Extensive structural remodeling of the injured spinal cord revealed by phosphorylated MAP1B in sprouting axons and degenerating neurons
Abstract
Spinal cord injury (SCI) results in loss of sensory and motor function because injured axons do not regenerate and neurons that die are not replaced. Nevertheless, there is evidence for spontaneous reorganization of spared pathways (i.e. sprouting) that could be exploited to improve functional recovery. The extent of morphological remodeling after spinal cord injury is, however, not understood. We have previously shown that a phosphorylated form of microtubule-associated protein-1B, MAP1B-P, is expressed by growing axons, but is detected in intact adult SC in fibers exhibiting a somatotopic distribution of myelinated sensory fibers. We now demonstrate that after adult SCI, MAP1B-P is up-regulated in other classes of axons. We used immunohistochemistry to show changing levels and distributions of MAP1B-P after a right thoracic hemisection of adult rat spinal cord. MAP1B-P labeling suggests rearrangements of the axonal circuitry that go well beyond previous descriptions. MAP1B-P-positive fibers are present in ectopic locations in gray matter in both dorsal and ventral horns and around the central canal. Double staining reveals that primary sensory and descending serotonergic and corticospinal axons are MAP1B-P positive. In white matter, high MAP1B-P expression is found on terminal enlargements near the injury, reflecting retraction of transected axons. MAP1B-P also accumulates in pre-apoptotic neuronal somata axotomized by the lesion, indicating association of MAP1B-P not only with axon extension and retraction, but also with neuronal degeneration. Finally, we provide evidence that MAP1B phosphorylation is correlated with activation of JNK MAP-kinase, providing a step towards unraveling the mechanisms of regulation of this plasticity-related cytoskeletal protein.
Introduction
Spinal cord injury triggers a cascade of cellular and molecular changes that exacerbate the initial damage, and eventually result in neurological deficits. Recovery is limited because of the very poor regenerative capacity of injured neurons. Nevertheless, spared systems within the central nervous system (CNS) are now believed to be capable of reorganization following injury: after incomplete spinal cord injury, sprouting by spinal and supraspinal systems has been described (Li & Raisman, 1995; Schwab & Bartholdi, 1996; Beattie et al., 1997; Christensen & Hulsebosch, 1997b; Krenz & Weaver, 1998; Brook et al., 2000; Hill et al., 2001; Ondarza et al., 2003; Mitsui et al., 2005a, b; for review, Raineteau & Schwab, 2001). Convincing evidence of sprouting by ascending and descending pathways associated with functional recovery was also shown by tracing of specific pathways (Weidner et al., 2001; Bareyre et al., 2004). However, to evaluate the full extent of reorganization within injured spinal cord, methods that are not based on labeling subsets of axons were lacking.
Sprouting or regeneration of damaged axons involves transformation of stable axonal segments into new growth cones. The contribution of cytoskeletal proteins to this process is crucial: their synthesis, organization and transport undergo quantitative and qualitative changes, recapitulating at least partially the pattern found during development. While this has been best demonstrated for regenerating peripheral axons, it is now accepted that some neuronal populations in the CNS are able to initiate an axonal growth program following injury (Tetzlaff et al., 1991).
The microtubule-associated protein MAP1B is expressed in neurons during development where it has a role in axonal growth (see References in Soares et al., 2002; Bouquet et al., 2004). MAP1B is also implicated in axonal regeneration in the adult, as seen in our in vitro study employing dorsal root ganglia (DRG) neurons (Bouquet et al., 2004) from MAP1B-null mutant mice (Meixner et al., 2000). MAP1B function is regulated by phosphorylation during development. We previously showed that a specific phosphorylated form, MAP1B-P, detected only in neurons and concentrated in the distal part of axons, is also associated with axonal plasticity in adult CNS (Nothias et al., 1996, 1997). High levels of MAP1B-P are found in spinal axons after neurodegenerative lesions (Soares et al., 1998), and in response to peripheral injury (Soares et al., 2002). Thus, we suggest that sprouting axons, even in the adult, are specifically labeled by MAP1B-P. Here, we used a MAP1B-P specific antibody (1B-P) as immunohistochemical marker to evaluate axonal reorganization in hemisectioned spinal cord. In addition, we found that MAP1B-P expression and distribution also undergo dramatic changes in axons and neuronal cell bodies bound to degenerate, suggesting its use as a marker for the predegenerating state of damaged neurons. Finally, we provide in vivo and in vitro evidence that MAP1B phosphorylation is correlated with activation of JNK MAP-kinase.
Materials and methods
Animals
Adult female Wistar rats (250 g; n = 32) were used in this study. Experimental procedures (authorization no. 75122) followed the European Communities Directive (86/609/EEC) and were approved by the Ethics Committee 3, Ile-de-France Region.
Surgery
Animals were anesthetized by i.p. injection of a solution containing xylazine (5 mg/kg; Rompun 2%, Bayer, France) and ketamine (100 mg/kg; Imalgène 500, Merieux, France). Laminectomy was performed at thoracic levels 8–9 (T8–T9) under visual guidance using an operating microscope. The skin was cut along the midline of the back, paravertebral muscles of the T8–T9 vertebrae were dissected out to expose the underlying spinal cord, the dura mater was opened and the spinal cord hemisectioned on the right side with iridectomy scissors. Muscles and skin were sutured, and the animals were left to recover, and returned to their home cages with free access to food and water. After survival times of 3–90 days post-lesion, animals were deeply anesthetized with pentobarbital, perfused transcardially with NaCl (0.9% at 37 °C), followed by 4% paraformaldehyde in 0.1 m phosphate buffer, pH 7.4 (PB). Spinal cords were dissected and cryoprotected in 30% sucrose in 0.1 m PB containing NaCl (0.9%; PBS). Serial sections (40 µm) were cut on a cryostat and maintained in PBS at 4 °C.
In some animals, the axonal tracer Dextran Biotin Amine (DBA 10000; 10%) was pressure-injected via a Hamilton syringe at 15 days post-lesion into the cortex (A:0.26, L:1.4, H:1.75/A:0.48, L:2, H:1.75/A:1, L:2.4, H:1.75) for tracing of right corticospinal tract (CST) projections. Animals were perfused 1 week thereafter.
Immunohistochemistry
Immunohistochemistry was performed as previously described (Soares et al., 2002). MAP1B and MAP1B-P immunostaining were performed using rabbit polyclonal antibody 1B-poly, and monoclonal antibody (mAb) 1B-P, respectively (hybridoma generously provided by Dr I. Fischer; Nothias et al., 1996). Neurofilaments were labeled with mAb SMI-31 (1 : 2000) and SMI-32 (1 : 4000; Interchim, France). Peptidergic primary sensory afferents were specifically labeled using a rabbit polyclonal antibody against calcitonin gene-related peptide (CGRP; 1 : 10 000, Peninsula, UK); non-peptidergic primary sensory afferents were specifically labeled using isolectin B4-FITC (IB4, Sigma, France). Macrophages that are also stained with IB4 are easily distinguished from IB4-positive axons through their morphology. Stable microtubules were detected in vitro by using monoclonal anti-acetylated tubulin antibody (1: 2000; Sigma) and beta3-tubulin by using monoclonal antibody Tuj-1 (1 : 1500; Babco, Richmond, CA, USA). Activated P-JNK was detected by a polyclonal antibody (1 : 400; Promega). Synaptophysin was detected by using a polyclonal antibody (1 : 1000, DAKO). Nuclear staining was peformed using DAPI staining. Sections were analysed on a standard light (Leica DMRB) or a confocal microscope (Leica SP1).
Relative quantification of MAP1B-P immunostaining in gray matter was performed using ImagePro-Plus software (Media Cybernetics, Silver Spring, MD, USA) on images of serial sections performed at 8, 15 and 30 days post-injury. On each section, separately for ipsi- and contralateral side to the lesion, the gray matter was divided into three areas. The number of pixels corresponding to MAP1B-P immunostaining was calculated for each area, and compared with the total surface area. For each region, the value was compared with that corresponding to the same region in a normal spinal cord and the ratio represented as histograms. Means for each area at each time point were calculated. Care was taken to ensure equal background staining intensities for each section examined.
Culture of dorsal root ganglia neurons
Adult dorsal root ganglia neurons were cultured as described (Bouquet et al., 2004). For some experiments, the following drugs were added after growing neurons for 24 h in vitro: SP600125, a specific inhibitor of activated P-JNK (Calbiochem), at 40 µm (this concentration was found to yield the maximum effect) – after 24 h, cells were fixed as described (Bouquet et al., 2004); and staurosporin (Sigma), at different concentrations (0–1 µm), used to induce apoptosis – cells were fixed 8 h after treatment. Immunocytochemistry on cell cultures were performed as described (Bouquet et al., 2004).
N2A cell experiments
N2A (neuroblastoma) cell line (generously provided by F. Propst) cultures were grown on poly lysine until confluence in DMEM−10% FCS (Invitrogen) at 37 °C and 5% CO2. SP600125 (40 µm) was added for 24 h. For Western blotting, wells were rinsed twice with PBS, and cells solubilized in lysis buffer [SDS 4%; Tris 0.125 m, pH 6.8; EDTA 12 mm; DTT 0.3%; glycerol 20%; anti-protease cocktail (Sigma); Na3VO4 1 mm]. Samples were sonicated, centrifuged and incubated for 10 min at 65 °C before loading on a 4% polyacrylamide gel (protein concentration was normalized for each sample). After transfer, nitrocellulose membranes were incubated overnight at 4 °C in BSA 5%–PBS–Tween 0.25%, then with polyclonal anti-total MAP1B in BSA 2%–PBS–Tween 0.25%, followed by goat anti-rabbit–alkaline phosphatase (1 : 3000). NBT/BCIP was used for the enzymatic detection.
MAP1B-P immunocytochemistry on N2A cells was done using the same procedure as described for primary DRG neuron cultures (see Bouquet et al., 2004).
Results
Distribution of MAP1B-P in intact adult spinal cord
In intact adult spinal cord, MAP1B-P is never detected in neuronal somata. MAP1B-P immunostaining within gray matter is limited to fibers exhibiting the somatotopic distribution of myelinated primary sensory fibers, i.e lamina I, intermediate gray matter, and ventral horn (Fig. 1‘control’, Fig. 3c; see also Soares et al., 2002). MAP1B-P is also found in white matter, in association with most ascending and descending pathways (1, 2-9), with the notable exception of the CST. Dorsal columns are strongly labeled, as well as dorsal and ventral roots, reflecting the presence of MAP1B-P in primary sensory afferents and motor axons, as described previously (Ma et al., 2000). Finally, the ventral funiculus is also highly stained around the midline, especially on lumbar sections.
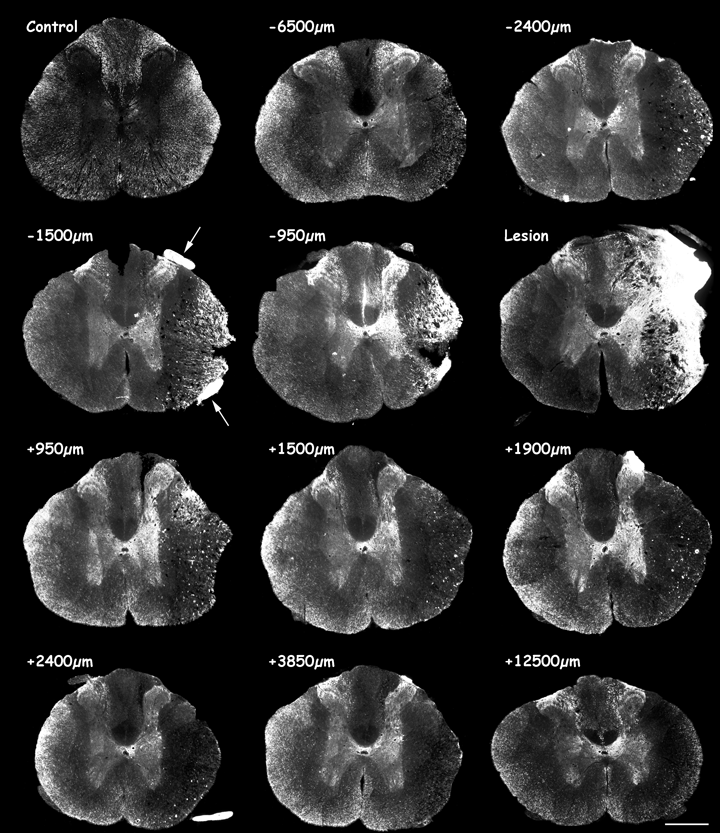
MAP1B-P immunofluorescence on coronal sections of adult rat spinal cord from an intact animal (control), and 8 days after a right lateral thoracic hemisection. Top left image shows a control section from an intact animal, followed by serial sections at varying distances rostro-caudal to the lesion site (‘Lesion’) as indicated. ‘Minus’ values correspond to distances rostral, and ‘plus’ values caudal to the injury site. Note that in lesioned spinal cord, MAP1B-P immunostaining in gray matter is higher than on the control section (from an intact animal), and is particularly strong around the lesion site. See high magnification in Fig. 3a. Scale bar, 600 µm.
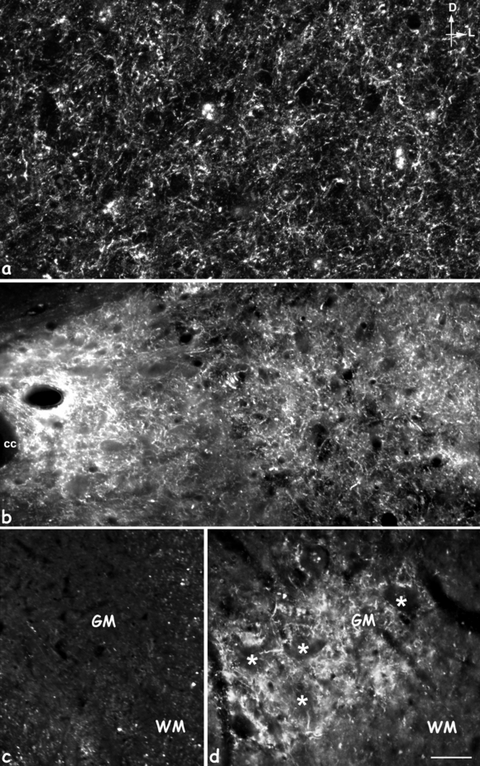
High magnification of MAP1B-P-immunostained fibers in gray matter (GM). (a,b) Intermediate layer at 8 days (a) and 15 days (b); (d) the ventral horn at the motoneuron level (stars) proximal to the lesion site, 15 days post-injury; (c) corresponds to the same region as in (d), but in control-intact spinal cord. No MAP1B-P-positive fibers are detected in intact GM (c). D, dorsal; L, lateral; cc, central canal; WM, white matter; GM: gray matter. Scale bars: a, 20 µm; b–d, 35 µm.
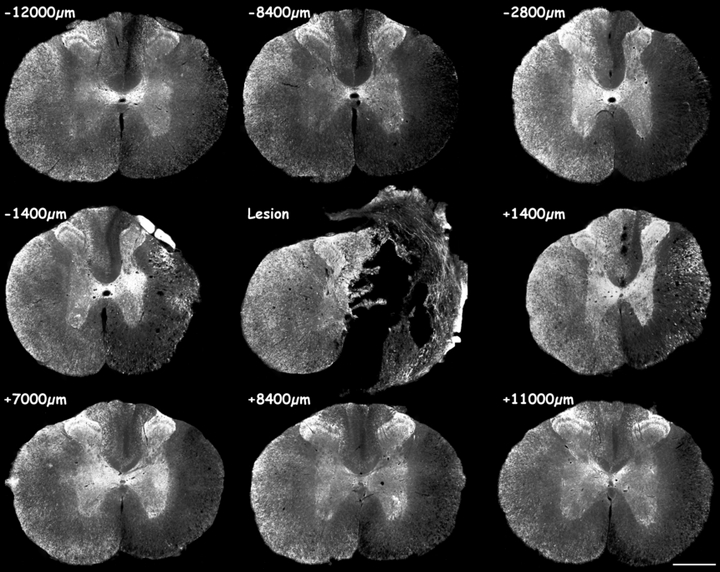
MAP1B-P immunofluorescence on serial sections of hemisectioned spinal cord, 15 days post-injury. Note the strong MAP1B-P staining in gray matter at all levels rostral and caudal to the lesion site. Numerous MAP1B-P-positive fibers are observed around the cavity formed at the lesion site. See at higher magnification Figs 3b and d. Scale bar, 600 µm.
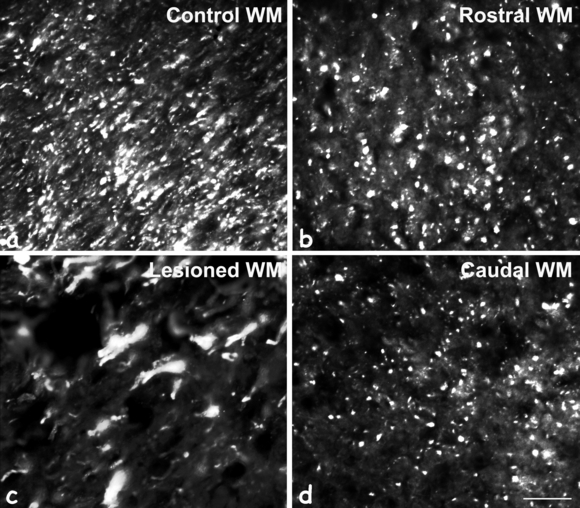
Higher magnification of MAP1B-P immunostaining in lateral white matter (WM). On control sections (a), most WM tracts contain MAP1B-P-positive fibers. Eight days after spinal cord injury, on rostral (b) and caudal (d) sections, some MAP1B-P-positive tracts have disappeared when compared with intact WM. (c) In WM rostral and close to the injury site (−950 µm), numerous axonal enlargements (swellings) are noted where MAP1B-P is accumulating. Scale bar, 100 µm.
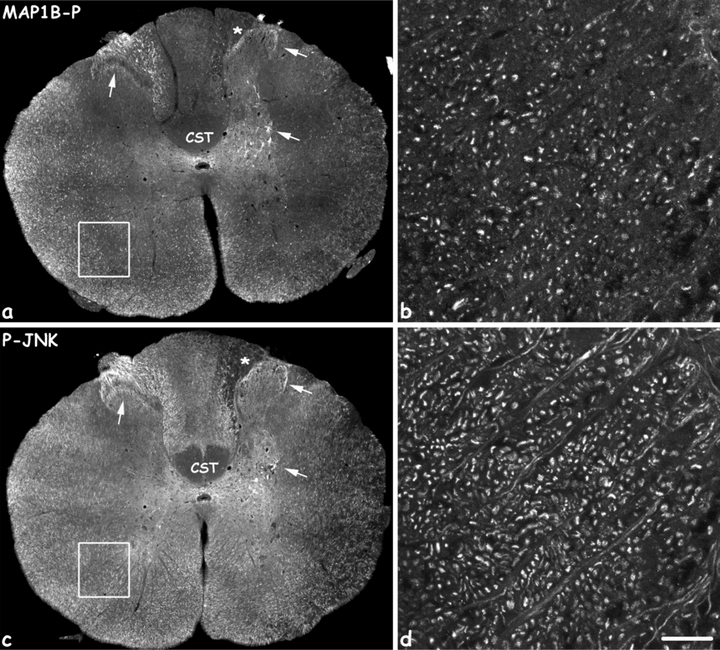
Co-localization of MAP1B-P (a,b) and phospho-JNK (P-JNK, c,d) within the same fiber types, 8 days after the injury. MAP1B-P and P-JNK staining was performed on adjacent sections. Note the identical distribution of both molecules in white and gray matter (arrows), and both ipsi- and contra-lateral to the spinal cord hemisection. b and d are high magnifications of the region delineated by white squares in a and c, respectively. CST, corticospinal tract. Scale bars, a and b: 400 µm; c and d: 20 µm.
Lamina II (L-II; substantia gelatinosa) is normally devoid of MAP1B-P. CGRP, expressed by small and medium-size DRG neurons, is present in unmyelinated and thinly myelinated primary afferents terminating in laminae I and II (outer part;Fig. 5m, left) of the dorsal horn. Small-diameter non-peptidergic neuron terminals that bind Griffonia simplicifolia lectin (isolectin B4; IB4) are also present within the inner part of lamina II (Fig. 5g, left; Kitchener et al., 1993). Neither of these fiber populations co-label with MAP1B-P.
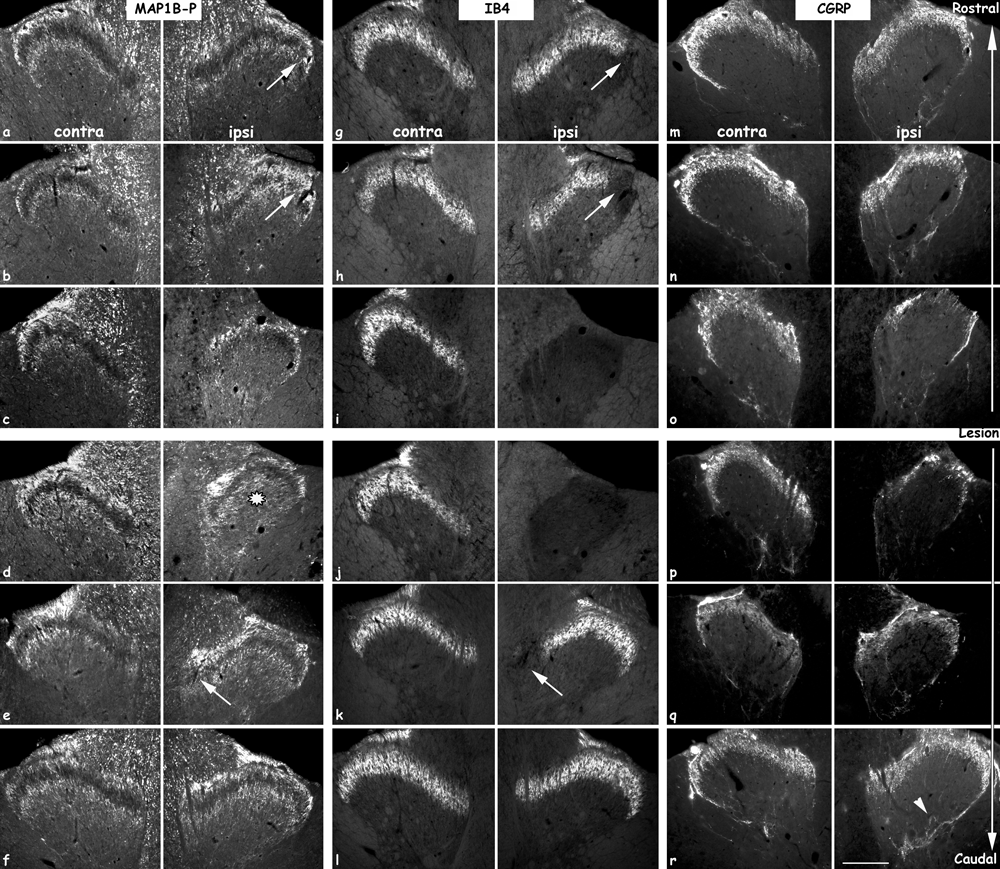
Distribution of the different primary sensory afferents in contra- (left colums) and ipsilateral (right colums) dorsal horn, 15 days after a spinal cord hemisection. Left panel, MAP1B-P-positive (myelinated) fibers; middle panel, IB4-positive (non-peptidergic) fibers; right panel, CGRP-positive (peptidergic) afferents. MAP1B-P and IB4 immunostaining was performed as double immunofluorescence on the same section, CGRP labeling was done on adjacent sections. Sections rostral to the lesion site (top part) were taken at: a, g, m, −12 000 µm, b, h, n, −7600 µ m, and c, i, o, −5600 µm; sections caudal to the lesion site (bottom part) at: d, j, p, +1400 µm, e, k, q, +4400 µm, and f, l, r, +10 000 µm. Intense MAP1B-P staining is observed on fibers in LI and LIII on both sides and at all rostro-caudal levels, as well as in deeper layers, particular on a section close to the injury (d, black star). On some sections, IB4-positive fibers are seen to be lacking in a part of LII (arrows in g, h and k), and are probably replaced by MAP1B-P-positive fibers (arrows in a, b and e, respectively). Note the decrease of all three types of primary sensory afferents in ipsilateral dorsal horn close to the lesion (c, i and o). The number of CGRP-positive fibers is increased in deeper layers (arrowhead in r). Scale bar, 190 µm.
Spatio-temporal evolution of MAP1B-P distribution after lateral hemisection at T8–T9
Lateral hemisection induces marked changes in MAP1B-P staining, rostral and caudal to the lesion, both ipsilaterally and, to a lesser extent, contralaterally to the lesion.
MAP1B-P levels are increased in fibers located within gray matter
8 days post-lesion. At the lesion epicentre (Fig. 1,‘Lesion’), the dorsal horn is disrupted, and the lesion site, where a cavity has not yet formed, is filled with numerous MAP1B-P-positive fibers, intermingled with non-neuronal cells that invade the lesion (for details and references, see also von Boxberg et al., 2006). On sections close to the injury, MAP1B-P staining is strongly increased, in particular in the median to ventral part of the gray matter (Fig. 1, −1500 µm, −2400 µm). At high magnification, MAP1B-P fibers are thin and their density is high (Fig. 3a). Staining is densest proximal to the injury, particularly in dorsal and ventral roots (arrows), where individual fibers cannot be distinguished. While more prominent ipsilateral to the hemisection, an increase in MAP1B-P labeling is also observed on the contralateral, unlesioned side. Further rostrally, the distribution of MAP1B-P-positive fibers in dorsal horn is not obviously affected (Fig. 1, −6500 µm), but staining density is increased in laminae I and III, and in L–X and the median portion of L–VII around the central canal on both sides of the spinal cord, and as in L–IX, around motoneuron pools. Just caudal to the injury (Fig. 1, +950 µm), MAP1B-P staining density and distribution in dorsal horn are comparable with those in control sections, but abnormal MAP1B-P staining is detected on numerous fibers in intermediate gray matter both ipsilateral and, to a lesser extent, contralateral to the lesion. Further caudally, gray matter is still abnormally highly positive for MAP1B-P in both the ventral horn and around the central canal ipsilateral to the lesion (Fig. 1, +12 500 µm).
15 days post-lesion At the lesion epicenter, a large cavity has developed (Fig. 2,‘Lesion’) that is surrounded by MAP1B-P-positive fibers. The distribution of highly MAP1B-P-positive fibers within the gray matter has extended both rostrally and caudally (Fig. 2, −2800 µm, see also Fig. 4) compared with at 8 days (Fig. 1, −2400 µm). A higher magnification of labeled fibers in the median and ventral part of the gray matter is shown in Fig. 3b and d. The increase in MAP1B-P immunostaining and its abnormal localization in gray matter is bilateral, although always more prominent ipsilateral to the lesion.
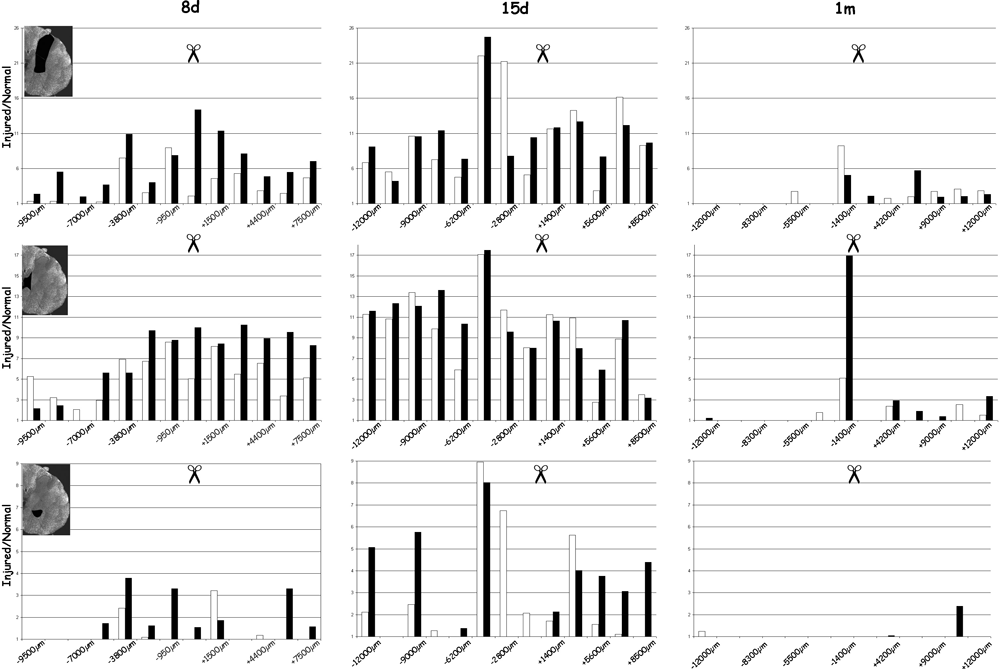
Quantification of MAP1B-P immunoreactivity in spinal cord gray matter, at 8 days (8d), 15 days (15d), and 1 month (1m) post-injury, evaluated from serial sections like those shown in 1, 2. Injury site is indicated by scissors. Density of positive pixels in gray matter was measured for three areas (delineated in black on the insets) comprising: top panel, dorsal horn and medio-lateral gray matter; middle panel, region around and lateral to the central canal; bottom panel, motoneuron area (ventral horn). White bars represent the left (contralateral) side, black bars the right side of the spinal cord (ipsilateral to the lesion). The number of positive pixels is summed for each region and reported to its surface. Pixel values obtained for injured spinal cord are then reported to those measured in the same region in normal (control) spinal cord. Only positive ratios are represented in these histograms. Note that at 8 days, sprouting is more prominent caudally than rostrally to the lesion; also note that the particularly high level and marked rostro-caudal extent of MAP1B-P immunoreactivity at 15 days, in both ipsi- and contralateral gray matter.
Quantification of the density of MAP1B-P immunostaining (Fig. 4) confirmed the qualitative results presented in 1, 2.
Thus, 8 days after the lesion (even as early as 3 days, not shown) a massive increase in MAP1B-P immunoreactivity is detected in gray matter, starting around the lesion site and gradually extending rostrally and caudally. At this time-point, mean values for lesion-induced MAP1B-P labeling are generally higher on caudal than on rostral sections, and highest overall values are observed around the central canal (middle panel), close to and caudal to the lesion site.
MAP1B-P immunostaining then reaches a peak at 15 days post-injury, in all three regions examined. Around the central canal, values are now higher for rostral sections, while they appear to decline on caudal sections. Thus, sprouting revealed by MAP1B-P labeling appears to be region- and time-dependent. L-X (see bottom panel) shows highly increased MAP1B-P immnoreactivity between 8 and 15 days post-lesion both rostral and caudal to the lesion site.
By one month, the rostro-caudal extent of MAP1B-P-positive fibers within gray matter has dramatically decreased in all three areas. Labeled fibers are mostly restricted to the region around and caudal to the lesion. The lesion cavity is still present, and as at 15 days, surrounded by some stained fibers (not shown). The distribution shown at 1 month persists up to 3 months (not shown).
Hemisection induces sprouting of both large and small caliber primary afferents into the dorsal horn
Using lesion paradigms that do not directly attain the spinal cord (peripheral lesion and rhizotomy), we previously demonstrated that increased MAP1B-P levels are associated with central sprouting of myelinated, but not of CGRP-positive fibers (Soares et al., 2002). We now investigated the distribution of primary sensory afferents after spinal cord hemisection, using MAP1B-P and CGRP as specific markers for large myelinated and thin peptidergic fibers, respectively. In addition, we used IB4 as a marker for thin non-peptidergic fibers. Figure 5 illustrates the distribution of all three labels at 15 days post-injury.
In L-II of the intact dorsal horn, no MAP1B-P staining and hence no co-localization with CGRP or IB4 is noted in thin primary sensory afferents. A co-localization of CGRP with MAP1B-P is seen only in very few fibers in L-I (see also Soares et al., 2002). Following a hemisection, CGRP levels are markedly decreased rostrally near the lesion site (Fig. 5o and p), and IB4-positive fibers have completely disappeared from L-II (Fig. 5i and j). MAP1B-P staining is also decreased in dorsal horn (Fig. 5c and d; laminae I and III). This decrease in specific staining for all types of primary sensory afferents is undoubtedly due to damage of the dorsal root projecting into the hemisectioned spinal segment.
On sections further rostral or caudal to the injury, IB4-positive fibers are absent from the lateral (Fig. 5g and h) and medial (Fig. 5k) parts of L-II. Interestingly, we noted that the area of L-II devoid of IB4-positive fibers is now occupied by MAP1B-P-positive fibers (compare Fig. 5b; 5e and Fig. 5h and Fig. 5k). Careful analysis of rostral and caudal sections suggests that MAP1B-P-positive fibers from adjacent roots not damaged by the hemisection have sprouted into the region of L-II that normally receives projections of IB4-positive fibers from the damaged root. Indeed, in cases where dorsal roots were spared by the lesion, MAP1B-P staining is dramatically increased in the dorsal root, and hence also in the whole dorsal horn (see for 8 days: Fig. 1, −1500 µm).
These changes in primary sensory afferent immunostaining within L-II were observed at all time points post-injury analysed.
Partial co-localization of MAP1B-P with CGRP can be detected in ectopic sprouts at the lesion site, early after injury (8 days: Fig. 6d- f), and at later post-injury stages (1–3 months after the lesion; not shown), in fibers crossing the midline in the dorsal-most part of the median gray matter above the central canal.
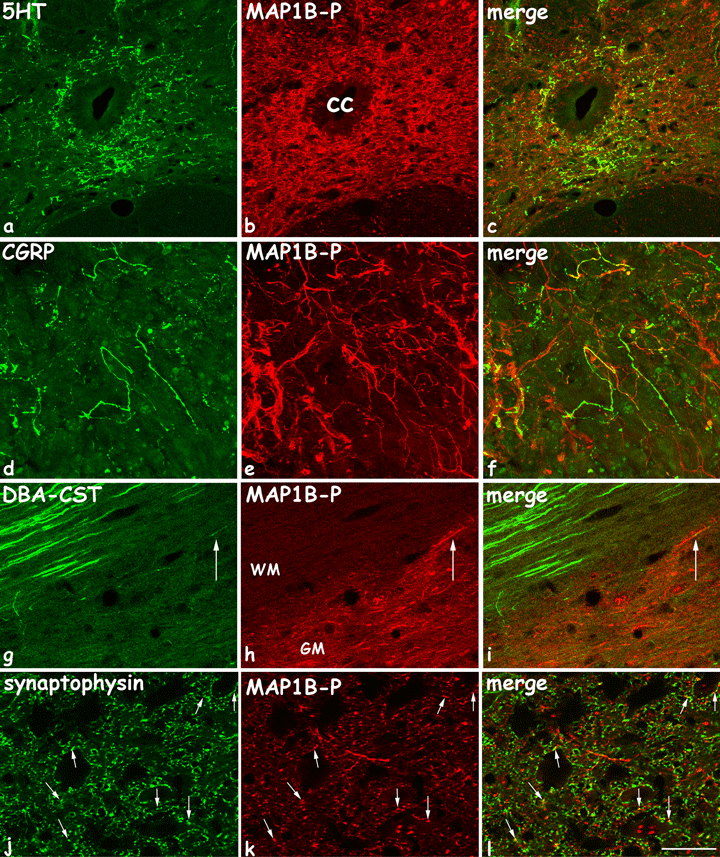
Confocal analysis of fibers double stained for: a–c, MAP1B-P and 5HT, coronal section 8 days post-lesion; d–f, MAP1B-P and CGRP, coronal section 8 days post-lesion; g–i, MAP1B-P and DBA-traced cortico-spinal tract (CST), horizontal section 21 days post-lesion (thoracic T8 level); and j–l, MAP1B-P and synaptophysine, 15 days post-lesion. 5HT-fibers are numerous around the central canal, and almost all coexpress MAP1B-P. CGRP-positive fibers are detected at the injury site, most of which are also MAP1B-P-positive. Axonal tracer DBA was injected into the contralateral motor cortex, which effectively labeled most fibers within the right CST in white matter, but only fibers ending in gray matter are double stained for MAP1B-P (arrows). Synaptophysin and MAP1B-P are tightly apposed and frequently co-localize (arrows). cc, central canal; WM, white matter; GM, gray matter. Scale bars, a–i, 55 µm; j–l, 20 µm.
Fibers expressing MAP1B-P after injury have multiple origins
Sprouting of primary sensory afferents accounts for some of the increase in gray matter MAP1B-P staining, as has been shown previously using other markers (Beattie et al., 1997; Krenz & Weaver, 1998). Sprouting of descending axons could also be involved, associated with the injury-induced reorganization of the local circuitry at each level of the spinal cord (Bareyre et al., 2004). We therefore investigated in which spinal afferents MAP1B-P is induced, using axon tracing and specific markers.
Double immunostaining with an anti-5HT antibody was performed to reveal serotoninergic Raphe-derived descending projections. In intact spinal cord, MAP1B-P is not detected in serotoninergic axons, but following hemisection, most 5HT-positive fibers around the central canal and in the intermediolateral area (as described for mouse in Inman & Steward, 2003) are also stained for MAP1B-P (confocal microscopy images: Fig. 6a–c). This result suggests that injury induces reorganization of the cytoskeleton within descending serotoninergic fibers, leading to their elongation.
Next, we performed tracing experiments by injecting BDA into the left sensorimotor cortex at 21 days after spinal cord injury. The CST is normally almost devoid of MAP1B-P staining. However, after the hemisection, we detected some MAP1B-P labeling in CST axons that had entered the gray matter (Fig. 6g–i). The actual number of MAP1B-P-positive CST axons may even be underestimated, as fiber tracing does not reveal the entire tract, as described by others (Hill et al., 2001; Bareyre et al., 2004).
Thus, we were able to correlate part of the injury-induced MAP1B-P immunoreactivity in gray matter to specific fiber tracts. Nevertheless, the massive increase in MAP1B-P-positive fibers suggests involvement of other classes of fibers, e.g. propriospinal fibers.
Synaptophysin, a vesicular protein present in presynaptic axons, has been used previously as a marker to show modifications in neuronal circuitry (Mitsui et al., 2005b). Thus, double immunostaining of synaptophysin and MAP1B-P has been undertaken on spinal cord sections, 15 days after the injury, and analysed via confocal microscopy. A tight apposition of both proteins that frequently co-localize was observed (Fig. 6j–l).
MAP1B-P immunoreactivity is decreased in white matter, rostral and caudal to the injury, but accumulates in axonal enlargements
Marked changes in MAP1B-P labeling were also observed in white matter. On rostral-most sections, the medial part of the dorsal funiculus is totally devoid of MAP1B-P staining as early as 8 days post-injury (see Fig. 9), indicating that ascending sensory fibers undergoing Wallerian degeneration (Warden et al., 2001) no longer express MAP1B-P.
In lateral parts of the white matter rostral and ipsilateral to the lesion, the number of MAP1B-P fibers located in the dorso- and ventro-lateral funiculi is reduced in comparison with the contralateral side (Fig. 1, −6500 µm; Fig. 9a; compare Fig. 7a and Fig. 7b). The same phenomenon was observed on caudal sections (compare Fig. 7a and Fig. 7d) at all time-points post-injury examined. This reduction is likely to reflect Wallerian degeneration of ascending tracts on rostral sections, and of descending tracts on caudal sections. Evidence for this was obtained by double staining for total MAP1B (with a polyclonal antibody; MAP1B-poly), and phosphorylated (SMI-31), or non-phosphorylated neurofilaments (SMI-32), which also showed a co-localization (not shown). On proximal sections, both rostral and caudal to the injury, we frequently observed enlarged fibers and large terminal bulbs that contained high amounts of MAP1B-P (Fig. 1, −2400 µm to +2400 µm; Fig. 7c), structures likely to correspond to terminal clubs in the course of retraction or retrograde degeneration. White matter tracts that undergo progressive loss of MAP1B-positive fibers exhibit progressive formation of microcavities, in which ‘swollen’, MAP1B-P-positive fibers are still occasionally detected. These microcavities, as we demonstrated at the light and electron microscopy level (von Boxberg et al., 2006), correspond to degenerating tracts associated with progression of secondary lesions.
Abnormal MAP1B-P accumulation in neuronal somata in gray matter close to the injury site
In intact tissue, MAP1B-P is exclusively located in axons. Following injury, we observed a strong MAP1B-P concentration in neuronal somata. In particular, on sections close to the injury site from 3 to 21 days post-lesion (Fig. 8; compare with control provided in Supplementary material, Fig. S1), Clarke's nucleus neurons are highly labeled. These cells are located caudal to the lesion (Lumbar segment 1) and project their axons rostrally in the dorsospinocerebellar tract, which is destroyed by the lateral hemisection, thus axotomizing Clarke's neuron (Fig. 8a, g and i). These cells are particulary vulnerable to spinal cord injury, and about half die by apoptosis within 2 months (Himes et al., 1994). MAP1B-P accumulation in the soma of these neurons may thus be indicative of changes in their cytoskeleton that precede their final degeneration. We also detected abnormal accumulation of other cytoskeleton components in the soma of these neurons, such as beta3-tubulin (Fig. 8b–d), and neurofilaments (Fig. 8e). Therefore, we investigated the expression of activated caspase-3 (Fig. 8i), known to be induced in neurons during early stages of apoptosis (Pettmann & Henderson, 1998). Indeed, activated caspase-3 co-localizes with MAP1B-P in neuronal somata (Fig. 8i), confirming our hypothesis that abnormal accumulation of MAP1B-P precedes death by apoptosis. TUNEL labeling performed at 1 week post-lesion revealed that MAP1B-P-positive Clarke's neurons in gray matter had not yet entered the final stage of apoptosis at this time-point, as no co-localization was observed (not shown).
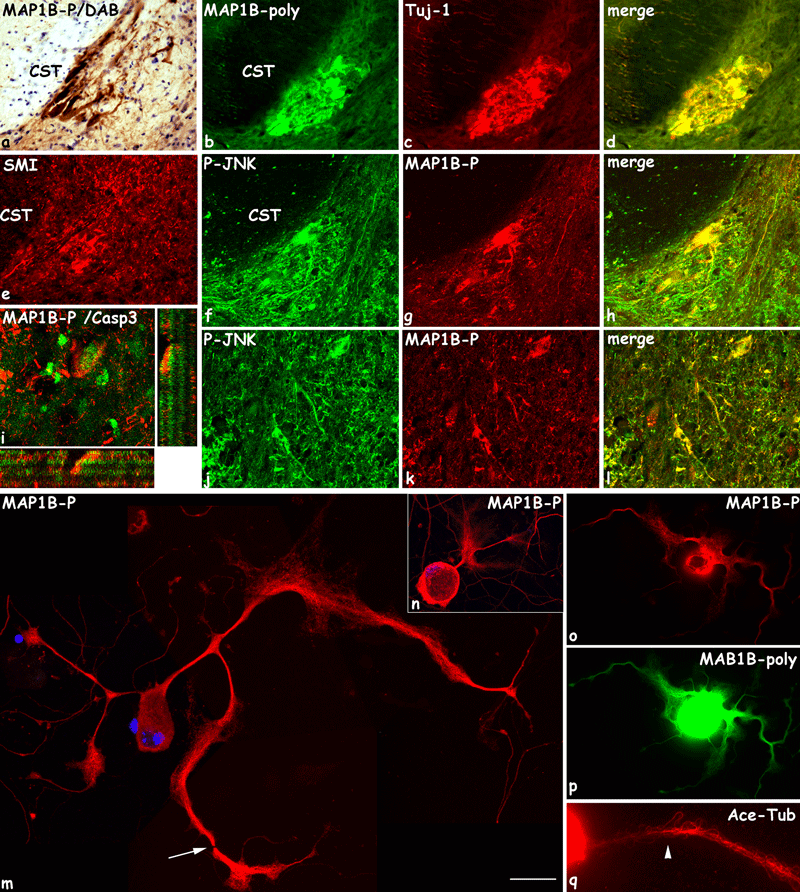
MAP1B-P accumulation in neuronal cell bodies in vivo (a–l) and in vitro (m–q). (a–l) Eight days after spinal cord hemisection, cell bodies of Clarke nucleus neurons ipsilateral to the lesion, located just caudal to the injury site on the basement of central white matter, are abnormally highly stained for MAP1B-P (a, g, i), total MAP1B (b, MAP1B-Poly), Tuj-1 (c), SMI (e), and phospho-JNK (P-JNK; f). These neurons, known to die by apoptosis, also express activated caspase-3 (i, green; confocal analysis, MAP1B-P in red). MAP1B-P and P-JNK also co-localize in other neuronal somata in gray matter (j–l). a: MAP1B-P immunostaining with diaminobenzidine-peroxydase reaction followed by Nissl conterstaining; b, c: double staining of total MA1PB (MAP1B-poly) and beta3-tubulin (tuj-1); d, merge image; f, g, j, k, double staining for MAP1B-P and phosphoJNK (P-JNK); h and l, merge images. CST, cortico-spinal tract. (m–q) Immunocytochemistry for MAP1B-P (m–o), total MAP1B (p), and acetylated tubulin (Ace-Tub, q) of cultured adult dorsal root ganglion neurons, 8 h after treatment with staurosporine. Neurite shafts undergo swelling and present interruptions (m, arrow). MAP1B-P staining, although high, no longer exhibits a proximo-distal gradient within neurites (m–o; compare with Fig. 10e); in some cells, MAP1B-P accumulates in the soma (n). In neurite enlargements, MAP1B-P staining seems to spread abnormally, and microtubules splay out (arrowhead in q). o and p, double immunostaining of the same cell. Scale bars, a–l: 90 µm; m: 55 µm; n–p: 80 µm; q: 50 µm.
To verify further our hypothesis that MAP1B phosphorylation is abnormally increased in axons and neuron cell bodies undergoing degeneration, we set up cultures of adult DRG neurons, in which apoptosis was artificially induced by addition of staurosporine (Boix et al., 1997). In control (untreated) neurons, total MAP1B is detected in all cytoplasmic compartments, cell bodies and neurites (Fig. 10d), whereas MAP1B-P is found only in neurites, distributed in a proximal to distal gradient (highest in growth cones; Fig. 10e). In staurosporine-treated cultures (Fig. 8m–q), MAP1B-P indeed accumulates in cell bodies, and in the proximal part of neurites (Fig. 8n) the proximo-distal gradient is lost. Processes that have not yet retracted or degenerated undergo swelling in their median part, with MAP1B-P appearing to spread out (Fig. 8m–o). Tubulin polymers appear also disorganized (Fig. 8q), and some neurites are seen to disintegrate (appearance of fissures along the neurites, Fig. 8m, arrow). These in vitro observations thus closely resemble the morphological state of affected neurons seen in vivo after a spinal cord lesion.
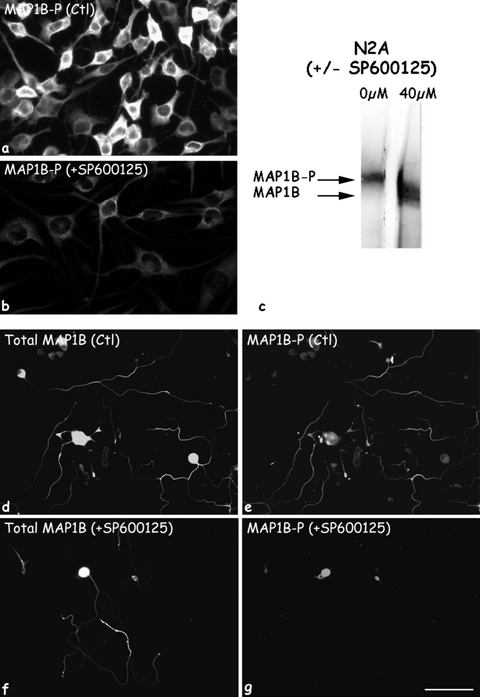
Effect of P-JNK inactivation on MAP1B-P levels. SP600125 inhibitor was applied for 24 h on cultures of a neuroblastoma (N2A) cell line (a,c), and of adult DRG neurons (d–g). In control (Ctl) cultures, both cell types are highly immunoreactive for MAP1B-P (a and e). For DRG neurons, total MAP1B staining is shown for comparison (d,f). Note that along DRG neurites, MAP1B-P exhibits a proximo-distal gradient (e). Addition of SP600125 induces a massive reduction in MAP1B-P levels, as revealed by both immunofluorescence staining (b,g), and by immuno-Western blotting of N2A protein extract (c), showing a shift in the MAP1B band from a highly phosphorylated to a non-phosphorylated form. Scale bars, a and b: 60 µm; d–g: 280 µm.
MAP1B-P staining co-localizes with activated phospho-JNK
In order to identify the specific kinase responsible for MAP1B phosphorylation after spinal cord injury, we performed double immunostaining for MAP1B-P and phospho-JNK (P-JNK), the activated form of the MAP kinase family member JNK. The P-JNK antibody used here recognizes activated JNK-1, -2 and -3. P-JNK is detected in both white and gray matter of spinal cord (Fig. 9c). Moreover, all axons labeled by MAP1B-P antibodies were also stained by P-JNK (Fig. 9b and d). Co-localization was also noted in neuronal cells bodies exhibiting MAP1B-P accumulation (Fig. 8j–l). These results show that after spinal cord injury, JNK might be involved in the specific phosphorylation of MAP1B that is detected by our 1B-P antibody.
The role of P-JNK in phosphorylation of MAP1B was further validated using cultures of adult DRG neurons treated with SP600125, a commonly used specific JNK inhibitor (Bennett et al., 2001). We noted that upon addition of this drug, neurite outgrowth was progressively affected, as had been shown for postnatal neurons (Lindwall & Kanje, 2005). Moreover, inhibition of JNK activity was followed by a drastic decrease in MAP1B-P levels, while the amount of total MAP1B remained unchanged (compare Fig. 10d and e with Fig. 10f and g). Thus, 24 h after treatment, MAP1B-P immunoreactivity was undetectable in the distal-most part of neurites (Fig. 10g).
The same result was obtained using cultured N2A cells, in which we detected high levels of MAP1B-P (Fig. 10a). A Western blot prepared from untreated N2A cells and reacted with the polyclonal antibody recognizing all MAP1B isoforms shows that most MAP1B is indeed phosphorylated (corresponding to the highest molecular weight band, Fig. 10c; see also our previous publications, Nothias et al., 1996; Ma et al., 2000; Alfei et al., 2004). Treatment of N2A cells with SP600125 results in a shift of the MAP1B band to a lower molecular weight, indicating that MAP1B-P is no longer present, in accordance with the immunostaining seen in Fig. 10g.
Discussion
Spontaneous axonal remodeling occurs in injured spinal cord, but its extent is not known. We previously provided evidence that the cytoskeletal protein MAP1B-P is a reliable and sensitive marker for plastic rearrangements of the axonal circuitry (Nothias et al., 1997; Soares et al., 1998; Ma et al., 2000). Here, using MAP1B-P immunohistochemistry on lesioned spinal cord, we show that (1) the extent of fibers undergoing morphological changes (probably sprouting and Wallerian degeneration) is in fact considerably greater than previously thought, affecting axons at substantial distances rostrally and caudally to the lesion; (2) MAP1B-P expression is also associated with apoptotic events, as it is accumulated in axotomized Clarke's nucleus neurons undergoing apoptosis; (3) MAP1B-P distribution is always closely associated with that of activated JNK, phospho-JNK. This suggests that enhanced JNK signaling after injury induces MAP1B phosphorylation, a hypothesis for which we provide further evidence in vitro.
Various experimental paradigms have been used to study morphological and functional consequences of spinal cord injury: electrolytic lesion (Li & Raisman, 1995), contusion (Hill et al., 2001; Inman & Steward, 2003; Mitsui et al., 2005a, b), dorsal or lateral hemisection (Christensen & Hulsebosch, 1997b; Fouad et al., 2001; Weidner et al., 2001; Ondarza et al., 2003; Bareyre et al., 2004; Ballermann & Fouad, 2006) and transection (Krenz & Weaver, 1998). All these studies demonstrated varying degrees of axonal sprouting, using techniques such as silver staining (Beattie et al., 1997), axon tracing (Li & Raisman, 1995; Krenz & Weaver, 1998; Hill et al., 2001; Inman & Steward, 2003; Bareyre et al., 2004) or immunohistochemistry for specific types of fibers (Christensen & Hulsebosch, 1997a; Krenz & Weaver, 1998; Brook et al., 2000; Ondarza et al., 2003). Markers specific to certain fiber types are likely to underestimate the full extent of sprouting. GAP-43, usually considered as a more general marker for axonal sprouting, has also been used to show spontaneous remodeling after spinal cord injury (Christensen & Hulsebosch, 1997b; Goldstein et al., 1997; Brook et al., 2000; Ondarza et al., 2003). However, GAP-43 staining noted within fibers in the dorsal horn is actually increased only during the first week post-injury, returning to control levels by 14 days (Christensen & Hulsebosch, 1997a). Thus, GAP-43 might be less sensitive as an intrinsic marker for axonal sprouting than MAP1B-P, whose expression is maximal at 2 weeks but remains detectable near the lesion for at least 3 months. Moreover, MAP1B-P staining reveals axonal remodeling in gray matter, not only around the lesion site, but up to several segments distant from the primary injury.
MAP1B-P shows massive structural remodeling of fibers that extends far beyond previous descriptions
We have previously shown that MAP1B-P reveals the capacity of central sprouting of primary sensory myelinated afferents in response to peripheral injury. These fibers also undergo sprouting after SC injury, as revealed by Krenz & Weaver (1998). Using MAP1B-P, we demonstrate here that sprouting of these fibers is more robust spatially, and more persistent temporally; spared dorsal roots proximal to the injury exhibited a massive increase in MAP1B-P staining detected at 3 days and up to 3 months post-injury. To confirm that these seemingly contradictory results were not due to differences in the experimental paradigms used (hemi- vs. complete transection), we also performed a complete spinal cord transection, which again yielded results similar to those described here (not shown). MAP1B-P is distributed in a proximo-distal gradient within axon shafts, and thus detected mainly at their terminals, as we have previously shown after a neurodegenerative injury in vivo (Soares et al., 1998) and here in vitro, in regenerating adult DRG neurites. This particular distribution might explain why after a hemisection, detection of MAP1B-P within corticospinal axons is limited to their projection areas within gray matter.
Interestingly, MAP1B appears also to play an important role in the maturation of neurites through establishment of the membrane skeleton during synaptogenesis in vitro (Kitamura et al., 2007). Furthermore, MAP1B-P has been used to demonstrate a correlation between functional recovery and enhanced plasticity after chondroitinase treatment (Galtrey et al., 2007). We thus suggest that remodeling of fibers after spinal cord injury, particularly massive by 2 weeks as revealed by MAP1B-P immunoreactivity, is also likely to reflect local synaptic modifications. This was also suggested previously to explain the significant electrophysiological alterations occurring after a chronic compressive injury (Nashmi & Fehlings, 2001). Accordingly, a co-localization of MAP1B-P with synaptophysin (a presynaptic marker) was observed within gray matter. The decrease in MAP1B-P immunoreactivity between 15 days and 1 month post-injury may indicate that the relatively high injury-related axonal plasticity is in fact limited in time. Bareyre et al. (2004) showed that transected CST is able to sprout and establish new contacts with short and long propriospinal neurons. Ultimately, however, only contacts with long propriospinal neurons are maintained that bridge cervical to lumbar segments, a process that can be compared with the refinement of axonal connections.
MAP1B-P staining and degenerative events
Our study reveals an association of increased MAP1B-P levels not only with axonal extension, but also with axonal degeneration in white matter, where highly MAP1B-P-positive terminal clubs are observed. These degenerative processes, occurring at progressive distances from the lesion, have been described before (Sayer et al., 2002, and references therein). Thus, spinal cord hemisection causes Wallerian degeneration of distal axon shafts, and injured axonal endings eventually retract (Hill et al., 2001; Houle & Jin, 2001). Loss of MAP1B-P-positive fibers in white matter then reflects the loss of ascending and descending axons (rostral and caudal to the lesion, respectively), a phenomenon seen to increase with time post-injury. A failure of retrograde transport might contribute to accumulation of axoplasm at the axon stump and, hence, terminal club swelling (Sayer et al., 2002). This is associated with important changes in the distribution of cytoskeletal proteins, as previously reported for phosphorylated neurofilaments (Petzold, 2005; our observations), or microtubules (for a review, see Moss & Lane, 2006).
Furthermore, we noted increased phosphorylation of MAP1B in somata of ‘suffering’ neurons that is clearly associated with injury-related apoptosis (i.e. Clarke's nucleus neurons; Himes et al., 1994), as revealed by double-labeling for activated caspase-3. As somata accumulating MAP1B-P were not detected by the TUNEL technique, we suggest that this phenomenon is indicative of an early state of degeneration, and perhaps a failure of anterograde transport. We were able to reproduce MAP1B-P accumulation in adult DRG neurons in vitro by treating them with staurosporin. This resulted in a shift from the normally proximo-distal distribution of MAP1B-P within their neurites towards an abnormal accumulation in cell bodies, accompanied by swelling and fragmentation of neurites. Abnormal MAP1B phosphorylation has also been reported for other neuronal pathologies, associated with aberrant phosphorylation of tau, such as at sites of neurofibrillary degeneration in Alzheimer's disease brains (Hasegawa et al., 1990; Ulloa et al., 1994; Good et al., 2004).
The two faces of JNK
MAP1B can be phosphorylated by two modes: while mode II phosphorylation (casein kinase II-dependent) is observed throughout developmental stages until adulthood, the developmentally regulated mode I is based on the action of proline-dependent kinases, such as GSK-3beta, cdk-5 and mitogen-activated protein kinases (MAPK; Pigino et al., 1997; Garcia-Perez et al., 1998; Lucas et al., 1998; Good et al., 2004; Kawauchi et al., 2005). Assuming that the 1B-P antibody recognizes MAP1B phosphorylated via mode I (see Discussion in Nothias et al., 1996; Soares et al., 1998; Ma et al., 2000), we further investigated a potential correlation between expression of MAP1B-P and activated c-Jun N-terminal kinases (JNKs). JNKs, of which three isoforms are known (JNK1–3), are part of the MAPK family. Various proteins in different cellular compartments are targets of JNKs, among which are microtubule-associated proteins (Chang et al., 2003; reviewed by Herdegen & Waetzig, 2001). There is increasing evidence that JNKs are potent effectors of apoptosis in the nervous system following a variety of injuries (Liu & Lin, 2005; Yin et al., 2005). On the other hand, JNKs are also involved in neuronal survival (for a review, see Liu & Lin, 2005), migration (Kawauchi et al., 2003) and axon growth (Chang et al., 2003; Xiao et al., 2006). Specific inhibition of JNK activity was shown to affect axonal regeneration from adult DRG neurons in vitro (Lindwall et al., 2004). Moreover, Chang et al. (2003) demonstrated that JNK-1-deficient mice exhibit progressive degeneration of neurites, associated with shorter microtubules and a reduced MAP1B (and MAP2) phosphorylation. The results presented here suggest that JNK signaling following spinal cord injury converges on phosphorylation of MAP1B. Thus, MAP1B-P and activated JNK co-localize in axons that undergo either sprouting or degeneration, and accumulate together in the somata of preapoptotic neurons. Furthermore, specific inhibition of JNK activity in vitro leads to a dramatic decrease in phosphorylation of the MAP1B epitope recognized by the 1B-P antibody. However, further studies are needed to elucidate the precise mechanism by which the JNK-regulated MAP1B phosphorylation state influences microtubule stability, leading to either axonal plasticity or degeneration.
In conclusion, our study shows that changes in MAP1B-P distribution after spinal cord lesion are well suited to monitoring the overall extent of the two forms of axonal remodeling, i.e. sprouting and degeneration. Furthermore, we present clues to the molecular mechanisms through which MAP1B is phosphorylated and, thus, to signaling pathways involved in structural modifications within the adult nervous system in response to injury.
Acknowledgements
We wish to thank Dr Jocelyne Caboche for advice on JNK proteins, and Dr Marion Murray for her reading of and helpful comments on the manuscript. We are very grateful to Dr Itzhak Fischer for providing the 1B-P antibodies. This research was supported by CNRS and UPMC, and by grants from IRME (Institut de Recherche pour la Moelle Epinière); ‘Combattre la Paralysie’; and AFM (Association Française contres les Myopathies, postdoctoral fellowship to S.S.).
Abbreviations
-
- CGRP
-
- calcitonin gene-related peptide
-
- CNS
-
- central nervous system
-
- CST
-
- corticospinal tract
-
- DGR
-
- dorsal root ganglion
-
- JNK
-
- c-Jun N-terminal kinases
-
- MAP1B-P
-
- phosphorylated form of microtubule-associated protein-1B.