ERP correlates of conscious error recognition: aware and unaware errors in an antisaccade task
Abstract
Event-related potential (ERP) studies identified the error-related negativity (Ne/ERN) and the error positivity (Pe) to be associated with performance errors. However, the functional significance of these components is not yet resolved. With the present study we intended to further investigate to what extent these components are related to error awareness. ERPs were recorded during an antisaccade task, and error awareness was obtained from accuracy ratings on each trial. In accordance with earlier findings, aware and unaware errors did not differ in Ne/ERN amplitude. Whereas the late Pe (400–600 ms) shows an increased parietal positivity for aware compared with unaware errors, the early Pe (200–300 ms) shows no dissociation between aware and unaware errors. These data lend further support to the view that the Ne/ERN and the (late) Pe reflect different processes in performance monitoring. In fact the present results provide a clear replication of [S. Nieuwenhuis et al. (2001) Psychophysiology, 38, 752–760], showing that the Pe is associated with error awareness and remedial action. Furthermore, it has been shown that this is only true for the late Pe, whereas the early Pe like the Ne/ERN is not modulated by error awareness.
Introduction
Processing of erroneous response outcomes is an essential source for learning and behavioural adaptation. Rabbitt (1966) first described the phenomenon of post-error slowing as behavioural adjustment after committing an error. In the event-related potential (ERP) a component has been identified that is specifically associated with error commission (Falkenstein et al., 1990, 1991; Gehring et al., 1993). The error negativity (Ne) or error-related negativity (ERN) is characterized by a fronto-central negative deflection shortly (< 100 ms) after the accidental execution of an incorrect response. Dipole source analysis (e.g. van Veen & Carter, 2002) and functional magnetic resonance imaging (fMRI) studies suggest that this component is generated by the anterior cingulate cortex (ACC, e.g. Ridderinkhof et al., 2004). Debener et al. (2005) recently combined electroencephalogram (EEG) and fMRI recordings, and found a direct coupling of single trial Ne/ERN amplitude and activity in the rostral cingulate zone (RCZ). Several studies report a smaller but also negative ERP component following a correct response execution, the correct-related negativity (Vidal et al., 2000, 2003). Typically, an erroneous response is also associated with a positive deflection, the error positivity (Pe; Falkenstein et al., 1991). This ERP component has a centro-parietal topography with a maximum between 200 and 400 ms after response execution.
In contrast to the behavioural effects Rabbitt (1966) earlier reported, the function of error-related brain activity and its connection to error-processing mechanisms are less clear. Originally, it has been suggested that the Ne/ERN represents an error-detection mechanism (mismatch hypothesis; Falkenstein et al., 1991). Holroyd & Coles (2002) posed with their reinforcement learning hypothesis an association between error processing and behavioural adaptation. According to this model, the Ne/ERN is generated when a negative reinforcement learning signal is conveyed to the ACC via the mesencephalic dopamine system and that this signal is used to modify performance. Alternatively, Carter et al. (1998) presented their response conflict hypothesis, suggesting that the Ne/ERN reflected a conflict signal between simultaneously active correct and incorrect response tendencies instead of mismatch. It has been stated that response conflict follows a different time course in correct (pre-response) and erroneous trials (post-response). Ridderinkhof et al. (2004) suggested that the RCZ is engaged and the Ne/ERN appears when the need for adjustments becomes evident, and provided a link between conflict and reinforcement learning theories.
Only a few studies examined the functional significance of the positive deflection following incorrect responses (Overbeek et al., 2005). Unless the Pe shares some characteristics with the stimulus-evoked P3, Leuthold & Sommer (1999) concluded that the Pe might reflect additional processing after errors, probably error detection or updating of the error context. Falkenstein et al. (2000) discussed several functional interpretations of the Pe. They argued against a role of the Pe in error correction, or the Pe as a delayed parietal P3, and concluded with the view that the Pe reflects additional processing after errors that is different from error detection or response checking. These processes could reflect conscious error recognition, adjustment of response strategies after an error, or subjective/emotional assessment of errors.
Nieuwenhuis et al. (2001) examined error processing in an antisaccade task with numerous errors that are not recognized but immediately corrected by the participants (Mokler & Fischer, 1999). Whereas error awareness did not influence Ne/ERN amplitudes, the Pe amplitude of unaware errors was fairly reduced compared with Pe amplitudes after aware errors. Importantly, only aware errors were associated with post-error slowing in the subsequent trial. This result was taken as evidence for the view that the Ne/ERN and Pe reflect separate error monitoring processes. Furthermore, the Pe appeared to be associated with conscious error recognition and remedial action. Endrass et al. (2005) also reported diminished Pe amplitudes for unaware errors compared with aware errors in a saccade countermanding task. Recently, it has been shown that the Pe but not the Ne/ERN was affected by participants' conscious error awareness in a manual response inhibition task (O'Connell et al., 2007). Hajcak et al. (2003) revealed that the Pe was related to autonomic response and post-error slowing, and suggested that the Pe may trigger both error awareness and subsequent compensation. Interestingly, Murphy et al. (2006) showed that the Pe but not error awareness was attenuated with extended wakefulness. Thus, as no trial-to-trial accuracy rating was obtained it is not possible to decide whether error awareness was actually intact during the extended wakefulness condition.
Source localization techniques were applied to determine neural generators contributing to Ne/ERN and Pe components, and support the view that they reflect different aspects of performance monitoring. Van Veen & Carter (2002) showed that the fronto-central N2 (on correct trials prior to the response) and the Ne/ERN (on erroneous trials immediately following responses) had a generator in the caudal ACC. For the Pe they revealed two subcomponents. The early Pe corresponded with caudal ACC activation, and the later Pe with rostal ACC and superior parietal cortex activation. Similar results were reported by van Boxtel et al. (2005). Partly overlapping regions were identified for the Ne/ERN and Pe with the low-resolution electromagnetic tomography (LORETA) method (Herrmann et al., 2004). Because source localization techniques should be interpreted cautiously, fMRI studies with higher spatial resolution are required for a more precise localization of the Pe. Although with this method a dissociation of the time course between Ne/ERN and Pe cannot be shown, it is possible to obtain a dissociation with experimental variation. Two fMRI studies employed the concept of error awareness (Nieuwenhuis et al., 2001), and compared aware and unaware errors. Hester et al. (2005) found equivalent activation of the ACC for aware and unaware errors. Explicit awareness of committing a response inhibition error and subsequent post-error adaptation were associated with bilateral prefrontal and parietal brain activation. Klein et al. (2007) reported a strong connection between error awareness and activation in the left anterior inferior insular cortex that might reflect increased awareness of the autonomic reaction to an error, or the increased autonomic reaction itself.
The objective of the present study was to further investigate the error awareness hypotheses of the Pe. Our goal was to replicate that error awareness selectively affects the Pe, whereas the Ne/ERN remains unchanged. Furthermore, we were also interested in the time course of error awareness and looked whether the early Pe was also sensitive to error awareness. To address this question we analysed the Pe with different time windows, starting immediately after the Ne/ERN. Hajcak et al. (2003) suggested that there is a connection between Pe and remedial action. Therefore, we investigated whether there was a correlation between ERP amplitudes and post-error slowing.
Materials and methods
Participants
Twenty-five individuals participated in the present experiment. They were either paid for study participation or received class credit points. The data of six participants were excluded from the current analysis because of excessive artefacts or low quality of eye movement correction. The remaining 19 subjects (10 female, nine male; mean age ± SD, 24.3 ± 3.14 years; all right-handed) were healthy, and had no history of psychopathological or neurological disorders. They gave written informed consent before the procedure started in accordance to the ethical guidelines of the Declaration of Helsinki.
Stimuli and procedure
The antisaccade task was presented on a 17-inch computer screen (60 cm viewing distance) using Presentation software for stimulus generation. The time course of stimulus presentation is displayed in Fig. 1. Each trial started with a fixation display including a fixation cross and two dotted square frames (each subtending 1.5° visual angle), located left and right to the fixation cross (distance 8°). Then the fixation cross disappeared and the peripheral squares either remained for the next 200 ms dotted or one of the square frames was printed with brighter broader lines (= cue) and turned back into the fixation display for the remaining 50 ms. Then the target stimulus (a filled circle, 1.1° in diameter) unpredictably appeared in the centre of the left or right dotted frame, and remained visible for 100 ms. Participants were instructed to execute a saccade to the horizontal mirror position of the peripheral circle as quickly and as accurately as possible. Nine-hundred milliseconds after the offset of the target stimulus participants were asked to indicate with a button press (within 1000 ms) whether they rated their performance as correct, incorrect or unsure.
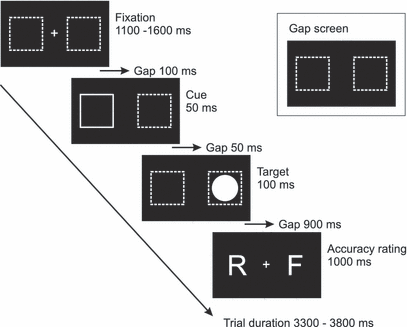
Schematic depiction of the experimental paradigm. Participants were instructed to execute a saccade to the horizontal mirror position of the peripheral circle (target). Subsequently, participants rated (accuracy rating) with a button press their previous response as correct (right button), incorrect (left button) or unsure (both buttons). Cue and target positions were either left or right.
A total of 800 trials were presented in a pseudorandomized order, including 100 trials without a cue, 100 trials with congruent cues (cue and target stimulus appeared on the same side) and 600 trials with incongruent cues (cue and target stimulus appeared on opposite sides). The cue was presented at the position where the saccade should be directed to in order to increase error rates (Fischer & Weber, 1996). To reduce predictability of the cue, it was presented congruent or incongruent with the target stimulus, or not at all. The experiment was preceded by 20 practice trials, and short breaks were given every 100 trials. The total duration of the experiment was 48 min.
EEG recording and analysis
EEG and electrooculogram (EOG) were recorded continuously from 65 electrodes, including Cz as recording reference with an EasyCap electrode system (Falk Minow Services, Munich, Germany), from 61 sites of an equidistant electrode position system (additional electrode locations: below the left and right eye: IO1 and IO2, Nasion, Neck). Impedances were kept below 5 kOhm. Data were digitized with a sampling rate of 500 Hz and amplified with a band pass of 0.01–100 Hz.
Eye movements were obtained from horizontal EOG cannels (LO1 and LO2). Target-locked segments (200 ms pre-onset and 2000 ms post-onset) were computed from the average referenced EEG, and LO2 EOG signals were subtracted from LO1 signals (rightward eye movements are reflected by negative deflections and leftward eye movements by positive deflections). Saccade onsets were determined in the horizontal EOG through visual inspection. The correctness of the position of the saccade onsets was double checked by a second evaluator. Trials containing saccades with a latency less than 70 ms or more than 500 ms after target onset were classified as invalid (Fischer et al., 1993). Overall, a percentage of 11.1% (SD 13.6) trials were excluded from further analysis due to early or late saccade onsets, low data quality or missing accuracy ratings. Figure 2A displays the average horizontal EOG waveforms for correct reactions, aware and unaware errors separately for leftward and rightward eye movements. Antisaccade errors are eye movements towards the target stimulus. Error trial participants rated as incorrect were classified aware errors and error trials rated as correct as unaware errors. Unsure ratings were too few to be analysed and therefore excluded from further analysis. Saccadic reaction times were determined as the latency between target onset and saccade onset.
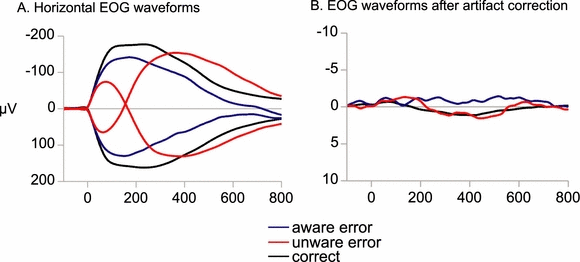
Grand average electrooculogram (EOG) waveforms (bipolar: LO1–LO2) are depicted time-locked to the saccade onset. (A) Original waveforms are given: rightward eye movements are reflected by negative; and leftward movements by positive defections for correct, aware and unaware errors. (B) Eye movement-corrected waveforms.
Prior to ERP analysis, eye movement artefacts were removed using the multiple source eye correction method (Surrogate Method, Berg & Scherg, 1994) implemented in BESA5 (Brain Electrical Source Analysis, MEGIS Software GmbH, Gräfelfing, Germany). Artefact corrected difference waveforms between left and right horizontal EOG channels are depicted in Fig. 2B. Epochs still containing large artefacts (exceeding ± 75 µV) were excluded from further analyses. A minimum of 15 trials in each condition was required for inclusion into statistical analysis.
ERPs were determined synchronously with the onset of the saccade (200 ms pre- and 1000 ms post-saccade onset). Averaged ERPs were filtered with a 30 Hz low-pass filter, and a baseline correction was applied with a pre-response interval from 200 to 100 ms. For statistical analysis Ne/ERN amplitudes were determined as the mean amplitude in the time window from 60 to 140 ms at the electrodes Fz, FCz and Cz. Mean Pe amplitudes were obtained from Fz, FCz, Cz, CPz and Pz for 100-ms time windows starting from 200 ms and ending at 600 ms post-response onset. For visual presentation, grand-average ERPs were filtered with a 12 Hz low-pass filter. Repeated measurement analysis of variances (anova) were computed with the factors Electrode (Ne/ERN: Fz, FCz, Cz; Pe: Fz, FCz, Cz, CPz, Pz) and Correctness (correct vs error) or Awareness (aware vs unaware error). Effects of Correctness were analysed by comparing correct with incorrect responses irrespective of error awareness. For all analyses, P-values were corrected with the Greenhouse–Geisser procedure, when appropriate [degrees of freedom (d.f.) > 1]. When significant main effects or interactions were obtained, Bonferonni-corrected P-values were reported for post hoc comparisons.
Because a relationship between ERP amplitudes and behavioural adjustments was expected, correlation coefficients (Pearson r) were computed for ERP amplitudes (from individual averages) and the post-error slowing scores. We used error minus correct difference amplitudes of the Ne/ERN, and both the difference amplitudes and raw amplitudes of error trials of the Pe.
Results
Behavioural data and post-error slowing
Table 1 presents the behavioural data. The comparison between aware errors and unaware errors revealed a trend for a higher proportion of unaware errors (t18 = 1.8, P = 0.093). The false alarm rate (correct reactions incorrectly classified as an error) was very low (2.6% SD = 4.3) and the percentage of trials with unsure ratings was 1.8% (SD = 2.4). Saccadic reaction times were significantly faster for erroneous saccades compared with correct antisaccades (aware errors t18 = 11.3, P < 0.001; unaware errors t18 = 6.9, P < 0.001). Aware and unaware error reaction times did not differ (t18 = 0.3, P = 0.74). Because eye movements were obtained from horizontal EOG channels, the size of saccades is given in µV and differed significantly between the three response categories (F2,36 = 18.9, P < 0.001, ɛ = 0.72). Correct antisaccades had significantly higher amplitudes than aware (P < 0.01) and unaware errors (P < 0.01), and aware errors had larger amplitudes than unaware errors (P < 0.01). Unless participants were not instructed to correct their errors, on average 64% erroneous saccades were followed by a corrective antisaccade. Unaware errors were corrected more frequently (t18 = 8.5, P < 0.001) and faster (t18 = 8.9, P < 0.001) than aware errors.
| Correct | Aware errors | Unaware errors | |
|---|---|---|---|
| Trials (%) | 73.1 ± 17.4 | 8.6 ± 7.5 | 13.0 ± 10.1 |
| Saccadic reaction time (ms) | |||
| Trial n | 265 ± 46 | 170 ± 40 | 174 ± 38 |
| Trial n + 1 | 268 ± 46 | 279 ± 40 | 257 ± 46 |
| Correction (%) | – | 32.8 ± 27.3 | 89.4 ± 17.0 |
| Correction time (ms) | – | 259 ± 51 | 147 ± 37 |
| EOG positivity (µV) | 83.6 ± 47.1 | 68.5 ± 40.1 | 37.9 ± 14.7 |
- Data are presented as means ± SD. EOG, electrooculogram.
We also analysed post-error slowing that should occur in trials following errors compared with trials following correct responses. For this analysis, we compared reaction times in succeeding correct trials (n + 1). A significant difference between the three response types was revealed (F2,36 = 14.3, P < 0.001, ɛ = 0.88). Reaction times in trials after aware errors were significantly slower than reaction times after correct responses (P < 0.05) and unaware errors (P < 0.001). Reaction times after unaware errors were actually faster than after correct reactions (P < 0.01).
ERP data
Figure 3A shows response-locked grand average waveforms for aware and unaware errors and correct trials. Difference waveforms (error minus correct) are depicted in Fig. 3B. For both aware and unaware errors, the Ne/ERN is larger compared with correct reactions (see also Fig. 4 for topography). This is confirmed by statistical analysis showing a significant main effect for Correctness and a significant Correctness × Electrode interaction (Table 2). Ne/ERN amplitudes were more negative for errors compared with correct reactions at Fz, FCz and Cz (all P-values < 0.001). The comparison of aware and unaware errors did not reveal a significant difference of the Ne/ERN amplitudes between these conditions. Also, the Awareness × Electrode interaction was not significant (F2,36 < 1). Additional paired t-tests confirmed that there was no significant differences between Ne/ERN amplitudes of aware and unaware errors at frontal midline electrodes (Fz: −3.33 vs −3.17 µV, P = 0.77; FCz: −3.39 vs −3.16 µV, P = 0.55; Cz: −0.90 vs −0.26 µV, P = 0.37). Unlike in the time window of the Ne/ERN, grand average waveforms in the later time windows of the Pe indicate pronounced differences between aware and unaware errors (Fig. 3). Separate anovas were computed for 100 ms windows starting from 200 ms and ending at 600 ms after response onset. Effects for the early Pe are reported in the time windows from 200 to 400 ms, and for the late Pe from 400 to 600 ms. As depicted in Fig. 3A, aware and unaware errors elicited more positive-going amplitudes compared with correct reactions. This difference is reflected by significant Correctness effects for all time windows (Table 2). This main effect was qualified by an interaction with Electrode in the first and the later two time windows. Post hoc comparisons (Table 3) revealed significant differences between erroneous and correct reactions at frontal electrode sites (Fz and FCz) in the time window from 200 to 300 ms, and pronounced differences at central and parietal electrode sites (Cz, CPz and Pz) from 400 to 600 ms.
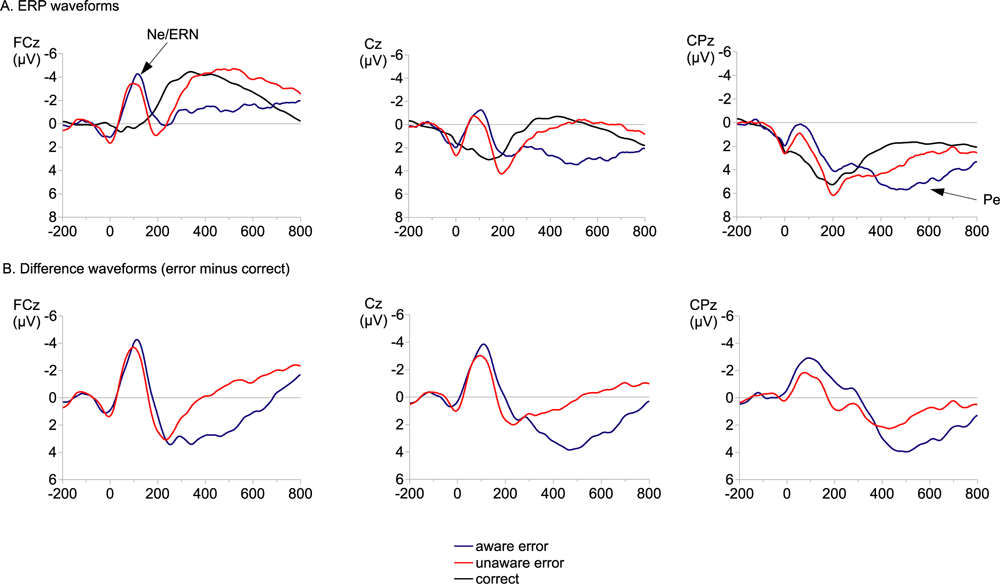
(A) Grand averaged event-related potentials (ERPs) elicited by aware errors, unaware errors and correct responses at FCz, Cz and CPz electrodes (baseline: −200 to −100 ms). (B) The difference waveforms aware error minus correct and unaware error minus correct at FCz, Cz and CPz (baseline: −200 to −100 ms). ERN, error-related negativity; Ne, error negativity.
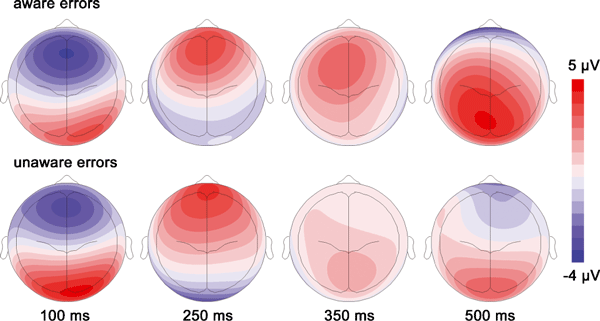
Scalp topographies (spherical spline interpolation, orthographic top view) of aware and unaware errors for different time points (Ne/ERN: 100 ms; Pe: 250 ms, 350 ms, 500 ms).
| Condition | Electrode | Condition × Electrode | ||||||
|---|---|---|---|---|---|---|---|---|
| d.f. | F-value | d.f. | F-value | (ε) | d.f. | F-value | (ε) | |
| anova results comparing correct and incorrect reactions, coded with the factor Correctness | ||||||||
| Ne/ERN (60–140 ms) | 1,18 | 19.96*** | 2,36 | 43.08*** | (0.65) | 2,36 | 7.40** | (0.69) |
| Pe (200–300 ms) | 1,18 | 8.82** | 4,72 | 72.49*** | (0.45) | 4,72 | 11.54** | (0.33) |
| Pe (300–400 ms) | 1,18 | 23.83*** | 4,72 | 56.15*** | (0.42) | 4,72 | 0.58 | (0.32) |
| Pe (400–500 ms) | 1,18 | 30.47*** | 4,72 | 55.76*** | (0.41) | 4,72 | 10.48** | (0.35) |
| Pe (500–600 ms) | 1,18 | 9.90** | 4,72 | 47.33*** | (0.40) | 4,72 | 10.27** | (0.34) |
| anova results comparing aware and unaware errors, coded with the factor Awareness | ||||||||
| Ne/ERN (60–140 ms) | 1,18 | 0.99 | 2,36 | 27.05*** | (0.64) | 2,36 | 0.69 | (0.59) |
| Pe (200–300 ms) | 1,18 | 0.59 | 4,72 | 40.03*** | (0.38) | 4,72 | 2.41 | (0.50) |
| Pe (300–400 ms) | 1,18 | 6.58* | 4,72 | 48.77*** | (0.40) | 4,72 | 10.20*** | (0.43) |
| Pe (400–500 ms) | 1,18 | 20.10*** | 4,72 | 52.36*** | (0.38) | 4,72 | 4.63* | (0.37) |
| Pe (500–600 ms) | 1,18 | 15.71*** | 4,72 | 43.51*** | (0.37) | 4,72 | 8.79*** | (0.67) |
- The Ne/ERN amplitude was analysed at Fz, FCz and Cz (Electrode), and the Pe at Fz, FCz, Cz, CPz and Pz (Electrode). *P < 0.05; **P < 0.01; ***P < 0.001; d.f., degrees of freedom; ε, Greenhouse–Geiser epsilon. ERN, error-related negativity; Ne, error negativity; Pe, error positivity.
| Bonferonni-corrected P-values | |||||
|---|---|---|---|---|---|
| Fz | FCz | Cz | CPz | Pz | |
| 60–140 ms | |||||
| Error vs correct | < 0.001 | < 0.001 | < 0.001 | – | – |
| Aware vs unaware error | n.s. | n.s. | n.s. | – | – |
| 200–300 ms | |||||
| Error vs correct | < 0.001 | < 0.001 | = 0.084 | n.s. | n.s. |
| Aware vs unaware error | n.s. | n.s. | n.s. | n.s. | n.s. |
| 300–400 ms | |||||
| Error vs correct | < 0.05 | < 0.001 | < 0.001 | < 0.05 | < 0.05 |
| Aware vs unaware error | < 0.01 | < 0.001 | < 0.01 | n.s. | n.s. |
| 400–500 ms | |||||
| Error vs correct | n.s. | n.s. | < 0.001 | < 0.001 | < 0.001 |
| Aware vs unaware error | < 0.05 | < 0.001 | < 0.001 | < 0.05 | < 0.05 |
| 500–600 ms | |||||
| Error vs correct | n.s. | n.s. | < 0.001 | < 0.001 | < 0.001 |
| Aware vs unaware error | n.s. | < 0.001 | < 0.001 | < 0.01 | < 0.01 |
Awareness main effects and Awareness × Electrode interactions were observed in the time windows from 300 to 600 ms (Table 2). Differences between aware and unaware errors were not found from 200 to 300 ms. In the second time window (300–400 ms) aware errors elicited more positive amplitudes than unaware errors at frontal and central electrode sites (Fz, FCz and Cz). Thereafter in the following two time windows enhanced Pe amplitudes were also obtained at parietal electrode sites (FCz, Cz, CPz and Pz) for aware errors compared with unaware errors.
Correlations between ERP amplitudes of aware and unaware errors and post-error slowing did not reveal significant associations, irrespective of using the difference or the raw Ne/ERN or Pe amplitudes.
Discussion
The current experiment was conducted to examine effects of error awareness on the error-related ERP components in an antisaccade task. We were especially interested in differential effects between aware and unaware errors on Ne/ERN and Pe. As expected we obtained errors participants were aware of (8.6%) and errors that participants did not report (13.1%).
Both error types were associated with a significant Ne/ERN. Importantly, aware and unaware errors did not differ in Ne/ERN amplitude. This result is in accordance with earlier findings (Nieuwenhuis et al., 2001; Endrass et al., 2005; O'Connell et al., 2007) that also reported no modulation of this early error monitoring correlate with error awareness. For the second error-related potential, the Pe, we found a robust error awareness effect for the later (400–600 ms) parietal positivity with an enhanced positivity for aware errors. In contrast, the frontal difference between errors and correct responses in the time window from 200 to 300 ms did not vary with error awareness. This indicates that an error awareness effect starts at about 300 ms after response onset (see also Fig. 3), with a pronounced positivity for aware errors and almost no positivity for unaware errors. The error awareness effect for the later Pe exactly replicates the findings that Nieuwenhuis et al. (2001) obtained in their antisaccade study. Also in accordance with earlier findings, we found a significant post-error slowing (in correct trials following an error compared with trials after correct responses) for aware errors and not for unaware errors. Because post-error slowing is an expression of remedial action following errors (Rabbitt, 1966), our results support the view that the Pe rather than the Ne/ERN is associated with remedial actions after erroneous responses.
However, aware and unaware errors differed also in correction rate, correction time and saccade amplitude. Unaware errors had higher correction rates, shorter correction times and lower saccade amplitudes than aware errors and may therefore remain undetected. In fact, unaware errors may share common properties with partial errors that are associated with smaller activation of the incorrect response as determined by muscle activation or measuring response force. Yet, both aware and unaware errors were associated with a comparable Ne/ERN that is in line with several studies reporting similar Ne/ERN amplitudes for full and partial errors (Scheffers et al., 1996; Vidal et al., 2000; Allain et al., 2004; Carbonnell & Falkenstein, 2006). Still, it might be criticized that unaware errors were not errors in the usual sense but rather normal antisaccades preceded by incorrect prosaccades. Therefore, latencies of correct antisaccades relative to stimulus onset were compared between unaware errors and correct trials. Results indicate that latencies of correct antisaccades following unaware errors were substantially prolonged compared with antisaccade latencies from correct trials (321 ms vs 265 ms, F1,18 = 13.6, P < 0.01). Together with pronounced Ne/ERN amplitudes, this suggests that undetected erroneous prosaccades actually were errors in the usual sense and therefore activated error-processing mechanisms.
Regarding error awareness, we found that participants recognized only half of their errors as incorrect (aware errors). Therefore the reliability of the accuracy ratings might be questioned. In contrast, on correct trials only 2.6% of the trials were classified as errors and 1.8% of all trials were rated as unsure. According to this the discrimination accuracy (d prime = 1.41) was quite good and participants feel sure about their performance in most of the trials. Therefore, unaware errors might actually be trials in which participants had no conscious representation of their erroneous performance.
Interestingly, these apparent differences between aware and unaware errors did not affect Ne/ERN amplitudes. Therefore, both error types are associated with the same error-processing mechanism in an early stage. Nieuwenhuis et al. (2001) suggested two error-processing mechanisms. An early process reflected by the Ne/ERN might be associated with the detection of incorrect motor commands through central processing pathways. In contrast, the Pe is suggested to reflect a later process that is strongly associated with the awareness of an erroneous response. The awareness might be formed by peripheral feedback about the previous response. Furthermore, it was suggested that the Pe might share common properties with the stimulus-evoked P3, and the two components might be different manifestations of the same biological and functional system. The current data clearly showed a dissociation of early and late error processing regarding error awareness. The Ne/ERN might reflect an early internal error-monitoring process based on automatic comparison of task requirements and internal motor commands, whereas the later Pe shows a strong connection with conscious error recognition. Thus, it might be speculated that both aware and unaware errors are internally detected what is reflected by the Ne/ERN, but then they differ in their mental representation and the subsequent compensatory mechanism. One reason why errors are not consciously represented might be that they are smaller in amplitude and, importantly, they are corrected very fast. Aware errors are corrected less frequently within the same trial but error compensation occurs in the next trial, which is reflected by post-error slowing.
In sum, the present results provide further evidence for a functional dissociation between the ERN and the Pe. Whereas aware and unaware errors are both followed by a Ne/ERN, aware errors were associated with a larger Pe (Nieuwenhuis et al., 2001; O'Connell et al., 2007). Considering early and late Pe (van Veen & Carter, 2002; Herrmann et al., 2004), a dissociation between aware and unaware errors was observed within the Pe. The early positivity with a fronto-central distribution starting immediately after the Ne/ERN does not differ between aware and unaware errors. The early Pe shares common scalp topography with the Ne/ERN and also common functional significance. The effect of conscious error recognition starts with the rising parietal positivity. Therefore, the two subcomponents of the Pe are differentially related to conscious error detection, and only the late Pe reflects error awareness.
Acknowledgements
This study was supported by grant Ka 815-4 of the Deutsche Forschungsgeimeinschaft. The authors thank Beate Schürmann and Franziska Preuße for their help with data collection and the two anonymous referees for their valuable comments on our paper.
Abbreviations
-
- ACC
-
- anterior cingulate cortex
-
- EEG
-
- electroencephalogram
-
- EOG
-
- electrooculogram
-
- ERN
-
- error-related negativity
-
- ERP
-
- event-related potential
-
- fMRI
-
- functional magnetic resonance imaging
-
- Ne
-
- error negativity
-
- Pe
-
- error positivity
-
- RCZ
-
- rostral cingulate zone.