiffele Laboratory for discussions, and Shu-huei Lin and Rocio Perez for technical support. E.C.B. is a participant in the MSTP training program at Columbia University. This work was supported by grant NS045014, Autism Speaks, The Irma T. Hirschl Fund and the John Merck Fund to P.S. and a Gatsby Pilot grant from the Columbia Neuroscience Initiatives.
Abbreviations
-
- DIV
-
- days in vitro
-
- E
-
- embryonic day
-
- GABAR
-
- GABAA receptor
-
- GluR
-
- glutamate receptor
-
- GluR2
-
- glutamate receptor subunit 2
-
- GluR2/3
-
- glutamate receptor subunit 2/3
-
- HA
-
- haemagglutinin
-
- NL
-
- neuroligin
-
- NL3ex
-
- neuroligin 3 extracellular domain antibody
-
- NL3in
-
- neuroligin 3 intracellular domain antibody
-
- NR1
-
- N-methyl-d-aspartate receptor subunit 1
-
- NRX
-
- neurexin
-
- P
-
- postnatal day
-
- PBS
-
- phosphate-buffered saline
-
- sh
-
- small hairpin
-
- SPM
-
- synaptic plasma membrane fraction
-
- VGAT
-
- vesicular GABA transporter