Small-conductance Cl– channels contribute to volume regulation and phagocytosis in microglia
Abstract
The shape and volume of microglia (brain immune cells) change when they activate during brain inflammation and become migratory and phagocytic. Swollen rat microglia express a large Cl– current (IClswell), whose biophysical properties and functional roles are poorly understood and whose molecular identity is unknown. We constructed a fingerprint of useful biophysical properties for comparison with IClswell in other cell types and with cloned Cl– channels. The microglial IClswell was rapidly activated by cell swelling but not by voltage, and showed no time-dependence during voltage-clamp steps. Like IClswell in many cell types, the halide selectivity sequence was I– > Br– > Cl– > F–. However, it differed in lacking inactivation, even at +100 mV with high extracellular Mg2+, and in having a much lower single-channel conductance: 1–3 pS. Based on these fundamental differences, the microglia channel is apparently a different gene product than the more common intermediate-conductance IClswell. Microglia express several candidate genes, with relative mRNA expression levels of: CLIC1 > ClC3 > ICln ≥ ClC2 > Best2 > Best1 ≥ Best3 > Best4. Using a pharmacological toolbox, we show that all drugs that reduced the microglia current (NPPB, IAA-94, flufenamic acid and DIOA) increased the resting cell volume in isotonic solution and inhibited the regulatory volume decrease that followed cell swelling in hypotonic solution. Both channel blockers tested (NPPB and flufenamic acid) dose-dependently inhibited microglia phagocytosis of E. coli bacteria. Because IClswell is involved in microglia functions that involve shape and volume changes, it is potentially important for controlling their ability to migrate to damage sites and phagocytose dead cells and debris.
Introduction
Patch-clamp studies have revealed the nearly ubiquitous presence of a swelling-activated chloride current, IClswell. Owing to its activation following exposure of cells to a hypotonic solution, the main function conjectured for IClswell is regulatory volume decrease (RVD). RVD involves coordinated activation of IClswell and a K+ channel(s), resulting in electroneutral KCl extrusion and passive water loss to restore cell volume (Roman et al., 1996; Mignen et al., 1999; Jentsch et al., 2002; d'Anglemont de Tassigny et al., 2003; Nilius & Droogmans, 2003; Sardini et al., 2003). Pharmacological studies support roles for IClswell in proliferation (Schumacher et al., 1995; Shen et al., 2000; Wondergem et al., 2001), migration (Ransom et al., 2001; Kim et al., 2004) and apoptosis (Okada et al., 2006). Several molecular candidates have been proposed as the volume-regulated anion channel (VRAC), with none gaining general acceptance. To help identify the endogenous channels, biophysical and pharmacological fingerprints are often constructed. IClswell have some general similarities in different cells, i.e. lack of voltage- and time-dependent activation, a requirement for intracellular ATP or a nonhydrolysable analogue, a broad permeability to anions (following Isenman sequence I, i.e. I– ≥ Br– ≥ Cl–) and mild outward rectification in symmetrical Cl– solutions. However, conflicting single-channel conductance values have been reported, from very small to intermediate outwardly rectifying (Jentsch et al., 2002; Nilius et al., 2003). Moreover, the degree of channel inactivation is highly variable and appears to depend on the cell type (Jentsch et al., 2002). Such differences might indicate heterogeneity in the molecular determinants. Two key problems in identifying heterologously expressed Cl– channels are the nearly ubiquitous expression of endogenous Cl– channels and the possible expression of multiple IClswell channels with some overlapping properties.
Microglia, the resident immune cells of the brain, possess a complex array of ion channels, including a swelling-sensitive Cl– channel that contributes to the membrane potential (Newell & Schlichter, 2005) and to proliferation (Schlichter et al., 1996) of rat microglia. A similar current in cultured murine microglia apparently helps maintain their ramified morphology (Eder et al., 1998), and supports chemokine-induced migration (Rappert et al., 2002). Although the same current is assumed to mediate volume regulation in microglia, this has never been tested and, because many properties of IClswell in microglia are unknown, limited comparisons could be made with previously described and cloned chloride channels. Furthermore, among the candidate genes proposed for IClswell, it is not known which are expressed in microglia. The present study was designed to fill several of these crucial knowledge gaps and to assess roles of this current in important microglia functions. First, we assessed several key properties of the current in order to facilitate comparisons with other cell types and cloned Cl– channels. Then, we quantitatively compared transcript expression of several Cl– channel genes that might underlie the current. Finally, we not only linked the microglial current to its classical function, regulatory volume decrease, but showed for the first time that it contributes to homeostatic volume regulation and to the essential microglia function of phagocytosis.
Materials and methods
Cell cultures
Microglia were isolated from brains of 2- to 3-day-old Wistar rats, as previously described (Fordyce et al., 2005; Newell et al., 2005; Kaushal et al., 2007). Rat pups were killed by cervical dislocation in accordance with guidelines from the Canadian Institutes of Health Research and the University Health Network. After carefully removing the meninges, whole brain tissue was mashed through a stainless steel sieve (100 mesh; Tissue Grinder Kit no. CD-1; Sigma), and then pelleted, re-suspended and seeded into flasks with Minimal Essential Medium containing 5% fetal bovine serum, 5% horse serum and 100 µm gentamycin (all from Invitrogen). Two days later, cellular debris, nonadherent cells and supernatant were removed and fresh medium was added to the flask. The mixed cultures were allowed to grow for 7–10 days and then shaken for 4 h on an orbital shaker at 8–10 Hz in a standard tissue culture incubator. The supernatant containing detached microglia was centrifuged and the cell pellet was resuspended for counting, and then plated according to the particular experiment. Microglia were plated at 3.5 × 104 cells per 15-mm-diameter glass coverslip for electrophysiology, and at 5.0 × 104 cells per well in 96-well (black-walled) plates (Corning, Acton, MA, USA) for the phagocytosis assay. Before experiments, the plated microglia were cultured for 1–3 days in Minimal Essential Medium with 100 µm gentamycin and a reduced serum concentration (2% fetal bovine serum) to decrease their spontaneous activation. This procedure yielded highly purified cultures of microglia (99–100%; see Fig. 4), as judged by labelling with FITC-conjugated isolectin B4 or tomato lectin (Sigma), or by immunofluorescence using the OX-42 monoclonal antibody (Serotec, Raleigh, NC, USA), which recognizes complement receptor 3. In addition, we previously demonstrated nearly 100% purity with quantitative real-time reverse transcriptase–polymerase chain reaction (qRT-PCR; Kaushal et al., 2007). From this unstimulated state, a variety of treatments can activate the microglia: activating NF-κB and p38 MAPK signalling pathways; up-regulating iNOS and production of reactive oxygen and nitrogen species; and causing them to kill healthy neurons (Fordyce et al., 2005; Kaushal et al., 2007).
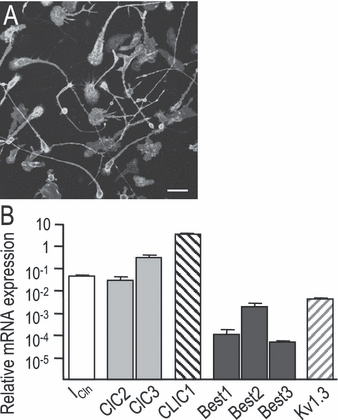
Expression of putative swelling-activated Cl– channel genes. (A) Demonstration of culture purity, showing that 100% of the cells in a representative microglia culture labeled with the microglia-specific marker, tomato lectin, conjugated to FITC. (B) Relative mRNA expression was monitored by qRT-PCR, normalized to the housekeeping gene HPRT-1 (see Materials and methods). Values shown are mean ± SD from four separate batches of microglia isolated from different rat litters. Scale bar, 25 µm.
Patch-clamp electrophysiology
Recordings were made in the whole-cell configuration with 4–5 MΩ-resistance pipettes pulled from thin-wall borosilicate glass capillaries (WPI, Sarasota, FL, USA). Currents were recorded with an Axopatch 200 integrating patch-clamp amplifier (Axon Instruments, Molecular Devices, Sunnyvale, CA, USA), digitized with a DigiData 1200 board, and acquired and analysed with pCLAMP version 8.0 software (Axon Instruments). The currents were filtered online using the low-pass Bessel filter of the amplifier at 5 kHz, except for noise analysis, when 10 kHz was used.
Whole-cell recordings were established with the standard bath solution, containing (in mm): NaCl, 125; KCl, 5; CaCl2, 1; MgCl2, 1; and HEPES, 10 (pH 7.4, 300 mOsm). Then, to minimize cation currents (e.g. the prevalent K+ currents) and isolate IClswell, the bath was changed to a Na+- and K+-free N-methyl-d-glucamine (NMDG+) solution (in mm: NMDG-Cl, 140; CaCl2, 1; MgCl2, 1; and HEPES, 10; pH 7.4, 300 mOsm). The pipette contained an NMDG+ solution (in mm): NMDG-Cl, 50; NMDG-aspartate, 70; CaCl2, 1; MgCl2, 1; HEPES, 10; EGTA, 10; and MgATP, 2 (pH 7.2, 300 mOsm), with low free Ca2+ (∼ 20 nm). The osmolarity of each solution was measured with a freezing-point depression osmometer and adjusted by adding sucrose, if necessary. IClswell was activated by applying a hypotonic solution (having 55% of the normal osmolarity, i.e. 165 mOsm) made with a 1 : 1 v/v dilution of the NMDG+ bath and a solution containing only (in mm): CaCl2, 1; MgCl2 1; and NMDG-HEPES, 10 (pH 7.4, 30 mOsm). For one type of experiment, a low-ionic-strength pipette solution was used in which the NMDG-aspartate was omitted and the osmolarity was adjusted with extra sucrose. Unless otherwise indicated, all chemicals were from Sigma-Aldrich (Oakville, ON, Canada).
Microglia on coverslips were rinsed with standard bath solution and mounted in the recording chamber, and recordings were made at room temperature. The junction potential between the bath and pipette solution before seal formation was −2 mV, and this value was not subtracted from the data in the figures. For most experiments (except for ionic selectivity; see below), the agar bridge was made with NaCl bath solution. This did not affect the junction potential when bath solutions were changed, except for the NMDG bath solution, for which the additional −4 mV junction potential was corrected in all recordings. For specific experiments, we used five classical Cl– channel blockers: the disulphonic stilbenes, 4,4′-diisothiocyanatostilbene-2,2′-disulphonic acid (DIDS) and 4-acetamido-4′-isothiocyanostilbene-2,2′-disulphonic acid (SITS); the indanylalkanoic acid IAA-94; and the fenamates, 5-nitro-2-(3-phenylpropylamino) benzoic acid (NPPB) and flufenamic acid (FFA; Sigma). We also tested an inhibitor of the ICln chloride channel, acyclovir (Furst et al., 2000), an inhibitor of the Na+–K+–Cl– symporter, bumetanide, and an inhibitor of the K+–Cl– cotransporter, [(dihydroindenyl)oxy]acetic acid (DIOA; Sigma).
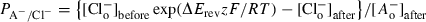 (1)
(1)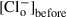 is the initial 74 mm Cl– concentration,
is the initial 74 mm Cl– concentration, 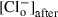 is the 4 mm Cl– concentration remaining after changing the external anion,
is the 4 mm Cl– concentration remaining after changing the external anion,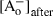 is the concentration of the test anion after the solution change and z is the valence. For each anion, several cells were tested and the permeability ratios were calculated and averaged. Importantly, for these experiments, a 3 m KCl agar bridge was used to prevent junction potential changes when the anion species was changed in the bath.
is the concentration of the test anion after the solution change and z is the valence. For each anion, several cells were tested and the permeability ratios were calculated and averaged. Importantly, for these experiments, a 3 m KCl agar bridge was used to prevent junction potential changes when the anion species was changed in the bath.qRT-PCR
Transcript levels were monitored by qRT-PCR, as previously described (Bustin & Nolan, 2004; Kaushal et al., 2007). Gene-specific primers (Table 1) were designed using the ‘Primer3Output’ program (http://frodo.wi.mit.edu/cgi-bin/primer3/primer3_www.cgi). RNeasy mini kits (Qiagen) were used to isolate RNA after degrading any contaminating DNA with DNaseI (0.1 U/mL, 15 min, 37 °C; Amersham Biosciences). A two-step reaction was performed according to the manufacturer's instructions (Invitrogen); i.e. total RNA (1 µg) was reverse-transcribed in a 20-µL volume using 200 U of SuperScriptII RNase H-reverse transcriptase, with 0.5 mm dNTPs (Invitrogen) and 0.5 µm oligo dT (Sigma). Amplification was performed on an ABI PRISM 7700 Sequence Detection System (PE Biosystems, Foster City, CA, USA) at 95 °C for 10 min, followed by 40 cycles at 95 °C for 15 s, 56 °C for 15 s and 72 °C for 30 s, ‘No-template’ and ‘no-amplification’ controls were included for each gene, and melt curves showed a single peak, confirming specific amplification (Bustin et al., 2004). The threshold cycle (CT) for each gene was determined and normalized against the housekeeping gene hypoxanthine guanine phosphoribosyl transferase (HPRT-1).
| Primer | Accession number | Sequence |
|---|---|---|
| HPRT-1* | NM_012583.2 | F: CAGTACAGCCCCAAAATGGT |
| R: CAAGGGCATATCCAACAACA | ||
| ClC-2 | X64139 | F: CCACCTTCTTCGCTGTTAGG |
| R: TTCTTCATCACGGTTCCACA | ||
| ClC-3 | XM_341428 | F: AGTGGAAAACATGGGCAGAG |
| R: GCAAAACTCAAAGCCCAAAA | ||
| CLIC1 | XM_345083 | F: GCTCCCGTTCCTGCTCTA |
| R: CGGGTTTGAGTTCTTGATGTAG | ||
| ICln | NM_031719 | F: AGGCGTCCGAACAGAAGA |
| R: CTGCTGGTGACAGCTTGC | ||
| Best1 | NM_001011940.1 | F: GTGGCAGAACAGCTCATCAA |
| R: CATCCCATCCACAGACAACA | ||
| Best2 | XM_344742.3 | F: ACCCCACTCCCTAGCATCTT |
| R: CCACTGGAAGGGAAGAACAC | ||
| Best3 | XM_001066317.1 | F: AGCGTATTTATGCCCAGGTG |
| R: GGCAGTGGAATGCTTGTGTA | ||
| Kv1.3 | M30312 | F: GCTCTCCCGCCATTCTAAG |
| R: TCGTCTGCCTCAGCAAAGT |
- * Housekeeping.
Monitoring volume regulation by flow cytometry
To examine the contribution of IClswell to regulating microglia volume, we measured forward light scatter using flow cytometry, as widely used (Downey et al., 1995; Ormerod et al., 1995; Khanna et al., 1999). For these experiments, microglia were harvested from the supernatants of flasks immediately after shaking for 4–8 h at 37 °C in the incubator. After harvesting the cells, they were suspended in the standard bath solution (same as for patch clamping) in the absence or presence of inhibitors of the Cl– channels or transporters (see above). For each condition and time-point, three replicate tubes containing microglia were sampled. After ∼ 10 min incubation with each inhibitor, a baseline reading was taken for each condition. The 30 mOsm dilution buffer (see above) was then added to each of the remaining samples (final osmolarity ∼ 55% of normal; ∼ 165 mOsm). Thus, because a 55% hypotonic dilution solution caused a reproducible swelling and a small but readily detectable RVD without cell death, it was used for subsequent experiments. The remaining samples were run in triplicate at specific times after exposure to the hypotonic solution. Experiments were conducted entirely at room temperature or the cells were maintained at 37 °C by keeping the tubes in a water bath until used. For each statistical replicate, volume regulation assays were performed on a batch of cells prepared from a different rat litter. Because the forward scatter values obtained by the flow cytometer are arbitrary, for each replicate they were normalized to untreated cells in the standard bath solution.
Phagocytosis assay
Microglia phagocytosis of bacteria (E. coli, labelled with FITC) was assessed using the Vybrant Phagocytosis Assay Kit according to the manufacturer's instructions (V-6694; Molecular Probes). In brief, fluorescent E. coli in suspension were added to each test well for 1 h, with or without a chloride channel blocker: NPPB or FFA. The E. coli suspension was then removed by vacuum aspiration, followed immediately by a 1-min treatment with trypan blue to quench the fluorescence of any E. coli adhering to the outside of the microglia. The total fluorescence of each well was then measured using a fluorescence plate reader (SPECTRAmax Gemini EM; Molecular Devices) at 480 nm excitation and 520 nm emission wavelengths. Each treatment was run in triplicate on the same plate and averaged to yield a single statistical replicate (n). For statistical comparisons, experiments were repeated on cells isolated from different rat litters. Phagocytosis indices were normalized to the solvent control (0.2% DMSO), which was the highest concentration used. The solvent control and untreated cells did not differ (P = 0.50, one-sample t-test).
Statistics
All statistics (Student's t-tests and anova) and curve fitting were conducted using Origin ver7.0 software (OriginLab, Northampton, MA, USA). To test for significant differences between anion permeabilities, and between relative mRNA levels, one-way anovas were used, followed by Tukey's test for multiple comparisons.
Results
Hypotonic shock activated a Cl– conductance
To investigate Cl– currents in rat microglia, both Na+ and K+ from the standard bath and pipette solutions were replaced with the bulky cation NMDG+. This eliminated several cation currents that are characteristic of microglia (Kv1.3, Kir2.1 and TRPM7), leaving an extremely small remaining current when the solutions were isotonic and external Cl– was 144 mm(Fig. 1A and B, trace 1). Then, when the hypotonic solution was perfused into the bath (55% normal osmolarity), a large current developed over the next couple of minutes; this current was outwardly rectifying despite similar internal (54 mm) and external (74 mm) Cl– concentrations. The volume sensitivity was further demonstrated by its decrease when the original isotonic solution was restored. These properties are entirely consistent with our initial description of this current in rat microglia (Schlichter et al., 1996). We also found that a 20% hypotonic solution activated the current, but more slowly and with a much more variable amplitude (not shown). The Cl– current activated spontaneously when a low ionic strength pipette solution was used (Fig. 1C). In this case, the current activated rapidly after break-in, reached a peak by ∼ 5 min and then spontaneously ran down by ∼ 25 min. Its subsequent volume sensitivity was confirmed by applying a hypertonic NMDG bath solution (350 mOsm), after which the current declined very rapidly. For the remainder of this study, we used the 55% hypotonic bath solution to activate the current because it produced a rapid response with a sufficiently stable plateau phase for testing treatments. Nevertheless, the amplitude was highly variable; i.e. after 5 min in this solution the mean current was 886 ± 561 pA (current density 42 ± 26 pA/pF; mean ± SD; n = 11). Of note, the amplitude did not simply correlate with cell size as measured by the whole-cell capacitance (R2 = 0.13; P > 0.2; n = 11). Its identity as a Cl– current was confirmed (Fig. 1B, inset) by a reversal potential (−12 mV) close to the Cl– Nernst potential (−10 mV), and a shift to −21 mV when the external Cl– concentration was increased from 74 to 144 mm (Nernst potential, −25 mV). All reversal potentials were calculated using the chloride activity coefficient. Note also the brief increase in current resulting from the increase in external Cl–. This was thus a swelling-activated Cl– current, and the next question was whether it was the same as IClswell in other cell types.
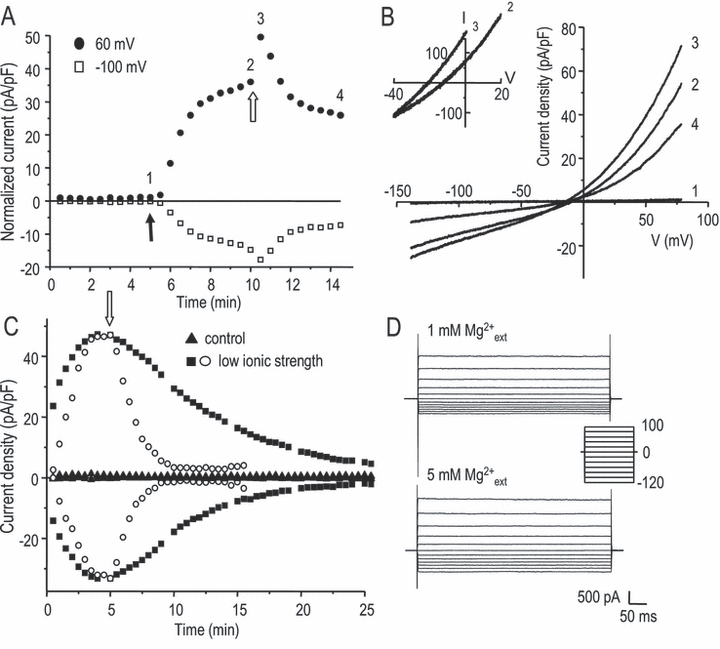
Activation of an osmosensitive Cl– current, IClswell, in primary rat microglia. (A) Representative whole-cell currents recorded in Na+- and K+-free solutions at +60 and −100 mV. After a 55% hypotonic bath solution was perfused in (closed arrow), a large current developed; this was then reduced after a slightly hypertonic bath solution (350 mOsm) was applied (open arrow). The transient increase after the open arrow is explained in the text. (B) Current-vs-voltage relationships measured at the time points indicated in panel A. Note the very small background current under isotonic conditions (trace 1), activation of an outwardly rectifying current by 55% hypotonic solution (74 mm Cl–, trace 2) and increase in the current immediately after the external Cl– concentration was increased (trace 3), i.e. after a hypertonic (∼ 350 mOsm) NMDG bathing solution with 144 mm Cl– was perfused in. The expanded traces (inset) show the shift in reversal potential when external Cl– was changed from 74 to 144 mm, providing evidence for its Cl– dependence. (C) Spontaneous activation of the current by low intracellular ionic strength. The pipette solution had the same Cl– concentration (54 mm), but the 70 mm NMDG-aspartate was replaced with sufficient sucrose to balance the osmolarity. Under these conditions, with the normal osmolarity bath solution (▪), the Cl– current developed and then ran down spontaneously. This current was more rapidly inhibited if a hypertonic bath solution (350 mOsm; open arrow) was perfused in (○). When both the osmolarity and ionic strength of the pipette solution were normal (), only a small background current was present. (D) Lack of voltage-dependent inactivation with either 1 or 5 mm extracellular Mg2+. Traces show a family of whole-cell currents at the time of maximal activation after applying the 55% hypotonic solution. Voltage steps were applied between −120 and +100 mV, from a holding potential of 0 mV.
Biophysical properties of IClswell in microglia
To assess whether the microglial IClswell is likely to be the same molecular entity as in other cell types, it is useful to compare biophysical properties that are most likely to arise from the protein sequence and contribute to a molecular fingerprint. IClswell in most cells inactivates at depolarized potentials (usually above +40 mV), and one cloned channel (ClC-2) shows voltage-dependent activation at hyperpolarized potentials (see Discussion). We found that the microglial whole-cell current had a current–voltage relation that was outwardly rectified but did not show voltage-dependent or time-dependent activation or inactivation (Fig. 1D). Because the degree of inactivation in some cells is affected by extracellular Mg2+, we supplemented the hypotonic bath solution with 4 mm MgCl2 (total Mg2+, 5 mm) after IClswell had reached its maximal amplitude (Fig. 1D). The elevated Mg2+ did not confer inactivation, even at +100 mV; thus, the microglial current apparently lacked voltage-dependent inactivation, and in this way differed from IClswell in many other cells.
Cl– channel selectivity among halide ions is a useful biophysical fingerprint, but has not been reported for the microglial current. We determined the channel's relative permeability to different anions under bi-ionic conditions when external Cl– was replaced with a test anion after IClswell had reached its maximal amplitude (Fig. 2). Using a modified Goldman–Hodgkin–Katz equation (see Eqn 1 in Materials and methods), the permeability of each test anion vs. chloride was calculated by measuring changes in reversal potential and averaged. The permeability sequence was I– = Br– > Cl– > F– > aspartate ≥ glutamate, where ‘>’ denotes a significant difference (P < 0.05) and ‘≥’ indicates a trend that did not reach statistical significance. This halide permeability sequence corresponds with Eisenman's sequence I, and is similar to IClswell in many other cell types.
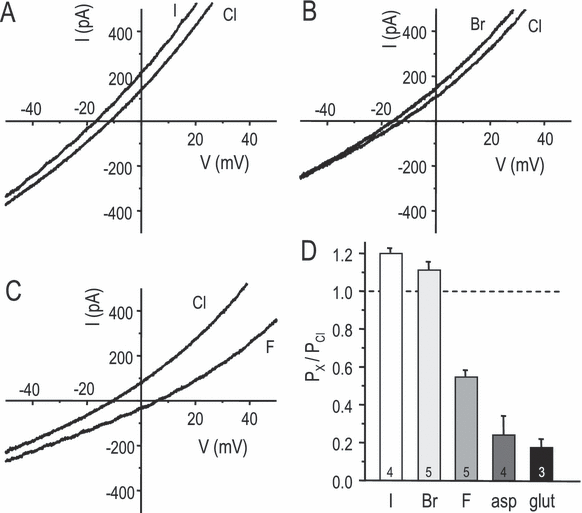
Anion selectivity of the swelling-activated Cl– current. (A–C) Representative traces showing the change in reversal potential when extracellular Cl– was replaced by I–, Br– or F–. (D) Summary of anion permeability relative to Cl– (mean ± SD; n indicated on each bar), calculated using a modified version of the Goldman–Hodgkin–Katz equation (see Eqn 1 in Materials and methods). The change in reversal potential was measured when Cl– in the extracellular solution was replaced with the test anion. All pair-wise comparisons were statistically significant (one-way anova, followed by Tukey's test; P < 0.05) except I– vs. Br– and aspartate vs. glutamate.
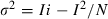 (2)
(2)
Single-channel conductance from noise analysis of IClswell. (A) A representative recording beginning immediately after the 55% hypotonic solution was perfused into the bath. Run-up of IClswell is seen during repeated voltage-clamp steps to +50 mV and then to −90 mV, followed by a ramp from −120 to +80 mV from a holding potential of 0 mV. This protocol was run every 5 s until the current reach its maximum amplitude; some traces have been omitted for clarity. For stationary noise analysis, the mean current and variance was calculated during each step to +50 mV. (B) In the same cell, an alternative method was used for stationary noise analysis. After IClswell had reached a plateau, the current was monitored at +50 mV as it was progressively blocked by a slowly increasing concentration of the Cl– channel blocker SITS (arrow). The mean current and variance were calculated from such records after correcting for baseline drift. (C) Mean-vs.-variance plots comparing the two methods on the same cell. Each data set (from panels A and B) was fitted to Eqn 2 (see Results). For this cell, the single-channel conductance was calculated as 1.10 pS during IClswell run-up (▪) and 3.15 pS from the SITS titration protocol (○).
It was important to compare this analysis with another approach because the assumption that current run-up represents a graded increase in open probability appears to be invalid for IClswell in some cells (Jackson & Strange, 1995b; Boese et al., 1996). In those studies, it was possible to also perform nonstationary noise analysis during the pronounced voltage-dependent inactivation of IClswell at highly positive potentials, and to compare results with stationary noise analysis done on current run-up. Their analysis showed unambiguously that run-up involves an increase in the number of active channels and that the single-channel conductance in C6 glioma cells is intermediate, with outward rectification (Jackson et al., 1995b). The same approach was not possible in the present study, because IClswell in microglia lacks voltage-dependent inactivation. In this, the microglia current is much like the current in T lymphocytes, which also did not inactivate and had an apparent single-channel conductance < 1 pS (Schumacher et al., 1995).
In our second approach, we used a chloride channel blocker (SITS). The main assumption was that an increase in blocker concentration reduces the IClswell amplitude by reducing the open probability. The blocker should have intermediate on–off rates; if the dissociation rate is too slow, the ‘apparent’ number of active channels will decrease and, if too fast, the ‘apparent’ single-channel conductance will decrease. If this assumption is met and the blocker concentration is increased slowly enough, the resulting current will be quasi-stationary for short time periods, allowing calculation of the single-channel conductance from the resulting mean-vs.-variance plot. To implement this approach, IClswell was first activated and stationary noise analysis performed on the current run-up (as above), and then a solution containing 2 mm SITS was added stepwise (∼ 10 µL/pulse added to the ∼ 400 µL bath) to gradually increase its concentration (Fig. 3B). Because SITS exhibited time- and depolarization-dependent block (Lewis et al., 1993; Nilius et al., 2003; present study), the membrane potential was continuously held at +50 mV to obtain a quasi-stationary record. Thus, as the drug concentration increased, the block remained near equilibrium. The mean current-vs.-variance plot of each record was constructed and compared with the stationary noise analysis from current run-up in the same cell (Fig. 3C). From the SITS titration method, the calculated single-channel conductance was 3.54 ± 1.31 pS (mean ± SD; n = 3); that is, it remained low but was significantly higher than from noise analysis during current run-up (P < 0.001, two-sample t-test). Because of this difference, we verified that the filtering bandwidth and duration of sampling were appropriate for measuring the SITS-induced variance, as follows. For the data above, we used a 20-kHz sampling rate and a 10-kHz low-pass filter, and the variance was sampled for periods of 1000 ms each as the SITS concentration slowly increased. Importantly, we found that the calculated single-channel conductance was not affected by decreasing the filter cutoff to 3000, 1000 or 500 Hz, or reducing the sampling period to 750 or 500 ms. Thus, even the higher concentrations of SITS appeared to change the probability of opening, rather than the ability to resolve single-channel currents. Lower frequencies or shorter durations decreased the apparent single-channel conductance, by ∼ 40% at 10 Hz and ∼ 50% at 100 ms.
Expression of putative IClswell genes
With this biophysical fingerprint in hand, we next monitored the relative expression of genes that have been proposed to underlie swelling-activated Cl– currents. qRT-PCR was used to compare mRNA expression levels in cultures of microglia that were shown to be essentially 100% pure (Fig. 4), after normalizing to the housekeeping gene, HPRT-1. Several known or putative Cl– channel genes were detected, with the following order of mRNA expression: CLIC1 > ClC3 > ICln ≥ ClC2 > Best2 > Best1 ≥ Best3 > Best4. For comparisons of transcript levels, ‘>’ denotes a significant difference (P < 0.05) and ‘≥’ indicates a trend that did not reach statistical significance. The potassium channel, Kv1.3, was included because it is a key candidate for the K+ component of the K+, Cl– and water loss during RVD. Kv1.3 is also a useful comparator as we can estimate the number of active Kv1.3 channels from our previous studies as 500–1000/cell, calculated by dividing the whole-cell Kv1.3 conductance (5–10 nS; Newell et al., 2005) by the single-channel Kv1.3 conductance (∼ 10 pS; Pahapill & Schlichter, 1992). Several of the genes were expressed at higher levels than Kv1.3 (CLIC1, ICln, ClC-2, ClC-3) and CLIC1 expression was higher than that of the housekeeping gene HPRT-1.
IClswell contributed to volume regulation in microglia
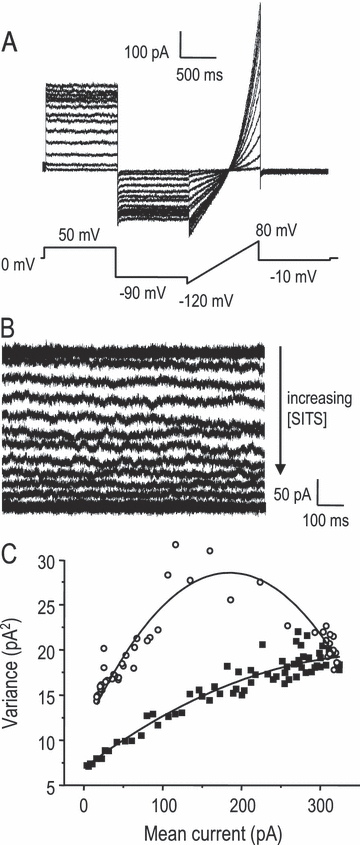
Because there are no potent, selective blockers of IClswell, the first step was to identify compounds for use in the functional assays (Fig. 5). We tested three classical Cl– channel blockers (IAA-94, NPPB and FFA), and inhibitors of ICln (acyclovir; Furst et al., 2000), the Na+–K+–Cl– symporter (bumetanide) and the K+–Cl– cotransporter (DIOA). For each drug, the concentration was chosen to produce > 50% inhibition of their respective targets. Effects of each drug on IClswell were determined at −50 mV, the approximate resting membrane potential of cultured microglia (Newell et al., 2005). For NPPB, IAA-94 and FFA, the predicted extent of IClswell block was ≥ 70% at the concentrations tested (Schumacher et al., 1995; Schlichter et al., 1996), and we observed nearly complete block of IClswell by 150 µm NPPB and 300 µm FFA, and ∼ 70% block by 500 µm IAA-94 (Fig. 5A). Essentially complete inhibition is predicted for ICln by acyclovir (Furst et al., 2000), the K+–Cl– cotransporter by DIOA, and the Na+–K+–Cl– symporter by bumetanide (Taouil & Hannaert, 1999), but block of IClswell was not anticipated. As expected, IClswell was not affected by 100 µm acyclovir (4 ± 5%, n = 4) or 10 µm bumetanide (−9 ± 11%, n = 3) but, surprisingly, 40 µm DIOA inhibited the current by 35 ± 9% (SD; n = 3). A similar DIOA concentration blocked an IClswell in nonpigmented ciliary epithelial cells (Botchkin & Matthews, 1995).
Blockers of IClswell inhibited homeostatic volume regulation. (A) Representative whole-cell current traces in response to voltage ramps during the plateau phase of the swelling-activated current, IClswell, to show effects of each compound used to study microglial volume regulation. Arrows indicate currents in the presence of each compound. IClswell was reduced by three well-known Cl– channel blockers with diverse chemical structures (150 µm NPPB, 300 µm FFA and 500 µm IAA-94) and, surprisingly, by the K+–Cl– cotransport inhibitor DIOA (40 µm). The current was not affected by either the Na+–K+–Cl– cotransport inhibitor bumetanide (10 µm) or the ICln inhibitor acyclovir (100 µm). (B) Isotonic volume regulation at room temperature. Ten minutes after applying each of the compounds in panel A to microglia in the isotonic bath solution, their volume (mean ± SD) was measured and normalized to drug-free controls. The hatched bars indicate classical Cl– channel blockers. Statistical differences from the controls were assessed by a one-way anova, followed by Tukey's test (*P < 0.05) for the number of replicates indicated on each bar.
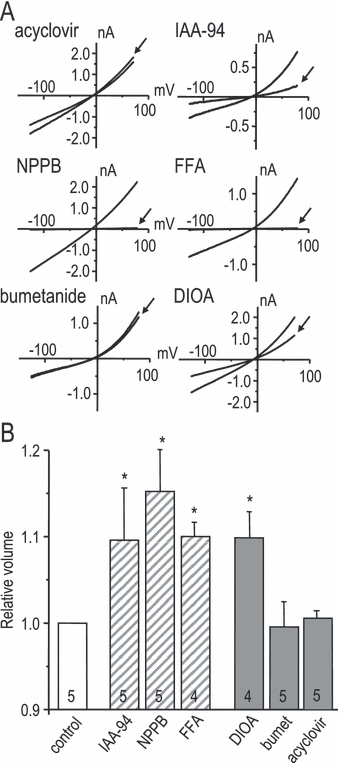
Before conducting RVD experiments, we used flow cytometry (see Materials and methods) to examine the normal volume of microglia suspended in an isotonic solution, and made a surprising observation: all compounds that reduced the current (IAA-94, NPPB, FFA and DIOA) significantly increased the baseline cell volume (measured 10 min after drug addition; Fig. 5B). Conversely, the compounds that did not affect the current (acyclovir and bumetanide) had no effect on homeostatic cell volume. RVD is usually considered the primary function of IClswell, and is recorded as cell swelling in hypotonic solution followed by a spontaneous return toward the initial volume. When transferred to 55% hypotonic solution, microglia swelled within 5 min to ∼ 155% of their original volume at room temperature, and ∼ 140% at 37 °C (Fig. 6A). RVD was slow and incomplete at both temperatures, with ∼ 26% recovery by 30 min after the hypotonic shock at room temperature. Again, the drugs that reduced IClswell inhibited volume recovery (IAA-94, NPPB, FFA and DIOA), while acyclovir and bumetanide had no effect (Fig. 6B). FFA reduced RVD by ∼ 50% but did not reach statistical significance based on the four experiments.
Blockers of IClswell inhibited the RVD. (A) Summary of the time course of cell swelling and spontaneous volume recovery after applying a 55% hypotonic solution at room temperature (▪) or at 37 °C (○). Values were normalized to the resting volume in isotonic solution and plotted as mean ± SD. (B) Effects of the compounds used in Fig. 5 on microglial RVD measured at room temperature. For each treatment, RVD was calculated as the difference in the relative volume at 5 min vs. 30 min after applying the hypotonic solution. The results were then normalized to drug-free control cells and plotted as mean ± SD. The hatched bars indicate classical Cl– channel blockers. Statistical differences from controls were assessed by a one-way anova, followed by Tukey's tests (*P < 0.05) for the number of replicates indicated on each bar.
IClswell contributed to phagocytosis
Phagocytosis is an important function of microglia and involves dramatic shape changes, probably accompanied by volume changes. Thus, given the role of IClswell in both homeostatic volume regulation and RVD, one might anticipate a role in phagocytosis. The two most effective IClswell blockers (NPPB and FFA) were tested while monitoring phagocytosis of fluorescent-labelled E. coli for 1 h at 37 °C. After quenching the fluorescence of any adhering E. coli (see Materials and methods), internalized bacteria were seen in most microglia (Fig. 7A). The assay was then conducted in multiwell plates, and showed that both IClswell blockers inhibited phagocytosis in a dose-dependent manner. The IC50 values were 13 µm for NPPB and 31 µm for FFA (Fig. 7B). These values compare quite well with the IC50 values we previously measured for IClswell block in microglia (∼ 30 µm for NPPB and ∼ 80 µm for FFA; Schlichter et al., 1996) and the concentrations needed to block the similar Cl– current in T lymphocytes (Schumacher et al., 1995), and argue for a specific role of IClwell in microglial phagocytosis.
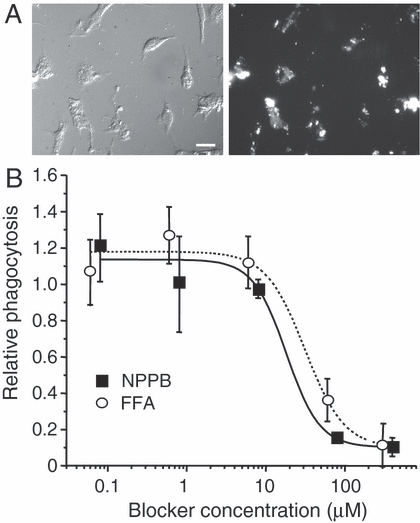
IClswell blockers inhibited phagocytosis. (A) Micrographs of microglia after exposure to fluorescent-labelled E. coli. Left, DIC image; right, fluorescence image of same field showing phagocytosed bacteria. (B) The Cl– channel blockers FFA and NPPB inhibited phagocytosis in a dose-dependent manner. Phagocytosis at different blocker concentrations was normalized to the DMSO control, and each point is the average of three replicates (mean ± SD). From fits to sigmoidal functions, the IC50 values for inhibiting phagocytosis were 31 µm for FFA and 18 µm for NPPB. Scale bar, 25 µm.
Discussion
Comparison with IClswell in other cells
Table 2 summarizes several salient features of IClswell in rat microglia and compares them with other cells (for recent reviews on IClswell, see Jentsch et al., 2002; d'Anglemont de Tassigny et al., 2003; Nilius et al., 2003; Sardini et al., 2003). The microglial current is similar in having time- and voltage-independent activation, a halide ion selectivity sequence of I– = Br– > Cl– > F–, and sensitivity to the blockers NPPB and FFA. Some IClswell can be activated by reduced intracellular ionic strength without cell swelling (Nilius et al., 1998), and this was true for the microglial current. Differences in pharmacology include poor block of the microglial current by SITS or DIDS (and no block by up to 10 mm external ATP; our unpublished observations). Biophysical differences include lack of inactivation and a smaller single-channel conductance. Most IClswell exhibit voltage-sensitive inactivation (Jackson & Strange, 1995a; Nilius et al., 2003), although the rate and degree vary (Jentsch et al., 2002), and may depend on extracellular Mg2+ (Braun & Schulman, 1996; Voets et al., 1997). Microglia lack inactivation, even at strongly depolarized potentials, and with the highest divalent cation concentration used in previous IClswell studies. To help identify the underlying channel, the single-channel conductance is often calculated from noise analysis of whole-cell currents; however, some studies found that the critical assumption that IClswell activation is due to a graded increase in Po was violated (Jackson et al., 1995b; Boese et al., 1996). From the differences in single-channel conductance based on stationary noise analysis during run-up (< 1 pS) and nonstationary analysis during inactivation (15 pS at 0 mV), it was concluded that current activation was due to a graded increase in the number of active channels, not in Po (Jackson et al., 1995b). Outward rectification and similar values (15–40 pS) have been observed from single-channel studies in myocytes and epithelial cells (Meyer & Korbmacher, 1996; Wang et al., 2005). As the microglial IClswell does not inactivate, we compared mean-vs-variance analysis with a new approach wherein Po was decreased by increasing the SITS concentration; both methods yielded a small conductance (∼ 1 and 3 pS). Overall, IClswell in microglia most resembles a current in human lymphocytes which has the same selectivity sequence, a small single-channel conductance and a similar pharmacological profile, which lacks inactivation and which can be activated by low ionic strength (Lewis et al., 1993; Schumacher et al., 1995).
| Microglia | Comments and comparisons |
|---|---|
| Voltage dependence | |
| Rectification: mildly outward rectifying1,2,3 | Many IClswell are more outwardly rectifying,6–9microglia are similar to T lymphocytes10, 11 |
| Activation: not time- or Vm-dependent1,2,3 | Similar to most IClswell6–9 |
| Activated by low internal ionic strength without swelling1 | Similar to some IClswell12 |
| Inactivation: none1,2, even at +100 mV, with or without high Mg2+o1 | Variable; some increased by high Mg2+o,13,14microglia are similar to T lymphocytes10, 11 |
| Single-channel conductance | |
| During run-up: 0.72 ± 0.17 pS1 | Some IClswell are outward rectifying (∼ 15–40 pS): during inactivation4,5 or from single-channel recordings15,16 |
| During SITS block: 3.54 ± 1.31 pS1 | Not tested on other IClswell |
| Permeability sequence | |
| I– ≥ Br–> Cl– > F– > aspartate ≥ glutamate1,11 | Eisenman sequence I (for halides); similar to many IClswell6–9 |
| Channel blockers | |
| ATPo | ATPo blocks many IClswell6–9 but not microglia |
| NPPB, IC50 ∼ 30 µm | NPPB blocks many IClswell6–9 |
| IAA-94, IC50 ∼ 200 µm2 | |
| FFA, IC50 ∼ 80 µm2 | FFA blocks many IClswell6–9 |
| DIDS, IC50 = 16.1 µm at +40 mV3 | Vm-dependent block 1,2; higher IC50 expected at –ve Vrest |
| SITS, IC50 = 71 µm at +40 mV3 | Most IClswell are more sensitive6–9 |
| DIAO (35 ± 9% block at 40 µm)1 | Not tested on other IClswell |
| Volume regulation: volume vs. control, isotonic1 and RVD1,2 (isotonic volume regulation not tested for other IClswell) | |
| Acyclovir (100 µm) | 1.00 (isotonic) and 1.03 (RVD) |
| IAA-94 (500 µm) | 1.10 (isotonic) and 0.46 (RVD) |
| NPPB (150 µm) | 1.15 (isotonic) and −0.10* (RVD) |
| FFA (300 µm) | 1.10 (isotonic) and 0.50 (RVD) |
| Bumetanide (10 µm) | 1.00 (isotonic) and 1.13 (RVD) |
| DIOA (40 µm) | 1.10 (isotonic) and 0.30 (RVD) |
| Inhibition of phagocytosis (role in phagocytosis not tested for other IClswell) | |
| NPPB, IC50 = 13 µm | |
| FFA, IC50 = 31 µm | |
- References: 1present study; 2Schlichter et al. (1996); 3Eder et al. (1998); 4Jackson et al. (1995b); 5Boese et al. (1996); 6Jentsch et al. (2002); 7d'Anglemont de Tassigny et al. (2003); 8Nilius et al. (2003); 9Sardini et al. (2003); 10Lewis et al. (1993); 11Schumacher et al. (1995); 12Nilius et al. (1998); 13Braun et al. (1996); 14Voets et al. (1997); 15Meyer et al. (1996); 16Wang et al. (2005). *The negative sign (−0.10) indicates an increase in volume.
Molecular candidates for the microglia swelling-activated channel
The identity of IClswell has long been debated, and it is often assumed that VRACs are the same gene product. However, the microglial IClswell appears to be a different entity based on differences in inactivation, single-channel conductance and pharmacology. It is thus valuable to compare the properties of the several candidate genes expressed in rat microglia. pICln is no longer considered a candidate (Jentsch et al., 2002; d'Anglemont de Tassigny et al., 2003), although it might regulate IClswell (Chen et al., 1999). We ruled out a contribution of ICln to the microglial current and volume regulation, as they were not affected by the ICln inhibitor acyclovir (Furst et al., 2000). ClC-2 is a small-conductance channel (∼ 2 pS; Weinreich & Jentsch, 2001) like the microglial IClswell, and is activated by cell swelling (Grunder et al., 1992), but differs in having a Cl– > Br– > I– permeability sequence and hyperpolarization-induced activation (Thiemann et al., 1992; Nilius et al., 2003). The properties of ClC-3 have been very difficult to determine as it is ubiquitously expressed, but it has been proposed that ClC-3 produces a highly outward-rectified current, with a Cl– > Br– > I– permeability sequence (Li et al., 2000; Jentsch et al., 2002), which is inconsistent with the microglial VRAC. Although it has been proposed that ClC-3 is a VRAC displaying inactivation and intermediate conductance (Duan et al., 1997; Wang et al., 2003), its properties, membrane expression and roles have been extensively debated (e.g. Li et al., 2000; Stobrawa et al., 2001; Nilius et al., 2003; Wang et al., 2005).
The contribution of the chloride intracellular channel (CLIC) family is controversial. Some investigators have proposed that soluble cytoplasmic CLIC1 molecules move into the plasma membrane (Tulk et al., 2000; reviewed in Ashley, 2003), while others doubt that it is a Cl– channel (Hartzell et al., 2005). Based on heterologous expression of CLIC1, its biophysical properties are also controversial, with a reported single-channel conductance from 6 to 160 pS, and selectivity sequences of F– > Cl– > I– or Br– ≈ Cl– > I– (reviewed in Ashley, 2003). Recently, CLIC1 was reported to form a small-conductance (6–7 pS) plasma membrane channel in rat microglia and the BV2 microglial cell line (Novarino et al., 2004). Although we observed higher expression of CLIC1 mRNA than the other Cl– channel genes examined, three observations argue against it encoding the microglial IClswell. First, single channels attributed to CLIC1 were seen in cell-attached recordings from microglia but no current was seen in whole-cell recordings (Novarino et al., 2004), whereas we found that IClswell could be activated by cell swelling for many minutes after going whole-cell. Second, the current from cloned CLIC1 is blocked by ≤ 10 µm IAA-94 (Valenzuela et al., 2000; reviewed in Ashley, 2003), while 500 µm produced only ∼ 70% block in the present study. Third, the limited information on the permeability sequence of cloned CLIC1 (reviewed in Ashley, 2003) is clearly at odds with our results.
We believe that the best candidate for the microglial IClswell lies within the recently discovered bestrophin gene family (reviewed in Hartzell et al., 2005). Transcripts for Best1, 2, 3 and 4 were found in rat microglia and, although their properties have not been well characterized, several features are apparently similar to the microglial current. Similarities to the microglial current include: (i) hBestl and mBest2 produce volume-sensitive Cl– currents with no voltage-dependent inactivation (reviewed in Fischmeister & Hartzell, 2005; Hartzell et al., 2005); (ii) two Xenopus bestrophins have a permeability sequence of I– > Br– > Cl– (Qu et al., 2003; Pifferi et al., 2006); (iii) the Drosophila gene, dBestl, has a conductance of 2 pS (Chien et al., 2006); and (iv) bestrophins are sensitive to high concentrations of SITS (complete block at 2 mm) and DIDS (∼ 50% block at 500 µm; Sun et al., 2002; Pifferi et al., 2006). One possible difference is the reported sensitivity of some cloned bestrophins to intracellular Ca2+ however, it is unclear whether any or all bestrophins require elevated Ca2+. Before attempting to use siRNA-mediated knockdown to help identify the channel, more information is needed about the biophysical and pharmacological properties of the cloned channels in mammalian cells. In addition, we have found that siRNA-mediated knockdown is extremely inefficient in primary microglia. That is, despite testing many methods (> 15 different transfection reagents, several retroviral and lentiviral constructs, electroporation and Amaxa™ nucleofection), none yielded effective transfection or infection, and most treatments damaged the microglia.
Roles of Cl– channels in microglia functions
Given the numerous candidate genes in rat microglia and the relatively poor pharmacological tools available (e.g. compared with K+ channel blockers), it has been difficult to ascribe a specific physiological function to a cloned Cl– channel. IClswell (Schlichter et al., 1996; present study) and a stretch-sensitive Cl– channel in microglia (Eder et al., 1998) have similar outward rectification, voltage-independent activation, lack of inactivation, and pharmacological profile; thus, we speculate that they are both mediated the same member of the bestrophin family. In contrast, little is known about a chemokine-induced Cl– current (Rappert et al., 2002) and, as explained above, the CLIC1-like channels (Novarino et al., 2004) are very likely a different gene product.
The present study adds important information about the roles of Cl– channels in microglia. The most widely studied role of Cl– channels in immune cells is the RVD that follows cell swelling. Our results support a role in RVD but also provide the first evidence that the current is involved in homeostatic volume regulation. Both forms of volume regulation were affected by the same three blockers of IClswell (NPPB, FFA and IAA-94) and, surprisingly, by the K+–Cl– cotransport inhibitor DIOA, which we found also inhibits IClswell. The latter finding raises questions about studies that use DIOA to invoke a role for K+–Cl– cotransport in microglia (e.g. Schilling et al., 2004). [The pharmacology argues against involvement of ICln (no effect of acyclovir) or the Na+–K+–Cl– symporter (no effect of bumetanide).] The effect of Cl– channel blockade on the resting microglia volume implies that some Cl– channels are active under resting conditions. This contention is supported by our earlier finding that Cl– channels strongly regulate the microglial membrane potential and that, owing to the very high membrane resistance, a small Cl– current is sufficient (Newell et al., 2005). A role for IClswell in homeostatic volume regulation might also account for the ability of FFA and NPPB to inhibit morphological changes in murine microglia (Eder et al., 1998). Phagocytosis by microglia is an essential function both in developmental refinement of the healthy CNS and for removing invading organisms and damaged cells and debris in the damaged CNS. Very little is known about involvement of ion channels in phagocytosis. Acidification of the phagosome involves the Cl– channel, CFTR, but phagocytosis is not affected in Cftr-null mice (Di et al., 2006). ClC-3-knockout mice showed defective phagocytosis by neutrophils and, because ClC-3 is a vesicular protein, its mechanism was thought to be through intracellular signalling (Moreland et al., 2006). Here, the inhibition of phagocytosis of E. coli particles and IClswell by similar concentrations of FFA and NPPB provides the first evidence for involvement of an IClswell in phagocytosis, but the lack of highly selective blockers means we cannot rule out other Cl– channels. Nevertheless, the apparent involvement of IClswell in volume regulation and phagocytosis raises the intriguing possibility that these roles are linked because phagocytosis is accompanied by dramatic shape changes and an increase in cell volume (Bos & de Souza, 2001), especially as osmolyte efflux might compensate for phagocytosis-induced volume increases (Warskulat et al., 1996). This hypothesis is also consistent with inhibition of phagocytosis by Kupffer cells (liver macrophages) and a mouse macrophage cell line by hyperosmotic solutions (Warskulat et al., 1996), and inhibition by classical Cl– channel blockers of release of osmolytes during phagocytosis (Wettstein et al., 2000).
Acknowledgements
We are grateful to Xiaoping Zhu for excellent technical assistance. Funded by operating grants to LCS from the Canadian Institutes of Health Research (MT-13657) and the Heart & Stroke Foundation, Ontario chapter (T4670; T5782). E.W.N. was supported by a National Institutes of Health (USA), Kirschstein Predoctoral National Research Service Award (no. F31NS049742).
Abbreviations
-
- CLIC
-
- chloride intracellular channel
-
- DIDS
-
- 4,4′-diisothiocyanatostilbene-2,2′-disulphonic acid
-
- DIOA
-
- [(dihydroindenyl)oxy]acetic acid
-
- FFA
-
- flufenamic acid
-
- HPRT-1
-
- hypoxanthine guanine phosphoribosyl transferase
-
- IAA-94
-
- indanylalkanoic acid
-
- IClswell
-
- swelling-activated Cl– current
-
- NMDG+
-
- N-methyl-d-glucamine
-
- NPPB
-
- 5-nitro-2-(3-phenylpropylamino) benzoic acid
-
- qRT-PCR
-
- quantitative real-time reverse transcriptase–polymerase chain reaction
-
- RVD
-
- regulatory volume decrease
-
- SITS
-
- 4-acetamido-4′-isothiocyanostilbene-2,2′-disulphonic acid
-
- VRAC
-
- volume-regulated anion channel