Functional specificity of human premotor–motor cortical interactions during action selection
Abstract
Functional connections between dorsal premotor cortex (PMd) and primary motor cortex (M1) have been revealed by paired-pulse transcranial magnetic stimulation (TMS). We tested if such connections would be modulated during a cognitive process (response selection) known to rely on those circuits. PMd–M1 TMS applied 75 ms after a cue to select a manual response facilitated motor-evoked potentials (MEPs). MEPs were facilitated at 50 ms in a control task of response execution, suggesting that PMd–M1 interactions at 75 ms are functionally specific to the process of response selection. At 100 ms, PMd–M1 TMS delayed choice reaction time (RT). Importantly, the MEP (at 75 ms) and the RT (at 100 ms) effects were correlated in a way that was hand-specific. When the response was made with the M1-contralateral hand, MEPs correlated with slower RTs. When the response was made with the M1-ipsilateral hand, MEPs correlated with faster RTs. Paired-pulse TMS confined to M1 did not produce these effects, confirming the causal influence of PMd inputs. This study shows that a response selection signal evolves in PMd early during the reaction period (75–100 ms), impacts on M1 and affects behaviour. Such interactions are temporally, anatomically and functionally specific, and have a causal role in choosing which movement to make.
Introduction
Paired-pulse transcranial magnetic stimulation (TMS), in which one TMS pulse is applied over each of two brain areas, makes it possible to interrogate functional cortical interactions on a subsecond timescale. In the motor system, paired-pulse TMS studies exploit the fact that stimulation of primary motor cortex (M1) elicits a motor-evoked potential (MEP), which can be used as an index of M1 excitability. One can test the causal influence of a connected area on M1 by first stimulating that area, and measuring consequent changes in the amplitude of MEPs (Ferbert et al., 1992; Netz et al., 1995; Di Lazzaro et al., 1999). The technique is thus complementary to combined TMS/neuroimaging approaches (Lee et al., 2003; Bestmann et al., 2005; O'Shea et al., 2007a), but its particular advantage is that it can reveal with subsecond resolution how activity changes in one brain area causally impact on activity in connected areas (O'Shea et al., 2007b).
Paired-pulse TMS has revealed resting-state physiological connections to M1 from premotor, parietal and supplementary motor areas of the same hemisphere (Civardi et al., 2001; Koch et al., 2006, 2007). Inter-hemispheric connections between dorsal premotor cortex (PMd) and M1 have also been demonstrated, with paired-pulse TMS facilitating (Baumer et al., 2006) or inhibiting MEPs (Mochizuki et al., 2004) as a function of stimulation intensity.
An important extension of the paired-pulse technique is to assess how resting-state connections are modulated by psychological context. There is evidence from the visual system, where phosphene induction is used to index visual cortex excitability, that resting-state V1–V5 connections are modulated by the performance of a visual motion detection task (Pascual-Leone & Walsh, 2001; Silvanto et al., 2005a, 2005b). According to this logic, interhemispheric PMd–M1 connections should be modulated when task demands recruit those circuits. The PMd plays an important role in the selection of responses for execution, specifically response selection based on learned stimulus–response mapping rules (Petrides, 1985; Passingham, 1993; Wise & Murray, 2000). PMd shows greater activation during tasks that require the selection of a different response on every trial, compared with tasks that instruct the same response on every trial (Schluter et al., 2001; Johansen-Berg et al., 2002; Thoenissen et al., 2002; Rushworth et al., 2003; Cavina-Pratesi et al., 2006; Grol et al., 2006). Although PMd is activated bilaterally during response selection, imaging and TMS studies suggest that left PMd exerts dominance over right PMd: TMS of either PMd disrupts response selection with the contralateral hand, but only left PMd TMS disrupts selection with the ipsilateral hand (Schluter et al., 1998; Schluter et al., 2001; Johansen-Berg et al., 2002).
We combined paired-pulse TMS with two tasks: a visuomotor choice reaction time (RT) task that emphasized response selection, and a control simple RT task that de-emphasized selection. With this task manipulation we aimed to isolate PMd–M1 interactions that were functionally specific to the process of response selection. We predicted that paired-pulse TMS would modulate MEPs early during the reaction period, because previous single-pulse TMS data suggest that this is when the PMd contributes to response selection (Schluter et al., 1998, 1999; Johansen-Berg et al., 2002). We further predicted that MEP modulation would be of greater magnitude or duration, and have greater relevance for selection behaviour, after TMS of the (dominant) left PMd than after TMS of the right PMd.
Materials and methods
Subjects
Eighteen healthy volunteers participated in this study (age range 23–30 years). In Experiment 1, ten subjects (six females) were tested in counter-balanced order on both task sessions. Two further subjects participated in only one of the two sessions, yielding a total of 11 subjects per session. In Experiment 2, eight subjects (five females) were tested in counter-balanced order on both task sessions. One further subject participated in only one of the two sessions. In total, seven subjects participated in all sessions of Experiments 1 and 2, so across-experiment analyses were performed on the data from those seven subjects. Six subjects (four female) were tested in Experiment 3, one of whom had participated in both sessions of Experiments 1 and 2 2 months previously. All subjects were right-handed and reported an absence of psychiatric or neurological disease in their known family history. All subjects gave written informed consent. The study was carried out under permission from the Central Oxford Research Ethics Committee (COREC, 05-Q1606-96) and in accordance with the Declaration of Helsinki.
Behavioural tasks
Two tasks were used: an experimental choice RT task (Select) (Experiments 1 and 3) and a control simple RT task (Execute) (Experiment 2). The Select task emphasized response selection: subjects had to select one of two button press responses with the index finger of their right or left hand on each trial, according to the identity of a visual cue. A small circle or large square instructed a right hand response, while a large circle or small square instructed a left hand response (Fig. 1A). These four stimulus–response mappings were counterbalanced across subjects. The fact that neither size nor shape instructed responses in a simple way meant that actions had to be selected with care, even after practice. By contrast, the Execute task de-emphasized selection: subjects made the same response on every trial regardless of which cue was presented. Thus, there was only one stimulus–response mapping, and the responding hand was always contralateral to the stimulated M1 (Fig. 1A). It has been shown that PMd, especially left PMd, is more active during the Select than the Execute task, and that single-pulse PMd TMS disrupts performance on the Select task more than the Execute task (Schluter et al., 1998, 1999; Johansen-Berg et al., 2002; Rushworth et al., 2003). In both tasks, visual stimuli were presented until the response was detected, and subjects were instructed to respond as quickly and accurately as possible. All experiments were controlled by Turbo Pascal software.
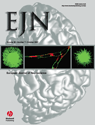
Experimental procedure. (A) Experimental set-up. During the Select task, a single shape stimulus was presented on each trial, and subjects made an index finger button-press response with the right or left hand according to a learned rule. There were four stimulus-response (S-R) mappings. For half of subjects, a large square or a small circle cued a left hand response, and a large circle or a small square cued a right hand response. For the other half of subjects, the S-R mappings were reversed. One TMS coil was placed over the dorsal premotor cortex (PMd) and the other over the contralateral primary motor cortex (M1). On paired-pulse trials, there was an 8-ms interval (IPI) between the first ‘conditioning’ TMS pulse (to PMd) and the second ‘test’ pulse (to M1). Motor-evoked potentials (MEPs) were recorded from the first dorsal interosseous (FDI) muscle contralateral to the stimulated M1. RTs were recorded from both hands. (B) Timecourse of a single trial. A visual shape stimulus was presented until the button press response. Following stimulus presentation, TMS was delivered according to the trial type. On single-pulse trials, a single TMS pulse was applied to M1; on paired-pulse TMS trials a ‘conditioning’ TMS pulse was applied to PMd 8 ms prior to the M1 pulse. TMS onset occurred at one of five SOAs: 50, 75, 100, 125 or 150 ms after the onset of the visual stimulus. MEPs and RTs were recorded following TMS. There was a variable intertrial interval (3.5–4.5 s).
Experiment 1: timing of PMd–M1 physiological interactions during response selection
Experiment 1 addressed three issues about interhemispheric PMd–M1 interactions during response selection: (i) timing, (ii) hemispheric asymmetry and (iii) behavioural relevance. Experiment 1 had two sessions, each conducted on different days. In one session the left PMd–right M1 (lPMd–rM1) pathway was tested; in the other session the right PMd–left M1 (rPMd–lM1) pathway was tested. Session order was counterbalanced across subjects. In both sessions, subjects performed the Select task while TMS was applied to the relevant PMd–M1. There were two types of TMS trials. On single-pulse trials, a single ‘test’ TMS pulse was applied over the relevant M1, and the resulting MEPs were recorded from the first dorsal interosseous (FDI) muscle of the contralateral hand. The peak-to-peak amplitude of the MEP and the RT recorded on each of those ‘single-pulse’ trials constituted the baseline measurements. On paired-pulse trials, the M1 ‘test’ pulse was preceded by a ‘conditioning’ TMS pulse delivered through a second TMS coil positioned over the contralateral PMd (Fig. 1A). The interval between the paired pulses (IPI) was 8 ms, based on previous resting-state MEP studies of PMd–M1 interactions that showed this IPI to be most effective (Mochizuki et al., 2004; Baumer et al., 2006). Physiological (MEP) and behavioural (RT) measurements from the baseline single-pulse trials were contrasted with those on paired-pulse trials to test the effect of the ‘conditioning’ TMS applied to PMd.
On each trial of the Select task, a visual shape stimulus was presented until a response was detected. MEP and RT data were recorded during the response period, followed by a variable intertrial interval (3.5–4.5 s). To establish the critical time window for PMd–M1 interactions, the M1 TMS pulse (the only one on single-pulse trials) was delivered at each of five stimulus-onset asynchronies (SOAs): 50, 75, 100, 125 or 150 ms after the onset of the visual stimulus (Fig. 1B). There were 20 single- and 20 paired-pulse TMS trials per SOA. In each condition, half of the trials required a left hand response and half required a right hand response. Although MEPs were recorded on every trial, they were always recorded from only one hand (that contralateral to the stimulated M1). This meant that on half of trials MEPs and RTs were recorded from the same hand (contralateral), while on the other half of trials RTs were recorded from the other hand (ipsilateral to the stimulated M1) (Fig. 1A). Only data from correct trials were analysed. In total there were ten trials per condition: TMS (single vs. paired-pulse) × SOA (50, 75, 100, 125, 150 ms) × Hand (contralateral vs. ipsilateral). In addition, a further six single-pulse TMS trials were presented at the start of the session. These were designed to allow MEP amplitudes to stabilize and were excluded from the analysis.
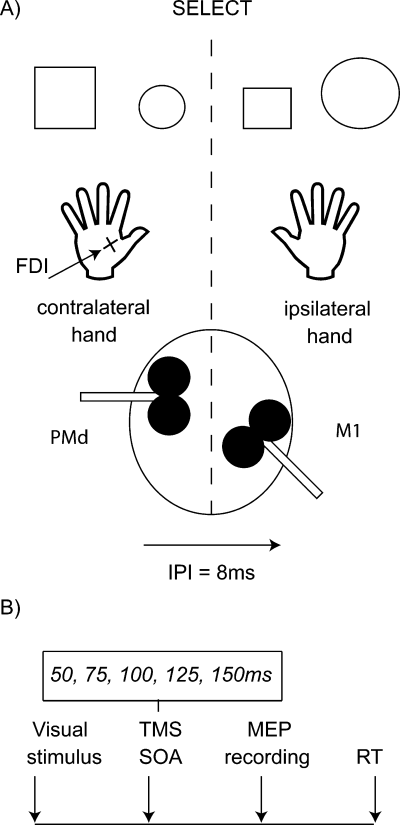
M1 TMS pulses were applied over the motor cortex ‘hotspot’, defined as the optimal scalp position at which the lowest intensity TMS evoked a just-noticeable twitch from the relaxed contralateral FDI muscle. PMd TMS was delivered at scalp coordinates 2 cm anterior and 1 cm medial from the ‘hotspot’. This procedure for targeting PMd has been used in a number of previous studies, which have shown that single-pulse TMS at these coordinates slows RT on the Select task used here (Schluter et al., 1998; Johansen-Berg et al., 2002). We verified the cortical locations of these TMS sites anatomically in nine subjects using Brainsight frameless stereotaxy (Rogue Research, Montreal, Canada)(Fig. 2). Individual subjects' structural MRI scans were registered to the Montreal Neurological Institute (MNI) 152-mean brain T1 template. This confirmed that PMd TMS was applied just anterior to the dorsal branch of the precentral sulcus [mean MNI coordinates: x = ± 28 (SEM ± 1.75), y = −5 (± 2.94), z = 71 (± 1.42)]. M1 TMS was applied over the motor hand hook in the central sulcus [mean MNI coordinates: x = ± 32 (SEM ± 2.36), y = −21 (± 2.61), z = 69 (± 1.72)]. Both locations correspond well with published probabilistic coordinates and sulcal landmarks for PMd and M1 (Fink et al., 1997; Yousry et al., 1997; Johansen-Berg et al., 2002; Chouinard et al., 2003; Amiez et al., 2006).
Stimulation sites. Each circle represents the MNI coordinates for an individual subject in Experiment 1 at which TMS was applied over PMd or M1. It is clear that PMd sites cluster above the precentral sulcus (pcs), while M1 sites cluster above the central sulcus (cs). Sections show the left hemisphere group average sagittal plane (PMd: x = −28; M1: x = −32). Dashed line denotes y = 0. Coordinate range for PMd: ± 21 < x < 37, −13 < y < 15, 63 < z < 76; and M1: ± 21 < x < 43, −5 < y <−32, 63 < z < 69.
Stimulation intensities were determined separately for each hemisphere. M1 TMS pulses were applied at the minimum intensity required to evoke an MEP of ∼1 mV peak-to-peak amplitude from the relaxed contralateral FDI muscle on ten consecutive trials. ‘Conditioning’ PMd TMS pulses were applied at 110% of the resting motor threshold (RMT) for M1 of that hemisphere. RMT was defined as the minimum intensity TMS required to evoke a ∼50-µV MEP from the relaxed contralateral FDI muscle on 5/10 trials. Mean stimulation intensities for M1 were 51.6 (SEM ± 0.29, left) and 51.1 (SEM ± 0.27, right), and for PMd were 44.4 (SEM ± 0.22, left) and 44.6 (SEM ± 0.21, right) of maximum stimulator output. TMS pulses were delivered using two monophasic Magstim 200 machines (Magstim Company, Carmarthenshire, Wales, UK). Pulses were applied to the PMd through a 50-mm figure-of-eight coil and to M1 through a 70-mm figure-of-eight coil. Both coils was held tangential to the skull, with the M1 coil handle orientated posteriorly at ∼45° and the PMd coil handle orientated laterally at ∼90° from the mid-sagittal axis (Fig. 1A).
MEPs were recorded from the contralateral FDI using Ag-AgCl electrodes and a tendon-belly montage. Electromyographic (EMG) responses were amplified, filtered and sampled using a CED 1902 amplifier, a CED 1401 analog-to-digital converter and a Pentium 4 computer running Signal (version 2.14) software (Cambridge Electronic Design Ltd, Cambridge, UK) on a PC running Windows 98. Signals were sampled at 10 000 Hz and band-pass filtered between 10 and 10 000 Hz.
Experiment 2: functional specificity of PMd–M1 physiological interactions
The aim of Experiment 2 was to establish whether the PMd–M1 physiological interactions (observed in Experiment 1) were functionally specific to the process of response selection, or whether similar effects would be observed during performance of a task with a more limited response selection component. Subjects performed the Execute task, which required them to make the same index finger button press response with the same hand on every trial, no matter which shape stimulus was presented. Subjects always responded with the hand contralateral to the stimulated M1, from which MEPs were recorded. So, unlike in Experiment 1, there was no factor of Hand, such that the total trial number was halved. Hence, both sessions (lPMd–rM1 and rPMd–lM1) were conducted on the same day, with order counterbalanced across subjects. Mean stimulation intensities for M1 were 47.6 (SEM ± 0.33, left) and 44.7 (SEM ± 0.27, right), and for PMd were 41.9 (SEM ± 0.23, left) and 43.4 (SEM ± 0.26, right). All other procedures were identical to Experiment 1.
Experiment 3: anatomical specificity of PMd–M1 physiological interactions
Experiment 3 aimed to establish whether the physiological effects of paired-pulse TMS depended on the anatomical location, PMd, at which the conditioning pulse was applied, or whether the same effects could be obtained by conditioning TMS elsewhere in the motor system. Subjects performed the Select task, and on paired-pulse trials both TMS pulses were delivered over left M1 through a single TMS coil (M1–M1 TMS). The interpulse interval and TMS intensities were the same as in Experiment 1, and all other procedures were identical. The mean stimulation intensity was 44.7 (SEM ± 0.5) for the conditioning pulse and 50.8 (SEM ± 0.45) for the test pulse.
Data analysis
All within-experiment analyses compared the effect of paired- vs. single-pulse TMS on mean MEP amplitudes (in mV) and mean RTs (ms). For all analyses between experiments, the data for each subject and condition were transformed into percentage change values [i.e.% MEP = paired-pulse/single-pulse × 100]. This transformation controlled for overall differences in MEP amplitude across experiments – caused by differences in task (Select vs. Execute, Experiment 1 vs. 2) or differences in the anatomical site at which conditioning TMS was applied (PMd–M1 vs. M1–M1 TMS, Experiment 1 vs. 3). For illustration purposes, all results are displayed in percentage change format. Two outlier data-points from Experiment 1 (1 MEP, 1 RT) that were more than two standard deviations from the mean were removed from all analyses. Paired-pulse TMS effects were identified using repeated-measures anovas with Huyn-Feldt correction and subsequent paired or one-sample t-tests corrected for multiple comparisons (Holm, 1979). Analyses were carried out using a within-subjects approach whenever possible (see Methods, Subjects). During the simple RT task (Execute, Experiment 2), subjects responded quickly (the earliest quartile of RTs occurred between 150 and 200 ms after trial onset). Hence, in the 150-ms SOA condition, subjects frequently responded prior to or during the TMS. To identify and eliminate any trials so contaminated by EMG activity, every trial for every subject and condition in every experiment was individually inspected. All trials showing evidence of EMG contamination were eliminated from the data set prior to analysis. This trial-by-trial inspection procedure revealed that the data for the 150-ms SOA condition of the Execute task (Experiment 2) were so frequently contaminated by voluntary muscle activity that they could not be analysed. In all other conditions, however, because MEPs occurred prior to EMG onset, individual trial data were only very rarely contaminated and hence rejected.
Results
Experiments 1 & 2
Functional specificity and timing of PMd–M1 physiological interactions
The aim of our study was to determine whether patterns of PMd–M1 functional connectivity differ during action choice (‘select’ task) vs. action execution (‘execute’ task). Hence, we carried out a between-experiments analysis on the percentage MEP data from Experiments 1 and 2, in order to compare directly the patterns of PMd–M1 functional connectivity observed under the two different task conditions.
A three-way anova was conducted on the percentage MEP data (for raw data see Supplementary material, Fig. S1) with one between-subjects factor of Task [Select (Experiment 1) vs. Execute (Experiment 2)], and two within-subjects factors of Hemisphere (lPMd–rM1 vs. rPMd–lM1) and SOA (50, 75, 100, 125 ms). In Experiment 2, all responses were made with the hand contralateral to the stimulated M1. Hence, for this across-experiments comparison, the data from Experiment 1 were pooled over the factor of hand before being submitted to analysis. There was a significant Task × SOA interaction (F3,15 = 5.490, P = 0.001), suggesting that paired-pulse TMS modulated MEP amplitude in both the Select and the Execute tasks, but that the relevant SOA differed between the tasks. The Task × Hemisphere interaction approached significance (F1,5 = 6.110, P = 0.056). There were no other effects, trends or interactions. To investigate further the Task × SOA interaction separate anovas were conducted on the data from each experiment.
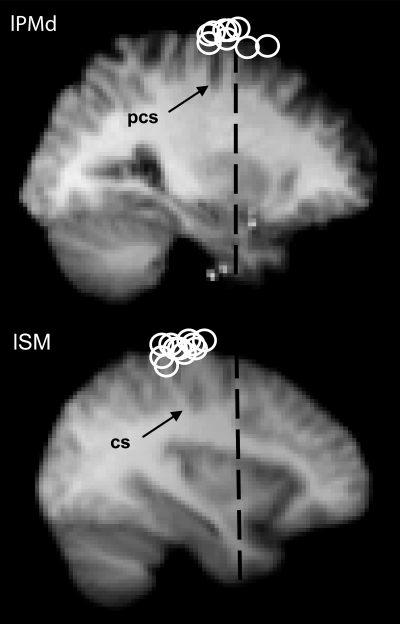
Mean MEP amplitude data from the Select task (Experiment 1) were analysed using a four-way repeated-measures anova with factors of Hemisphere (rPMd–lM1 vs. lPMd–rM1), TMS (single- vs. paired-pulse), SOA (50, 75, 100, 125, 150 ms) and Hand (contralateral vs. ipsilateral). The factor of Hand was included because although MEPs were always recorded from the hand contralateral to M1 stimulation, on half of correct trials the response was made with the other (ipsilateral) hand. The TMS × SOA interaction was significant (F4,32 = 2.854, P = 0.039). There were no other effects, trends or interactions. Paired-samples t-tests (pooled over Hemisphere and Hand) showed that paired-pulse TMS facilitated MEP amplitude significantly at 75 ms (t8 = −2.513, P = 0.036), and there was a non-significant trend towards facilitation at 50 ms (t9 = −1.944, P = 0.084) (Fig. 3A). MEP inhibition at 125 and 150 ms was not significant (P > 0.22).
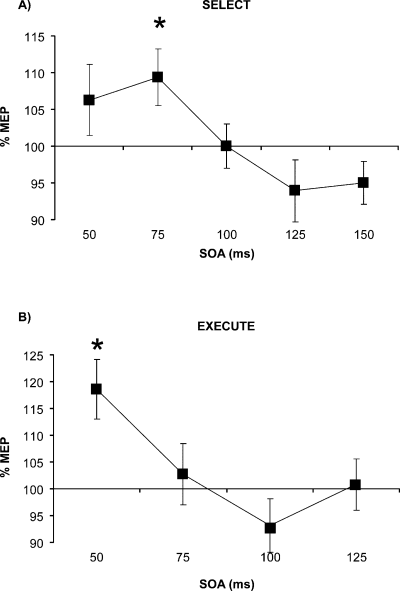
Timing and functional specificity of PMd–M1 interactions. Graphs show the mean percentage change in MEP amplitude (% MEP) on paired-pulse compared with single-pulse TMS trials at different SOAs during the Select and Execute tasks (paired-pulse/single-pulse × 100). In combination, the patterns of MEP facilitation across the two tasks suggest that the timing of PMd–M1 interactions is early (50, 75 ms), and that PMd–M1 interactions at 75 ms are functionally specific to the cognitive processes of response choice. The data in both panels have been combined over factors of Hemisphere (lPMd–rM1, rPMd–lM1 sessions), and in A have also been combined over Hand (contralateral, ipsilateral). (A) Select task. Paired-pulse TMS facilitated MEPs at the 75-ms SOA, indicating that PMd–M1 interactions occur at that time interval. (B) ‘Execute task’. Paired-pulse compared with single-pulse TMS facilitated MEPs at 50 ms (*P < 0.05, error bars = 1 SEM).
Mean MEP amplitude data from the Execute task (Experiment 2) were analysed using a three-way repeated-measures anova with the same factors of Hemisphere, TMS and SOA (50, 75, 100, 125 ms). There was a main effect of SOA (F3,24 = 7.092, P = 0.023) and a marginally significant TMS × SOA interaction (F3,24 = 2.998, P = 0.051). A paired-samples t-test revealed significant paired-pulse MEP facilitation at the 50-ms SOA (t8 = 4.202, P = 0.003) (Fig. 3B). The tendency towards MEP inhibition at 100 ms was not significant (P > 0.21).
These results reveal the timing of task-dependent interhemispheric PMd–M1 interactions, and establish their functional specificity by contrasting the patterns of MEP modulation across the Select and the Execute tasks. Paired-pulse TMS significantly facilitated MEP amplitude when applied at an SOA of 50 ms in the Execute task (with a non-significant trend in the Select task). In the Select task only, significant MEP facilitation occurred at 75 ms only, suggesting that such facilitation is functionally specific to the process of response selection.
No difference in PMd–M1 physiological interactions as a function of Hemisphere or Hand
Although the between-experiment anova showed that the Task × Hemisphere interaction approached significance (P = 0.056), separate within-experiment analyses found no main effect of Hemisphere in either task (Experiment 1: Select task P = 0.133; Experiment 2: Execute task P = 0.14). More importantly, there was no evidence of hemispheric asymmetry in the pattern of functional connectivity during either task: in both between- and within-experiment analyses, none of the interactions between Hemisphere and TMS approached significance (all P > 0.16). Further exploratory analyses on the MEP data separated by Hemisphere found no evidence to support our a priori hypothesis that conditioning TMS of the left PMd would have a greater effect on MEPs than conditioning TMS of the right PMd. Rather, the results suggest that the timing of interhemispheric functional connectivity is the same for both the lPMd–rM1 and the rPMd–lM1 pathway.
There was no evidence that paired-pulse MEP modulation differed as a function of which hand was selected to make the response (all P > 0.77). In fact, when all data from the critical 75-ms SOA were analysed (all sessions and subjects), the magnitude of the percentage MEP effect showed a significant positive correlation between: (1) trials in which the contralateral hand was selected to respond; and (2) trials in which the ipsilateral hand was selected to respond (Spearman's r = 0.23, n = 100, P = 0.021) (Fig. 4). In other words, paired-pulse MEP modulation was similar regardless of which hand was used to respond, suggesting that the MEP facilitation effect by itself is not a correlate of the response selection process.
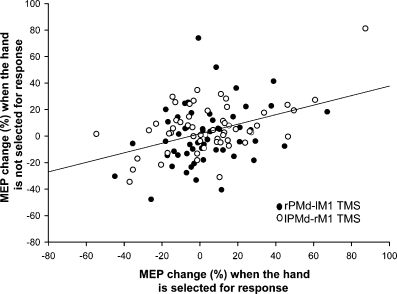
No difference in PMd–M1 interactions during the Select task as a function of which hand is selected to make the response. Graph shows the mean percentage change in MEP amplitude (% MEP) caused by paired-pulse TMS at the 75-ms SOA of the Select task (Experiment 1). The x-axis shows data from trials in which the response was made with the same hand from which MEPs were recorded (i.e. the hand contralateral to M1); the y-axis shows data from trials in which the response was made with the other hand (ipsilateral to M1). The significant positive correlation between the two types of data recorded in each session shows that conditioning PMd TMS facilitated MEPs at 75 ms irrespective of which hand was selected to make the response. Black circles are data from the rPMd–lM1 session; white circles are data from the lPMd–rM1 session. Each circle represents the mean percentage MEP change for a single subject at a single SOA. Data are from all 11 subjects and all five SOAs.
Behavioural relevance of PMd–M1 physiological interactions
We have previously shown that single-pulse PMd TMS applied at an SOA of 100 ms during the Select task can delay choice RT (Schluter et al., 1998; Johansen-Berg et al., 2002; O'Shea et al., 2007a). Whereas right PMd TMS slows RTs with the left hand only, left PMd TMS slows RTs with either hand (Johansen-Berg et al., 2002; Schluter et al., 1998). This reflects the established functional dominance of left vs. right PMd for response selection (Rushworth et al., 2003). Based on these previous findings, in the present study we had strong a priori directional predictions about the nature of the behavioural effect we expected to be produced by PMd–M1 TMS. First, we predicted that PMd–M1 TMS applied at the 100-ms SOA would delay RTs. Second, we predicted a greater effect of left than right PMd TMS. Finally, we expected the RT delay to occur on trials in which the hand contralateral (but not ipsilateral) to the stimulated M1 was selected to make the response. That is, in the present interhemispheric paired-pulse design, M1 TMS was applied on every trial, eliciting MEPs from the contralateral hand and itself affecting responses made with that hand. Hence, the present RT analysis aimed to measure the additional impact of the PMd TMS pulse on RTs with the contralateral hand (Fig. 1A).
Mean RTs from the Select task (Experiment 1) (supplementary Table S1) were analysed using a four-way repeated-measures anova with factors of Hemisphere (rPMd–lM1 vs. lPMd–rM1), TMS (single- vs. paired-pulse), Hand (contralateral vs. ipsilateral) and SOA (50, 75, 100, 125, 150 ms). The four-way interaction of Hemisphere × TMS × Hand × SOA was significant (F4,28 = 5.856, P < 0.001). To explore this further, separate follow-up anovas were conducted on the data separated by Hemisphere. A three-way anova on the data from the left PMd session (lPMd–rM1) found a main effect of SOA (F4,36 = 5.122, P = 0.002) and a three-way interaction of Hand × TMS × SOA (F4,36 = 4.453, P = 0.005). We separated the data according to Hand and ran two paired-samples t-tests at the 100-ms SOA, as this was the condition for which we had strong a priori predictions. As expected, paired-pulse TMS delayed RTs significantly on trials in which responses were made with the hand contralateral to the stimulated M1 (left hand) (t9 = −1.834, P = 0.05, one-tailed) (Fig. 5). There was no difference between single- and paired-pulse RTs when responses were made with the hand ipsilateral to the stimulated M1 (right hand) (P > 0.67). The same anova applied to the RT data from the right PMd session (rPMd–lM1) found no significant effects or interactions (three-way interaction of Hand × TMS × SOA: P = 0.146). Thus, paired-pulse TMS of lPMd–rM1 at 100 ms significantly delayed RTs with the contralateral hand, replicating established findings of left PMd dominance for response selection behaviour.
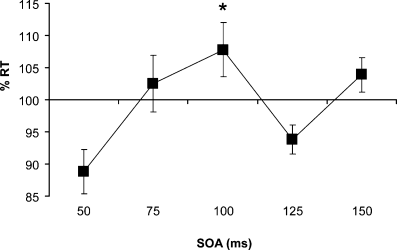
Conditioning TMS of left PMd at 100 ms delayed choice RT with the contralateral hand. The graph shows the percentage change in reaction time (%RT) after paired-pulse PMd–M1 TMS compared with single-pulse M1 TMS in the Select task of Experiment 1. Paired-pulse TMS at 100 ms caused a significant delay in RTs on trials in which the response was made with the hand contralateral to the stimulated M1 (*P < 0.05, error bars = 1 SEM).
As Fig. 5 shows clearly, the RT slowing effect was specific to the 100-ms SOA condition. There was no evidence of an RT delay in any other condition. Although faster RTs occurred at the 50-ms SOA, behavioural deficits rather than facilitations are the gold standard for claiming that TMS has causally impacted on cognitive function (O'Shea & Walsh, 2007). Hence, while the 100-ms RT delay can be clearly ascribed to a functional interference effect of the TMS, the faster RTs at 50 ms probably reflect a non-specific alerting effect caused by the acoustic and somatosensory artefacts of the TMS discharge, a phenomenon that is often reported (e.g. Schluter et al., 1998).
To investigate the potential significance of PMd–M1 physiological interactions for response selection behaviour, we tested for a relationship between the paired-pulse RT and MEP effects. As conditioning PMd TMS facilitated MEPs (at 75 ms) and delayed RTs (at 100 ms), we expected a positive correlation between the two effects. To enable statistical correlations to be computed, the data were transformed into percentage MEP and percentage RT values (see Data Analysis). Analyses of the MEP data had shown that the pattern of PMd–M1 functional connectivity did not change as a function of which hemisphere was stimulated or which hand was selected to make the response (all P > 0.16; Fig. 4). By contrast, the RT effect was both hemisphere- and hand-specific, significant only after lPMd but not rPMd TMS, and occurring only on trials in which responses were made with the contralateral hand (Fig. 5). Hence, correlation analyses were carried out on the data separated by Hand.
We first analysed trials in which responses were made with the contralateral hand (left hand in the lPMd–rM1 session; right hand in the rPMd–lM1 session) (Fig. 1A). As expected, there was a significant positive correlation between the percentage MEP effect (at 75 ms) and percentage RT effect (at 100 ms) (Spearman's r 0.434, n = 19, P = 0.032, one-tailed). That is, when responses were made with the contralateral hand, conditioning TMS of left or right PMd both facilitated MEPs (at 75 ms) and slowed RTs (at 100 ms) (Fig. 6). This slowing effect on the hand contralateral to the stimulated M1 made it easier to select responses on trials in which the visual stimulus instructed a response with the hand ipsilateral to the stimulated M1. Thus, on those trials, the relationship was reversed: there was a significant negative correlation between percentage MEP (at 75 ms) and percentage RT (at 100 ms) effects (Spearman's r = −0.407, n = 19, P = 0.042, one-tailed; Fig. 6). Importantly, similar analyses performed on MEP data from the 50-ms SOA condition found no significant correlations (all P > 0.138). This confirms that only those PMd–M1 physiological interactions that were specific to the Select task (at 75 ms) were significantly correlated with response selection behaviour.
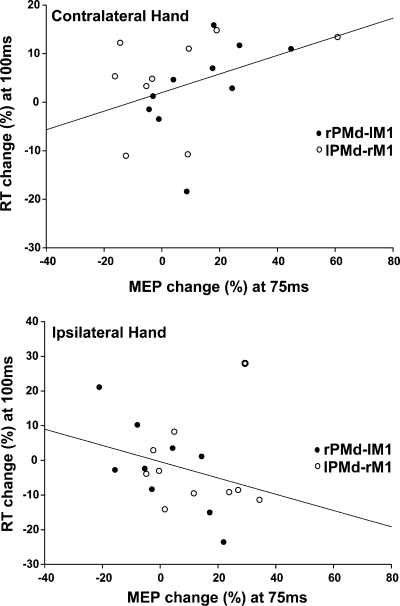
Physiological and behavioural effects of PMd–M1 TMS during the Select task correlate in a hand-specific manner. The graph plots the percentage change in MEPs at 75 ms (x-axis) against the percentage change in reaction times at 100 ms (y-axis) caused by paired-pulse compared with single-pulse TMS. MEP and RT changes correlated significantly in a hand-specific manner. On trials in which subjects selected a response with the hand contralateral to the stimulated M1, the degree of MEP facilitation (at 75 ms) correlated positively with the RT change (at 100 ms). On trials in which subjects selected a response with the hand ipsilateral to the stimulated M1, the degree of MEP facilitation (at 75 ms) correlated negatively with the RT change (at 100 ms). Black circles are data from the rPMd–lM1 session; white circles are data from the lPMd–rM1 session. Each circle represents the mean percentage MEP and percentage RT changes for a single subject.
In summary, the physiological and behavioural effects of paired-pulse TMS correlated significantly, suggesting that these two effects shared a common origin. That this relationship was hand-specific strongly suggests that these correlations are a functional marker of the process of response selection. Whereas the MEP effect at 75 ms was not hand-specific, the RT effect at 100 ms was. The hand-specificity of the RT effect, combined with the inverse correlation patterns between the RT and MEP modulation for the contralateral vs. ipsilateral hand, suggests that the computational state of PMd evolves during this time window (75–100 ms) to generate a response selection decision that causally impacts on M1 and mediates manual response behaviour.
Experiments 1 & 3
Anatomical specificity of PMd–M1 physiological interactions
To determine whether the demonstrated patterns of functional connectivity during the Select task depended specifically on inputs from PMd, we compared the percentage MEP data from Experiments 1 and 3. In Experiment 3, both TMS pulses were applied to left M1 so the analysis compared those data with data from the rPMd–lM1 session of Experiment 1.
An anova with one between-subjects factor (Experiment: 1 vs. 3) and two within-subjects factors (SOA, Hand) revealed only a main effect of Experiment (F1,14 = 25.573, P < 0.001), indicating that the pattern of paired-pulse modulation during the Select task differed significantly depending on whether conditioning TMS was applied to PMd or M1 (compare Fig. 7 with Fig. 3A). This analysis shows that PMd input is critical to the pattern of functional connectivity identified in Experiment 1: an entirely distinct pattern of modulation was produced when paired-pulse TMS was confined to M1.
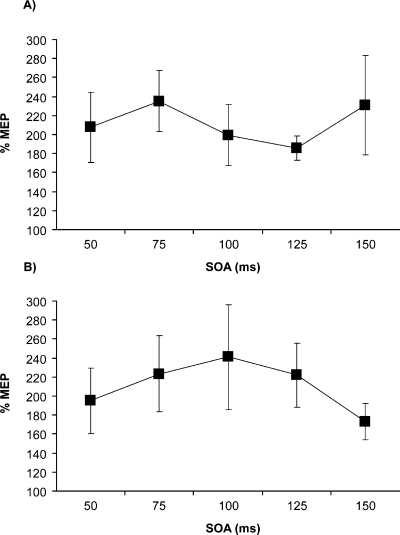
Anatomical specificity of PMd–M1 interactions during the Select task. Graphs show the mean percentage change in MEP amplitude (% MEP) at different SOAs on paired-pulse compared with single-pulse TMS trials of Experiment 3. Single or paired pulses of TMS were applied to left M1 while subjects performed the Select task. (A) % MEP facilitation on trials in which responses were made with the same hand from which MEPs were being recorded. (B) % MEP facilitation on trials in which responses were made with the other hand. Paired-pulse TMS of M1 significantly facilitated MEPs, but this generalized pattern of facilitation differed significantly from the temporally specific modulation caused by paired-pulse TMS of PMd–M1 (compare with Fig. 4A) (error bars = 1 SEM).
Discussion
We set out to establish whether the physiological connections between PMd and contralateral M1 that have been demonstrated at rest would be modulated when cognitive demands recruit those circuits. Previous studies have shown that MEP amplitude (M1 excitability) can be altered by applying a ‘conditioning’ TMS pulse to the contralateral PMd 8 ms prior to the M1 pulse (Mochizuki et al., 2004; Baumer et al., 2006). Hence, we investigated whether the causal impact of the PMd pulse on M1, at this same interpulse interval, would change during the process of response selection, a function for which the PMd is specialized (Passingham, 1993; Murray et al., 2000; Passingham & Toni, 2001; Toni et al., 2001; Thoenissen et al., 2002; Petrides, 2005; Amiez et al., 2006).
Timing of PMd–M1 physiological interactions during response selection
In Experiment 1 we found that the impact of the conditioning PMd TMS pulse on M1 excitability during the Select task varied over time. Paired-pulse TMS significantly increased the amplitude of MEPs when applied 75 ms after the onset of the response instruction cue (Fig. 3A). Conditioning PMd TMS at later times did not cause a significant change in the effect of the M1 test pulse. The changing impact of TMS-induced activity in PMd on M1 suggests that endogenous changes in PMd–M1 functional connectivity occur early during the task, consistent with a process of response selection.
The early timing of the physiological modulation identified by the present study (75 ms) is consistent with previous behavioural TMS studies that have disrupted response selection performance by applying single-pulse PMd TMS early (100–140 ms) during the reaction time period (Johansen-Berg et al., 2002; Schluter et al., 1998, 1999; Mochizuki et al., 2005). Such previous behavioural findings were interpreted as evidence for an early period of response selection, mediated by PMd, followed by a later period of response execution, involving M1. The present study stimulated both of these areas in quick succession, whilst measuring the effects on M1 excitability, thus providing physiological evidence about the direction of causality and the timing of PMd–M1 interactions during response selection.
The early timing is consistent with single unit recording studies in macaques which have shown that PMd neurons encode the significance of visual cues for response selection, and that PMd neurons are active at approximately similar early time periods from the onset of the cue to move (Boussaoud & Wise, 1993; Okano, 1992; Johnson et al., 1996; Wise et al., 1997; Cisek et al., 2003). In comparison to PMd, M1 neurons begin to encode the movement to be made at slightly later, but overlapping, time periods.
Perhaps most notably, the MEP facilitation at 75 ms replicates the timing of PMd–M1 interactions reported by Koch et al. (2006), who combined a similar paired-pulse TMS protocol with an auditory choice reaction time task. The evidence for similar timing of PMd–M1 interactions in these two different studies using two different stimulus modalities (auditory and visual) strengthens the claim that these interactions reflect processes of response selection. However, unlike Koch and colleagues, we conducted an additional experiment to establish empirically whether these state-dependent PMd–M1 interactions were functionally specific to the cognitive process of response selection.
Functional specificity of PMd–M1 physiological interactions
To establish functional specificity, we manipulated response selection demands by using two tasks. Whereas on a given trial of the Select task (Experiment 1) subjects had to select one of two responses based on four different stimulus–response mappings, in the Execute task (Experiment 2) subjects had to select the same response on every trial whichever stimulus was presented (one stimulus–response mapping). Positron emission tomography (PET) and functional magnetic resonance imaging (fMRI) studies that have compared similar pairs of tasks have shown significantly greater activation of PMd in tasks that emphasize response selection over execution(Schluter et al., 2001; Johansen-Berg et al., 2002; Rushworth et al., 2003; Amiez et al., 2006).
Correspondingly, the comparative analysis of Experiments 1 and 2 showed that the effect of paired-pulse TMS differed significantly between the two tasks. Conditioning TMS of PMd facilitated MEPs in both tasks, but at different times. In the Execute task, MEP facilitation occurred at 50 ms (Fig. 3B). In the Select task, significant MEP facilitation occurred 75 ms after the response instruction cue, although there was also a (non-significant) tendency towards facilitation at the 50-ms SOA (Fig. 3A). Most importantly, the significant dissociation between the tasks establishes the functional specificity of PMd–M1 interactions at the 75-ms SOA. As MEP facilitation at 75 ms occurred in the choice RT task, but not in the simple RT task, this argues that the PMd–M1 interactions observed at 75 ms reflect processes specific to response choice.
Although response selection demands in the Execute task were minimal, they may not have been entirely absent. The blocked nature of the Execute task would have enabled subjects to prepare the same response in advance on every trial, rather than having to select a response only when the visual cue was presented. Nevertheless, subjects still had to select when to respond (at the appearance of the visual cue, which had a variable onset). The early timing of the MEP facilitation (50 ms) may reflect the first arrival of visual input to PMd neurons – signalling the onset of the visual cue and the activation of a preselected response. Activity changes occur at approximately similar latencies in macaque PMd neurons when responses are selected under the simplest of conditions, but do not occur until several tens of milliseconds later when a more complex learned conditional visuomotor association rule is used to select between competing response options (Cisek & Kalaska, 2005). Response preparation is itself associated with changes in PMd activity (Wise & Mauritz, 1985; Schluter et al., 1999; Toni et al., 1999; Thoenissen et al., 2002; Cavina-Pratesi et al., 2006; Mars et al., 2007). This may account for the small degree of fMRI activation typically observed in bilateral PMd during simple RT tasks (Jenkinson & Smith, 2001; Schluter et al., 2001; Rushworth et al., 2003; O'Shea et al., 2007a).
Behavioural relevance of PMd–M1 physiological interactions
More direct evidence that PMd–M1 interactions at 75 ms reflect processes of response selection comes from the analysis of the behavioural data. A previous paired-pulse study of PMd–M1 interactions did not report any behavioural consequences of TMS on choice RT (Koch et al., 2006). However, the Select task used in our study featured four stimulus–response mappings, and thus had greater response selection demands than the simple audio-motor response task used by Koch et al. Hence, our task may have had greater sensitivity to detect behavioural effects.
In the Select task, a conditioning TMS pulse applied to left PMd at 100 ms slowed RTs when responses were made with the hand contralateral to the M1 TMS pulse. Note that this slowing effect of paired-pulse PMd–M1 TMS is measured relative to the effect of single-pulse TMS of M1 alone, identifying the locus of behavioural interference as PMd (Fig. 5). A similar delaying effect of single-pulse PMd TMS at 100 ms on choice RT has been reported in a number of previous studies (Schluter et al., 1998, 1999; Johansen-Berg et al., 2002). In all of those studies, the RT delay was greater in the Select than the Execute task, was more prominent in the hand contralateral to the stimulated PMd, and was greater and more bilateral (affecting responses with either hand) after left than right PMd TMS. Note that in the present study, RT slowing selectively affected the hand contralateral to the stimulated M1 (and thus ipsilateral to the stimulated PMd). That this occurred after left (but not right) PMd TMS thus replicates the known behavioural dominance of left (over right) PMd for the selection of responses to be made with the ipsilateral hand.
To investigate the potential significance of PMd–M1 physiological interactions for response selection behaviour, we tested for a relationship between the Select task-specific MEP effect (at 75 ms) and the RT slowing effect (at 100 ms). On trials in which responses were made with the M1-contralateral hand, conditioning TMS of left or right PMd both facilitated MEPs (at 75 ms) and slowed RTs (at 100 ms) (Fig. 6). This slowing effect on the contralateral hand made it easier to select a response when the visual cue instructed a response with the other hand. Hence, on those ipsilateral hand response trials, the MEP/RT relationship was inverted, reflected in a significant negative correlation (Fig. 6). That the relationship was hand-specific strongly suggests that these MEP/RT correlations are a functional marker of the process of manual response choice. The two measures appear to be differentially sensitive: while the MEP effect did not differ as a function of which hand was selected to make the response, the RT effect was hand-specific. Thus, the two measures appear to reflect the computational state of PMd at two distinct phases – a state prior to selection (at 75 ms), and a later state (at 100 ms) by which time the manual response choice is evident and causally affects behaviour. The hand-specific pattern of correlation suggests that both effects share a common origin that is related to a particular cognitive process, response selection. They further suggest that the nature of PMd–M1 interactions evolves during this interval (75–100 ms) – from a state in which the response selection decision is not yet evident (at 75 ms), to one in which the selection decision causally impacts on M1 and can be read out in behaviour (at 100 ms). Cisek & Kalaska (2005) have described a series of neural events in the macaque PMd that unfold between 50 and just over 100 ms after cue presentation that are related to different aspects of response selection. The present PMd TMS-induced MEP changes at 50 and 75 ms, and the behavioural change at 100 ms, suggest a similar evolution of response selection processes in human PMd from the time at which the response instruction cue is initially registered to the time at which the response is selected.
No hemispheric asymmetry in PMd–M1 interactions
Koch et al. (2006) reported that conditioning stimulation of left PMd had a greater impact on right M1 than did right PMd TMS on left M1. Given the established behavioural dominance of left PMd over right PMd for response selection (Schluter et al., 1998, 2001; Johansen-Berg et al., 2002; Mochizuki et al., 2005), we also expected that conditioning TMS of left PMd would have a greater physiological effect than conditioning TMS of right PMd. However, we were unable to find any physiological evidence for hemispheric dominance. Arguably, the interhemispheric nature of the TMS protocol means it is not optimized to detect hemispheric asymmetries. More importantly, however, the present replication of similar MEP modulation in both hemispheres underlines the importance of the 75-ms time-point, first identified by Koch and colleagues, for PMd–M1 physiological interactions during response selection.
Anatomical specificity
In Experiment 3 we confirmed that the observed pattern of paired-pulse MEP modulation during the Select task depended critically on PMd inputs. When the identical paired-pulse TMS protocol was applied to M1, it produced a generalized pattern of MEP facilitation that was entirely distinct from the temporally specific modulation produced by PMd–M1 TMS (Fig. 7). This confirms that, while M1 may be susceptible to prior conditioning pulses applied via a variety of routes (indirectly via PMd, or directly via M1 itself), there is only a brief window during the RT period when manipulations of PMd activity impact on M1 activity. These results complement and extend the demonstration that intracortical facilitation (ICF) and short intracortical inhibition (SICI) effects produced by paired-pulse TMS of M1 do not resemble the effect on MEPs produced by paired-pulse TMS of PMd–M1 (Koch et al., 2006).
Conditioning stimulation of PMd may exert its influence on contralateral M1 via a number of anatomical routes. One possible route is via a direct transcallosal projection linking left and right PMd, followed by an intrahemispheric connection between PMd and M1. Tracer injection studies in macaques have demonstrated transcallosal projections linking homotopic regions of PMd in each hemisphere (Marconi et al., 2003; Boussaoud et al., 2005), and there are strong intrahemispheric projections from PMd to M1 (Dum & Strick, 2005; Miyachi et al., 2005). Using implanted intracortical microwires, it has been shown that direct electrical stimulation of the macaque ventral premotor cortex can facilitate the impact of ipsilateral M1 stimulation on electromyographic activity (Cerri et al., 2003; Shimazu et al., 2004). However, it has not been possible to examine whether such effects are modulated by cognitive state, because the animals in those preparations were anaesthetized. An alternative route by which conditioning TMS of PMd may exert its effect is via a direct projection from PMd to contralateral M1. Tracing evidence has revealed direct transcallosal projections from PMd to contralateral M1 in the macaque monkey brain (Marconi et al., 2003; Boussaoud et al., 2005). Similar direct and indirect transcallosal pathways are likely to exist in the human brain. Recently, we have used diffusion-weighted imaging and tractography to investigate white matter pathways in human subjects who have participated in paired-pulse TMS experiments (Boorman et al., 2007). We have shown that the diffusion anisotropy of white matter adjacent to PMd, and in its transcallosal projection region, is correlated with the size of the PMd conditioning effect on MEPs.
Conclusions
A couple of recent studies have applied paired-pulse TMS to M1 at very short intervals to identify cortico-cortical functional connectivity changes over a subsecond time-course during the preparation of a movement (Cattaneo et al., 2005; Prabhu et al., 2007). The anatomical origin of the modulatory influence over M1 in these studies is not clear. The present study demonstrates that changes in functional connectivity occur in the pathway linking PMd and contralateral M1 when a response is being selected. These findings confirm important aspects of the timing of PMd–M1 interactions first identified by Koch et al. (2006). Importantly, however, the present study further demonstrates that: these state-dependent PMd–M1 interactions are functionally specific to a particular cognitive process, response selection; are anatomically specific to the PMd–M1 pathway; and have a causal impact on response selection behaviour.
Acknowledgements
This study was funded by the MRC, UK, with additional support from the Stevenson Junior Research Fellowship, University College Oxford (J.O'S.), the Wellcome Trust (H.J-B. E.D.B.), the MRC (studentship to C.S.) and the Royal Society (M.F.S.R.).
Abbreviations
-
- EMG
-
- electromyographic
-
- FDI
-
- first dorsal interosseous muscle
-
- IPI
-
- interpulse interval
-
- M1
-
- primary motor cortex
-
- MEP
-
- motor-evoked potential
-
- MNI
-
- Montreal Neurological Institute
-
- PMd
-
- dorsal premotor cortex
-
- RT
-
- reaction time
-
- SOA
-
- stimulus-onset asynchrony
-
- TMS
-
- transcranial magnetic stimulation.