Enhanced production and dendritic growth of new dentate granule cells in the middle-aged hippocampus following intracerebroventricular FGF-2 infusions
Abstract
Declined production and diminished dendritic growth of new dentate granule cells in the middle-aged and aged hippocampus are correlated with diminished concentration of fibroblast growth factor-2 (FGF-2). This study examined whether increased FGF-2 concentration in the milieu boosts both production and dendritic growth of new dentate granule cells in the middle-aged hippocampus. The FGF-2 or vehicle was infused into the posterior lateral ventricle of middle-aged Fischer (F)344 rats for 2 weeks using osmotic minipumps. New cells born during the first 12 days of infusions were labeled via daily intraperitoneal injections of 5′-bromodeoxyuridine (BrdU) and analysed at 10 days after the last BrdU injection. Measurement of BrdU+ cells revealed a considerably enhanced number of new cells in the subgranular zone (SGZ) and granule cell layer (GCL) of the dentate gyrus (DG) ipsilateral to FGF-2 infusions. Characterization of β-III tubulin+ neurons among newly born cells suggested an increased addition of new neurons to the SGZ/GCL ipsilateral to FGF-2 infusions. Quantification of DG neurogenesis at 8 days post-infusions via doublecortin (DCX) immunostaining also revealed the presence of an enhanced DG neurogenesis ipsilateral to FGF-2 infusions. Furthermore, DCX+ neurons in FGF-2-infused rats exhibited enhanced dendritic growth compared with their counterparts in vehicle-infused rats. Thus, subchronic infusion of FGF-2 is efficacious for stimulating an enhanced DG neurogenesis from neural stem/progenitor cells in the middle-aged hippocampus. As dentate neurogenesis is important for hippocampal-dependent learning and memory and DG long-term potentiation, strategies that maintain increased FGF-2 concentration during ageing may be beneficial for thwarting some of the age-related cognitive impairments.
Introduction
Ageing in the hippocampus is associated with a dramatic decline in dentate gyrus (DG) neurogenesis (Kuhn et al., 1996; Nacher et al., 2003; Rao et al., 2005). Studies further demonstrate that the major age-related diminution in DG neurogenesis occurs by middle age (Heine et al., 2004; Rao et al., 2005, 2006). Therefore, the potential link between decreased DG neurogenesis and hippocampal-dependent cognitive impairments during ageing has been a subject of intense inquiry (Klempin & Kempermann, 2007). Although there is no universal consensus in the field regarding this issue (Bizon & Gallagher, 2003; Merrill et al., 2003; Bizon et al., 2004), a series of correlative studies imply that dramatically decreased DG neurogenesis contributes to hippocampal-dependent cognitive deficits observed during ageing. First, increased DG neurogenesis after exposure of middle-aged animals to an enriched environment has been found to be associated with increased capability for spatial memory (Kempermann et al., 2002). Second, aged rats with unimpaired spatial memory abilities exhibit higher levels of DG neurogenesis than aged rats with impaired spatial memory (Drapeau et al., 2003; Dupret et al., 2005). Third, enhanced DG neurogenesis following physical exercise in middle-aged mice was found to be associated with better learning and memory performance (van Praag et al., 2005). Fourth, lowering corticosterone levels during middle age enhances DG neurogenesis as well as spatial memory performance (Montaron et al., 2006). Fifth, a recent study shows that, in aged rats with preserved spatial memory, learning enhances the survival of new cells generated before the onset of learning paradigm, suggesting involvement of newly born neurons in the aged hippocampus in memory processing (Drapeau et al., 2007). Additionally, manipulations that decrease DG neurogenesis in adult animals lead to impairments in some of the hippocampal-dependent memories (Shors et al., 2001; Madsen et al., 2003; Rola et al., 2004) and altered DG long-term potentiation, a cellular prototype considered to underlie learning and memory (Wang et al., 2000; Schmidt-Hieber et al., 2004; Ge et al., 2007; Zhao et al., 2007).
Because of the association between DG neurogenesis and hippocampal-dependent cognitive function during ageing described above, there is an enormous interest in boosting DG neurogenesis in the middle-aged and aged hippocampus via a variety of anti-aging strategies. These comprise infusions of neurotrophic factors, increased physical exercise, diet restriction, environmental enrichment, and grafting of glial progenitors and neural stem cells (Kuhn et al., 1997; van Praag et al., 1999, 2005; Lichtenwalner et al., 2001; Jin et al., 2002, 2003; Kempermann et al., 2002; Lee et al., 2002; Aberg et al., 2003; Bondolfi et al., 2004; Cao et al., 2004; Kronenberg et al., 2006; Olson et al., 2006; Hattiangady et al., 2007). Intriguingly, recent studies on DG neurogenesis in middle-aged and aged rats suggest that most of the regulatory events of neurogenesis are not altered with ageing. These include neuronal fate–choice decision of newly born cells, the migration and long-term survival of newly born neurons (Rao et al., 2005), and the stability in the numbers of putative neural stem/progenitor cells (NSCs) in the subgranular zone (SGZ) of the DG (Hattiangady & Shetty, 2007). However, far fewer NSCs proliferate in the SGZ during middle age (McDonald & Wojtowicz, 2005; Rao et al., 2006; Hattiangady & Shetty, 2007). Decreased proliferation of NSCs at middle age is likely due to age-related changes in the DG microenvironment, particularly an imbalance in the positive and negative regulators of neurogenesis. Indeed, recent studies demonstrate that critical neurotrophic factors and signaling proteins that are widely acknowledged as positive regulators of NSC proliferation exhibit considerable decline at middle age. These comprise fibroblast growth factor-2 (FGF-2), insulin-like growth factor-1, vascular-endothelial growth factor, brain-derived neurotrophic factor, neuropeptide Y, and phosphorylated cyclic-AMP response-element-binding protein and γ-aminobutyric acid (Shetty, 2004; Stanley & Shetty, 2004; Hattiangady et al., 2005; Shetty et al., 2005). Moreover, age-related changes have been observed in the neurogenic niches of the SGZ. These include increased distance between vascular niches and NSCs, and altered density of FGF-2 receptors on putative NSCs and other supporting cells (Chadashvili & Peterson, 2006; Hattiangady & Shetty, 2007). Additionally, several negative regulators of neurogenesis, such as the concentration of glucocorticoids, exhibit upregulation at middle age (Cameron & McKay, 1999). Thus, it appears that the microenvironment of the DG at middle age becomes adverse for maintaining greater levels of neurogenesis. Therefore, to enhance neurogenesis in the middle-aged hippocampus, it may be necessary to either increase the concentration of one or more of the positive regulators of DG neurogenesis or suppress the accumulation of negative regulators of neurogenesis in the DG milieu.
This study examined whether increased FGF-2 concentration in the milieu boosts both production and dendritic growth of new dentate granule cells in the middle-aged hippocampus of male Fischer 344 (F344) rats. The choice of FGF-2 was based on findings from earlier studies that FGF-2 is one of the key mitogenic factors of NSCs (Ray et al., 1993; Vescovi et al., 1993; Gage et al., 1995; Palmer et al., 1995; Vaccarino et al., 1995), and infusions of FGF-2 into the brain increase adult neurogenesis (Kuhn et al., 1997; Tao et al., 1997; Jin et al., 2003). The selection of middle-aged rats for enhancement of DG neurogenesis was based on our earlier finding that a dramatic decrease in DG neurogenesis occurs by middle age, and the decrease between middle age and old age is minimal (Rao et al., 2005). With the aim of making the infused substance available to the entire septo-temporal axis of the hippocampus, we infused FGF-2 or vehicle solution artificial cerebrospinal fluid (ACSF) into the posterior lateral ventricle of middle-aged (12 months old) F344 rats for 2 weeks using osmotic minipumps. New cells and neurons born during the first 12 days of infusions were labeled via daily intraperitoneal injections of 5′-bromodeoxyuridine (BrdU) and measured at 10 days after the last BrdU injection using BrdU immunostaining and optical fractionator counting method, and a dual immunofluorescence method for BrdU and β-III tubulin. In order to ascertain the status of DG neurogenesis at 8 days after the conclusion of infusions, the extent of newly born neurons in the DG was measured using doublecortin (DCX) immunostaining and the optical fractionator counting method. The effects of FGF-2 infusions on growth and maturation of dendrites in newly born dentate granule (DCX+) cells were analysed using Neurolucida (Microbrightfield, Colchester, VT, USA).
Materials and methods
Animals
Middle-aged 12-months-old male F344 rats were purchased from the National Institute of Ageing colony at Harlan Sprague–Dawley (Indianapolis, IN, USA). F344 rats were chosen in this study because the genetic background of this strain is known, the normal life span and development of these rats are reasonably well defined (Coleman et al., 1977) and age-related changes in the amount of DG neurogenesis, NSC number and hippocampal microenvironment have been characterized in this strain (Kuhn et al., 1996; Hattiangady et al., 2005; Rao et al., 2005, 2006; Shetty et al., 2005; Hattiangady & Shetty, 2007). All animal experiments were conducted in accordance with NIH guidelines for the care and use of laboratory animals (NIH publications no. 80-23), and all protocols were approved by the animal studies subcommittee of the Durham Veterans Affairs Medical Center & the Duke University Institutional Animal Care & Use Committee. The various experiments and analyses performed in this study are detailed in Fig. 1.
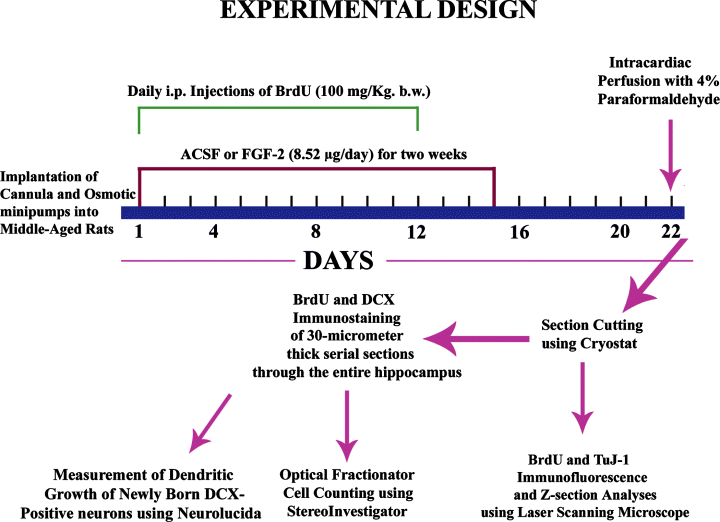
Schematic of major experiments performed in this study. The sequence of experiments mainly include implantation of cannula and osmotic minipumps into middle-aged rats, infusions of artificial cerebrospinal fluid (ACSF) or fibroblast growth factor-2 (FGF-2) into the posterior lateral ventricle for 2 weeks, daily intraperitoneal injections of 5′-bromodeoxyuridine (BrdU) for 12 days commencing from Day 1 of ACSF or FGF-2 infusions, intracardiac perfusion of rats with 4% paraformaldehyde at 10 days after the last of 12 BrdU injections (equivalent to 8 days after the completion of FGF-2 infusions). The tissues were processed for BrdU and doublecortin (DCX) single immunostaining, and the immunopositive cells in the SGZ and GCL were counted via the optical fractionator method using Stereoinvestigator. Moreover, sections were processed for BrdU and TuJ-1 dual immunofluorescence, and the fractions of newly born cells that differentiate into neurons were measured using confocal microscopic analyses. In addition, dendritic growth of newly born DCX+ neurons was measured using Neurolucida.
Intracerebroventricular infusions of FGF-2 or ACSF
Alzet minipumps (model 2002) were purchased from DURECT (Cupertino, CA, USA). These minipumps are designed to deliver 0.5 µL of fluid every hour (i.e. 12 µL/day) at 37 °C for 14 days. The minipumps were filled with either FGF-2 or ACSF in sterile conditions. The FGF-2 was dissolved in ACSF at a concentration of 0.71 µg/mL so that 8.5 µg of FGF-2 will be infused every day. Each filled minipump was attached to a brain infusion cannula via vinyl tubing (Brain infusion kit, DURECT), immersed in sterile saline and incubated at 37 °C for 6 h. The chosen middle-aged animals were divided into two groups: animals receiving FGF-2; and animals receiving vehicle ACSF. Each animal belonging to these groups was then anesthetized with a cocktail of ketamine (50 mg/mL), xylazine (4.5 mg/mL) and acepromazine (0.4 mg/mL) at a dose of 1.25 mL/kg b.w., and fixed into a stereotaxic apparatus with the plane of the incisor bar set at 3.7 mm below the interaural line. In each rat, the dorsal surface of the skull was exposed and a burr hole was drilled into the skull using aseptic techniques and the following co-ordinates: antero-posterior, 3.7 mm caudal to bregma; and lateral, 4.1 mm right lateral to the midline. A subcutaneous tunnel was made between the scapulae through a small midline incision on the back of the neck for placement of the osmotic minipump. Following this, a 5-mm-long stainless steel cannula connected via vinyl tubing to an osmotic pump containing either FGF-2 or ACSF was implanted into the right posterior lateral ventricle 3.7 mm deep to the pial surface. The cannula was fixed firmly to the skull bone through application of Loctite glue (Alzet) and dental cement, and the skin incisions were closed using surgical clips. Animals belonging to the FGF-2 treatment group received 8.5 µg FGF-2 every day (i.e. 0.355 µg of FGF-2/h in 0.5 µL of ACSF) for 14 days, whereas animals belonging to the ACSF (or vehicle group) received only ACSF (0.5 µL/ h) for 14 days (Fig. 1). To prevent potential infections of the surgical site, rats receiving FGF-2 or ACSF infusions were treated with tetracycline antibiotic in their drinking water.
Analyses of the addition of new cells in animals receiving FGF-2 and ACSF infusions
For visualizing the addition of new cells to different hippocampal regions in animals receiving intracerebroventricular FGF-2 or ACSF infusions, intraperitoneal injections of BrdU were given (once daily for 12 days; Sigma, St Louis, MO, USA) at a dose of 100 mg/kg b.w. (Fig. 1) The injections commenced on the day of implantation of minipumps, and stopped on the 12th day after the implantation of minipumps. Ten days after the 12th BrdU injection (i.e. on the 22nd day after the implantation of minipumps in FGF-2- and ACSF-infused groups), animals were deeply anesthetized with halothane, the implanted osmotic pumps were removed by cutting the vinyl tubing attached to the cannula, and rats perfused transcardially with 4% chilled paraformaldehyde in 0.1 m phosphate buffer (PB). The removed osmotic minipumps were cut open to evaluate whether the minipumps had delivered the fluid as desired. All minipumps appeared to have delivered the filled fluid as desired because they contained only a small residual volume of fluid (20–40 µL). This is expected because the minipump filled with 200 µL of fluid employed in this study is designed to pump out 12 µL of fluid every day (at a rate of 0.5 µL/h) for 14 days, which is equivalent to a delivery of 168 µL out of the total 200 µL initially filled. Following perfusion, the brains were collected, postfixed in 4% paraformaldehyde overnight at 4 °C and cryoprotected in 30% sucrose solution.
Processing of tissue sections for BrdU and DCX immunostaining
Thirty-micrometer-thick cryostat sections were cut coronally through the entire hippocampus and collected serially in PB. Every 15th section through the entire hippocampus was selected in each of the animals and processed for BrdU immunostaining (Fig. 1). The sections were treated with 0.1 m Tris-buffered saline (TBS) containing 0.6% hydrogen peroxide to remove the endogenous peroxidase, washed thoroughly in TBS, incubated in formamide (50%) solution prepared in 2 × saline sodium citrate buffer for 2 h at 65 °C, washed in TBS, and incubated in 2 N HCl for 60 min at 37 °C. Following this, the sections were then neutralized with borate buffer (0.1 m, pH 8.5), washed in TBS, blocked in 10% normal horse serum (NHS), incubated overnight at 4 °C in the mouse monoclonal BrdU antibody (1 : 16; Roche, Indianapolis, IN, USA) and washed in TBS. The subsequent visualization procedure was performed by the avidin-biotin complex (ABC) method (Elite ABC kit; Vector, Burlingame, CA, USA) with diaminobenzidine as the chromogen (Shetty & Turner, 2000; Rao & Shetty, 2004). Sections were mounted on gelatin-coated slides, air-dried, counterstained with hematoxylin, dehydrated, cleared and coverslipped. Because 30-µm-thick sections were stained using the free-floating BrdU-immunostaining protocol, there was clear penetration of BrdU antibody throughout the thickness of the section.
Another series (every 15th through the entire hippocampus) of sections was selected in each of the animals and processed for DCX immunostaining (Fig. 1). The sections were treated with 0.1 m phosphate-buffered saline (PBS) containing 20% methanol and 3% hydrogen peroxide, washed in PBS, blocked in 10% NHS and incubated overnight at 4 °C in the DCX antibody (1 : 200; Sc-8066, Santa Cruz Biotechnology, Santa Cruz, CA, USA). As per the manufacturer's information, the DCX antibody used in this study is an affinity-purified goat polyclonal antibody, which was raised against a peptide mapping at the carboxy terminus of DCX of human origin. Following primary antibody treatment, sections were washed in PBS, incubated in the biotinylated horse anti-goat IgG (Vector) for 1 h, washed in PBS and incubated in the ABC reagent (Vector) for 1 h. Following this, the peroxidase reaction was visualized using Vector gray (Vector) as the chromogen.
Quantification of BrdU+ newly born cells and DCX+ newly born neurons in the DG
The BrdU+ cells and DCX+ neurons located in the SGZ and the granule cell layer (GCL) were quantified in the hippocampi ipsilateral to FGF-2 or ACSF infusions (n = 5/group). For this, the BrdU+ cells and DCX+ neurons in these regions were counted from every 15th section through the entire anterior-posterior extent of the hippocampus (450 µm apart). The counting was performed using the StereoInvestigator system (Microbrightfield, Williston, VT, USA) consisting of a color digital video camera (Optronics, Muskogee, OK, USA) interfaced with a Nikon E600 microscope (Fig. 1). In each hippocampus, BrdU+/DCX+ cells were counted from 50–400 randomly and systematically selected frames (each measuring 40 × 40 µm) in every 15th section using the 100 × oil immersion lens. The numbers and densities of frames were determined by entering the parameter grid size (60 × 60 µm) in the optical fractionator component of the StereoInvestigator system. Thirty-micrometer-thick sections were cut through the hippocampus for these studies. However, with BrdU/DCX immunostaining, sections appeared to exhibit significant shrinkage along the Z-axis. The thickness of sections in different regions of the DG was measured using the StereoInvestigator system incorporating XYZ stage controller equipped with Z-axis position control (LEP Electronic Products, Hawthorne, NY, USA). This revealed that, in all groups, the average thickness was reduced to 33% of the initial thickness in sections processed for BrdU immunostaining and 40% in sections processed for DCX immunostaining.
Thus, at the time of data collection, the thickness of sections was 10 µm for BrdU-immunostained sections and 12 µm for DCX-immunostained sections. For cell counting, the contour of GCL and SGZ was first delineated in every section using the tracing function of the StereoInvestigator. The optical fractionator component was then activated, and the number and location of counting frames and the counting depth for that section was determined by entering parameters such as the grid size (60 × 60 µm), the thickness of the top guard zone (4 µm) and the optical dissector height (i.e. 8 µm). A computer-driven motorized stage then allowed the section to be analysed at each of the counting frame locations. In every counting frame location the top of the section was set, after which the plane of the focus was moved 4 µm deeper through the section (guard zone). This plane served as the first point of the counting process. Continuing to focus down, all BrdU+ cells/DCX+ neurons that came into focus in the next 8-µm section thickness were counted if they were entirely within the counting frame or touching the upper or right side of the counting frame. Based on the above parameters and cell counts, the StereoInvestigator program calculated the total number of BrdU/DCX-positive cells per DG by utilizing the optical fractionator formula, as described in our previous reports (Rao & Shetty, 2004; Hattiangady et al., 2007; Shetty et al., 2005).
Analyses of neuronal differentiation of newly born cells via BrdU and β-III tubulin dual immunofluorescence and confocal microscopy
To visualize fractions of BrdU+ cells that differentiated into β-III tubulin+ neurons in different groups, dual immunofluorescence methods for demonstrating BrdU and β-III tubulin were performed. A minimum of three representative sections were processed in every chosen animal (n = 4/group). Sections were first processed for various BrdU preincubation treatments as described earlier, washed in TBS, blocked in normal goat serum (NGS), and incubated overnight in a rat BrdU antibody solution (1 : 200, Serotec). Sections were washed in TBS and incubated in goat anti-rat IgG tagged with Alexa Fluor 594 (1 : 100, Molecular Probes). Following this, sections were washed in PBS, blocked in 10% NHS and treated overnight with mouse TuJ-1 antibody solution (1 : 1000; Chemicon), which binds to β-III tubulin in neurons. Sections were rinsed in PBS, and then treated with biotinylated horse anti-mouse IgG (1 : 200, Vector), washed in PBS, and incubated in streptavidin fluorescein (1 : 150, Molecular Probes), and washed again in PBS. The sections were coverslipped with slow fade/anti-fade mounting medium (Molecular Probes) and examined using a laser confocal microscope (LSM-410 Carl Zeiss) to estimate the fractions of BrdU+ cells expressing β-III tubulin. For determination of the co-expression of BrdU with β-III tubulin, BrdU+ cells in the SGZ were individually examined using Z sectioning at 1-µm intervals. The average number of BrdU+ cells examined per animal for β-III tubulin expression was 67 for the ACSF-infused group and 144 for the FGF-2-infused group. The optical stacks of at least 10 images were used for determination of dual antigen labeling.
Analyses of cells positive for glial fibrillary acidic protein (GFAP) in the dentate SGZ
Representative sections from each animal in ACSF- and FGF-2-infused groups were immunostained for GFAP (Shetty et al., 2005). Briefly, all sections were incubated in 3% hydrogen peroxide for 30 min, washed in PBS three times, and blocked in NGS for 30 min. Sections were then incubated overnight in the GFAP primary antibody solution (rabbit polyclonal, 1 : 1000; Dako), washed in PBS, incubated in biotinylated anti-rabbit IgG for 60 min and processed further using ABC method, and immunoreaction was visualized using Vector gray as the chromogen. Sections were counterstained using neutral red and dehydrated. The numerical density of GFAP+ cells per unit volume of the SGZ was then quantified in three sections from every animal belonging to the ACSF- and FGF-2-infused groups (n = 4/group) using StereoInvestigator. The SGZ (two-cell-thick zone) was first marked in each section (Hattiangady & Shetty, 2007), and a frame size of 40 × 40 µm and a grid size of 60 × 60 µm were then chosen for stereological cell counting using the optical fractionator principle. After counting cells in all three sections in each animal, data on total measured volume and the total number of counted cells were collected, and from these the average count per unit volume was determined for each animal (Shetty & Hattiangady, 2007).
Measurement of the dendritic maturation of newly generated DCX + neurons
We measured the maturation of newly generated (i.e. DCX+) neurons in the SGZ/GCL ipsilateral to ACSF or FGF-2 infusions (Fig. 1), based on the orientation of dendrites in relation to the GCL. This was accomplished in each group by measuring the percentages of DCX+ neurons exhibiting only vertical dendrites emanating from the soma and extending into the dentate molecular layer through the GCL (relatively mature newly born neurons), and DCX+ neurons exhibiting horizontally orientated soma/dendrites in the SGZ or basal dendrites (immature newly born neurons). For these measurements, 400 DCX+ neurons in each group (100 neurons/animal, n = 4/group) were examined. For further analyses of the dendritic growth of DCX+ neurons, morphological measurements of well-differentiated DCX+ neurons (i.e. neurons with vertically orientated and branching dendrites that are not truncated close to soma, not overlapping with dendrites of neighboring neurons and located mostly in the middle of the section thickness) were performed (Fig. 1). This was done at 1150 × magnification, using a semiautomatic neuron tracing system (Neurolucida; Microbrightfield) linked to a Nikon microscope (Shetty & Turner, 1998). In each group, 40 DCX-positive neurons (n = 10/animal) were traced in their entirety and data for various morphological measurements were calculated, including the cell body area, number of dendritic nodes and ends, and total dendritic length. To measure the extent of dendritic growth away from the soma and the branching of dendrites at different distances from the soma, the concentric circle analysis of Sholl (1953) was performed using the NeuroExplorer component of the Neurolucida program.
Statistical analyses
For every parameter, the value was first calculated separately for each animal before the means and standard errors were determined for the total number of animals included per group. Comparison of various quantitative parameters across the two groups utilized unpaired, two-tailed t-test.
Results
Effects of FGF-2 infusions on the number of newly born cells in the hippocampus
Newly born cells in the hippocampus were visualized through BrdU immunostaining. In hippocampi ipsilateral to ACSF or FGF-2 infusions, newly born cells were observed in all regions of the hippocampus, including the DG and CA1 and CA3 subfields (Fig. 2, A1 and B1). Of the two groups, the density appeared much greater in the FGF-2-infused group (ipsilateral to FGF-2 infusion) than the ACSF-infused group (Fig. 2, A1 and B1). In hippocampi contralateral to ACSF or FGF-2 infusions, newly born cells were scarce but comparable between the two groups (data not shown), which appeared consistent with the density of newly born cells reported for naive middle-aged control animals following 12 daily injections of BrdU (Rao et al., 2005). Examination of the SGZ and GCL revealed a greater density of newly born cells in hippocampi ipsilateral to FGF-2 infusions, in comparison to hippocampi ipsilateral to ACSF infusions (Fig. 2, A2, A3, B2 and B3). Quantification of the absolute number of newly born cells (i.e. BrdU+ cells) that are added to the GCL and SGZ over a period of 12 days revealed that the addition of new cells is increased by 98% in hippocampi ipsilateral to FGF-2 infusions, in comparison to hippocampi ipsilateral to ACSF infusions (Fig. 2C).
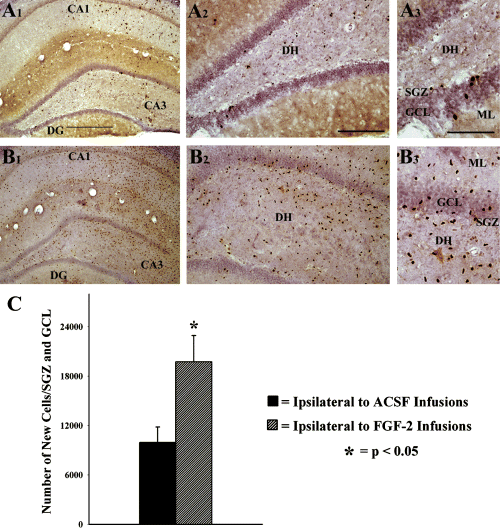
Distribution of newly born cells in the hippocampus following unilateral infusions of artificial cerebrospinal fluid (ACSF) or fibroblast growth factor-2 (FGF-2) for 2 weeks into the posterior lateral ventricle, visualized using BrdU immunostaining. (A1) BrdU+ (newly born) cells in a section from the hippocampus ipsilateral to ACSF infusions, (B1) BrdU+ cells in a section from the hippocampus ipsilateral to FGF-2 infusions. (A2 and B2) Magnified views of a dentate gyrus (DG) region from (A1) and (B1) illustrating the density and distribution of newly born cells in different layers. (A3 and B3) Enlarged views of a region from (A2) and (B2) showing the distribution of newly born cells in the subgranular zone (SGZ), the granule cell layer (GCL) and the dentate hilus (DH). Scale bars: 500 µm (A1 and B1); 200 µm (A2 and B2); 100 µm (A3 and B3). The bar chart in (C) illustrates the total number of BrdU+ newly born cells added to the SGZ and GCL ipsilateral to ACSF or FGF-2 infusions over a period of 12 days during infusions. Note that the total numbers of newly born cells in the SGZ and GCL are considerably greater in the SGZ and GCL ipsilateral to FGF-2 infusions. ML, molecular layer.
Effects of FGF-2 infusions on net neurogenesis in the dentate SGZ and GCL
Newly born neurons in the SGZ and GCL were visualized via BrdU and β-III tubulin dual immunofluorescence and confocal microscopy (Fig. 3, A1–D3). The percentages of new (BrdU+) cells expressing the neuronal marker β-III tubulin were first calculated for both groups. This revealed that the overall neuronal differentiation of newly born cells in the SGZ and GCL was 15% in hippocampi ipsilateral to ACSF infusions and 21% in hippocampi ipsilateral to FGF-2 infusions (Fig. 3E). Extrapolation of BrdU cell counts using the BrdU–β-III tubulin ratio suggested that a far greater number of new neurons are added to the SGZ and GCL in hippocampi ipsilateral to FGF-2 infusions (Fig. 3F). The overall increase was 178% in hippocampi ipsilateral to FGF-2 infusions, in comparison to hippocampi ipsilateral to ACSF infusions. Thus, the effects of FGF-2 infusions on dentate neurogenesis in the middle-aged hippocampus are considerable.
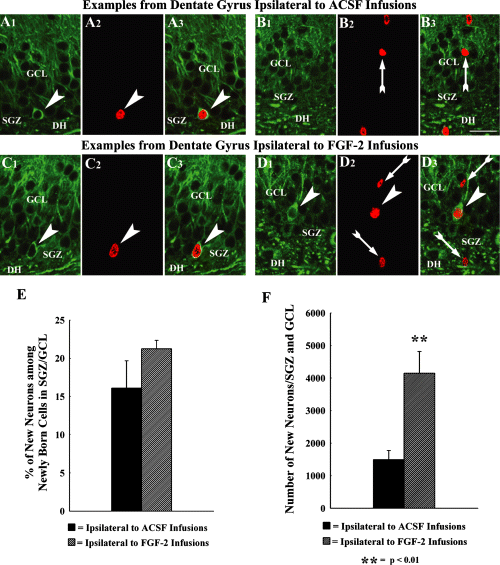
Neuronal differentiation of newly born cells in the subgranular zone (SGZ) and the granule cell layer (GCL), as determined by TuJ-1 and BrdU dual immunofluorescence and confocal microscopy. The top panel (A1–B3) illustrates examples of 1-µm-thick optical images from dentate gyrus (DG) ipsilateral to artificial cerebrospinal fluid (ACSF) infusions, whereas the lower panel (C1–D3) shows examples of 1-µm-thick optical images from DG ipsilateral to fibroblast growth factor-2 (FGF-2) infusions. Arrowheads denote newly born BrdU+ cells (red nuclei in A2, C2 and D2) that differentiate into TuJ-1-immunopositive neurons (green soma in A1, C1 and D1) in the SGZ (A1–A3 and C1–C3) and GCL (D1–D3). Arrows indicate newly born BrdU+ cells (red nuclei in B2, B3 and D2, D3) that lack TuJ-1 immunoreactivity (presumably non-neuronal cells). DH, dentate hilus. Scale bar: 25 µm. The bar chart in (E) compares the percentages of newly born cells that differentiate into TuJ-1-immunopositive neurons between SGZ and GCL ipsilateral to ACSF infusions, and SGZ and GCL ipsilateral to FGF-2 infusions. The bar chart in (F) illustrates net neurogenesis, based on calculation of the total number of neurons among BrdU+ cells using percentages of cells expressing TuJ-1. Note that the net neurogenesis is 178% greater in the SGZ and GCL ipsilateral to FGF-2 infusions than SGZ and GCL ipsilateral to ACSF infusions.
Effects of FGF-2 infusions on the status of dentate neurogenesis, as measured by DCX immunohistochemistry
The status of DG neurogenesis at 10 days after the last of 12 daily BrdU injections (equivalent to 8 days after the conclusion of FGF-2 infusions) was analysed through DCX immunostaining, and the optical fractionator counting of DCX+ neurons in the SGZ and GCL. In both groups, DCX immunostaining demonstrated newly formed neurons in the SGZ and GCL (Fig. 4). The cell bodies of virtually all DCX+ neurons were located in the SGZ or the inner third of the GCL. In hippocampi ipsilateral to FGF-2 infusions (Fig. 4, B1–B3), the density of DCX+ neurons appeared greater than the density in hippocampi ipsilateral to ACSF infusions (Fig. 4, A1–A3). Assessment of the status of DG neurogenesis via quantification of the number of DCX+ neurons demonstrated that the addition of new neurons to the SGZ and GCL is increased by 138% in hippocampi ipsilateral to FGF-2 infusions (Fig. 4C).
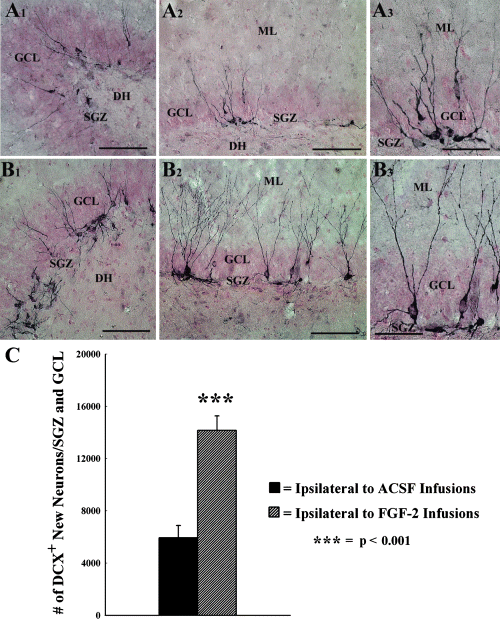
Distribution of newly born neurons in the subgranular zone (SGZ) and the granule cell layer (GCL) at 8 days following 2-week infusions of artificial cerebrospinal fluid (ACSF) or fibroblast growth factor-2 (FGF-2) into the posterior lateral ventricle, visualized by doublecortin (DCX) immunostaining. (A1 and A2) DCX+ neurons in representative regions of the crest and upper blade of the SGZ and GCL ipsilateral to ACSF infusions. (B1 and B2) DCX+ neurons in representative regions of the crest and upper blade of the SGZ and GCL ipsilateral to FGF-2 infusions. (A3 and B3) Magnified views of a region from (A2) and (B2) demonstrating the morphology and extent of dendrites in individual neurons. DH, dentate hilus; ML, molecular layer. Scale bars: 100 µm (A1, A2, B1 and B2); 50 µm (A3 and B3). The bar chart in (C) compares the status of neurogenesis (based on the total number of DCX+ newly born neurons) between SGZ and GCL ipsilateral to ACSF infusions, and SGZ and GCL ipsilateral to FGF-2 infusions. Note that the amount of neurogenesis is 138% greater in the SGZ and GCL ipsilateral to FGF-2 infusions than SGZ and GCL ipsilateral to ACSF infusions.
Effects of FGF-2 infusions on putative NSCs in the dentate SGZ
To evaluate whether intracerebroventricular FGF-2 administration induced upregulation of DG neurogenesis in the middle-aged hippocampus is linked to an increase in the number of putative NSCs, the density of cells positive for GFAP in the dentate SGZ was examined. The choice of GFAP for quantifying the density of NSCs in the SGZ is based on earlier findings that GFAP-immunoreactive cells in the neurogenic SGZ mostly represent NSCs (Komitova & Eriksson, 2004; Hattiangady & Shetty, 2007). Examination of putative NSCs using GFAP immunostaining in the dentate SGZ revealed no apparent changes in this population of cells between ACSF-infused and FGF-2-infused rats. Both density and morphology of GFAP+ cells in the SGZ appeared similar in rats receiving ACSF or FGF-2 infusions (Fig. 5). Furthermore, quantification of GFAP+ cells per mm3 of SGZ revealed an average of 585 cells (585 ± 51) in rats receiving ACSF infusions and 606 cells (606 ± 29) in rats receiving FGF-2 infusions, suggesting that the density of GFAP+ cells in the SGZ does not change with FGF-2 infusions. Thus, subchronic FGF-2 administration into the posterior lateral ventricle does not increase the population of GFAP+ cells (i.e. putative NSCs) in the SGZ of the hippocampus.
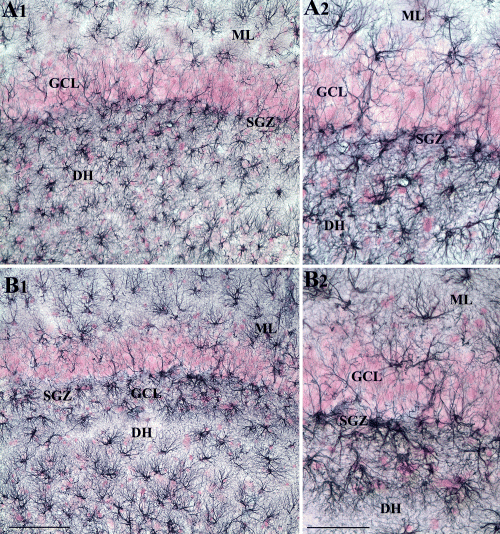
Distribution of GFAP-immunopositive cells in the subgranular zone (SGZ) at 8 days following 2-week infusions of ACSF or FGF-2 into the posterior lateral ventricle. (A1) GFAP+ astrocyte-like cells in the SGZ and upper blade of the granular cell layer (GCL) ipsilateral to ACSF infusions. (B1) GFAP+ astrocyte-like cells in the SGZ and upper blade of the GCL ipsilateral to FGF-2 infusions. (A2 and B2) Magnified views of regions from (A1) and (B1), respectively. DH, dentate hilus; ML, molecular layer. Scale bars: 100 µm (A1 and B1); 50 µm (A2 and B2).
Measurement of dendritic maturation of newly generated neurons in the DG
The maturation of dendrites from DCX+ neurons located in the SGZ or inner third of the GCL were quantified in both the groups. In rats infused with ACSF, 79% (mean ± SEM = 79.0 ± 1.6%) of DCX+ neurons exhibited horizontally orientated or basal dendrites, an immature feature of granule cells in rodents (Seress & Pokorny, 1981; Marti-Subirana et al., 1986; Lubbers & Frotscher, 1988; Jones et al., 2003). The remaining (21.0 ± 1.6%) DCX+ neurons exhibited vertically orientated dendrites that traversed both GCL and the inner and middle regions of the molecular layer. In rats receiving FGF-2 infusions, 61% (61 ± 4.1%) of newly formed neurons exhibited horizontally orientated or basal dendrites, and 39% (39.0 ± 4.1%) exhibited dendrites that are orientated vertically and traversed both GCL and the inner and middle regions of the molecular layer. In some, several dendrites extended into the outer third of the molecular layer. Overall, in hippocampi ipsilateral to FGF-2 infusions, there was an 86% increase in the number of newly born neurons with vertically orientated dendrites, in comparison to hippocampi ipsilateral to ACSF infusions. Previous studies suggest that neurons with vertically orientated dendrites are relatively mature neurons among the population of DCX+ neurons in the DG (Rao & Shetty, 2004; Rao et al., 2005). Considering this, greater fractions of such neurons in the FGF-2-infused group implies that the dendritic maturation of neurons during the DCX expression phase is enhanced in middle-aged rats receiving 2-week infusions of FGF-2 into the lateral ventricle.
Next, the overall dendritic growth in relatively mature DCX+ neurons belonging to the two groups (10 neurons/animal; 40 neurons per group) were quantified. The chosen neurons exhibited vertically orientated and branching dendrites that are not truncated close to the cell body, not overlapping with dendrites of neighboring neurons and located mostly in the middle of the section thickness. Comparison of data between the two groups revealed that relatively mature newly born neurons in hippocampi ipsilateral to FGF-2 infusions have a greater number of dendritic nodes (89% increase) and dendritic endings (90% increase), and an increased total dendritic length (77% increase), in comparison to similar neurons in hippocampi ipsilateral to ACSF infusions (Fig. 6C). The concentric circle analysis of Sholl revealed that in newly born neurons belonging to hippocampi ipsilateral to ACSF infusions, the majority of dendrites (62–63%) end within 150 µm distance from the soma (Fig. 6D). The remaining dendrites mostly end at 150–200 µm distance from the soma, and only a few extend up to 200–250 µm distance from the soma. In contrast, in rats receiving FGF-2 infusions, only 42% of dendrites end within the 150 µm distance from the soma; the remaining 58% of dendrites mostly end at 150–250 µm distance from the soma with a few extending up to 250–300 µm distance from the soma (Fig. 6D). Thus, dendritic segments in the FGF-2-infused group are longer, in comparison to the ACSF-infused group. This was also supported by the observation that, in the FGF-2-infused group, the total dendritic intersections are greater at 150–250 µm and 200–250 µm distances from the soma, in comparison to the ACSF-infused group (Fig. 6D). Furthermore, neurons in the FGF-2-infused group exhibited a greater number of dendritic nodes at 150–250 µm and 200–250 µm distances from the soma, in comparison to their counterparts in the ACSF-infused group (Fig. 6D), suggesting that secondary and tertiary dendrites in the FGF-2-infused group exhibit enhanced branching near their termination.
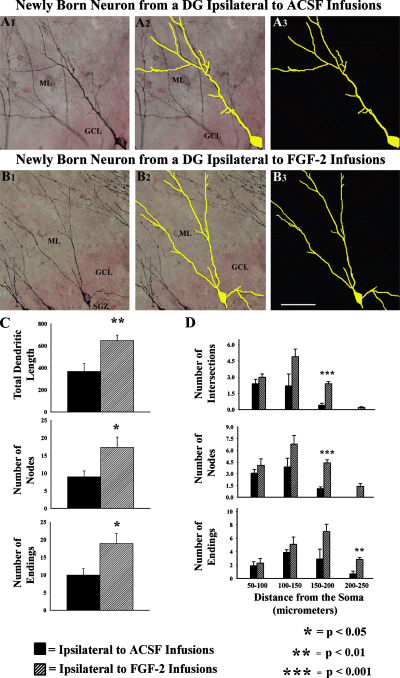
Dendritic growth of DCX-immunopositive newly born neurons with vertically orientated dendrites (i.e. relatively mature neurons among the DCX population) in the SGZ and granule cell layer (GCL). (A1 and B1) An example of DCX+ neuron with vertically orientated dendrites from the SGZ and GCL ipsilateral to artificial cerebrospinal fluid (ACSF; A1) or fibroblast growth factor-2 (FGF-2; B1) infusions. (A2 and B2) Tracings (in yellow color) over the soma and the entire dendritic tree of these neurons, performed using Neurolucida. (A3 and B3) Tracings to highlight the branching pattern of these neurons. Note that the neuron from SGZ and GCL ipsilateral to FGF-2 infusions (B1–B3) exhibits a more extensive apical dendritic tree than the neuron from SGZ and GCL ipsilateral to ACSF infusions (A1–A3). DG, dentate gyrus; ML, molecular layer. Scale bar: 50 µm. The bar charts in (C) show the total dendritic length, and numbers of dendritic nodes and endings of relatively mature DCX+ neurons from SGZ and GCL ipsilateral to ACSF or FGF-2 infusions. Note that all of these parameters of dendritic growth are considerably greater in neurons from the SGZ and GCL ipsilateral to FGF-2 infusions than neurons from SGZ and GCL ipsilateral to ACSF infusions. The bar chart in (D) shows Sholl's concentric circle analysis of apical dendrites of relatively mature DCX+ neurons from SGZ and GCL ipsilateral to ACSF or FGF-2 infusions. Note that the apical dendrites of neurons in SGZ and GCL ipsilateral to FGF-2 infusions exhibit extensive dendritic growth with a greater number extending beyond 200 µm distances from the soma than those in SGZ and GCL ipsilateral to ACSF infusions.
Discussion
The results provide novel evidence that subchronic infusion of FGF-2 into the posterior lateral ventricle is proficient for substantially augmenting both production and dendritic growth of new dentate granule cells in the middle-aged hippocampus. Prior reports revealed that dramatically waned neurogenesis in the middle-aged and aged hippocampus is a consequence of increased quiescence of NSCs, which was evinced by proliferation of far fewer NSCs in the SGZ, albeit the overall population of NSCs in the SGZ does not diminish during the course of ageing (McDonald & Wojtowicz, 2005; Hattiangady & Shetty, 2007). Additionally, no changes were perceived in other regulatory events of neurogenesis such as neuronal fate–choice decision of newly born cells, migration of newly born neurons, and short- or long-term survival of newly added neurons (Rao et al., 2005, 2006). In the framework of the above findings, the present observations underscore that it is possible to improve the overall addition of new neurons from NSCs in the middle-aged hippocampus with an exogenous supply of mitogenic factors such as FGF-2. Declined DG neurogenesis during ageing seems to be linked to both performance deficits on hippocampus-dependent tasks, hippocampal volume reduction and altered DG long-term potentiation (Kempermann et al., 2002; Drapeau et al., 2003, 2007; van Praag et al., 2005; Driscoll et al., 2006; Montaron et al., 2006; Zhao et al., 2007). From this perspective, increasing the FGF-2 concentration in the microenvironment of the aged hippocampus may perhaps be advantageous for thwarting age-related impairments in long-term potentiation and hippocampal-dependent learning and memory functions.
Rationale for selecting FGF-2 for enhancing neurogenesis in the middle-aged hippocampus
Our choice of middle-aged rats for enhancement of DG neurogenesis in this study was based on our earlier findings that DG neurogenesis dramatically decreases by middle age, and the decrease between middle age and old age is minimal (Rao et al., 2005). In addition, our preceding studies showed considerable diminution in the concentration of several neurotrophic factors and signaling proteins that are considered positive regulators of NSC proliferation in the hippocampus by middle age (Shetty et al., 2004, 2005; Hattiangady et al., 2005). Moreover, there were alterations in vascular niches as early as middle age, typified by diminished density of capillaries and an increased expanse between capillaries and NSCs in the SGZ (Hattiangady & Shetty, 2007). Additionally, it was found that numbers of putative NSCs (i.e. cells expressing markers such as Sox-2, GFAP and vimentin in the SGZ) remain constant during the course of ageing. Thus, the foremost deterioration in neurogenesis occurs by middle age, and modifications in the DG microenvironment likely underlie this decrease. While NSCs in the SGZ are sensitive to the concentration of a variety of neurotrophic factors in their milieu, FGF-2 stands out as one of the key mitogenic factors influencing NSCs and the extent of neurogenesis in the adult hippocampus (Ray et al., 1993; Vescovi et al., 1993; Gage et al., 1995; Palmer et al., 1995; Vaccarino et al., 1995; Craig et al., 1996; Lichtenwalner et al., 2001; Jin et al., 2003). For example, in vitro studies show increased proliferation of hippocampal NSCs in the presence of FGF-2 (Vicario-Abejon et al., 1995; Bull & Bartlett, 2005), and in vivo studies establish that exogenous infusion of FGF-2 into the adult brain increases neurogenesis in the subventricular zone (Kuhn et al., 1997) and/or the hippocampus (Tao et al., 1997; Jin et al., 2003). Furthermore, endogenously synthesized FGF-2 seems to be essential for fueling the proliferation and differentiation of NSCs in the adult hippocampus after brain injury (Yoshimura et al., 2001). Moreover, NSCs residing in regions of the adult brain that generate only glia can also produce neurons after exposure to FGF-2 in vitro (Palmer et al., 1999). In addition, FGF-2 promotes the formation of neuronal circuits as well as survival of neurons in organotypic hippocampal slices in vitro (Nakagami et al., 1997). Besides, FGF-2 neurotrophic activity in hippocampal astrocytes including those in the SGZ declines dramatically at middle age (Shetty et al., 2005). Thus, the choice of FGF-2 for stimulating neurogenesis in the middle-aged hippocampus was based on the favorable influence of this protein on proliferation of NSCs and neurogenesis in the adult brain, and reduced concentration of this protein in the middle-aged and aged hippocampus.
Extent of FGF-2-mediated increase in DG neurogenesis and technical considerations
We ascertained that infusions of 8.5 µg of FGF-2 every day (at a rate of 0.355 µg/h) for 2 weeks considerably increase the production of new cells during the infusion period in the hippocampus ipsilateral to FGF-2 infusions. Investigation of neuronal differentiation of newly born cells in the SGZ and GCL, and measurement of neurogenesis based on fractions of neurons among newly born cells within respective groups revealed 178% improvement in net neurogenesis in hippocampi ipsilateral to FGF-2 infusions, in comparison to hippocampi ipsilateral to ACSF infusions. Furthermore, evaluation of the status of DG neurogenesis through measurement of cells immunopositive for DCX (a marker of newly born neurons) showed that the DG neurogenesis at 8 days after the conclusion of FGF-2 infusions is increased by 138% in contrast to hippocampi ipsilateral to ACSF infusions. Based on previous studies showing that DCX+ cells in the DG of F344 rats represent neurons that are mostly added during the 2 weeks prior to death (Rao & Shetty, 2004; Rao et al., 2005) and that DCX is expressed in new neurons as early as 3 h after birth (Kempermann et al., 2003; Plümpe et al., 2006), it is tempting to suggest that the increase observed with DCX analyses in this study reflects enhanced neurogenesis that occurred predominantly after the conclusion of FGF-2 infusions. This is because animals in this study were killed at 8 days after the conclusion of FGF-2 infusions. However, as this study did not examine whether FGF-2 exposure affects the kinetics of development and the duration of DCX expression in newly born neurons, definitive conclusions about the birth date of DCX+ neurons examined at 8 days after the conclusion of FGF-2 infusions need further studies in the future. Nevertheless, DCX analyses performed in this study reveal the presence of an enhanced DG neurogenesis ipsilateral to FGF-2 infusions.
A previous study in mice has suggested increased production of new cells in the aged hippocampus following short-term (3-day) infusions of a much lower dose of FGF-2 (1.2 µg/day) into the anterior lateral ventricle (Jin et al., 2003). However, it was unclear whether the effects were seen throughout the hippocampus, as only a small segment of the hippocampus (between bregma −1.34 mm and bregma −2.46 mm) was included in the analyses. Furthermore, as neuronal differentiation of newly born cells was not quantified, it was unknown whether net neurogenesis was increased following FGF-2 infusions. Additionally, a study by Kuhn et al. (1997) reported no changes in hippocampal neurogenesis following infusions of much lower doses of FGF-2 (360 ng/day) into the anterior lateral ventricle of adult rats. Considering these, in our study we chose to test the effects of a much higher dose (8.5 µg/day) of FGF-2 for longer duration on DG neurogenesis in the middle-aged hippocampus. Although our quantitative results imply positive effects with the selected dose of FGF-2, it is still uncertain whether this dose denotes the optimal dose of FGF-2 that induces a maximal increase in DG neurogenesis in the middle-aged hippocampus. Greatly increased FGF-2 concentration in the hippocampal milieu may inhibit neuronal differentiation of newly born cells. Indeed, long-term treatment of adult hippocampal progenitors with FGF-2 in vitro has been shown to produce upregulation of glycogen synthase kinase-3β, a signaling component downstream of Notch that inhibits the neuronal fate–choice decision by progenitors during development (Ruel et al., 1993; Marcus et al., 1998; Tatebayashi et al., 2003). Thus, long-standing exposure of progenitors to elevated intensity of FGF-2 might hinder adult neurogenesis by maintaining progenitors as uncommitted cells or by altering their lineage potential to gliogenic (Johe et al., 1996; Tatebayashi et al., 1999). Some modification in lineage potential of newly born cells into uncommitted or glial fates might have occurred in the current study. Yet this change does not appear to be specific to FGF-2 infusions as the translation rate of newly born cells into neurons was diminished in both FGF-2- and ACSF-infused groups in comparison to values found for naive control rats in our prior study (Rao et al., 2005). Furthermore, it has been proposed that increased concentration of FGF-2 might maintain the neuronally committed progenitors in the cell cycle for protracted periods and thereby inhibit them from undergoing full differentiation (Tatebayashi et al., 2003). This scenario, however, appears unlikely in the current study because the overall dendritic growth of newly differentiated neurons was considerably enhanced in the FGF-2-infused group. Nevertheless, further studies with multiple doses are essential in the future to establish the most favorable dose of FGF-2 that persuades a maximal increase in DG neurogenesis in the middle-aged hippocampus.
Influence of FGF-2 on dendritic maturation of newly generated neurons in the DG
In addition to their effects on proliferation and differentiation of NSCs, FGF-2 has positive effects on neuronal morphogenetic differentiation processes, which include elongation and branching of axonal processes (Patel & McNamara, 1995; Kalil et al., 2000). Within the hippocampus, FGF-2 has been shown to promote bifurcation and growth of axonal branches without affecting the elongation rate of primary axons causing increased complexity of axonal trees, but not dendrites (Aoyagi et al., 1994; Patel & McNamara, 1995). In this study, we found that FGF-2 infusions into the middle-aged hippocampus increase the dendritic growth of newly born DCX+ dentate granule cells located in the SGZ or inner third of the GCL. A vast majority of DCX+ neurons in the DG of ACSF-infused rats exhibited horizontally orientated or basal dendrites, an immature feature of granule cells in rodents (Seress & Pokorny, 1981; Marti-Subirana et al., 1986; Lubbers & Frotscher, 1988; Jones et al., 2003; Ribak et al., 2004; Rao et al., 2005; Ribak & Shapiro, 2006). In contrast, a greater fraction of DCX+ newly born granule cells in the DG of FGF-infused rats exhibited vertically orientated dendrites, a feature of relatively mature neurons among the DCX+ population of neurons (Rao & Shetty, 2004; Rao et al., 2005), which traversed both GCL and inner and middle thirds of the molecular layer. A greater fraction of newly born neurons with vertically orientated dendrites in the FGF-2-infused group implies that dendritic maturation of neurons during the DCX expression phase is enhanced by the presence of FGF-2 in the DG milieu. Quantification and comparison of overall dendritic growth in relatively mature DCX+ neurons revealed that DCX+ neurons in rats receiving FGF-2 infusions exhibited increases in total dendritic length, the number of nodes and endings, and elongated secondary and tertiary dendrites than their counterparts in the ACSF-infused group. Thus, our study provides the first evidence that exogenous application of FGF-2 into the middle-aged brain greatly influences the dendritic growth of newly born DCX+ dentate granule cells. Because dendritic growth of newly born neurons is retarded in the middle-aged hippocampus (Rao et al., 2005, 2006), an enhanced growth of newly born neurons mediated by FGF-2 is likely helpful for rapid incorporation of these newly born neurons into the functional circuitry of the hippocampus, which in turn may ameliorate impairments in long-term potentiation and learning and memory observed during old age.
Potential mechanisms of increased neurogenesis after FGF-2 infusions
Although it is recognized that FGF-2 is a potent mitogenic factor for NSCs (Temple & Qian, 1995; Vicario-Abejon et al., 1995) and actions of FGF-2 are mediated via FGF receptors (FGFRs), direct evidence for a role of FGF-2 in adult neurogenesis in vivo is still being established. The mammalian FGF receptor family (FGFR) consists of a group of four trans-membrane proteins (FGFR1–FGFR4) with intrinsic tyrosine kinase activity (Coumoul & Deng, 2003; Zhao et al., 2007). However, the specific receptors that mediate functions of FGF-2 in adult neurogenesis are still unclear. While a previous in vitro study suggests FGFR1 and FGFR2 as potential candidates (Kalyani et al., 1997), a recent study suggests a critical role for FGFR1 in adult neurogenesis in vivo, as FGFR1 expression is largely restricted to the hippocampus in the adult brain (Belluardo et al., 1997) and severe impairments in dentate neurogenesis follow deletion of the FGFR1 gene (Zhao et al., 2007). Previous studies have shown that FGFR1 is necessary for hippocampal growth, because it promotes proliferation of hippocampal NSCs during development (Ohkubo et al., 2004). Additionally, it has been observed that mice deficient in expression of FGFR1 mRNA in the hippocampal DG exhibit considerably diminished NSC proliferation and neurogenesis (Pieper et al., 2005). In light of decreases in both FGF-2 neurotrophic activity and FGFR2 in hippocampal astrocytes during middle age and old age (Shetty et al., 2005; Chadashvili & Peterson, 2006), it is plausible that infused FGF-2 binds to FGFR1 expressed on NSCs and/or neurogenic astrocytes in the SGZ, which in turn stimulates their proliferation and increases neurogenesis in the middle-aged hippocampus. Because it is likely that adult neurogenesis in the SGZ originates from putative NSCs that are GFAP+ (Steiner et al., 2006), it is also possible that FGF-2 administration increases the density of putative NSCs by inducing increased proliferation in GFAP+ NSCs in the SGZ. However, quantification of GFAP+ cells in the SGZ in this study revealed comparable numbers between ACSF- and FGF-2-infused groups. This suggests that FGF-2 administration does not increase the overall density of putative NSCs in the SGZ, but likely promotes increased production of new neurons (or DCX-expressing transit amplifying cells) from existing GFAP+ cells. However, some direct effects of FGF-2 on DCX-expressing transit amplifying cells cannot be ruled out because a recent study suggests that considerable fractions of DCX+ newly born neurons exhibit proliferation (Plümpe et al., 2006). Under normal conditions, NSCs in the middle-aged and aged hippocampus display increased quiescence, and based on Ki-67 and Sox-2 immunostaining, it was found that only 4–8% of NSCs proliferate in the middle-aged hippocampus on a given day (Hattiangady & Shetty, 2007). Although depletions in FGF-2 concentration and FGFR2 have been observed in the middle-aged and aged rat hippocampus (Shetty et al., 2005; Chadashvili & Peterson, 2006), it was unclear whether decreased proliferation of NSCs is due to depletion of FGF-2 per se or changes in FGFRs. From the current results, it appears that the defect is mostly in the reduced concentration of FGF-2 in the hippocampal milieu than widespread alterations in FGFRs, as exogenous application of FGF-2 considerably increased neurogenesis. However, the precise mechanisms of this increase could not be deciphered in this study as analyses were done on cohorts of cells that are born over a period of 12 days during FGF-2 infusions. Specific studies on proliferation and survival following FGF-2 infusions are required in the future to understand these issues.
Acknowledgements
This research was supported by grants from the National Institute for Ageing (NIH-NIA grant RO1 AG20924 to A.K.S.), the National Institute of Neurological Disorders and Stroke (RO1 NS 043507 RO1 NS54780 to A.K.S.), and the Department of Veterans Affairs (VA Merit Review Award to A.K.S.). We thank Dr Bing Shuai for excellent technical assistance in this study.
Abbreviations
-
- ABC
-
- avidin-biotin complex
-
- ACSF
-
- artificial cerebrospinal fluid
-
- BrdU
-
- 5′-bromodeoxyuridine
-
- DCX
-
- doublecortin
-
- DG
-
- dentate gyrus
-
- F344
-
- Fischer 344
-
- FGF-2
-
- fibroblast growth factor-2
-
- FGFR
-
- FGF receptor
-
- GCL
-
- granule cell layer
-
- GFAP
-
- glial fibrillary acidic protein
-
- NGS
-
- normal goat serum
-
- NHS
-
- normal horse serum
-
- NSC
-
- neural stem/progenitor cell
-
- PB
-
- phosphate buffer
-
- PBS
-
- phosphate-buffered saline
-
- SGZ
-
- subgranular zone
-
- TBS
-
- Tris-buffered saline.