Gaze pursuit, ‘attention pursuit’ and their effects on cortical activations
Abstract
A moving object draws our attention to it and we can track the object with smooth pursuit eye movements (SPEM). Gaze and attention are usually directed to the same object during SPEM. In this study we investigated whether gaze and attention can be divided during pursuit. We explored the cortical control of ocular tracking and attentive tracking and the role of focused and divided attention. We presented a sinusoidally moving target for pursuit and simultaneously a stationary target for fixation. Gaze could be directed to the pursuit target and attention to the fixation target or vice versa, or gaze and attention were directed to the same (moving or stationary) target. We found that gaze (overt) and attentive (covert) pursuit similarly activated the cortical oculomotor network. Gaze pursuit showed higher activations than attentive pursuit. Activations, specific to the dissociation of attention from gaze and independent of eye movements, were found solely in the posterior parietal cortex. A cue indicating a forthcoming attention task activated large parts of the cortical SPEM network, as a kind of preparatory mechanism. We did not find any attention-related regions outside the well-known visuo-oculomotor network. We conclude that attention control during gaze pursuit and gaze fixation occur within the cortical SPEM network, supporting the premotor theory of attention [Rizzolatti, G., Riggio, L., Dascola, I. & Umilta, C. (1987) Neuropsychologia, 25, 31–40].
Introduction
The fovea is the location on the retina with the highest visual acuity. Objects of interest are projected onto the fovea with the help of eye movements. Relevant moving objects like a prey, a potential predator or a fast-moving vehicle need to be scrutinized and this is facilitated by smooth pursuit eye movements (SPEM). Before execution of visual pursuit the SPEM system needs to first detect the moving object; visual attention should then be focused on it, its velocity needs to be processed and a motor signal to the extraocular muscles has to be prepared to allow pursuit. Although it has been argued (Kathmann et al., 1999) that SPEM were executed automatically and thus do not depend on attention, other authors have confirmed the importance of attention for accurate pursuit performance (Wyatt & Pola, 1987; Hutton & Tegally, 2005). Furthermore, it has been shown that, during SPEM, visual attention is located close to the focus of gaze (Van Donkelaar & Drew, 2002).
During steady gaze fixation it has been shown that the focus of attention can be separated from the focus of gaze (Posner, 1980). The effects of such visual attention shifts were investigated using positron-emission tomography (PET; Corbetta et al., 1993), PET and functional magnetic resonance imaging (fMRI; Coull & Nobre, 1998) and fMRI (O'Craven et al., 1997; Buchel et al., 1998; Corbetta et al., 1998, 2000; Culham et al., 1998, 2001; Gitelman et al., 1999; Wojciulik & Kanwisher, 1999; Hopfinger et al., 2000; Nobre et al., 2000; Perry & Zeki, 2000; Jovicich et al., 2001; Yantis et al., 2002; Astafiev et al., 2003). For investigation of attentional processes during fast, goal-directed eye movements to a peripheral target (saccades), several of these studies compared effects of attention shifts without saccades (so-called covert shifts of attention, with gaze fixation) to attention shifts in parallel with saccades (overt shifts of attention; equivalent to normal saccades; Astafiev et al., 2003; Corbetta et al., 1998; Gitelman et al., 2000; Nobre et al., 2000; Perry & Zeki, 2000; Beauchamp et al., 2001; Yantis et al., 2002). Bilateral activations during covert shifts of attention and during saccades were located in parts of the premotor cortex [frontal eye fields (FEF) and supplementary eye fields (SEF)], parts of the parietal cortex [involving intraparietal sulcus and/or superior parietal lobule (SPL); Beauchamp et al., 2001; Astafiev et al., 2003] and, in addition, in parts of the posterior temporal cortex (the motion-sensitive region MT+, corresponding to MT/MST in monkey; Corbetta et al., 1998; Nobre et al., 2000). Thus, as a common result, the cortical network for directing the focus of visual attention seemed to overlap widely with the network for saccadic eye movements. This finding supports the premotor theory of attention, which postulates a strict link between covert orienting of attention and programming explicit ocular movements: attention is orientated to a given point when the oculomotor program for moving the eyes to this point is ready to be executed (Rizzolatti et al., 1987). The above-mentioned findings led to the conclusion that covert attention shifts and saccades are subserved by similar neural mechanisms.
The human cortical pursuit network has been studied using fMRI (Berman et al., 1999; Kimmig et al., 1999; Petit & Haxby, 1999; Schmid et al., 2001; Rosano et al., 2002; Tanabe et al., 2002). However, to our knowledge there is only one fMRI study which investigated covert attentive tracking, but not during pursuit eye movements (Culham et al., 1998). An investigation of continuous, covert attentive tracking (‘attention pursuit’) in relation to SPEM (gaze pursuit) is lacking so far. Thus, the role of attention during overt and covert pursuit remains a matter of debate, although this issue is of major importance in everyday life: we frequently pursue something with our eyes but at the same time we must be able to attend to the motion of something else (e.g. pursuing a ball and attending to a neighbouring player; pursuing an approaching car and attending to an approaching vehicle from another direction).
We examined covert shifts of attention during ongoing SPEMs. We investigated cortical activations during (i) pursuit of a moving target and (ii) fixation of a stationary target. In either condition, attention and gaze directions could be focused on the same target location (termed the focused attention condition) or attention direction could be divided from the gaze direction by shifting attention to a second target (termed the divided attention condition). The second target could also be moving or stationary. Thus, we were able to investigate overt gaze pursuit, covert attention pursuit and the effects of focused and divided attention.
We asked how covert shifts of attention during SPEM influence the activation of the cortical pursuit network and the oculomotor performance. Which brain regions control covert attention pursuit? Does attention modulate the cortical pursuit network in general or does attention activate specific regions in addition to the SPEM system? Is the pursuit attention system similar to the saccadic attention system found in other studies?
Materials and methods
Visual stimulation
Visual stimulation was generated by a computer and back-projected via a video beamer (PLUS Vision, Tokyo, Japan; resolution 1024 × 768 pixels) onto a translucent screen at the back of the scanner gantry. The light of the beamer was reduced by polarizing filters and by darkening the translucent screen such that subjects in the scanner saw nothing except the two visual stimulation dots, thereby excluding unwanted motion stimuli on the retina resulting from eye movements. Subjects viewed the stimuli via a mirror mounted on the scanner headcoil.
The visual stimulus consisted of two red circular dots (0.5° of visual angle, 4° vertical distance). Visual stimulation was performed in a block design. Each trial consisted of a cue period (12.5 s), a stimulation period (25 s) and a rest period (10 s; Fig. 1).
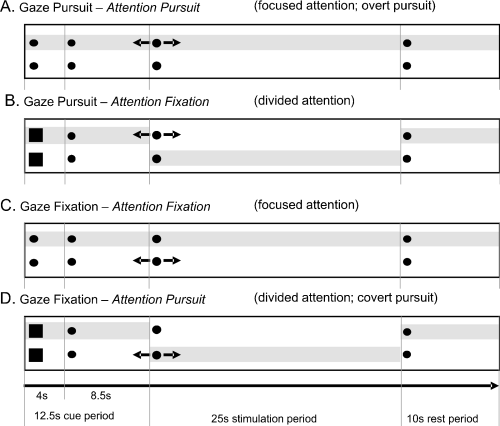
Schematic time course of experimental tasks A–D. Each trial started with a 12.5-s cue period, followed by a 25-s stimulation period and terminated with a 10-s rest period. Gaze always directed to the upper dot; likewise attention (attention locus indicated by shaded area, not shown in the experiment). If the squared cue appeared, attention had to be directed to the lower dot during the next stimulation sequence. Black arrows indicate sinusoidal motion in the horizontal plane during stimulation.
During the cue period, the two stationary circular dots changed to somewhat larger squares in 50% of the trials. This cue indicated that the subject should shift attention to the lower dot during the subsequent stimulation period. After 4 s the squares switched back to circular dots for a duration of 8.5 s, which served as washout period for any cue-related blood oxygen level-dependent (BOLD) changes, thereby avoiding contamination of cue-related BOLD activations with subsequent stimulation-related activations.
During stimulation, either the upper or the lower dot moved sinusoidally in the horizontal direction (amplitude ±5° at 0.16 Hz, peak velocity 5°/s). Four different stimulus conditions were tested: (A) gaze and attention directed to the moving upper dot, the lower dot being stationary (‘gaze pursuit, attention pursuit’; Fig. 1A); (B) gaze pursuit of the moving upper dot, but attention directed to the stationary lower dot (‘gaze pursuit, attention fixation’; Fig. 1B); (C) attention and gaze directions located on the stationary upper dot with the lower dot moving (‘gaze fixation, attention fixation’; Fig. 1C); and (D) gaze located on the upper dot, but attention directed to the moving lower dot (‘gaze fixation, attention pursuit’; Fig. 1D). The labelling A–D for the four stimulus conditions will be kept throughout the text and figures.
During the rest condition the two dots remained stationary.
Please note that in stimulus conditions (A) ‘gaze pursuit, attention pursuit’ and (C) ‘gaze fixation, attention fixation’ the directions of attention and gaze were focused on the upper dot (focused attention conditions), while in conditions (B) ‘gaze pursuit, attention fixation’ and (D) ‘gaze fixation, attention pursuit’ the directions of attention and gaze were divided (divided attention conditions; attention on lower dot, gaze on upper dot). Furthermore, condition (A) ‘gaze pursuit, attention pursuit’ denotes an overt pursuit task. The condition (D) ‘gaze fixation, attention pursuit’ refers to a covert pursuit task.
One stimulus condition was tested per trial. The four tasks (A–D) were repeated three times in a pseudo-random order, resulting in 12 trials per series. Each subject performed three series.
Two instructions were given to the subjects prior to measurements in the MR scanner: (i) ‘Always keep gaze directed on the upper dot during the whole experiment’ (as subjects had their heads immobilized in the scanner, gaze direction was identical with eye direction); (ii) ‘Keep visual attention directed on the upper dot unless the cue appears (the two dots change to squares). If the cue does appear, covertly shift attention to the lower dot during the subsequent stimulation period and then shift attention back to the upper dot’ (Fig. 1).
Note that we controlled for gaze direction but not for attention direction. In this experimental approach we investigated whether the task of shifting attention away from gaze was able to modulate the cortical BOLD response. If differences occur they can at least in part be related to the process of dividing attention and gaze directions. Methods to control the attention direction (e.g. by prompting subjects to indicate random dimmings or colour changes of the attention target) have the disadvantage that they can only yield discrete samples of attention direction. Intermittent shifts of attention remain undiscovered and uncontrolled. In addition, such methods may cause popout effects, visual distraction, etc., and thereby further complicate the paradigm.
To control for effects of the cue's change in form and size we applied an inverted task in a control experiment. In this inverted paradigm subjects always had to shift attention covertly to the lower dot during the stimulation period. If the cue appeared, attention and gaze directions remained on the upper dot during the stimulation period.
Subjects were trained to perform the four tasks before fMRI measurements to ensure that they correctly understood the tasks and the meaning of the cue. Training of the subjects, monitoring of eye movements during the scanning sessions, and interview after the scanning session ensured that subjects performed the task correctly.
fMRI
Measurements were performed on a 1.5T Avanto (Siemens, Erlangen, Germany) MR Scanner. Functional imaging was performed with T2*-weighted gradient-recalled EPI (echoplanar imaging) sequences (response time, 2.5 s; echo time, 50 ms; flip angle, 90°; field of view, 22 × 22cm2; matrix size, 64 × 64 × 28; voxel size, 3.44 × 3.44 × 5 mm3). Anatomical images of the head and brain were obtained using high-resolution T1-weighted 1-mm isovoxel MP-RAGE (magnetization-prepared rapid acquisition gradient echo) sequences (192 slices; response time, 2000 ms; echo time, 3.22 ms; field of view, 25.6 × 25.6 cm2; flip angle, 8°; voxel size, 1 × 1 × 1 mm3). Shimming was performed for the entire brain using an auto-shim routine for magnetic field homogeneity. The stimulation protocol for each stimulation sequence consisted of 12 25-s stimulation intervals each preceded by a 12.5-s cue period and succeeded by a 10-s period of rest. This protocol produced 228 EPI volumes per series. Each subject had to perform three series. Data acquisition was performed in 28 slices per volume containing the whole brain except the cerebellum. Thus, measurement time was little more than half an hour for each subject. Subjects had their heads immobilized in the MR headcoil. Effects of the gradient noises were reduced by sound-dampening headphones.
Eye tracking
Eye movement measurements were performed in parallel with the fMRI measurements using the Freiburg infrared MR Eye-tracker (methods in detail in Kimmig et al., 1999). A multichannel computer program (LabVIEW®; National Instruments, Austin, TX, USA) was used to acquire and display the signals derived from the MR Eye-tracker. The sampling frequency was 1000 Hz and the best spatial resolution was 0.2° of visual angle. The stimulus position was displayed and recorded in parallel with the eye movement data. The MR scanner provided a TTL pulse at the beginning of each volume acquisition. This pulse was used to trigger both our stimulation and the eye movement acquisition programs. Calibration of eye position was performed prior to and after each run. For calibration, subjects shifted their eyes repeatedly from the central fixation point towards targets at lateral locations of ±5°.
Subjects
The study was approved by the Ethics Committee of the University of Freiburg and was in accordance with the Declaration of Helsinki. Written informed consent was obtained from the 12 healthy right-handed subjects. Eight subjects participated in the experiment (three males, five females). Four subjects performed exclusively the control experiment with the inverted cue task (two males, two females). Subjects' ages ranged between 24 and 35 years and vision was normal or corrected to normal. Right-handedness was controlled using an adapted Edinburgh handedness inventory (Oldfield, 1971). Subjects were compensated for participation in the study.
Data analysis
fMRI data was analysed using the statistical parametric mapping (SPM) software SPM2 (http://www.fil.ion.ucl.ac.uk/spm/). Head motion was corrected via the realignment preprocessing tool of SPM2, and data were spatially normalized to the Montreal Neurological Institute (MNI) EPI template brain and interpolated to 2 × 2 × 2 mm voxel size. Data were then spatially smoothed with a 6-mm isotropic Gaussian kernel, full width at half maximum. All brain activation findings are reported in the MNI coordinate system. Statistical analysis was performed using the general linear model of SPM2; group analysis involved calculating a fixed-effects analysis, taking into account the number of subjects (n = 8). Main contrasts were calculated for each of the four stimulation conditions vs. the rest condition (e.g. ‘gaze pursuit, attention fixation’ minus ‘rest’) and for the cue condition vs. the rest condition. Differential contrasts (Table 1) were calculated for gaze pursuit with divided vs. focused attention, for gaze fixation with divided vs. focused attention, for the cue condition vs. the gaze fixation conditions (‘cue’ minus ‘gaze fixation, attention fixation’ and ‘cue’ minus ‘gaze fixation, attention pursuit’), attention pursuit vs. attention fixation and divided attention vs. focused attention tasks. All contrasts were family-wise error (FWE)-corrected. Activations with P ≤ 0.05 were considered significant. Activation locations were identified via the SPM toolbox WFU pickatlas (Lancaster et al., 2000; Tzourio-Mazoyer et al., 2002; Maldjian et al., 2003, 2004).
| Attention pursuit | Attention fixation | Differential contrast | |
|---|---|---|---|
| Gaze pursuit | Focused attention (A) | Divided attention (B) | B − A |
| Gaze fixation | Divided attention (D) | Focused attention (C) | D − C |
| Attention pursuit (A + D) | Attention fixation (B + C) | (A + D) − (B + C) | |
| Divided attention (B + D) | Focused attention (A + C) | (B + D) − (A + C) |
- A, gaze pursuit, attention pursuit; B, gaze pursuit, attention fixation; C, gaze fixation, attention fixation; D, gaze fixation, attention pursuit.
We used the statistical parametric mapping software SPM2. Today SPM is the commonly used standard for brain data analysis and allows for robust FWE-corrected results. Due to the limited number of subjects in this study, the analysis was confined to fixed-effects SPM. To ensure that the results were not just the effect of too many degrees of freedom included in an SPM fixed-effects analysis, we performed in addition a statistical nonparametric analysis (SnPM; uncorrected t-threshold of t = 3; Holmes et al., 1996). Nonparametric tests are generally more robust but less sensitive than parametric tests and yield more distributed clusters of activation. However, the two methods should reveal similar activations in the well-known regions of the smooth pursuit system.
For eye-movement data analysis we used a semiautomatic MatLab (The Math-Works Inc., Natick, MA, USA)-based analysis program, with which we calibrated the data, detected saccades, blinks and artifacts. Then we calculated SPEM gain in the pursuit conditions (gain defined as ratio of eye velocity to target velocity), the mean saccade amplitude and the saccadic frequency in all stimulation tasks.
Results
Eye-movement data
Original eye movement traces of the ‘gaze pursuit, attention pursuit’ task and the ‘gaze pursuit, attention fixation’ task are shown in Fig. 2A and B. The performance of gaze pursuit was very good despite the requirement for attention to be directed away from the gaze pursuit target (Fig. 2B). SPEM gain in both SPEM tasks was close to unity, indicating that subjects followed the moving dot accurately (Fig. 2C). There was no significant difference in SPEM gain in the ‘gaze pursuit, attention pursuit’ and the ‘gaze pursuit, attention fixation’ tasks (P > 0.9). Saccades occurred at slightly higher frequency during gaze pursuit sequences than during rest and gaze fixation sequences without, however, reaching statistical significance (Fig. 2D; P > 0.12). The saccade amplitude was significantly higher during pursuit (Fig. 2E; F2,7 = 19.25; P < 0.0001) than rest and gaze fixation. These saccades were, however, very small in all conditions (max. 1.2° of visual angle). According to our previous study one would expect no effect for saccade amplitudes between 2 and 10°, and possibly no effect for the small amplitudes of ∼ 1° (Kimmig et al., 2001).
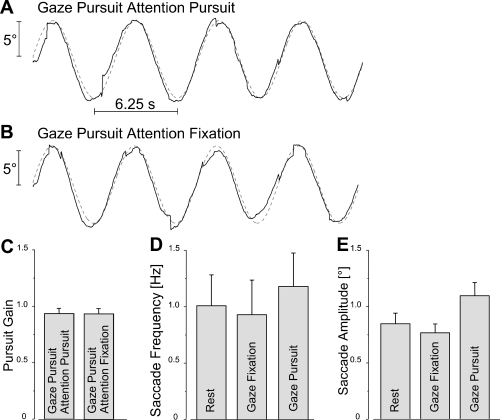
Eye movement data. (A) Original SPEM trace overlaid on sinusoidal stimulus signal (amplitude ±5°, frequency 0.16 Hz, peak velocity 5°/s). Gaze and attention direction focused on the stimulus. (B) SPEM following the same stimulus signal as in A, but attention directed to a stationary target. (C) Gaze pursuit gain of n = 8 subjects in the two pursuit tasks (A and B) close to unity, independent of attention direction. (D) Saccadic frequency in the rest, gaze-fixation and gaze-pursuit periods. Saccadic frequency during gaze pursuit was slightly, but not significantly, higher than during rest and gaze fixation. (E) Mean saccade amplitude in the rest, gaze-fixation and gaze-pursuit periods. Generally, amplitudes were very small (∼ 1.2° of visual angle). Error bars are SEM; n = 8.
fMRI data
Gaze pursuit with attention on the pursuit target (‘gaze pursuit, attention pursuit’ minus ‘rest’) activated the well-known pursuit network: cuneus, precuneus (PCU), MT+, posterior parietal cortex (PPC), posterior cingulate gyrus (pCG), SEF, FEF and putamen as part of the basal ganglia (Fig. 3A; for an overview of local activation maxima see Table 2). Covert shifts of attention (to the lower stationary dot) during SPEM (‘gaze pursuit, attention fixation’ minus ‘rest’) similarly activated cuneus, PCU, MT+, PPC, pCG, SEF, FEF and putamen (Fig. 3B; Table 2).
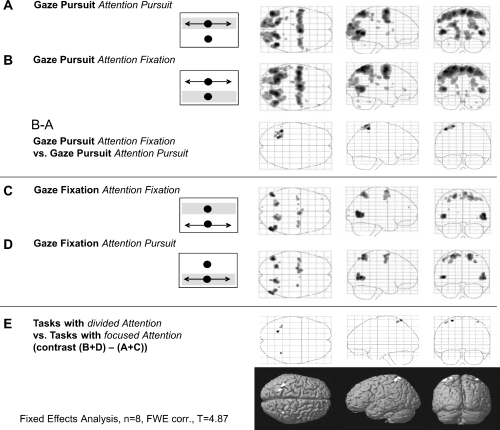
Functional data of eight subjects shown by glass brains in the horizontal, sagittal and coronal planes for (A) ‘gaze pursuit, attention pursuit’; (B) ‘gaze pursuit, attention fixation’ and the differential contrast B − A; (C) ‘gaze fixation, attention fixation’; (D) ‘gaze fixation, attention pursuit’; and (E) the differential contrast ‘divided attention’ minus ‘focused attention’ (B + D) − (A + C). Insets delineate the stimulation setup: black arrows indicate sinusoidal motion of the corresponding dot. Shaded bars indicate attention location (not shown in the experiment).
| Anatomical area | Gaze pursuit, attention pursuit | Gaze pursuit, attention fixation | Gaze fixation, attention fixation | Gaze fixation, attention pursuit | Cue | ||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| BA/f R | x | y | z | T | BA/f R | x | y | z | T | BA/f R | x | y | z | T | BA/f R | x | y | z | T | BA/f R | x | y | z | T | |
| Superior frontal gyrus | |||||||||||||||||||||||||
| R | 6/FEF | 20 | −8 | 70 | 6.1 | ||||||||||||||||||||
| L | 6/FEF | −20 | −6 | 62 | 5.42 | 6/FEF | −12 | 0 | 70 | 6.29 | 6/FEF | −20 | −2 | 72 | 7.05 | ||||||||||
| Middle frontal gyrus | |||||||||||||||||||||||||
| R | 6/FEF | 32 | −4 | 54 | 5.7 | ||||||||||||||||||||
| L | 6/FEF | −28 | −6 | 58 | 11.38 | 6/FEF | −18 | −8 | 66 | 6.77 | 6/FEF | −28 | −4 | 52 | 9.48 | ||||||||||
| Medial frontal gyrus | |||||||||||||||||||||||||
| R | 6/SEF | 4 | −4 | 68 | 10.85 | 6/SEF | 6 | −2 | 70 | 6.83 | |||||||||||||||
| L | 6/SEF | −2 | −4 | 64 | 12.56 | 6/SEF | −4 | 4 | 56 | 5.72 | 6/SEF | −6 | −6 | 68 | 6.15 | 6/SEF | −4 | 8 | 50 | 5.97 | |||||
| Inferior frontal gyrus | |||||||||||||||||||||||||
| R | 44 | 62 | 12 | 14 | 5.35 | 44 | 52 | 42 | 0 | 6.54 | |||||||||||||||
| Precentral gyrus | |||||||||||||||||||||||||
| R | 6/FEF | 32 | −6 | 56 | 8.22 | 6/FEF | 28 | −4 | 48 | 6.71 | |||||||||||||||
| L | 6/FEF | −42 | −10 | 54 | 8.92 | 6/FEF | −40 | −6 | 58 | 12.87 | 6/FEF | −34 | −6 | 50 | 5.91 | 6/FEF | −44 | −6 | 58 | 6.26 | 6/FEF | −44 | −4 | 46 | 5.84 |
| Postcentral gyrus | |||||||||||||||||||||||||
| L | 7/40 | −34 | −38 | 60 | 6.49 | ||||||||||||||||||||
| Superior parietal lob. | |||||||||||||||||||||||||
| R | 7 | 20 | −60 | 64 | 10.25 | 7 | 20 | −62 | 64 | 12.38 | 7 | 18 | −60 | 60 | 8.77 | 7 | 18 | −62 | 58 | 7.2 | 7 | 28 | −62 | 62 | 7.67 |
| L | 7 | −20 | −60 | 62 | 10.25 | 7 | −20 | −56 | 70 | 12.84 | 7 | −22 | −56 | 60 | 7.21 | 7 | −24 | −58 | 66 | 7.2 | 7 | −18 | −66 | 58 | 7.28 |
| Inferior parietal lob. | |||||||||||||||||||||||||
| R | 36 | −50 | 52 | 6.45 | |||||||||||||||||||||
| Supramarginal gyrus | |||||||||||||||||||||||||
| L | −54 | −26 | 20 | 5.83 | −48 | −40 | 30 | 7.1 | |||||||||||||||||
| Precuneus | |||||||||||||||||||||||||
| R | 10 | −56 | 64 | 5.6 | |||||||||||||||||||||
| L | −12 | −72 | 58 | 7.83 | −16 | −62 | 66 | 11.51 | −12 | −72 | 60 | 8.35 | |||||||||||||
| Cuneus | |||||||||||||||||||||||||
| R | 17/V1 | 16 | −96 | 0 | 6.93 | 19 | 26 | −76 | 32 | 7.18 | |||||||||||||||
| L | 17/18 | −20 | −92 | 28 | 9.34 | 17/18 | −14 | −92 | 16 | 5.9 | |||||||||||||||
| Lingual gyrus | |||||||||||||||||||||||||
| R | 18 | 10 | −70 | 0 | 7.27 | 18 | 10 | −70 | 0 | 5.67 | |||||||||||||||
| L | 18 | −10 | −72 | −6 | 8.51 | 18 | −10 | −70 | −6 | 6.32 | |||||||||||||||
| Fusiform gyrus | |||||||||||||||||||||||||
| L | 18 | −28 | −74 | −10 | 5.32 | ||||||||||||||||||||
| Superior occipital gyrus | |||||||||||||||||||||||||
| R | 18 | 26 | −92 | 16 | 6.47 | ||||||||||||||||||||
| Middle occipital gyrus | |||||||||||||||||||||||||
| R | 19 | 34 | −84 | 20 | 8.96 | 19 | 34 | −84 | 18 | 7.4 | 19 | 42 | −84 | 6 | 5.46 | ||||||||||
| L | 39/MT+ | −46 | −74 | 8 | 10.34 | 37/MT+ | −46 | −66 | 4 | 8.05 | 39/MT+ | −40 | –74 | 6 | 5.32 | ||||||||||
| Inferior occipital gyrus | |||||||||||||||||||||||||
| R | 18 | 46 | −78 | −6 | 6.58 | ||||||||||||||||||||
| Middle temporal gyrus | |||||||||||||||||||||||||
| R | 39/MT+ | 50 | −74 | 10 | 6.77 | 37/MT+ | 46 | −62 | 0 | 11.2 | 39/MT+ | 52 | −72 | 10 | 8.47 | 19/MT+ | 52 | −76 | 2 | 7.52 | 37/MT+ | 50 | −68 | 2 | 6.42 |
| L | 39/MT+ | −48 | −72 | 8 | 10.84 | 37/MT+ | −44 | −64 | 6 | 11.42 | 39/MT+ | −46 | −68 | 6 | 7.98 | ||||||||||
| Putamen | |||||||||||||||||||||||||
| L | 26 | −2 | 2 | 5.68 | 26 | −2 | 6 | 6.25 | |||||||||||||||||
| Cingulate gyrus | |||||||||||||||||||||||||
| L | −14 | −22 | 38 | 5.43 | −14 | −20 | 38 | 6.85 | |||||||||||||||||
- Coordinates show the local maximum of an activated voxel cluster in MNI space; fR = functional region; T = T-value at voxel level.
To detect activation specifically related to the shift of ‘attention direction’ away from ‘gaze direction’ (i.e. the effect of divided attention) during gaze pursuit we calculated the difference contrast of ‘gaze pursuit, attention fixation’ minus ‘gaze pursuit, attention pursuit’ (Fig. 3, the B − A contrast; Table 2), which revealed significant differential activations in the SPL and inferior parietal lobule (IPL) and in the postcentral gyrus, predominantly in the left hemisphere.
Gaze fixation during motion of a peripheral dot with attention directed to the stationary fixation dot (‘gaze fixation, attention fixation’ minus ‘rest’; Fig. 3C, Table 2) also activated parts of the SPEM network, namely MT+, PPC, FEF and SEF. However, these activations were lower and more circumscribed than those during pursuit. Significant activations were not observed in primary visual areas, PCU, pCG and the putamen. A very similar activation pattern (MT+, PPC, FEF and SEF) was found during gaze fixation with attention directed to the moving lower dot (‘gaze fixation, attention pursuit’ minus ‘rest’; Fig. 3D, Table 2).
To investigate the effect of attention shifts away from gaze (i.e. the effect of divided attention) during gaze fixation we calculated the contrast of ‘gaze fixation, attention pursuit’ minus ‘gaze fixation, attention fixation’ (comparing Fig. 3D and C). We did not observe any significant differential activations for the contrast D−C (not shown). Similarly, the differential contrast of both tasks with attention pursuit vs. both tasks with attention fixation [Fig. 3, the (A + D) − (B + C) contrast] did not show significant activations.
Finally, the differential activation contrast of tasks with divided attention, ‘gaze pursuit, attention fixation’ and ‘gaze fixation, attention pursuit’ (Fig. 3, B + D) vs. tasks with focused attention, ‘gaze pursuit, attention pursuit’ and ‘gaze fixation, attention fixation’ (Fig. 3, A + C) showed activation in a part of the PPC in both hemispheres [Fig. 3E, Table 3, differential contrast (B + D) − (A + C)]. Please note that this contrast was independent of eye movements and therefore represents attention-specific activation in terms of divided attention.
| Anatomical area | Gaze pursuit, attention fixation vs. gaze pursuit, attention pursuit(Fig. 3, contrast B − A) | Divided attention vs. focused attention [Fig. 3, contrast (B + D) − (A − C)] | Cue vs. Gaze fixation, attention pursuit | Cue vs. Gaze fixation, attention fixation | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| x | y | z | T | x | y | z | T | x | y | z | T | x | y | z | T | |
| Superior frontal gyrus | ||||||||||||||||
| R | 28 | 62 | 6 | 7.42 | ||||||||||||
| L | −28 | 64 | 6 | 6.5 | ||||||||||||
| Middle frontal gyrus | ||||||||||||||||
| R | 34 | 32 | 38 | 5.66 | ||||||||||||
| L | −28 | 6 | 60 | 5.79 | ||||||||||||
| Medial frontal gyrus | ||||||||||||||||
| R | 4 | 20 | 50 | 5.8 | ||||||||||||
| Inferior frontal gyrus | ||||||||||||||||
| R | 56 | 16 | 32 | 7.94 | ||||||||||||
| Superior parietal lobule | ||||||||||||||||
| R | 26 | −50 | 74 | 4.37 | 36 | −64 | 54 | 9.97 | 34 | −68 | 50 | 6.33 | ||||
| L | −24 | −56 | 70 | 6.38 | −24 | −58 | 70 | 5.82 | −36 | −56 | 44 | 8.29 | ||||
| Inferior parietal lobule | ||||||||||||||||
| R | 34 | −58 | 46 | 5.3 | ||||||||||||
| L | −42 | −48 | 62 | 6.67 | −30 | −58 | 40 | 5.26 | ||||||||
| Postcentral gyrus | ||||||||||||||||
| R | 36 | −48 | 64 | 4.83 | 40 | −48 | 64 | 5.15 | ||||||||
| Precuneus | ||||||||||||||||
| R | 4 | −68 | 56 | 9.02 | ||||||||||||
| Cuneus | ||||||||||||||||
| R | 6 | −98 | 12 | 8.17 | ||||||||||||
| L | −24 | −96 | −6 | 6.88 | ||||||||||||
| Fusiform gyrus | ||||||||||||||||
| L | −28 | −82 | −18 | 7.38 | ||||||||||||
| Middle occipital gyrus | ||||||||||||||||
| L | −24 | −94 | 14 | 7.38 | ||||||||||||
| Inferior occipital gyrus | ||||||||||||||||
| R | 40 | −86 | −6 | 8.77 | ||||||||||||
- Coordinates represent the local maximum of an activated voxel cluster in MNI space; T = T-value at voxel level.
We did not find attention-related regions outside the well-known visuo-culomotor network.
The task of dividing attention and gaze directions during the next stimulation period (indicated by the cue) led to activation of PCU, MT+, PPC, FEF and SEF in the cue period (Fig. 4A, Table 2).
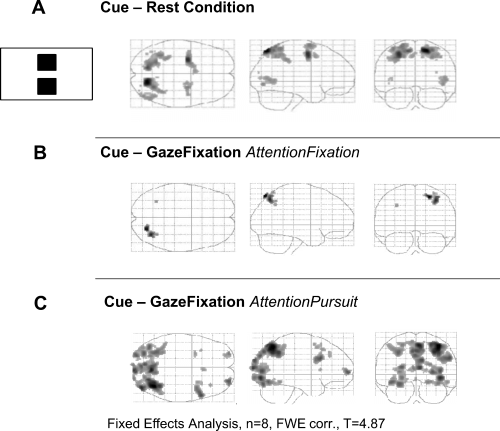
Functional data of eight subjects shown by glass brains in the horizontal, sagittal and coronal planes. (A) Effect of the task to divide attention and gaze directions in the subsequent stimulation period, ‘cue’ minus ‘rest’ condition. (B) Differential contrast ‘cue’ minus ‘gaze fixation, attention fixation’. (C) Differential contrast ‘cue’ minus ‘gaze fixation, attention pursuit’.
The cue consisted in a change of the two dots from circles to somewhat larger squares. In the control experiment the squares indicated to the subject not to shift attention, which did not lead to any specific activation in the ‘cue’ minus ‘rest’ contrast. Thus, the cue's change in form and size per se could not explain the activations related to upcoming attention shifts.
The differential contrast ‘cue’ minus ‘gaze fixation, attention fixation’ showed activation in a more lateral part of PPC (Fig. 4B; Table 3). The differential contrast ‘cue’ minus ‘gaze fixation, attention pursuit’ yielded similar activations in PPC, but additional activations in PCU, striate cortex, FEF, SEF and the superior frontal gyrus (Brodmann area 10; Fig. 4C, Table 3).
As expected, the SnPM analysis revealed more scattered clusters of activation. The analysis showed activations in the well-known regions of the SPEM network but also scattered activations in unhypothesized brain regions. An overview of the activated clusters resulting from the SnPM analysis is given in Tables 4 and 5. In the regions of interest for SPEM the results corresponded with those of the SPM analysis, with respect to the main contrasts as well as to the differential contrasts. Therefore, we will focus the discussion on these regions. Scattered activations found solely by the SnPM analysis in unhypothesized regions may be found in the table, but will not be discussed further given uncertainties about their neurobiological relevance.
| Anatomical area | Gaze pursuit, attention pursuit | Gaze pursuit, attention fixation | Gaze fixation, attention fixation | Gaze fixation, attention pursuit | Cue | ||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| BA/fR | x | y | z | pT | BA/fR | x | y | z | pT | BA/fR | x | y | z | pT | BA/fR | x | y | z | pT | BA/fR | x | y | z | pT | |
| Superior frontal gyrus | |||||||||||||||||||||||||
| L | 8 | −40 | 18 | 54 | 4.10 | ||||||||||||||||||||
| L | −30 | −2 | 68 | 3.70 | |||||||||||||||||||||
| L | 6/SEF | −6 | 6 | 56 | 3.49 | ||||||||||||||||||||
| Middle frontal gyrus | |||||||||||||||||||||||||
| R | 6/FEF | 32 | −6 | 52 | 4.72 | 6/FEF | 36 | −8 | 48 | 5.08 | 6 | 32 | −8 | 48 | 3.55 | 6 | 30 | −6 | 50 | 5.78 | |||||
| R | 6/FEF | 42 | −6 | 54 | 4.72 | ||||||||||||||||||||
| Middle frontal gyrus | |||||||||||||||||||||||||
| L | 6/FEF | −28 | −8 | 52 | 4.88 | 6/FEF | −28 | −8 | 52 | 5.37 | 10 | −36 | 52 | 26 | 3.70 | 6 | −40 | −2 | 60 | 3.35 | 6 | −28 | −6 | 54 | 6.13 |
| L | 6 | −34 | −6 | 48 | 3.41 | 6/FEF | −26 | −6 | 48 | 3.04 | 6 | −44 | 0 | 38 | 4.25 | ||||||||||
| Medial frontal gyrus | |||||||||||||||||||||||||
| R | 6/SEF | 2 | −4 | 62 | 4.95 | ||||||||||||||||||||
| L | 6/SEF | −10 | −10 | 62 | 4.27 | 6/SEF | −2 | −2 | 62 | 5.58 | |||||||||||||||
| Inferior frontal gyrus | |||||||||||||||||||||||||
| L | 44 | −54 | 12 | 18 | 4.77 | 44 | −52 | 8 | 16 | 3.99 | |||||||||||||||
| Precentral gyrus | |||||||||||||||||||||||||
| R | 6 | 40 | −10 | 48 | 4.66 | ||||||||||||||||||||
| R | 42 | −6 | 58 | 3.94 | |||||||||||||||||||||
| Precentral gyrus | |||||||||||||||||||||||||
| L | 6 | −44 | −6 | 56 | 4.84 | 6 | −50 | −6 | 54 | 3.95 | 6 | −36 | −4 | 46 | 4.60 | ||||||||||
| L | 36 | 0 | −50 | 3.97 | |||||||||||||||||||||
| Postcentral lobe | |||||||||||||||||||||||||
| L | −30 | −40 | 44 | 4.02 | |||||||||||||||||||||
| Superior parietal lobule | |||||||||||||||||||||||||
| R | 18 | −64 | 66 | 4.15 | 7 | 18 | −60 | 68 | 3.49 | 7 | 22 | −72 | 58 | 5.53 | |||||||||||
| L | −22 | −58 | 56 | 5.23 | 7 | −22 | −56 | 58 | 4.93 | 7 | −26 | −58 | 60 | 3.46 | 7 | −12 | −72 | 58 | 5.18 | ||||||
| L | 7 | −12 | −72 | 58 | 4.15 | ||||||||||||||||||||
| Inferior parietal lobule | |||||||||||||||||||||||||
| L | −30 | −38 | 42 | 5.08 | −30 | −44 | 46 | 3.43 | 40 | −34 | −50 | 50 | 5.46 | ||||||||||||
| L | −30 | −48 | 52 | 4.44 | |||||||||||||||||||||
| Precuneus | |||||||||||||||||||||||||
| R | 24 | −56 | 54 | 4.39 | 7 | 22 | −56 | 54 | 5.54 | 22 | −52 | 54 | 4.63 | 7 | 20 | −54 | 54 | 3.14 | |||||||
| R | 7 | 14 | −48 | 52 | 4.50 | ||||||||||||||||||||
| L | −24 | −78 | 26 | 3.91 | 7 | −20 | −56 | 54 | 3.89 | ||||||||||||||||
| L | −16 | −70 | 38 | 3.19 | |||||||||||||||||||||
| Cuneus | |||||||||||||||||||||||||
| R | 19 | 24 | −78 | 30 | 3.63 | ||||||||||||||||||||
| Lingual gyrus | |||||||||||||||||||||||||
| L | 18 | −10 | −70 | −6 | 4.43 | ||||||||||||||||||||
| Middle temporal gyrus | |||||||||||||||||||||||||
| R | 37/MT+ | 44 | −62 | 4 | 4.65 | 39/MT+ | 42 | −58 | 6 | 4.98 | 44 | −60 | 4 | 4.17 | 46 | −60 | 2 | 3.40 | 44 | −60 | 2 | 4.78 | |||
| L | 39/MT+ | −42 | −60 | 6 | 5.40 | 37/MT+ | −44 | −66 | 6 | 3.86 | 37/MT+ | −44 | −66 | 6 | 3.47 | 37/MT+ | −42 | −66 | 4 | 4.00 | |||||
| L | −46 | −60 | −4 | 4.00 | |||||||||||||||||||||
| Middle occipital gyrus | |||||||||||||||||||||||||
| R | 19 | 34 | −84 | 18 | 5.02 | 19/MT+ | 48 | −78 | −8 | 5.41 | 19/MT+ | 48 | −72 | 6 | 3.81 | ||||||||||
| R | 32 | −74 | 26 | 3.52 | 36 | −86 | 18 | 4.00 | |||||||||||||||||
| L | 37/MT+ | −42 | −72 | 6 | 4.10 | −38 | −74 | 6 | 5.11 | ||||||||||||||||
| Inferior occipital gyrus | |||||||||||||||||||||||||
| R | 46 | −80 | −6 | 4.83 | |||||||||||||||||||||
| Inferior temporal gyrus | |||||||||||||||||||||||||
| R | 37/MT+ | 54 | −72 | −2 | 5.18 | ||||||||||||||||||||
| Putamen | |||||||||||||||||||||||||
| R | 26 | 0 | 2 | 4.83 | 26 | 0 | 6 | 5.90 | |||||||||||||||||
| Lateral globus pallidus | |||||||||||||||||||||||||
| L | 24 | −12 | −4 | 3.91 | |||||||||||||||||||||
| Cingulate gyrus | |||||||||||||||||||||||||
| L | −14 | −20 | 38 | 4.83 | |||||||||||||||||||||
| R | 22 | 6 | 38 | 3.87 | |||||||||||||||||||||
- Coordinates show the local maximum of an activated voxel cluster in MNI space; fR = functional region; pT = pseudo T-value at voxel level.
| Anatomical area | Gaze pursuit, attention fixation vs. gaze pursuit, attention pursuit | Divided attention vs. focused attention | Cue vs. Gaze fixation, attention pursuit | Cue vs. Gaze fixation, attention fixation | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| BA | x | y | z | pT | BA | x | y | z | pT | BA | x | y | z | pT | BA | x | y | z | pT | |
| Middle frontal gyrus | ||||||||||||||||||||
| R | 9 | 50 | 14 | 32 | 4.55 | |||||||||||||||
| Medial frontal gyrus | ||||||||||||||||||||
| L | −8 | 52 | −8 | 3.31 | ||||||||||||||||
| Inferior frontal gyrus | ||||||||||||||||||||
| R | 32 | 8 | 30 | 4.05 | 46 | 52 | 28 | 20 | 3.24 | 38 | 8 | 30 | 4.05 | |||||||
| Precentral gyrus | ||||||||||||||||||||
| L | −20 | −14 | 56 | 4.25 | ||||||||||||||||
| Postcentral gyrus | ||||||||||||||||||||
| R | 5 | 30 | −48 | 72 | 4.39 | |||||||||||||||
| Superior parietal lobule | ||||||||||||||||||||
| R | 5 | 38 | −48 | 66 | 4.38 | 5 | 38 | −50 | 66 | 4.00 | 7 | 30 | −68 | 60 | 4.48 | 7 | 38 | −64 | 56 | 7.03 |
| R | 7 | 32 | −66 | 46 | 3.45 | 30 | −66 | 44 | 4.61 | |||||||||||
| L | 7 | −26 | −56 | 70 | 4.37 | −40 | −50 | 62 | 3.59 | −22 | −72 | 58 | 4.89 | |||||||
| L | −26 | −44 | 74 | 4.09 | ||||||||||||||||
| Inferior parietal lobule | ||||||||||||||||||||
| R | 34 | −46 | 58 | 3.24 | 46 | −58 | 56 | 3.22 | 36 | −54 | 42 | 5.05 | ||||||||
| R | 40 | 48 | −40 | 60 | 3.31 | |||||||||||||||
| Supramarginal gyrus | ||||||||||||||||||||
| R | 40 | 62 | −48 | 36 | 3.26 | |||||||||||||||
| Precuneus | ||||||||||||||||||||
| R | 10 | −76 | 56 | 4.47 | ||||||||||||||||
| L | 7 | −18 | −44 | 78 | 3.61 | −6 | −66 | 66 | 3.38 | |||||||||||
| Cuneus | ||||||||||||||||||||
| R | 19 | 6 | −92 | 32 | 3.56 | 19 | 6 | −94 | 30 | 4.57 | ||||||||||
| L | −30 | −90 | 25 | 4.57 | ||||||||||||||||
| Lingual gyrus | ||||||||||||||||||||
| R | 26 | −94 | −16 | 4.55 | ||||||||||||||||
| Fusiform gyrus | ||||||||||||||||||||
| L | −28 | −80 | −18 | 4.22 | 19 | −44 | −70 | −18 | 4.83 | |||||||||||
| Middle occipital gyrus | ||||||||||||||||||||
| R | 28 | −62 | 2 | 3.61 | 19 | 40 | −82 | 6 | 4.56 | |||||||||||
| L | −24 | −92 | 14 | 4.57 | ||||||||||||||||
| Inferior occipital lobule | ||||||||||||||||||||
| L | −40 | −58 | −10 | 4.25 | −40 | −62 | −8 | 4.02 | ||||||||||||
| Cingulate gyrus | ||||||||||||||||||||
| R | 24 | −14 | −6 | 50 | 3.55 | 24 | 8 | 32 | 14 | 3.70 | 10 | −8 | 30 | 3.41 | ||||||
| Insula | ||||||||||||||||||||
| L | −36 | 8 | 6 | 3.76 | −34 | 14 | 10 | 3.63 | −32 | 16 | 10 | 4.45 | ||||||||
| Thalamus | ||||||||||||||||||||
| L | −2 | −12 | 12 | 5.35 | ||||||||||||||||
- Coordinates represent the local maximum of an activated voxel cluster in MNI space; pT = pseudoT-value at voxel level.
Discussion
This is the first fMRI study to investigate the effects of dissociating visual attention and gaze directions during SPEM, simulating natural behaviour of attention shifts to objects in motion. Our intention was to describe those parts of the brain that control visual attention during SPEM.
Gaze pursuit with focused vs. divided attention
Our data showed that gaze pursuit with the task to divide attention from gaze direction was as good as gaze pursuit with the task to focus attention on the gaze direction. Two explanations can be given for this oculomotor result: (i) visual attention can indeed be divided from gaze during SPEM without significant changes in gaze pursuit performance; or (ii) subjects did not execute the task correctly, such that attention and gaze directions remained focused in both the ‘gaze pursuit, attention pursuit’ and the ‘gaze pursuit, attention fixation’ tasks.
Irrespective of the tasks to divide or to focus attention and gaze directions, our fMRI data revealed that SPEM always activated a similar cerebral network. However, the gaze pursuit task with divided attention led to a general tendency for more cortical activation in the whole SPEM system and significantly higher activations in the left PPC (SPL, IPL and postcentral gyrus; contrast Fig. 3, the B − A contrast). Thus, additional processing appeared to be required for the task to shift attention to a stationary target while maintaining gaze pursuit. Our data indicate that subjects tried to divide attention and gaze directions during gaze pursuit. This attempt did not influence gaze pursuit performance. The additional cortical processing might be due to the effort to keep gaze and attention directions divided, to switching the attention focus repeatedly between the two targets, or to the suppression of saccades to the stationary target.
Previous studies investigating focused attention vs. distributed or divided attention during fixation tasks also describe an increased parietal activity for divided attention (Jovicich et al., 2001; Nebel et al., 2005; Sturm et al., 2006). The study of Jovicich et al. (2001) even demonstrated a correlation between the number of attention targets and the amount of PPC activation. Yantis et al. (2002) concluded from their data that activation in PPC was transient in nature and correlated with the shift of attention, not with maintaining attention at a defined location. Hemispheric differences should not be overestimated in our data set. Shulman et al. (2002) reported stronger activations in the left PPC during preparation (cue period) and execution (test period) of attention tasks of different dimensions or motion direction. Furthermore, attentional switching between global and local aspects of visual stimuli led to activation of the left medial parietal cortex (Fink et al., 1997). Gitelman et al. (1999), on the other hand, reported more activation in the right parietal cortex during attention shifting to the right and left visual hemifield.
Gaze fixation with focused vs. divided attention
Furthermore, we found that the task of attending to a moving target while fixating a stationary target activated the same cortical network as did pursuit eye movements (FEF, SEF, PPC, MT+; Fig. 3D). Essentially the same result was obtained when both attention and gaze directions had to be kept on the stationary target (Fig. 3C) while a second target was moving. One interpretation of the latter result is that our subjects were in fact not able to keep attention on the ‘boring’ stationary target, but inevitably shifted attention to the moving target. A more complex explanation would be that some of the regions are active independent of the attentional state or that different functions are processed within the same region (e.g. fixation or suppression of eye movements vs. execution of eye movements within FEF).
Attention pursuit vs. attention fixation
Both the attention pursuit tasks (Fig. 3A and D) as well as the attention fixation tasks (Fig. 3B and C) consisted of a gaze pursuit and a gaze fixation task. The differential contrast (Fig. 3E) was therefore independent of eye movements. Furthermore, both gaze pursuit conditions (Fig. 3A and B) led to strong activations, compared to which the activations in the gaze fixation tasks (Fig. 3C and D) were rather small. This might partially explain why the differential contrast of attention pursuit tasks vs. attention fixation tasks [Fig. 3E, (A + D) − (B + C)] revealed no significant activations. We further concluded that neither attention pursuit nor attention fixation activated cortical regions outside the SPEM system, but rather attention pursuit and attention fixation seemed to be processed by similar cortical areas within the SPEM system. Similar mechanisms seem also to subserve the saccadic system, as previously shown.
Culham et al. (1998) showed that, during fixation of a stationary target, attentive tracking and attentive ‘saccadic’ shifts activated the same cortical regions, IPS, postcentral sulcus, SPL, cuneus, FEF and precentral sulcus. However, they did not investigate attention directly during SPEM. While our task enabled pursuit-like attentive tracking during SPEM, our activations were also similar to those described by Culham et al. (1998). This indicates that attentive processes related to saccade-like shifts and to pursuit-like tracking operate in the same cortical structures.
Divided vs. focused attention (independent of eye movements)
Interestingly, when calculating the contrast specifically related to attention shifts (independent of gaze pursuit or gaze fixation) we obtained activation exclusively in the PPC. Such a contrast was not calculated in previous studies which mostly compared covert shifts of attention and saccades or attentive vs. passive viewing, thereby modulating the cortical visuomotor system as a whole. Furthermore, effects evoked by eye movement suppression could not be excluded by such previous designs (Culham et al., 1998; Perry & Zeki, 2000; Beauchamp et al., 2001; Yantis et al., 2002). Our result is furthermore in accordance with the study of Wojciulik & Kanwisher (1999), which stated that posterior parietal areas around the IPS play a general role in visual selective attention independent of the kind of the attentional task.
Note that we did not find attention-related regions outside the well-known visuo-culomotor network.
Overt and covert pursuit
The ‘gaze fixation, attention pursuit’ task (covert pursuit) activated similar cortex regions as did the ‘gaze pursuit, attention pursuit’ task (overt pursuit). This result indicates that overt pursuit and covert pursuit are processed by similar neural mechanisms. These mechanisms seem also to subserve the saccadic system (Corbetta et al., 1998; Beauchamp et al., 2001). Furthermore, the activations of the overt pursuit task were stronger than those of the covert pursuit task, a further analogy to overt and covert saccadic shifts (Beauchamp et al., 2001). Taken together, our data indicate that the premotor theory of attention (Rizzolatti et al., 1987) may also be applicable to the pursuit system.
Cortical processing in the cue condition
Furthermore, we found that most parts of the network active during SPEM could also be activated in the cue period (i.e. during fixation of a stationary target) by the simple task to divide attention and gaze directions during the subsequent stimulation period. This task led to bilateral activations of FEF, SPL, IPL, PCU and MT+. It remains undetermined whether these activations were due to attentional load or the preparation of the attention shift, to the suppression of eye movements or to untimely execution of the attention shift during the cue period. Among preparatory processes, visuospatial short-term memory might play a role (i.e. subjects had to keep in mind the task of dividing attention and gaze directions during the following stimulation period). It has been reported that posterior parietal and prefrontal regions are involved in the retention of visuospatial information (Munk et al., 2002; Todd & Marois, 2005). Furthermore, similar activations as in our experiment were found during attention shifts, preparation of saccades and preparation of pointing hand movements following a cue indicating target location (Hopfinger et al., 2000; Astafiev et al., 2003). Chawla et al. (1999) reported that baseline activity in the motion-sensitive area MT+ was enhanced by selective attention to the attribute ‘motion’ (even without a moving stimulus). These authors favoured the hypothesis that attention modulates sensitivity of neuronal populations to inputs by changing background activity.
Finally, the activations of the cue condition are different from those evoked by the gaze fixation conditions. The ‘cue’ minus ‘gaze fixation, attention fixation’ contrast showed a higher activation in the PPC, predominantly of the right side. A similar result was obtained in the ‘cue’ minus ‘gaze fixation, attention pursuit’ contrast, which in addition yielded activations in other regions of the SPEM network (such as precuneus, primary visual areas and FEF). It seems that preparing to divide attention from gaze directions takes higher efforts than actually executing the task. In the latter differential contrast, area BA 10 in the prefrontal cortex appeared active on both sides. Previously it has been shown that the frontal polar cortex of BA 10 participates in sustained control processes and plays a role in forming and maintaining an attentional set or task mode (Koechlin et al., 1999; Sakai & Passingham, 2003; Velanova et al., 2003). This seems to be important in the cue condition but becomes irrelevant during execution of the task in the ‘gaze fixation, attention pursuit’ condition.
In conclusion, our study shows that the modulation of visual attention is fully integrated in the cortical oculomotor network processes. Attention modulations operate within this network; as a kind of superordinate system they are independent of eye movements but if eye movements are present attention modulations act on pursuit and saccades in a similar way. The process of dividing attention from gaze and its preparation is specifically controlled by the PPC. Whether, in a divided attention task, continuous attentive tracking is at all possible or whether attention is inevitably shifted between the attention targets remains to be shown in future studies.
Acknowledgements
This project was supported by a PhD exchange grant for S.O. by the Eltem program in Neuroscience, supported by the University of Basel, as part of Neurex, the Neuroscience network in the upper Rhine Valley. We would like to thank A. Sprenger for his advice with the analysis of eye movements and Frank Huethe, Sascha Schmidt, Markus Klarhoefer and Martin Braun for technical assistance.
Abbreviations
-
- A
-
- gaze pursuit, attention pursuit
-
- B
-
- gaze pursuit, attention fixation
-
- BA
-
- Brodmann area
-
- BOLD
-
- blood oxygen level-dependent
-
- C
-
- gaze fixation, attention fixation
-
- D
-
- gaze fixation, attention pursuit
-
- EPI
-
- echoplanar imaging
-
- FEF
-
- frontal eye fields
-
- fMRI
-
- functional magnetic resonance imaging
-
- FWE
-
- family-wise error
-
- IPL
-
- inferior parietal lobule
-
- MNI
-
- Montreal Neurological Institute
-
- MT+
-
- motion-sensitive region
-
- pCG
-
- posterior cingulate gyrus
-
- PCU
-
- precuneus
-
- PPC
-
- posterior parietal cortex
-
- SEF
-
- supplementary eye fields
-
- SnPM
-
- statistical nonparametric mapping
-
- SPEM
-
- smooth pursuit eye movements
-
- SPL
-
- superior parietal lobule
-
- SPM
-
- statistical parametric mapping