Distinct, parallel pathways link the medial mammillary bodies to the anterior thalamus in macaque monkeys
Abstract
Mammillary body neurons projecting to the thalamus were identified by injecting retrograde tracers into the medial thalamus of macaque monkeys. The source of the thalamic projections from the medial mammillary nucleus showed strikingly different patterns of organization depending on the site of the injection within the two anterior thalamic nuclei, anterior medialis and anterior ventralis. These data reveal at least two distinct modes by which the primate medial mammillary bodies can regulate anterior thalamic function. Projections to the thalamic nucleus anterior medialis arise mainly from the pars lateralis of the medial mammillary nucleus. A particularly dense source is the dorsal cap in the posterior half of the pars lateralis, a subregion that has not previously been distinguished. In contrast, neurons spread evenly across the medial mammillary nucleus gave rise to projections more laterally in the anterior thalamic nuclei. A third pattern of medial mammillary neurons appeared to provide the source of projections to the rostral midline thalamic nuclei. In contrast, the labeled cells in the lateral mammillary nucleus were evenly spread across that nucleus, irrespective of injection site. In addition to the established projection to anterior dorsalis, the lateral mammillary nucleus appears to project lightly to a number of other thalamic nuclei, including lateralis dorsalis, anterior medialis, anterior ventralis, and the rostral midline nuclei, e.g. nucleus reuniens. These anatomical findings not only reveal novel ways of grouping the neurons within the medial mammillary nucleus, but also indicate that the mammillothalamic connections support cognition in multiple ways.
Introduction
A vital clue to the functions of the mammillary bodies comes from its principal output, the massive, unidirectional projections to the anterior thalamus. These projections, which mainly arise from the larger, medial mammillary nucleus, comprise the mammillothalamic tract (or bundle of Vicq d'Azyr). This tract is a central link in hippocampal–diencephalic circuits now widely thought to support memory (Delay & Brion, 1969; Gaffan, 1992; Aggleton & Brown, 1999). Clinical evidence for the importance of the mammillothalamic tract comes from detailed analyses of thalamic strokes, which reveal a very high association between tract damage and amnesia (Von Cramon et al., 1985; Gentilini et al., 1987; Van der Werf et al., 2000, 2003). Other support comes from descriptions of mammillary body damage in humans with amnesia (Dusoir et al., 1990; Tanaka et al., 1997; Hildebrandt et al., 2001). The conclusion that mammillothalamic connections are vital for memory is reinforced by studies into the effects of selective mammillary body and mammillothalamic tract lesions in monkeys and rats (Aggleton & Mishkin, 1985; Parker & Gaffan, 1997; Vann & Aggleton, 2003). Even though they comprise one of the largest sources of anterior thalamic afferents in the primate brain (Powell et al., 1957) and seem to be necessary for normal memory, remarkably little is known about the mammillary body projections to the thalamus in the primate brain.
So far, studies of monkeys have shown that the two main mammillary nuclei (medial and lateral) have different termination patterns within the three anterior thalamic nuclei. The much larger medial mammillary nucleus projects to both nucleus anterior medialis and nucleus anterior ventralis, while the lateral mammillary nucleus projects to nucleus anterior dorsalis (Veazey et al., 1982b; Xiao & Barbas, 2002b). Both electrophysiological and anatomical evidence suggest that the two largest anterior thalamic nuclei (anterior medialis and anterior ventralis) have different roles in supporting memory (Vertes et al., 2001; Xiao & Barbas, 2002a, b). If so, then the medial mammillary inputs to these two thalamic nuclei might be expected to differ, potentially reflecting distinct mnemonic functions. This prediction was tested in the present study.
Fluorescent retrograde tracers were injected into the anterior thalamic nuclei and the adjacent nucleus lateralis dorsalis in six rhesus monkeys (Macacca mulatta). Additional information came from six cynomolgus monkeys (Macaca fascicularis) with injections of horseradish peroxidase (HRP) close to the thalamic midline. The injection placements made it possible to address a number of questions. First, whether there are qualitative differences in the patterns of inputs from the mammillary bodies to nuclei anterior medialis and anterior ventralis. Second, whether the mammillary bodies project to any additional thalamic nuclei. Candidates include the midline thalamic nuclei (Veazey et al., 1982b) and nucleus lateralis dorsalis, the latter sometimes classified within the anterior thalamic nuclear complex (Van Groen & Wyss, 1992; Bentivoglio et al., 1993). The study also examined the source of inputs to nucleus medialis dorsalis. Although this thalamic nucleus has repeatedly been implicated in memory processes (Markowitsch, 1982; Aggleton & Mishkin, 1983; Gold & Squire, 2006), its connections suggest markedly different functions to those of the mammillary bodies and anterior thalamic nuclei.
Materials and methods
Data were collected from two different series of tracer injections, one using the retrograde transport of fluorescent dyes [fast blue (Fb) and diamidino yellow (Dy)], the other using the retrograde transport of HRP. By using two different fluorescent tracers in the same animal it was possible to inject multiple thalamic sites and make direct comparisons of retrogradely labeled cells that project to different thalamic targets. In addition, this refinement reduced the number of animals necessary to complete the study. Although there are a number of minor variations in some of the experimental procedures, as well as possible differences in the sensitivity of the various tracers, both series are described as they complement each another to provide a more complete picture. Data from these animals also contributed to two earlier studies (Aggleton et al., 1986; Saunders et al., 2005), and the numbering of the individual cases corresponds to that previously published. All experimental procedures were carried out with the approval of the NIMH, Bethesda, and with strict adherence to the NIH Guide for Care and Use of Laboratory Animals, such that the ‘Principles of laboratory animal care’ (NIH Publication no. 86-23, revised 1985) were followed.
Fluorescent tracer injections
Six adult rhesus monkeys received injections of two fluorescent tracers, Dy (Keizer et al., 1983) or Fb (Kuypers et al., 1980), with different tracers being placed into different thalamic sites in the same animal. The animals were tranquilized with ketamine hydrochloride (10–15 mg/kg, intramuscular injection) and surgical anesthesia was maintained with gas (isoflurane 1–4%, to effect). They were placed in a specialized head holder and, following aseptic procedures, dorsal bone and dural flaps were opened to expose the midline. The wall of the left hemisphere was then gently retracted, and a 5–10-mm portion of the corpus callosum and the underlying fornix were split longitudinally to expose the thalamic midline. An injection of either Fb (Sigma) or Dy (Sigma) was made through a 5-µL Hamilton syringe fitted with a 28-gauge needle. Both tracers were injected as 3% suspensions in distilled water. In three cases (BRh3, BRh4, BRh6), a fiber pathway (fornix or extreme capsule) was cut unilaterally immediately prior to the injections (Table 1).
| Case | Species | Injection site, tracer, surgery | Mammillary body label | ||||
|---|---|---|---|---|---|---|---|
| LMB | MMBb | MMBm | MMBl | MMBlcap | |||
| ACy1 | Cynomolgus | AM, Mid, MD, Reuns (HRP) | ++ | + | + | ++ | ++++ |
| ACy2 | Cynomolgus | AM, Mid (HRP) | ++ | 0 | + | +++ | ++++ |
| ACy3 | Cynomolgus | MD, Mid (HRP) | (+) | 0 | 0 | 0 | 0 |
| ACy5 | Cynomolgus | MD, Mid (HRP) | (+) | 0 | 0 | 0 | 0 |
| ACy7 | Cynomolgus | MD, Mid (HRP) | 0 | 0 | 0 | 0 | 0 |
| ACy26 | Cynomolgus | Reuns, Mid, AM (HRP) | ++ | 0 | + | ++++ | + |
| BRh1 | Rhesus | MD (Fb l) | 0 | 0 | 0 | 0 | 0 |
| BRh2 | Rhesus | AM (Fb r) | ++ | 0 | ++ | ++ | ++++ |
| MD, Mid (Dy r) | 0 | 0 | 0 | 0 | 0 | ||
| BRh3 | Rhesus | AV, AM (Dy l, Fx) | + | (+) | ++ | ++ | +++ |
| MD (Fb l, Fx) | – | – | – | – | – | ||
| AM, MTT (Fb r, ECx) | ++ | ++ | +++ | +++ | +++ | ||
| MD (Dy r, ECx) | – | – | – | – | – | ||
| BRh4 | Rhesus | AV, AM (Fb r, Fx) | + | + | +++ | ++ | +++ |
| MD (Dy r, Fx) | – | – | – | – | – | ||
| AM, Mid (Dy l, ECx) | + | 0 | ++ | ++ | ++++ | ||
| MD (Fb l, ECx) | – | – | – | – | – | ||
| BRh5 | Rhesus | AV (Fb l) | ++ | + | +++ | ++++ | ++ |
| LD (Fb r) | – | – | – | – | – | ||
| LD (Dy l) | + | 0 | 0 | 0 | 0 | ||
| BRh6 | Rhesus | LD (Dy r, Fx) | + | 0 | 0 | 0 | 0 |
| MD, Mid (Fb, Fx, ECx) | (+) | – | – | – | – | ||
| LD (Dy l, ECx) | + | 0 | 0 | 0 | 0 | ||
- Abbreviations: AM, anterior medial thalamic nucleus; AV, anterior ventral thalamic nucleus; LD, lateral dorsal thalamic nucleus; LMB, lateral mammillary nucleus; MD, nucleus medialis dorsalis; Mid, midline thalamic nuclei; MMBb, medial mammillary nucleus pars basalis; MMBl, medial mammillary nucleus pars lateralis; MMBlcap, medial mammillary nucleus pars lateralis cap; MMBm, medial mammillary nucleus pars medialis; MTT, mammillothalamic tract; Reuns, nucleus reuniens. Type of tracer (Dy, diamidino yellow; Fb, fast blue; HRP, horseradish peroxidase); hemisphere of injection site (l, left; r, right); and surgery in same hemisphere as injection (ECx, amygdala and extreme capsule cut; Fx, fornix transection). The density of retrograde label (lower) is marked on a scale from zero to ++++; the (+) indicates just a couple of cells, and ‘−’ indicates that repeated use of the same tracer in that animal precludes any conclusion.
To minimize the use of animals, some monkeys received two (BRh2), three (BRh5, BRh6) or even four (BRh3, BRh4) injections of the two fluorescent tracers, each in different thalamic sites (Table 1). In BRh2, single injections of the two different tracers (Dy, Fb) were directed at either the anterior thalamic nuclei (Fb) or nucleus medialis dorsalis (Dy) in opposite hemispheres. In animals BRh3 and BRh4, separate injections were placed in the anterior thalamic nuclei and nucleus medialis dorsalis in the same hemisphere, but using different tracers (Fb and Dy). This procedure was then repeated in the opposite hemisphere, ensuring that the anterior thalamic nuclei were injected with Fb in one hemisphere and Dy in the other hemisphere (Table 1). In case BRh5, bilateral injections (one Fb, the other Dy) were placed in nucleus lateralis dorsalis in opposite hemispheres, while a further injection of Fb was placed in the anterior thalamic nuclei in the same hemisphere as that with the Dy injection. Finally, in BRh6, two injections (one Fb, the other Dy) were placed in nucleus lateralis dorsalis in opposite hemispheres, while an injection of Fb was placed in the midline and so involved the most medial portion of medialis dorsalis in both hemispheres (Table 1).
Three of the rhesus monkeys (BRh3, BRh4 and BRh6) had additional surgical procedures immediately before the tracer injections, i.e. as part of the same surgery (Table 1). In all three cases this involved unilateral removal of the fornix and, in the other hemisphere, a unilateral cut in the ventral amygdalofugal pathway/extreme capsule. Details of these concurrent surgical procedures have been provided previously (Aggleton et al., 2005; Saunders et al., 2005). The cut in the ventral amygdalofugal pathway was lateral and dorsal to the amygdala, and so did not interfere with any structures or tracts in the present study. Even though the fornix lesions will have removed hippocampal inputs to the anterior thalamic nuclei and the mammillary bodies, the surgery is most unlikely to have affected direct projections from the mammillary bodies to the anterior thalamus as it was completed just prior to tracer injection, i.e. in the same surgery. Furthermore, transection of the fornix in rhesus monkeys does not result in a long-term loss of neurons within the mammillary bodies, despite evidence of atrophy due to a loss of neuropil (Loftus et al., 2000).
After a postoperative period of between 5 and 10 days the animals were deeply anesthetized with sodium pentobarbital. They were then perfused intracardially with saline followed by approximately 2 L of 4–6% paraformaldehyde in 0.1 m cacodylate buffer (pH 7.4). The brains were then removed and placed in a series of cryoprotectant solutions consisting of first 10% and then 20% glycerol in 0.1 m cacodylate buffer with 2% dimethylsulfoxide and 4–6% paraformaldehyde (pH 7.4, 4 °C). Four−6 days after the perfusion the brains were rapidly frozen by immersion in −75 °C isopentane, and then cut at 40 µm in the coronal plane on a freezing microtome (Rosene et al., 1986). Three, one-in-10 series of sections were mounted immediately onto subbed slides, dried and stored in the dark at 4 °C.
HRP injections
A single injection of HRP was made into the medial part of the thalamus in six adult cynomolgus monkeys weighing 3.5–6.8 kg at the time of surgery. The surgical procedures used to visualize the dorsal thalamus closely matched those for the fluorescent injections. All injections were then made under visual guidance. In three cases (ACy1, ACy2, ACy26) the injection tract was in or very close to the midline at the level of the anterior thalamic nuclei. In the remaining three cases (ACy3, ACy5, ACy7) the HRP injections were targeted at the magnocellular part of nucleus medialis dorsalis.
Four cases received a single injection of 40% HRP (Sigma, type IV) with a 1-µL Hamilton syringe. The volume injected ranged from 0.13 µL (ACy2) to 0.15 µL (ACy3, ACy5) to 0.22 µL (ACy1). In animal ACy7 a 10% solution of HRP in Tris buffer was delivered iontophoretically through a glass pipette, while in ACy26 the injection consisted of 0.09 µL of a 4% solution of HRP (Sigma, type VI) conjugated with wheat germ agglutin.
Two days after surgery the monkeys were deeply anesthetized with Nembutal and perfused intracardially with 0.9% saline followed by a solution of 1% paraformaldehyde and 1.25% glutaraldehyde in 0.1 m phosphate buffer (pH 7.2). The brains were stored in 30% sucrose buffer at 4 °C for 3–4 days and then cut in 50-µm coronal sections. A one-in-five series was then treated according to the protocols of either Hardy & Heimer (1977) or Mesulam (1978). Alternate sections were dehydrated, counterstained with thionine and coverslipped, while the remaining sections were just dehydrated and coverslipped.
Anatomical designations
The primate mammillary bodies are located at the base of the brain in the posterior hypothalamus (Fig. 1A) and comprise two main nuclear groups, the medial and lateral mamillary nuclei (Rose, 1939; Veazey et al., 1982a). By far the larger of the two is the medial mammillary nucleus, which is surrounded by a dense, fiber capsule (Fig. 1B–D). The major afferent tract is the postcommissural fornix, which can be traced running into the rostral part of the medial mammillary nucleus. The mammillothalamic tract, the primary efferent tract, exits from the dorsal half of the same nucleus (Fig. 1A). Using the descriptions of the cynomolgus monkey brain by Veazey et al. (1982a), three cytoarchitectonic divisions can be observed within the medial mammillary nucleus: pars medialis, pars basalis and pars lateralis (Fig. 1B and 1D, see also Rose, 1939). Pars medialis is distinctive as it contains the largest neurons within the medial mammillary nucleus, while the cells in pars basalis are among the smallest in the posterior hypothalamus (Fig. 1B and D). The third component, pars lateralis, is the largest subdivision and occupies the lateral half of the medial nucleus. The medial and central parts of pars lateralis are distinguished from pars medialis by the lower density of cells, mainly as a consequence of the large number of fibers in this more lateral part of the medial nucleus. As a consequence there is not a sharp border with pars medialis. In the caudal half of the medial mammillary nucleus the more dorsal cells in pars lateralis form a distinctive ‘cap’ where they appear more densely aggregated (Fig. 1). This cap is sometimes more visible in myelin stained sections due to a reduction in fiber density (Fig. 1C).
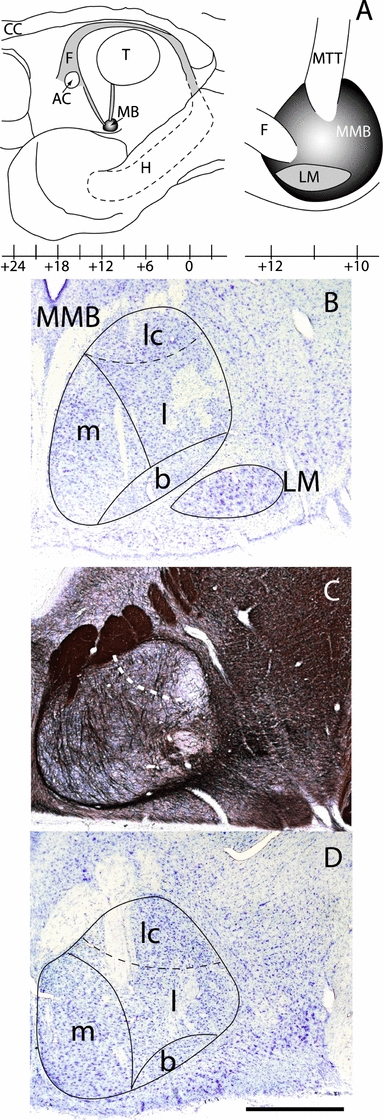
(A) Schematic drawings showing the position of the mammillary bodies in the primate brain. Left: parasagittal view of the medial surface of the adult rhesus monkey brain showing the position of the mammillary bodies (MB) near the base of the brain. Right: detail showing the mammillary bodies from a lateral view, with the fornix (F) entering the rostral part of the structure and the mammillothalamic tract (MTT) exiting dorsally. (B–D) Photomicrographs of coronal sections from the rhesus macaque showing the cytoarchitectonic and myeloarchitectonic appearance of the MB. (B and D) Nissl-stained sections showing subdivisions within the mammillary bodies. (C) Myelin-stained (Gallyas) section showing the arrangement of tracts at the mid level of the structure. White dashed line indicates the ventral border of the ‘cap’ region within the pars lateralis Abbreviations: AC, anterior commissure; b, medial mammillary nucleus, pars basalis; CC, corpus callosum; H, hippocampus; l, medial mammillary nucleus pars lateralis; lc, ‘cap’ region of the medial mammillary nucleus pars lateralis; LM, lateral nucleus of the mammillary bodies; m, medial mammillary nucleus pars medialis. MMB, medial nucleus of the mammillary bodies; T, thalamus. Scale bar: 1 mm.
The other nuclear group, the lateral mammillary nucleus, lies lateral and ventral to the medial mammillary nucleus (Fig. 1B). The lateral nucleus, which is shorter in the anterior–posterior plane than the medial nucleus (Fig. 1A), contains relatively large cells that stain intensely for Nissl substance. These cells are uniformly distributed, and so no cytoarchitectonic subdivisions have been described within the primate lateral nucleus. Although a third mammillary nucleus, nucleus intercalatus, has been reported by some authors (Veazey et al., 1982a), there is disagreement over its existence (Crosby & Showers, 1969; Nauta & Haymaker, 1969). This region, which lies lateral to pars lateralis of the medial mammillary nucleus, is indistinct as it is perforated by many fibers. While the study by Veazey et al. (1982a) recognized a separate nucleus intercalatus, it was rarely visible in the present study and so is omitted from descriptions of the data.
The nomenclature and borders of the thalamic nuclei, including those of the midline nuclei, are all taken from Olszewski (1952), as this monograph provides the most complete cytoarchitectonic description of all of the thalamic nuclei in the macaque brain. The anterior thalamic nuclei comprise three major nuclei (anterior medialis, and anterior dorsalis anterior ventralis). Immediately caudal to nucleus anterior dorsalis lies nucleus lateralis dorsalis, which is often grouped with the anterior thalamic nuclei (Bentivoglio et al., 1993). For this reason, nucleus lateralis dorsalis also received injections of retrograde tracers.
Results
Although the study used two different species of macaque monkey and three different retrograde tracers, there was a high degree of internal consistency across cases. For this reason, the results are grouped by injection site (2, 3) rather than by species or tracer. In describing the pattern of results priority is given to those cases in which the data are the most straightforward. Key factors are the precision of the injection site and whether there are injections using the same tracer in a different site in the same animal (Table 1). When considering any potential involvement from injections in the contralateral hemisphere it should be noted that while the projections from the medial mammillary nucleus to the thalamus are thought to be ipsilateral, those from the lateral mammillary nucleus are known to be bilateral (Cruce, 1975; Veazey et al., 1982b). The mammillary body label is depicted on a series of coronal sections, with the anterior–posterior level of these sections matched against Fig. 1.
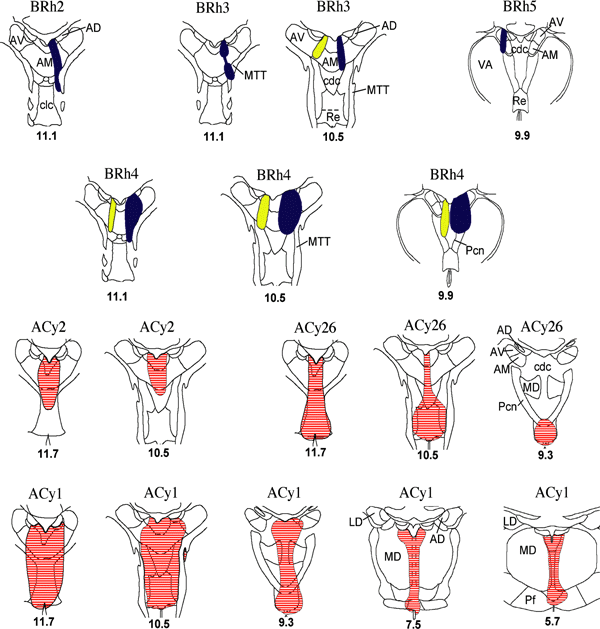
Location and extent of injections of retrograde tracers (HRP, Fb, Dy) in the rostral medial thalamus. The extent of the HRP (red horizontal lines, bottom two rows), Dy (yellow) and Fb (blue) injections are shown on coronal sections. The numbers below each section refer to their anterior–posterior level from Olszewski (1952; see also Fig. 1A). Abbreviations: AD, nucleus anterior dorsalis; AM, nucleus anterior medialis; AV, nucleus anterior ventralis; cdc, nucleus centralis densocellularis; clc, nucleus centralis latocellularis; LD, nucleus lateralis dorsalis; MD, nucleus medialis dorsalis; MTT, mammillothalamic tract; Pcn, nucleus paracentralis; Pf, nucleus parafascicularis; Re, nucleus reuniens; VA, nucleus ventralis anterior.
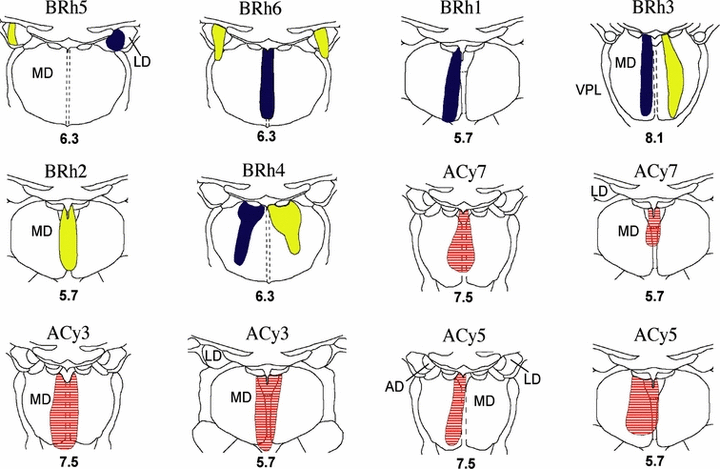
Location and extent of injections of retrograde tracers (HRP, Fb, Dy) into the caudal medial thalamus. The extent of the HRP (horizontal lines), Dy (yellow) and Fb (blue) injections are shown on coronal sections. The numbers refer to the anterior–posterior level of the sections from Olszewski (1952; see also Fig. 1A). Abbreviations: AD, nucleus anterior dorsalis; LD, nucleus lateralis dorsalis; MD, nucleus medialis dorsalis; VPL, nucleus ventralis posterior lateralis.
Injections in nucleus anterior medialis
Very striking evidence of a topographic projection from the medial mammillary nucleus was found in case BRh2 after an injection of Fb placed in the mid anterior–posterior level of nucleus anterior medialis just lateral to the midline (Fig. 2). There was an extremely clear dorsoventral gradient of retrograde label within the medial mammillary nucleus (Fig. 4). While the dorsal third of the medial mammillary nucleus contained a high density of labeled cells, there was only a diffuse presence throughout the rest of the medial mammillary nucleus. This more dense label was largely confined to pars lateralis, and was especially obvious in the dorsal cap of pars lateralis (4, 5). Very light Fb label was also present in the dorsal half of the medial mammillary nucleus in the contralateral hemisphere. Finally, a few Fb-labeled cells were spread diffusely across the lateral nucleus of the mamillary bodies in both hemispheres.
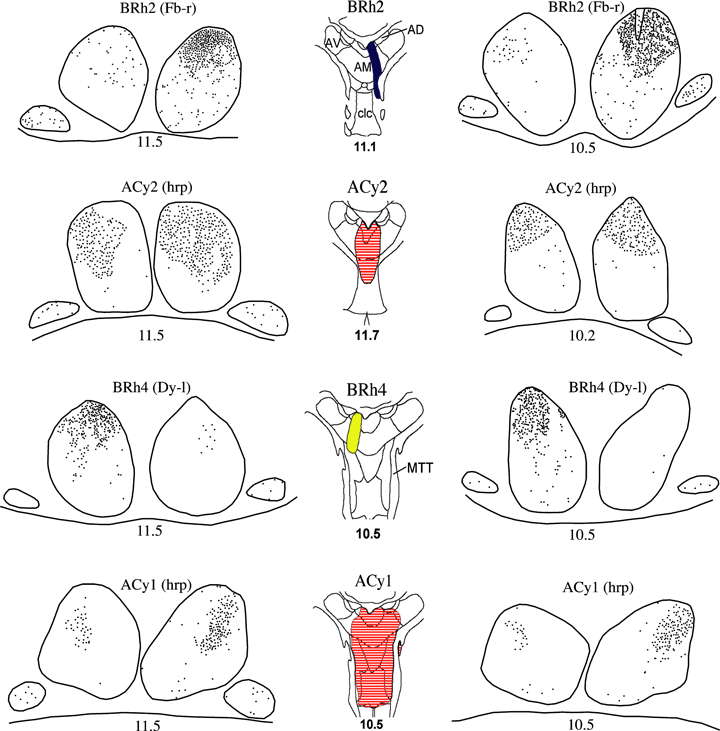
Series of drawings of the mammillary bodies showing the location of retrogradely labeled cells (black dots) in four cases with tracer injections centered in the medial ATN. The figures down the middle depict the center of each injection site (from Fig. 2). The numbers refer to the anterior–posterior levels from Olszewski (1952; see also Fig. 1A). Abbreviations: AD, nucleus anterior dorsalis; AM, nucleus anterior medialis; AV, nucleus anterior ventralis; clc, nucleus centralis latocellularis; Dy, diamidino yellow; Fb, fast blue; HRP, horseradish peroxidase; MTT, mammillothalamic tract.
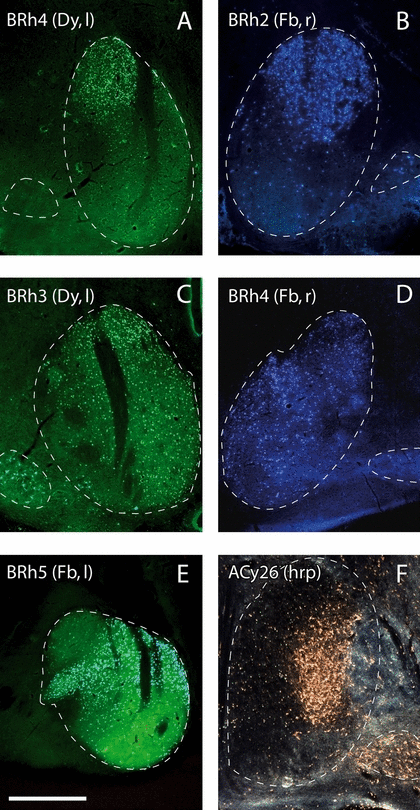
(A–F) Series of photomicrographs showing examples of retrogradely labeled cells in the mammillary bodies after fluorescent tracer injections of either fast blue (Fb, B, D, E), diamidino yellow (Dy, A, C) or horseradish peroxidase (HRP, F) in the thalamus. The white dashed lines show the borders of the medial and lateral mammillary nuclei. Scale bar: 1 mm.
All cases in which the tracer injection was centered in the nucleus anterior medialis resulted in label in the pars lateralis of the medial mammillary nucleus with a clear dorsoventral gradient in the density of labeled cells. In case BRh4, the Dy injection (Fig. 2) was centered in the caudal part of the nucleus anterior medialis, resulting in a number of labeled cells throughout the medial mammillary nucleus on the ipsilateral side, with the exception of pars basalis, which contained few if any cells. As with the more rostral injection there was a clear gradient as the density of label increased appreciably in the dorsal half of the nucleus (4, 5). The Dy label in the dorsal cap within pars lateralis was especially dense (4, 5). Very small numbers of Dy-labeled cells were present in the lateral nucleus of the mamillary bodies in both hemispheres.
Similar patterns of label came from two cases with HRP injections (ACy1, ACy2) that involved nucleus anterior medialis (Fig. 2). In case ACy2, the needle tract was in the midline portion of rostral nucleus anterior medialis, but also involved nucleus centralis densocellularis. Near mirror image patterns of mammillary body label were present in both hemispheres, presumably reflecting the spread of HRP into medial nucleus anterior medialis in both hemispheres (Fig. 2). The very large majority of retrograde label in ACy2 was in the pars lateralis of the medial mammillary nucleus. In the rostral third of the medial mammillary nucleus this label was grouped in a central region so that it involved the lateral parts of pars medialis and medial part of pars lateralis (Fig. 4). At mid anterior–posterior levels pars lateralis contained a great many retrogradely labeled cells that were present almost throughout this subdivision. The dorsal portion of pars medialis also contained many labeled cells, which contrasted with the rest of pars medialis and pars basalis as they contained extremely few labeled cells. At slightly more caudal levels the dense retrograde label was almost completely confined to the dorsal portion of pars lateralis, completely filling the dorsal cap. Because some sections in case ACy2 were counterstained for Nissl, it was possible to confirm that every cell contained HRP retrograde label in the dorsal cap region of pars lateralis. At this more caudal level there were only a handful of labeled cells distributed across pars medialis. The lateral nucleus of the mammillary bodies contained a scattering of labeled cells in both hemispheres. These cells appeared to be evenly distributed across the lateral nucleus, except for a lack of label at the most caudal portion of this nucleus.
The needle placement in ACy1 was very similar to ACy2 as it crossed centralis densocellularis and midline anterior thalamic nuclei (Fig. 2). The HRP injection was, however, just a little more caudal and a little deeper. Although a much greater amount of HRP was injected into the thalamus, the pattern of retrograde label within the mammillary bodies indicated a restricted region of uptake. Apart from the most rostral level of the mammillary bodies, where labeled cells were scattered evenly across the medial mammillary nucleus, the dense retrograde label was almost entirely confined to pars lateralis (Fig. 4). Here, the label was very heavily concentrated in the lateral and dorsal parts of pars lateralis. This label filled much of the dorsal cap in the caudal half of the structure, only avoiding its most medial part. Occasional labeled cells were also found in pars basalis and pars medialis within the medial mammillary nucleus, but these were very few in number. The lateral nucleus of the mammillary bodies contained a scattering of labeled cells, with no obvious topography.
While case BRh3 had an injection of Fb centered in the nucleus anterior medialis, the injection tract clearly severed the mammillothalamic tract (Fig. 2). The fibers of the mammillothalamic tract were densely filled, and in contrast to the localized label within the pars lateralis there was dense label throughout the medial mammillary nucleus. The lateral nucleus of the mammillary bodies nucleus also had numerous labeled cells throughout (Fig. 6).
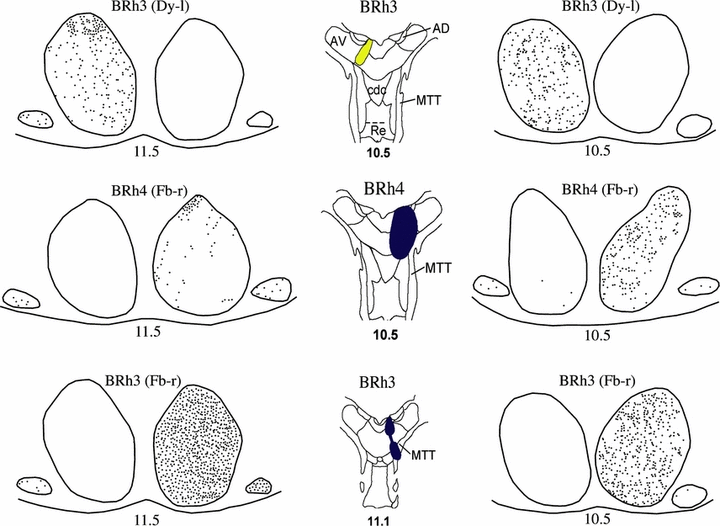
Series of drawings of the mammillary bodies showing the location of retrogradely labeled cells (black dots) in three cases with tracer injections centered in the anterior nuclei. The figures down the middle depict the center of each injection site (from Fig. 2). The numbers refer to the anterior–posterior levels from Olszewski (1952; see also Fig. 1A). Abbreviations: AD, nucleus anterior dorsalis; AV, nucleus anterior ventralis; cdc, nucleus centralis densocellularis; Dy, diamidino yellow; Fb, fast blue; MTT, mammillothalamic tract; Re, nucleus reuniens.
Injections in nucleus AV
In case BRh4 an injection of Fb was centered in the mid anterior–posterior level of nucleus anterior ventralis, and is likely to have extended into adjacent parts of nuclei anterior dorsalis and anterior medialis (Fig. 2). There were large numbers of labeled cells distributed throughout the medial mammillary nucleus (5, 6). The lowest density was in the pars basalis, and the highest density was in the more dorsal portions of pars medialis. Labeled cells were found across pars lateralis, and in contrast to the pattern observed after the nucleus anterior medialis injections there was no obvious increase or decrease of label in the dorsal cap. Small numbers of Fb-labeled cells were found evenly distributed across the lateral nucleus of the mammillary bodies in both hemispheres (5, 6). A very similar pattern of retrograde label was found across the medial mammillary nucleus in case BRh3, which had a Dy injection involving the more medial parts of nucleus anterior ventralis (5, 6).
In case BRh5 an injection of Fb was made in the caudal part of anterior ventralis, but it also encroached into caudal nucleus anterior medialis and some of caudal nucleus anterior dorsalis (Fig. 2). Considerable label was found in all three subregions of the medial mammillary nucleus (5, 7). At rostral levels, pars basalis contained the fewest cells, while pars lateralis and medialis contained very similar densities of labeled cells. At mid anterior–posterior levels label was present across all subregions, but there was a denser band across the ventral and central parts of pars lateralis, along with dorsal pars medialis (5, 7). As a consequence the dorsal cap showed a decrease in the density of label. At the most caudal levels very dense packing of labeled cells was observed across the medial mammillary nucleus. Scattered cells were found across the lateral mammillary nucleus in both hemispheres. In addition, a light scattering of Fb-labeled cells was found across the medial mammillary nucleus in the contralateral hemisphere. This label was most evident close to the periphery of the nucleus, i.e. near to its outer edges, and so included all three subregions.
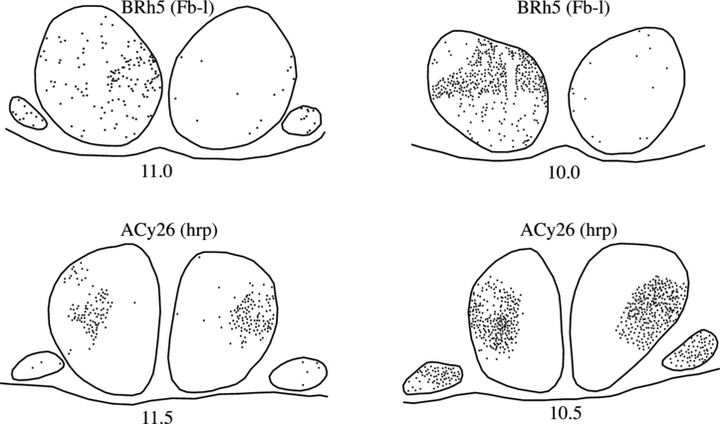
Series of drawings of the mammillary bodies showing the location of retrogradely labeled cells (black dots) in two cases with injections centered in the caudal nucleus AV (BRh5) or the rostral midline thalamus, including nucleus reuniens (ACy26). The figures down the middle depict the center of each injection site (from Fig. 2). The numbers refer to the anterior–posterior levels from Olszewski (1952; see also Fig. 1A). Abbreviations: Fb, fast blue; HRP, horseradish peroxidase.
Injections in the rostral midline thalamic nuclei
In one case (ACy26) an injection of HRP was centered in the rostral thalamic midline just ventral to nucleus anterior medialis, so that the tract passed through the latter nucleus (Fig. 2). The needle tip was placed in ventral nucleus centralis latocellularis, and so the core of the injection involved nucleus reuniens along with some of nucleus anterior medialis. Large numbers of HRP-retrogradely labeled cells were observed in both the medial mammillary nucleus and lateral nucleus of the mammillary bodies (5, 7). A dense aggregation of labeled cells was restricted to pars lateralis of the medial mammillary nucleus. These labeled cells were, for the most part, restricted to the ventral, central and lateral parts of pars lateralis (5, 7). This aggregation was present throughout the anterior–posterior extent of the nucleus. Little or no label was observed in the dorsal cap of pars lateralis. Compared with other cases, larger numbers of labeled cells were scattered across the lateral nucleus of the mammillary bodies, with a slight increase in the medial half of this nucleus. The same patterns of mammillary body label were observed in both hemispheres (Fig. 7).
Injections in nucleus lateralis dorsalis
Two animals received bilateral injections of fluorescent tracers centered in nucleus lateralis dorsalis (BRh5, BRh6; Fig. 3). BRh5 had Dy in the left and Fb in the right nucleus lateralis dorsalis, as well as Fb in nucleus anterior ventralis (Table 1). Potential mammillary inputs to nucleus lateralis dorsalis can therefore only be inferred from the Dy injection in BRh5. While no Dy-positive cells were seen in the medial mammillary nucleus, a few Dy-positive cells were present in the lateral nucleus of the mammillary bodies.
BRh6 had bilateral injections of Dy in nucleus lateralis dorsalis, and an injection of Fb along the midline involving the midline thalamic nuclei and the nucleus medialis dorsalis (Table 1). Once again, only a very few Dy cells were found in lateral nucleus of the mammillary bodies and none in medial mammillary nucleus.
Injections in MD and/or caudal midline nuclei
Animals with injections placed in nucleus medialis dorsalis helped to clarify the findings from the two cases with multiple tracer injections (BRh3, BRh4) involving the anterior thalamic nuclei and nucleus medialis dorsalis (Table 1, 2, 3). Three cynomolgus monkeys had single injections of HRP centered in magnocellular nucleus medialis dorsalis (ACy3, ACy5, ACy7). There was variable medial spread across the midline, but little evidence of rostral spread into the anterior thalamic nuclei (Fig. 3). Additional information came from three cases with fluorescent tracer injections (BRh1, BRh2, BRh6), where nucleus medialis dorsalis was the only target for one specific tracer. In none of these six cases was there evidence of a projection from the medial mammillary nucleus. In view of the placement of these injections (2, 3), it can be concluded that the medial mammillary nucleus does not project to either nucleus medialis dorsalis or nucleus centralis intermedialis, the midline thalamic nucleus running between the left and right nucleus medialis dorsalis at this level.
The findings for the lateral nucleus of the mammillary bodies nucleus were not so consistent after the injections involving nucleus medialis dorsalis. No labeled cells were observed in the lateral nucleus of the mammillary bodies nucleus in cases ACy7 (HRP), BRh1 (Fb) or BRh2 (Dy). In case BRh6, with a Fb injection into midline thalamus and nucleus medialis dorsalis, a couple of cells were present in lateral nucleus of the mammillary bodies. Consistent with this finding, an extremely small number of HRP-positive cells were seen in the lateral nucleus of the mammillary bodies in cases ACy3 and ACy5. A common feature of the injections in these three cases was the greater involvement of the midline thalamic nuclei at the level of the rostral half of nucleus medialis dorsalis. This pattern of results suggested the presence of an extremely light projection to one or more midline thalamic nuclei, either at the level of nucleus medialis dorsalis or immediately rostral to this nucleus (e.g. centralis intermedialis, centralis superior or centralis densocellularis).
Discussion
The thalamus is the principal efferent target of the mammillary bodies (Powell et al., 1957; Xiao & Barbas, 2002b), and so the mammillothalamic projections must reflect core aspects of mammillary body function. Indeed, it has been supposed that every mammillary body neuron might project to the thalamus (Hopkins, 2005), and the density of label found in pars medialis and pars lateralis of the medial mammillary nucleus would seem to support this notion for these subregions at least. Previous studies have confirmed that the medial mammillary nucleus projects ipsilaterally to the thalamic nuclei anterior medialis and anterior ventralis, while the lateral mammillary nucleus projects bilaterally to nucleus anterior dorsalis (Veazey et al., 1982b; Xiao & Barbas, 2002b). The present study has not only shown that the pattern of connections is more complex than previously supposed, but has also revealed novel organizational properties within the medial mammillary nucleus. The latter finding is of especial interest as the medial mammillary nucleus is the major contributor to the mammillothalamic tract, and the medial mammillary nucleus is most implicated by the evidence concerning the mammillary bodies and memory (Victor et al., 1971; Vann & Aggleton, 2004).
Injections of retrograde tracers into the medial thalamus in both rhesus and cynomolgus macaque monkeys produced consistent patterns of mammillary body label. The pars lateralis of the medial mammillary nucleus provided the large majority of the many neurons projecting to the medial part of nucleus anterior medialis (Fig. 8). In more caudal parts of the mammillary nucleus, these efferent neurons were largely confined to the ‘dorsal cap’ of pars lateralis (see Fig. 5A and B). In some cases every neuron within this subregion appeared to contain label, an observation consistent with the belief that there are no interneurons in the mammillary bodies (Hopkins, 2005). Although this ‘dorsal cap’ has not been previously described (e.g. Crouch, 1934; Crosby & Showers, 1969), it is visible in Nissl-stained sections, as the cells pack more closely together (Rose, 1939, Fig. 16), reflecting the relative decrease in white matter (Fig. 1). Although other connectional data from studies of primates have not yet separated this subregion within pars lateralis, it seems likely that the dorsal cap will prove to have other, distinct anatomical features given the importance of the thalamic projections for mammillary body function.
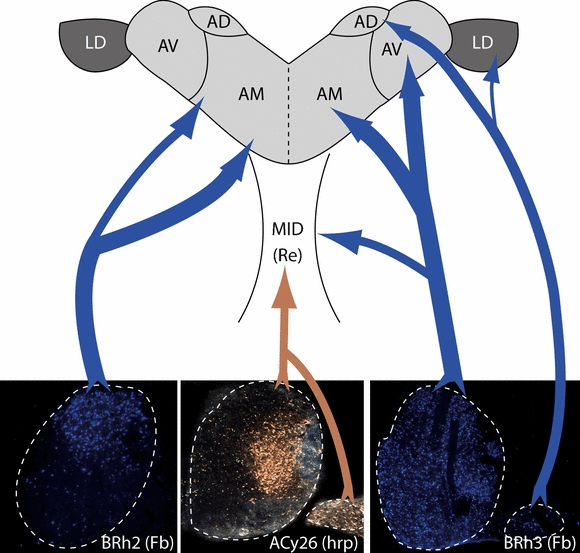
Summary diagram showing the origin of the major projections of the mammillary bodies to the thalamus. Case BRh3 shows how all regions within the medial (MMB) and lateral (LMB) mammillary nuclei contribute to the thalamic projections. Much of the projection to anterior medialis (AM) originates from the dorsal or ‘cap’ region of the pars lateralis in the MMB (e.g. BRh2). The projection to the nucleus anterior ventralis (AV) has a more distributed origin in the MMB. Additional projections originate from the central and lateral regions of pars lateralis of MMB, and may project to the midline nuclei, including nucleus reuniens (Re, ACy26). There is a bilateral projection from LMB to nucleus anterior dorsalis (AD; Veazey et al., 1982b), and potentially a light input to nucleus lateralis dorsalis (LD). Abbreviations: Fb, fast blue; HRP, horseradish peroxidase; Mid, rostral midline thalamic nuclei.
More lateral injections within the anterior thalamic nuclei, involving nucleus anterior ventralis and the border with nucleus anterior medialis, produced a very different pattern of mammillary body efferents (Fig. 8). In these cases, retrograde label was evenly distributed across the entire medial mammillary nucleus. As a consequence, the cytoarchitectonic borders within the medial mammillary nucleus (Rose, 1939; Veazey et al., 1982a) were not marked by any obvious changes in label density. It appears therefore that the projections from both pars medialis and pars basalis primarily terminate in nucleus anterior ventralis, while projections from pars lateralis terminate in both nucleus anterior medialis and nucleus anterior ventralis, with the former projection being appreciably denser (Fig. 8). This pattern is consistent with case RM9 described by Veazey et al. (1982b), where the injection of amino acids spread over the midline so that just pars medialis of the medial mammillary nucleus was involved in one hemisphere. Projections from this very restricted source were traced to the anterior thalamic nuclei, with appreciably denser label present in nucleus anterior ventralis than nucleus anterior medialis. This pattern accords with the present overview as it not only supports the evidence of a topography within the medial mammillothalamic projections, but also that pars medialis only projects lightly to nucleus anterior medialis, i.e. pars lateralis is the main source of this input.
One previous study has described mammillary body neurons projecting to the anterior thalamic nuclei in monkeys (Xiao & Barbas, 2002b). An injection of either Dy or fluoro-ruby was placed in nucleus anterior medialis in four rhesus monkeys (Xiao & Barbas, 2002b). That study confirmed the huge numbers of neurons in the medial mammillary nucleus that project to the anterior thalamus as some individual thalamic injections labeled over 50% of the cells in the entire medial mammillary nucleus. While only brief descriptions of the location of mammillary body label were provided (Xiao & Barbas, 2002b), the label was typically distributed evenly across all subdivisions of the medial mammillary nucleus, i.e. there was no dense concentration of label within pars lateralis or evidence of a distinct dorsal cap. Thus, the pattern of label described by Xiao & Barbas (2002b) corresponds much more closely to that seen with the more lateral anterior thalamic injections, e.g. cases BRh3Dyl and BRh4Fbr, even though their injections were centered in the anterior medalis. There are several possible explanations for this apparent discrepancy. First, in some of the cases of Xiao & Barbas (2002b) there may have been uptake of label by the immediately adjacent mammillothalamic tract, so giving an even, dense pattern of retrograde label (as in case BRh3Fbr). Second, there is some evidence that the anterior medialis injections described by Xiao & Barbas (2002b) may have been appreciably larger than indicated. Only the core of each injection site was depicted, while considerable retrograde label was reported in sites not typically linked to the anterior medalis, e.g. in the basolateral amygdala and the hippocampal subfields CA3 and CA4. If their injections had, in fact, been larger and so included nucleus anterior ventralis, then their study agrees more closely with the present one.
Evidence was also found in the present study for mammillary body projections to the rostral midline nuclei of the thalamus. Numerous labeled cells were found in the medial and lateral mammillary bodies after an HRP injection (case ACy26) that involved nucleus reuniens. A dense patch of label was found in the central and lateral portions of pars lateralis in the medial mammillary nucleus, and this location was different to that seen after immediately dorsal injections in the midline part of nucleus anterior medialis (e.g. case ACy2). This difference suggests a separate projection to nucleus reuniens or the midline nuclei above. Consistent with this interpretation, Veazey et al. (1982b) noted appreciable anterograde label in the rostral midline nuclei, especially after an injection in the region of the lateral mammillary nucleus, but these authors were uncertain of its origin. As some of this midline thalamic label was in fibers (Veazey et al., 1982b), care must be taken over the possibility that some HRP was picked up by fibers of passage. Nevertheless, the appearance of a third, different pattern of label within the medial mammillary nucleus again emphasizes the potential heterogeneity of function within this nucleus.
The lateral mammillary nucleus appeared to project to several thalamic nuclei. It has long been known that there are dense, bilateral projections from the lateral mammillary nucleus to nucleus anterior dorsalis (Fry et al., 1963; Veazey et al., 1982b), but the present study suggested additional, light projections to the rostral midline nuclei, anterior medialis, anterior ventralis and lateralis dorsalis. The proximity of anterior dorsalis to anterior ventralis means, however, that any input to the latter nucleus requires additional confirmation. The possible projection from the lateral mammillary nucleus to lateralis dorsalis is especially interesting, as drawings from the study by Veazey et al. (1982b) indicate such a projection, although it is not stated in the text. Furthermore, electrophysiological studies with rats link these two nuclei together as both the lateral mammillary nucleus and lateralis dorsalis contain ‘head-direction’ cells (Taube, 1998). Label in the lateral mammillary nucleus was always scattered across the structure. This lack of topography accords both with the diffuse pattern of hippocampal terminations across this nucleus (Aggleton et al., 2005) and the absence of recognized subdivisions within the nucleus.
The most important finding in the present study was the strikingly different patterns of mammillary body label after tracer injections in medial anterior medialis and those more lateral injections involving anterior ventralis. This finding introduces the idea that the medial mammillary nucleus nucleus has at least two functions, and that these map onto different processes within nuclei anterior ventralis and anterior medialis. Previous studies of the inputs to the rat (Hopkins, 2005) and monkey (Aggleton et al., 2005) mammillary bodies indicate that the projections from the subiculum are distributed in a horizontal fashion. In contrast, the inputs from the tegmentum are distributed in the dorsal axis, i.e. orthogonal to those from the subiculum (Hopkins, 2005). It can be seen that the diffuse origin of the inputs from the medial mammillary nucleus nucleus to anterior ventralis should ensure that this connection is influenced by all of the subicular projections. In contrast, the projections to anterior medialis should be more selectively influenced by the caudal subiculum, as this part projects most densely upon the dorsal mammillary bodies (Aggleton et al., 2005).
The notion that nuclei anterior medialis and anterior ventralis have distinct functions receives further anatomical support. Of the two thalamic nuclei, anterior medialis is preferentially connected with the prefrontal cortex (Kievet & Kuypers, 1977; Carmichael & Price, 1995; Barbas et al., 1991; Xiao & Barbas, 2002a). Furthermore, the dense thalamic afferents from the hippocampus terminate bilaterally in anterior medialis, but only ipsilaterally in anterior ventralis (Aggleton et al., 1986). Other evidence of functional differences between these two thalamic nuclei comes from electrophysiological studies in rats showing a much higher percentage of neurons in anterior ventralis (40%) that discharge rhythmically and in synchrony with hippocampal theta (Albo et al., 2003) than those in nucleus anterior medialis (5.7%). Similarly in the medial mammillary nucleus nucleus, a subpopulation of cells also show theta-related activity (Kocsis & Vertes, 1994; Kirk, 1998), and they presumably project to anterior ventralis. The relaying of theta from the medial mammillary nucleus nucleus to anterior ventralis is seen as one way that the mammillary body–anterior thalamus partnership might support memory (Kirk, 1998; Hasselmo et al., 2002; Vann & Aggleton, 2004; Vertes et al., 2004). This activity leaves a separate mnemonic role for nucleus anterior medialis (Aggleton et al., 2005) and its medial mammillary inputs from pars lateralis. Clues to this role come from the substantial prefrontal connections of anterior medialis (Kievet & Kuypers, 1977; Carmichael & Price, 1995; Barbas et al., 1991; Xiao & Barbas, 2002a), placing it in a strategic position to integrate hippocampal, medial mammillary body and prefrontal activity.
Acknowledgement
The authors thank the invaluable assistance of Lorraine Woods for the figure preparation, and the BBSRC for support to S.D.V.
Abbreviations
-
- Dy
-
- diamidino yellow
-
- Fb
-
- fast blue
-
- HRP
-
- horseradish peroxidase