Nitric oxide synthase expression in the central nervous system of Sepia officinalis: an in situ hybridization study
Abstract
We recently reported the molecular cloning of nitric oxide synthase (NOS) mRNA from Sepia officinalis (SoNOS) using a strategy that involves hybridization of degenerate PCR primers to highly conserved NOS regions, combined with a RACE procedure. Here, in situ hybridization study has been performed on serial sections of the cuttlefish central nervous system to reveal localized specific staining of cell bodies in several lobes of the brain. Staining was found in many lower motor centres, including cells of the inferior and superior buccal lobes (feeding centres); in some higher motor centres (anterior basal and peduncle lobes); in learning centres (vertical, subvertical and superior frontal lobes); and in the visual system [medulla and deep retina (optic lobe)]. Positive staining was also found in the olfactory lobe. NOS-expressing cells have been detected also in the interbasal lobe. Double labelling experiments, performed on consecutive sections, showed that neurons containing NOS immunoreactivity were also positive in in situ hybridization staining. All these data support the presence of NOS in several systems in the cuttlefish brain.
Introduction
During the last decade molluscs have emerged as useful models for studies of roles and functions of nitric oxide (NO) and its producing enzyme, nitric oxide synthase (NOS) (Di Cosmo, 2003; Palumbo, 2005; Palumbo & d'Ischia, 2006). Following the pivotal papers of Winlow's group (Moroz et al., 1993, 1994, 1995) on the gastropod Lymnaea stagnalis, the presence of NOS and the functions of NO have been investigated in other gastropods, in bivalves and in cephalopods. As a result, a number of physiological roles have been attributed to NO in the mollusc central nervous system (CNS) mainly in the context of behaviour and plasticity (Teyke, 1996; Katzoff et al., 2002; Korneev et al., 2005).
Among invertebrates, cephalopods have one of the most highly developed nervous systems, and exhibit a number of complex behaviours, including a marked ability to learn (Hanlon & Messenger, 1996). Their CNS is functionally differentiated into distinct lobes (Boycott, 1961; Young, 1971; Budelmann, 1995) and contains a variety of putative neurotransmitters (for a review, see Messenger, 1996). Chichery & Chichery (1994), Kimura et al. (1997) and Gillette et al. (1997), using NADPH diaphorase histochemistry, localized putative NOS activity in the CNS, respectively, of Sepia officinalis, Loligo bleekeri, and in the CNS and peripheral nervous system of Octopus vulgaris.
Robertson et al. (1994, 1996) observed that intramuscular injections of NOS inhibitors completely block both touch and visual learning in Octopus, suggesting the involvement of NO in learning mechanisms in cephalopods. Tu & Budelmann (1994, 1999, 2000a, b) provided the first direct, physiological evidence in cephalopods that NO can affect the resting activity of nerve fibres.
Previous studies from our groups have described the presence of a neuronal isoform of NOS in the CNS of S. officinalis (Palumbo et al., 1999; Di Cosmo et al., 2000) along with the excitatory glutamate/NO/cyclic GMP signalling pathway (Palumbo et al., 1999). Subsequent efforts to elucidate molecular aspects of NOS and its product culminated in the cloning of Sepia NOS (SoNOS) (Scheinker et al., 2005). All these data provided an improved background to study the anatomical distribution and functional significance of NOS and the multiple physiological roles of NO, including regulation of neuronal plasticity in a convenient model system, the cephalopod brain (Di Cosmo et al., 2000).
Herein, we report the complete and detailed ‘footprint’ of SoNOS expression in Sepia brain. Moreover, in order to evaluate colocalization of NOS immunoreacitivity and SoNOS mRNA, double labelling experiments have been performed.
Materials and methods
Animals
Adult male Sepia officinalis (500–700 g, n = 6) were collected in the Bay of Naples and kept in both a light- and a temperature-controlled environment for 2 days. Dissections were performed using cold anaesthesia and the CNS was collected and immediately fixed.
In situ hybridization and immunohistochemistry
CNS was fixed overnight at room temperature by immersion in 4% paraformaldehyde in phosphate-buffered saline (PBS). Tissues were dehydrated in ethanol, cleared in xylene and embedded in paraffin (Paraplast). In situ hybridization experiments were conducted on 7-µm sagittal sections with digoxigenin-11-UTP-labelled riboprobes (Roche Diagnostics, Basel, Switzerland), according to the manufacturer's instructions and as previously described (Simeone et al., 1995). The antisense SoNOS digoxigenin riboprobe was prepared from the cDNA fragment encoded by the last 150 amino acids and the 3′ untranslated region of SoNOS (Scheinker et al., 2005). Eighty nanograms of probe per slide at 60 °C were used. Control experiments were run in parallel using the corresponding sense probe.
In order to perform double labelling experiments, 5-µm consecutive sections were placed on alternate slides; one of these was used for in situ hybridization analysis and the other for immunohistochemistry.
For immunohistochemistry, sections were treated as described in Di Cosmo et al. (2000). Briefly, sections were deparaffinized, rehydrated and then washed in 0.1 m phosphate buffer, pH 7.4, containing 0.9% NaCl (PBS). The sections were treated with 0.3% H2O2 to block the endogenous peroxidase. After a 20-min incubation with normal goat serum, to reduce background staining, the sections were placed overnight in a moist chamber at 4 °C with the primary antibody: a rabbit polyclonal anti-NOS (1 : 250) raised against the C-terminal sequence (amino acid residues 1414–1429) of the rat NOS-I protein. Afterwards, the sections were rinsed in several baths of PBS and incubated for 1 h at room temperature with a biotinylated goat antirabbit (Pierce, Milan, Italy) diluted 1 : 200 in PBS. Finally, after two rinses in PBS, the slides were incubated with an avidin–peroxidase conjugate (Pierce) for 1 h at 1 : 200 dilution followed by DAB visualization. The sections were dehydrated and mounted with BioMount (British BioCell International, London, UK).
The range in number of in situ positive neurons was estimated taking into account the number of sections from a specific lobe, the 7-µm distance among consecutive sections and the average diameter of positive neurons. This was randomly performed on sections from four distinct animals and the results were subject to statistical analyses.
Results
Localization of SoNOS mRNA-expressing cells
The CNS of Sepia officinalis is highly centralized (Fig. 1) with the brain arranged around the oesophagus. The brain is subdivided into supraoesophageal and suboesophageal parts, laterally connected by the perioesophageal magnocellular lobes. The large optic lobes are joined to the supraoesophageal mass by short optic tracts, on which lie the peduncle and olfactory lobes (Boycott, 1961; Young, 1974, 1976, 1977; Messenger, 1979). Anterior to the brain lie the buccal lobes.
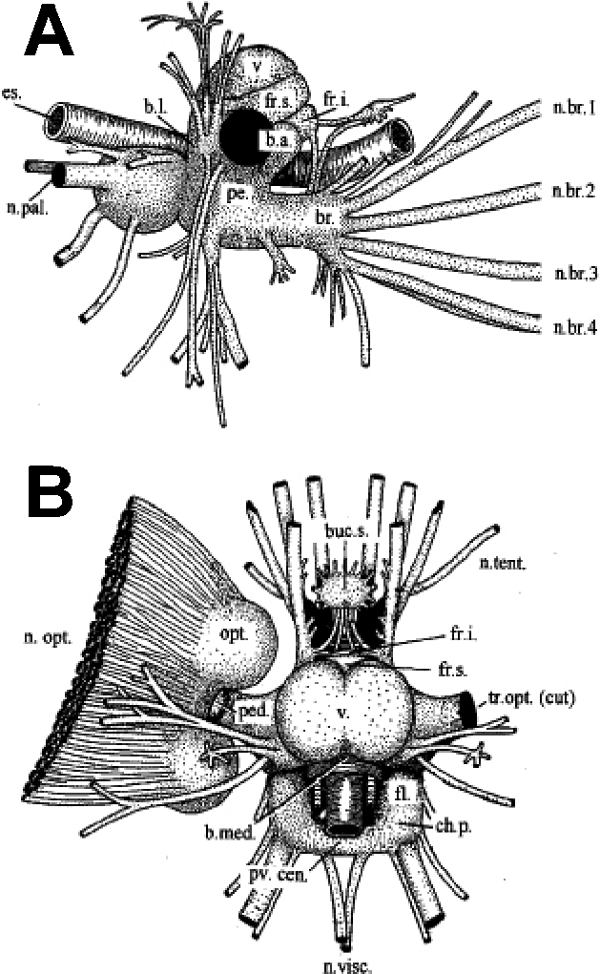
Sepia brain, with right optic lobe cut away. (A) Lateral aspect, with nerves omitted on the left side. (B) Dorsal view. (Redrawn from Tompsett, 1939.)
The technique used here labels neuronal cell bodies and very occasionally fibres, and the distribution of SoNOS mRNA-expressing cell bodies in the brain, as well as their abundance, is summarized in Table 1. Moreover, a summary diagram showing the distribution of labelled neurons is given in Fig. 2. No labelling of glial cells was observed throughout the brain. At least for the animals analysed, no differences in NOS expression between preparations was observed. Whether NOS expression varies within individual cells for the duration of the cell's lifetime remains to be investigated. When the nonsense probe was used in control experiments, no staining was observed.
| Brain region | Positivity | Positive neurons (n) |
|---|---|---|
| Subesophageal lobes | ||
| Brachial lobe and prebrachial lobe | + | 2000–2100 |
| Anterior chromatophore lobe | – | |
| Anterior pedal lobe | + | 1200–1300 |
| Lateral pedal lobe (anterior) | – | |
| Lateral pedal lobe (posterior) | + | 700–900 |
| Posterior pedal lobe | ++ | 6000–7000 |
| Anterior palliovisceral lobe | ++ | 8000–9000 |
| Central palliovisceral lobe | ++ | 20000–22000 |
| Posterior chromatophore lobe | – | |
| Fin lobe | – | |
| Latero-ventral palliovisceral lobe | ++ | 6000–6500 |
| Visceral lobe | ++ | 3000–3300 |
| Magnocellular lobes | ||
| Ventral magnocellular lobe | + | |
| Dorsal magnocellular lobe | – | |
| Posterior magnocellular lobe | – | |
| Supraesophageal lobes | ||
| Anterior anterior basal lobe | ++ | 18000–20000 |
| Posterior anterior basal lobe | + | 2500–3000 |
| Median basal lobe | + | 800–1000 |
| Lateral basal lobe | – | |
| Peduncle lobe | + | |
| Dorsal basal lobe | ++ | 10000–12000 |
| Dorso-lateral lobe | + | 2000–2500 |
| Subpedunculate lobe | – | |
| Interbasal lobe | ++ | 3000–4000 |
| Vertical-Superior frontal system | ||
| Superior frontal lobe | – | |
| Post-frontal lobe | + | 500–700 |
| Vertical lobe | ++ | 12000–13000 |
| Subvertical lobe | ++ | 10000–11000 |
| Precommissural lobe | – | |
| Optic lobe | ||
| Outer granular layer | – | |
| Plexiform layer | No cells | |
| Inner granular layer | + | 1000–1500 |
| Medulla | + | 2000–2500 |
| Olfactory system | ||
| Olfactory lobe | + | 2800–3000 |
| Buccal system | ||
| Superior buccal lobe | + | 1300–1500 |
| Inferior buccal lobe | – | |
| Inferior frontal lobe | + | 2000–2300 |
- The numbers of neurons (n) are given as a range (analyses performed on 4 animals). Positivity: –, lobes with no cells staining; +, lobes with sparse positive staining; ++, lobes with extensive positive staining.
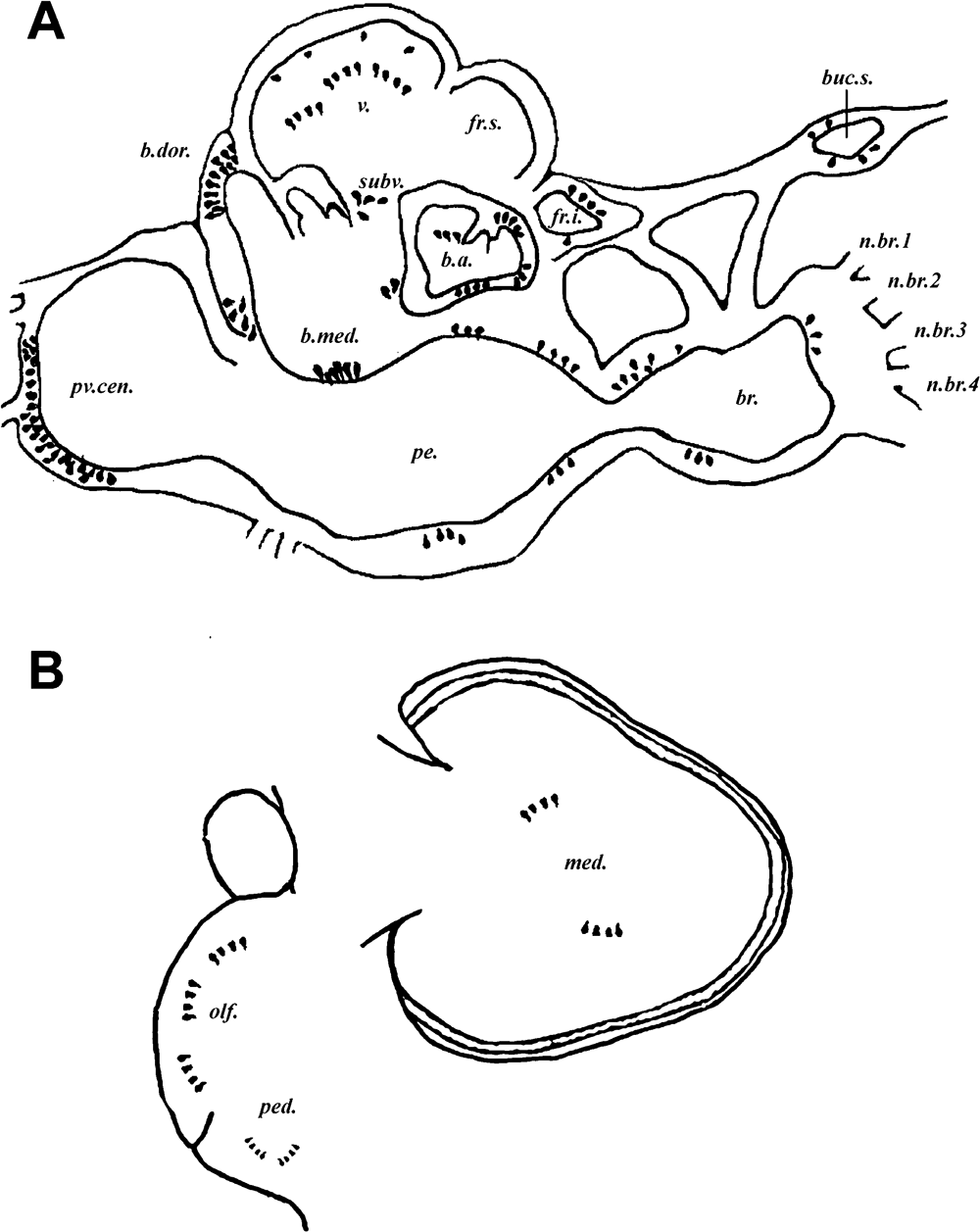
Summary figure of Sepia brain showing in situ hybridization staining. (A) Sagittal section showing distribution in supra- and suboesophageal masses. (B) Distribution in optic and optic tract lobes.
Suboesophageal lobes
The suboesophageal lobes comprise the anterior, middle and posterior suboesophageal masses. Functionally, they contain lower motor centres, whose cells provide the final common pathways to the peripheral effectors, and intermediate motor centres, whose fibres have at least one further synapse in the periphery. Electrical stimulation of the lower motor centres produces movements of the parts they innervate: these movements are essentially local (Boycott, 1961).
In the anterior suboesophageal mass, the expression of SoNOS mRNA is limited to cell bodies of different size of the prebrachial lobe (Fig. 3A). Some small neurons lie close to the neuropil. Only a few positive neurons are present in the brachial lobe.
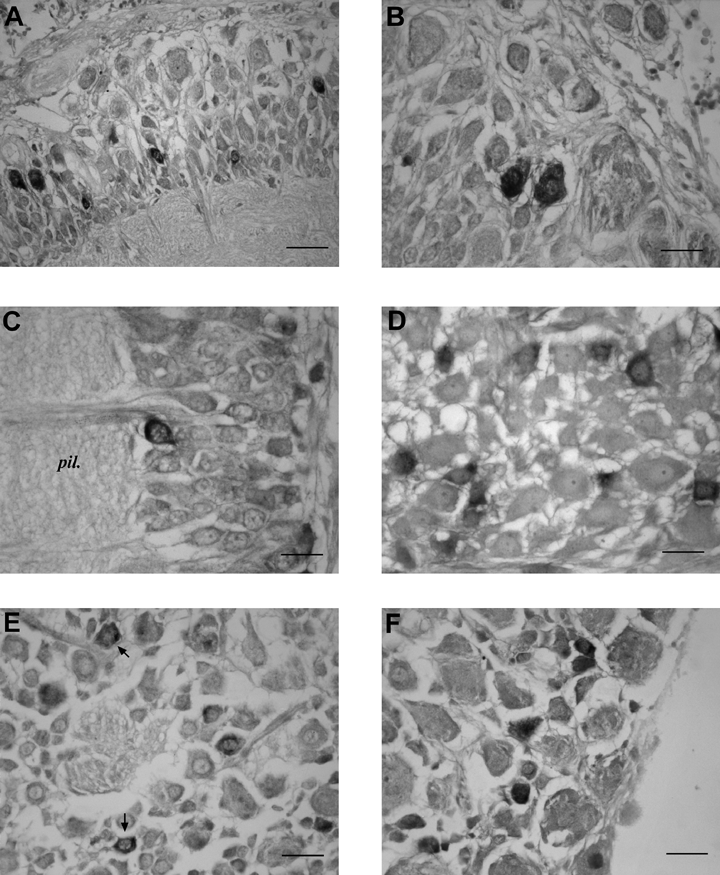
SoNOS mRNA-expressing neurons in the suboesophageal lobes. (A) Prebrachial lobe. Several positive neurons of different size are present in the cell layer. (B) Anterior pedal lobe. Positive staining of two small cells, probably interneurons. (C) Lateral pedal lobe. A positive neuron lining the neuropil. (D) Posterior pedal lobe. Small neurons expressing SoNOS mRNA in the cortical layer. Note the negative large neurons. (E) Positive staining of small neurons (arrows) in the central part of palliovisceral lobe. (F) Latero-ventral palliovisceral lobe. Positive staining of small cell bodies. Scale bars, 35 µm (A), 30 µm (B–F).
In the middle suboesophageal mass, SoNOS mRNA is expressed in cell bodies of all the pedal lobes. In the anterior pedal lobe, a few small positive cell bodies can be found in the innermost region of the cell layer (Fig. 3B). These cells are probably microneurons (Young, 1971).
Few SoNOS mRNA-expressing cell bodies are present in the dorsal region of the lateral pedal lobe (Fig. 3C). Several large neurons expressing SoNOS mRNA are observed in the posterior pedal lobe although many small SoNOS-expressing cells are present in the most postero-dorsal region of this lobe (Fig. 3D). No positive cells are seen in the anterior chromatophore lobe.
In the posterior suboesophageal mass SoNOS mRNA-expressing cells are distributed in all regions of the palliovisceral lobe; this part of the brain also showed strong NOS-like immunoreactivity (Di Cosmo et al., 2000). In both the anterior and the central palliovisceral lobe, too, many small cells (diameter 10–20 µm) can be seen (Fig. 3E). In the latero-ventral palliovisceral lobe, as already reported (Scheinker et al., 2005), positive cell bodies are found, localized in the inner region of the lobe (Fig. 3F). Finally in the visceral lobe (Young, 1976), SoNOS mRNA-expressing neurons are also present. These cells appear to be organized in columns reaching the neuropil. Neither the fin lobe nor the posterior chromatophore lobes express SoNOS mRNA.
Magnocellular lobes
In the dorsal and ventral regions of the ventral magnocellular lobe a few SoNOS-expressing cells are present. The dorsal and the posterior magnocellular lobes contain few or no SoNOS mRNA-expressing cells.
Supraoesophageal lobes
The supraoesophageal lobes comprise: the basal lobes, namely the higher motor centres of Boycott (1961); the lobes of the dorsal basal system; and the vertical–superior frontal system, the so-called silent areas involved in learning (Sanders & Young, 1940; Boycott, 1961; Messenger, 1973).
The anterior basal lobe comprises two separate regions: anterior and posterior (Young, 1977). In the anterior anterior basal lobe many SoNOS mRNA-expressing neurons are seen in the lateral and ventral portions. These neurons vary in size (Fig. 4A–D). The largest cells lie ventrally (Fig. 4B) and the smallest dorsally (Fig. 4C). Other SoNOS mRNA-expressing neurons (20–30 µm in diameter) are seen in the antero-dorsal and antero-ventral regions of the antero-median lobule (Fig. 4D). In the posterior anterior basal lobe, the spine region contains numerous positive small cells (Fig. 4E and F).
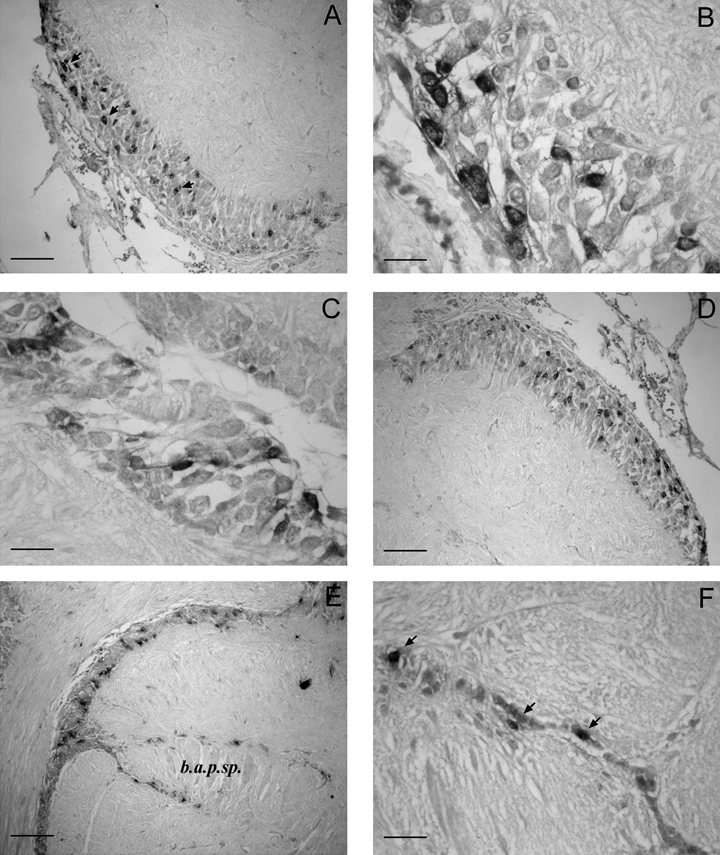
SoNOS mRNA-expressing neurons in the anterior basal lobes. (A) Many SoNOS mRNA-expressing neurons (arrows) are scattered in the ventral portion of the lobe. (B and C) Higher magnification of the ventral and dorsal neurons, respectively. Note the different size of these cells that characterize the different regions. (D) Antero-dorsal region of the antero-median lobule: numerous small positive neurons are present in the cell layer. (E) Spine region of the posterior anterior basal lobe. Note the small neurons positive in the cell layer. (F) Higher magnification of the spine region contains numerous positive small cells (arrows). Scale bars, 90 µm (A, D, E ), 30 µm (B, C), 35 µm (F).
In the median basal lobe few cells stain weakly in the postero-ventral region (Fig. 5A and B) while no SoNOS mRNA-expressing cells are seen in the lateral basal lobe. In the peduncle lobe staining is virtually absent: there are very few weakly staining cell bodies. They are seen only in the spine region, lying next to the well-defined boundary of the spine.
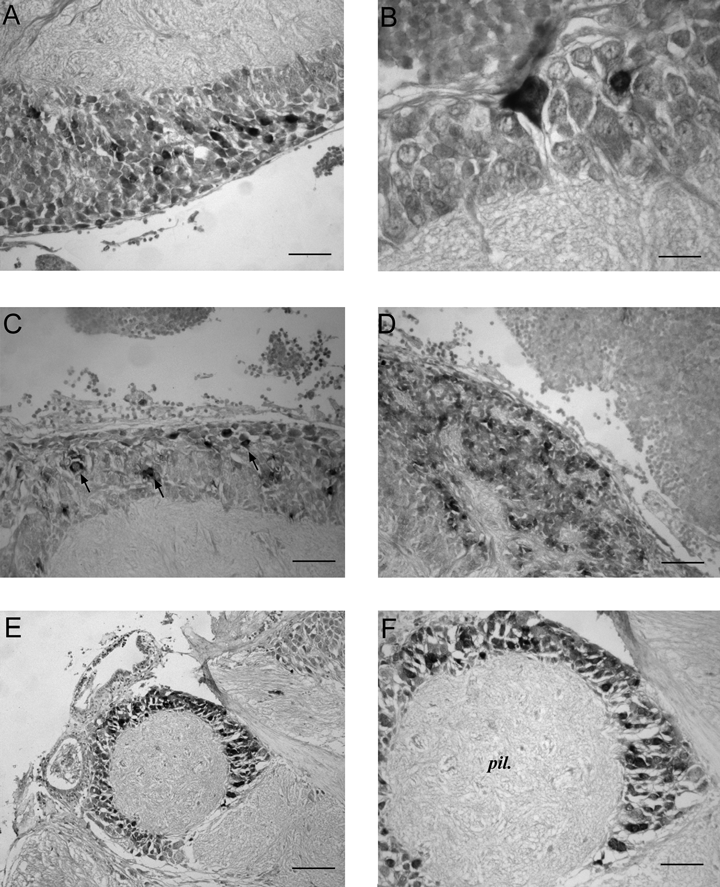
SoNOS mRNA-expressing neurons in the basal lobes. (A) Median basal lobe. Packed small positive neurons are present in the cell layer of this lobe. (B) A higher magnification of a large pear-shaped neuron in cortex of the median basal lobe. (C) Dorsal basal lobe. Positive staining of scattered cells (arrows) in the cell layer of the posterior region of the lobe. (D) Dorsolateral basal lobe. Cluster of many small in situ positive neurons. (E) Interbasal lobe. Positive cells are distributed in the cortex of this lobe. (F) Higher magnification of interbasal lobe. Positive staining of several cells in the cell layer of the lobe. Scale bars, 40 µm (A, C, D, F), 20 µm (B), 80 µm (E).
In the anterior region of the dorsal basal lobe, there are some strongly positive cell bodies (10–15 µm in diameter), just behind the subvertical lobe. In the posterior region of the lobe there is a cluster of positive cell bodies in the outer cell layer (Fig. 5C). In the dorso-lateral lobe, small cell bodies show a very strong positivity for NOS mRNA (Fig. 5D). Interestingly, many SoNOS mRNA-expressing cells of different sizes are detected in the interbasal lobe (Fig. 5E and F).
Vertical–superior frontal system
This comprises the superior frontal, the vertical and the subvertical lobes. These lobes are involved in the establishment and retrieval of memories (Boycott, 1961; Messenger, 1973; Young, 1979).
No SoNOS mRNA-expressing cell bodies are present in the superior frontal lobe. In the vertical lobe, the large cells lying around the edge of the lobe (Young, 1979) do not express SoNOS mRNA. By contrast, a large number of smaller SoNOS mRNA-expressing cell bodies, which displayed immunopositivity (Di Cosmo et al., 2000), were detected at the inner edge of the lobe (Fig. 6A) and throughout the neuropil (Fig. 6B). No SoNOS mRNA-expressing amacrine cells are detected. In the subvertical lobe, positive cell bodies are observed (Fig. 6C) in both the posterior and the anterior regions of the lobe (Fig. 6D). Some SoNOS mRNA-expressing neurons have been detected in the precommissural lobe.
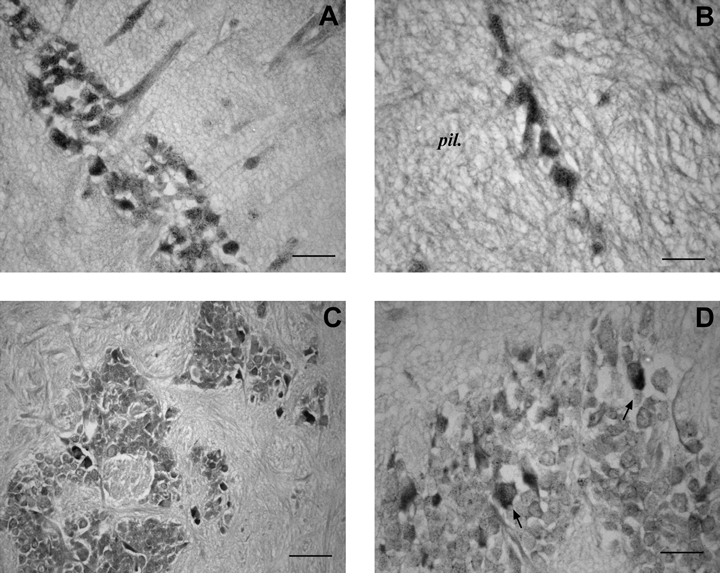
SoNOS mRNA-expressing neurons in the vertical–superior frontal system. (A) Small SoNOS mRNA-expressing cell bodies at the inner edge of the lobe. Note that positivity, although faint, is also present in the first part of axons running to neuropil. (B) Magnification of small positive neurons in the neuropil of the vertical lobe. (C) Positive cell bodies are present in the posterior region of the subvertical lobe as scattered cells. (D) Subvertical lobe. Positive neurons in the anterior region of the lobe (arrows). Scale bars, 35 µm (A, D), 25 µm (B), 50 µm (C).
Optic lobes
The optic lobe comprises an outer region, the ‘deep retina’ of Cajal (1917), and a central medulla. The deep retina is composed of layers of outer and inner granule cells separated by a plexiform zone that itself comprises four layers of tangential fibres and four layers of radial fibres (Young, 1974). The deep retina is a visual analysing system, and the medulla is part motor centre, part memory store (Boycott, 1961; Young, 1974).
The deep retina. No staining is seen in the outer granule cell layer and only few SoNOS mRNA-expressing neurons are present in the inner granule cell layer (Fig. 7A).
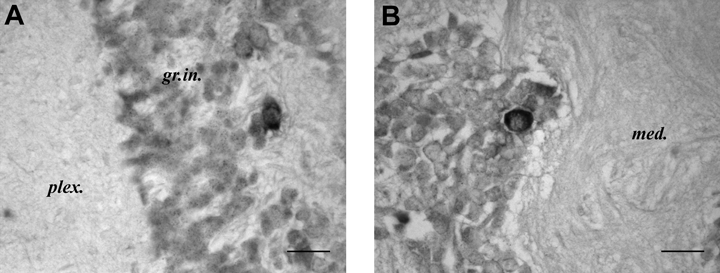
SoNOS mRNA-expressing neurons in the optic lobe. (A) Few SoNOS mRNA-expressing neurons are present in the inner granule cell layer. (B) Strong positive neuron in the medulla. Scale bars, 25 µm (A, B).
The medulla. In this region, very few cells stain positively with NOS mRNA probe; however, those that stain do so strongly (Fig. 7B).
Olfactory system
In all lobules of the olfactory lobe there are a few weakly staining cells (Fig. 8).
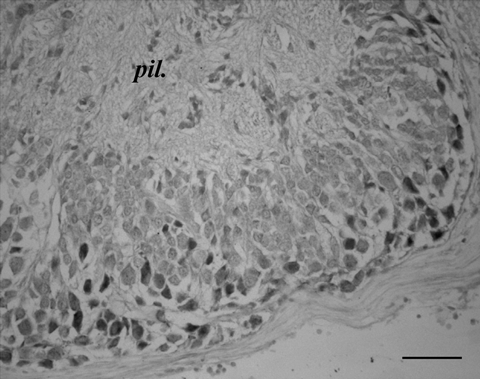
SoNOS mRNA-expressing neurons in olfactory system. Cell bodies stain positively in the cortical layer of the lobe. Scale bar, 60 µm.
Buccal system
This comprises the buccal lobes and the inferior frontal lobe. The buccal lobes control movements of the jaws and radula (Messenger & Young, 1999). The inferior frontal lobes influence the buccal lobes but also serve to allow fibres from the arms and mouth to influence one another (Boycott, 1961).
In this system SoNOS mRNA-expressing cells are present only in the superior buccal and inferior frontal lobes. In the superior buccal lobe, a few NOS-positive cell bodies can be seen located in the outer and inner region of the cell layer (Fig. 9A). The inferior frontal lobe shows several NOS mRNA-containing neurons. These are located mostly in the posterior wall of the lobe adjacent to the neuropil (Fig. 9B). The inferior buccal lobe does not contain NOS-expressing cell bodies.
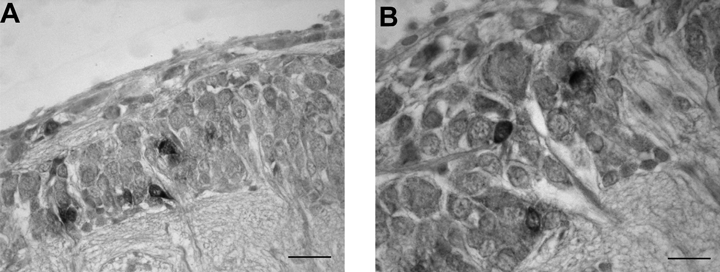
SoNOS mRNA-expressing neurons in the buccal system. (A) SoNOS mRNA-expressing cells in the superior buccal lobe. These cell bodies are located in the outer and inner region of the cell layer. (B) NOS mRNA-containing neurons located in the posterior wall of the lobe. Scale bar, 30 µm (A), 25 µm (B).
Double labelling
We tested the hypothesis of possible colocalization of SoNOS mRNA and the translated NOS, using the probe for SoNOS and NOS antibody on alternate 5-µm sections. Apart from the immunostaining of fibres, clearly absent in in situ staining, this analysis revealed a broad overlap between the immunostaining and in situ hybridization staining in almost all the lobes investigated. Indeed, some neurons apparently showed both immuno and in situ staining, as a result of neurons straddling two consecutive 5-μm sections.
In the suboesophageal mass an almost complete overalapping was evident in brachial lobes (Fig. 10A and B) as well as in pedal and palliovisceral lobes. Occasionally, some discrepancies were observed in the palliovisceral lobe, where some neurons were immunopositive, in situ negative.
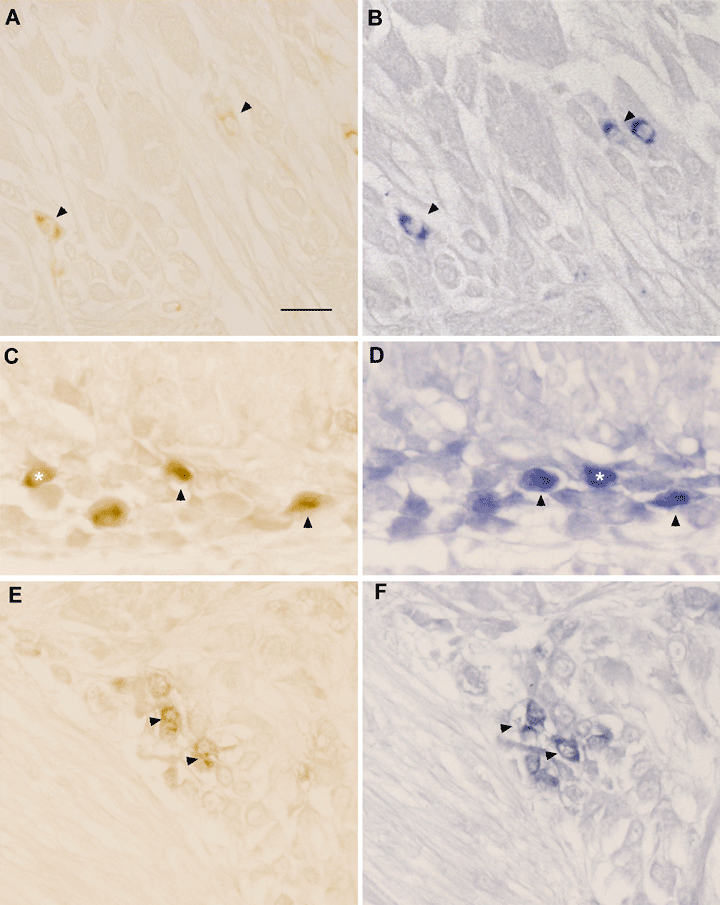
Double NOS immunostaining and in situ staining on consecutive sections (left and right panels, respectively). (A and B) Stained neurons on the dorsal part of anterior suboesophageal lobe. Arrowheads indicate copresence of immuno- and in situ staining. Note the presence of neurons which show either immuno- or in situ staining. (C and D) Immuno- and in situ staining of neurons in the median basal lobe. Colocalization is present in several neurons (arrowheads). Again copresence is not evident in some neurons (asterisks), probably due to the consecutive section strategy. (E and F) Copresence of immunostaining and in situ staining in a cluster of neurons in the subvertical lobe. Note the almost complete overlap of staining (arrowheads). Scale bar, 30 µm.
In the supraoesophageal mass, again, the distribution of immuno- and in situ positive neurons was almost identical. Complete colocalization was observed in the basal lobes (Fig. 10C and D) as well as in the lobes of the vertical lobe system (Fig. 10E and F). Exceptions were the interbasal and peduncle lobes. Indeed, immunoreactivity was virtually absent in the interbasal lobe, compared with in situ hybridization, whereas, in spite of immunoreactivity, no or only faint in situ staining was observed in the peduncle lobe.
In the optic lobes, neurons of the medulla showing strong immunopositivity were also in situ-positive.
In olfactory lobes, there was a complete overlap, although the in situ staining was fainter than immunostaining. Finally, the same neurons immunopositive to NOS in the buccal system also showed in situ positivity.
Discussion
NOS detection methodologies
In the past decade three different techniques, namely diaphorase staining (Chichery & Chichery, 1994), immunohistochemistry (Di Cosmo et al., 2000) and in situ hybridization (Scheinker et al., 2005), have been employed to determine NOS distribution in Sepia brain. Chichery & Chichery (1994), by using diaphorase, were the first to show that this enzyme is selectively localized in the spine region of both the peduncle and the posterior anterior basal lobes. Immunohistochemical data (Di Cosmo et al., 2000) confirmed the occurrence of NOS in both regions but suggested the presence of the enzyme in many other regions of the brain previously reported as negative for NADPH-diaphorase. Recently, the molecular cloning of Sepia NOS gave us the opportunity to set up a preliminary in situ hybridization staining (Scheinker et al., 2005), which has been considerably integrated and expanded in the present study.
In spite of the gross homogeneity of the results obtained by the different approaches, each of these techniques, diaphorase, antibodies and mRNA probes, has provided peculiar contributions to the overall picture of NOS distribution in the nervous system of Sepia. The first obvious, technical difference is that diaphorase and immunohistochemistry are employed to localize the protein, and in the case of the diaphorase, the functioning protein. Conversely, in situ hybridization detects mRNA.
Although discrepancies were observed utilizing these methodologies, several lines of evidence make us confident that, presumably, differences depend on the levels of transcript/translated enzyme rather then methodological artefacts.
First, it appears that only diaphorase staining is susceptible to technical pitfalls, the staining intensity depending, for example, on the time of fixation and the poor ability of this technique to detect NOS uniquely when utilized in invertebrates (Leake et al., 1995; Moroz & Gillette, 1996). Nonetheless, diaphorase staining has been generally confirmed using antibodies.
Second, the possibility of nonspecific staining by using mammalian NOS antibody in immunohistochemical study (Di Cosmo et al., 2000) seems unlikely. The nucleotide sequence of Sepia nNOS (Scheinker et al., 2005) has revealed that the peptide that was used as epitope to raise antibodies, corresponding to the C-terminus region of a mammalian nNOS (1414–1429 of rat neuronal NOS), shares 62% sequence identity and almost 80% similarity with the region 1076–1091 of SoNOS.
Third, the results of immunoreactivity and in situ hybridization colocalization on alternate sections show, with a few exceptions, that the protein recognized by the antibody is localized in the same neurons containing Sepia nNOS mRNA.
We thus suggest that the different staining observed in some lobes comparing immunostaining and in situ techniques probably reflect different functional states of the neurons in specific lobes. In other words, it would reflect a slow or fast turnover of either the transcript or the protein. Obviously, this might represent a way to regulate the action of this enzyme, stabilizing the transcript or inducing its fast translation. In the case of immunonegative, in situ-positive brain regions, an alternative possibility could be the presence of a regulatory action of endogenous antisense mRNA derived from NOS preudogenes, as first documented in another mollusc (Korneev et al., 1999; Korneev & O'Shea, 2002). Finally, in the case of immunopositive, in situ-negative brain regions, although there is close overlap of data using antibodies (Di Cosmo et al., 2000) and mRNA probes, we do not exclude the antibody used in the 2000 study might cross-react with some unknown protein, as shown by Western blotting (see Di Cosmo et al., 2000), and occasionally give rise to staining in some cells that are negative for NOS mRNA, as observed in the present paper.
Discrepancies are quite evident in the interbasal and peduncle lobes. If the interbasal lobe shows strong labelling using in situ hybridization and no staining when antibodies are used, the situation is completely reversed in the peduncle lobe, in which, by contrast, faint staining was previously reported. Why this occurs in these lobes and the turnover rates of mRNA and protein remain open to debate.
NOS in Sepia brain
In situ hybridization, like immunohistochemistry (Di Cosmo et al., 2000), suggests that NOS is unevenly distributed throughout Sepia brain, indicating that in the CNS of cephalopods NOS is essential for some but not all systems. Combined lines of evidence indicate that NOS is present in the vertical lobe system, in the optic lobe, in the basal lobes, including the peduncle lobe, in the olfactory lobes, in the buccal system, and is widespread in the suboesophageal mass.
Perhaps the most interesting finding of studies on NOS in Sepia brain is that the pathway of cell bodies and fibres from the subvertical lobe to vertical lobe is one of the most intensely labelled as ‘nitrergic way’, because of the presence of NOS mRNA and the translated enzyme. We have found that SoNOS mRNA is present in many lobes of this system and, previously, hundreds of NOS-positive neurons forming the roof of the subvertical lobe were shown to send their information to the vertical lobe, as revealed by the huge number of positive fibres in the vertical lobe neuropil (Di Cosmo et al., 2000). These fibres originated almost exclusively from those cells, given the low positivity in other neurons afferent to the vertical lobe.
This suggests that this enzyme and its product are implicated in the learning capabilities of cephalopods (Di Cosmo, 2003). In fact, the vertical lobe system is part of an accessory system involved in establishing and accessing visual memories (Boycott, 1961; Young, 1979). In this context the papers of Robertson et al. (1994, 1996) must assume particular significance: they showed, in Octopus, that visual as well as tactile learning could be abolished by intramuscular injection of the NOS inhibitor L-NAME, although they admitted that they had no evidence regarding the site of action of L-NAME. Nevertheless, the indication that known learning centres in Sepia are utilizing NO is very interesting, because NO is thought to act in an area of the mammalian brain important for memory: the hippocampus (Haley et al., 1992; Zhuo et al., 1993; Bannerman et al., 1994; Malenka, 1994). NOS is also known to be involved in learning in the honeybee (Muller, 1996) and it has been shown to be present in the mushroom bodies of the locust (O'Shea et al., 1998). Moreover this enzyme has been shown unequivocally to be involved in the learning in other molluscs, Lymnaea (Korneev et al., 2005) and Aplysia (Katzoff et al., 2002). Unfortunately, there are no data regarding the role of NO in the LTP generation which has been recently shown to be present at glutamatergic synapses in the vertical lobe of Octopus vulgaris (Hochner et al., 2003).
In the optic lobe there are many cell bodies staining positive for NOS mRNA, although is not yet clear whether the positive fibres in both the inner (deep retina) and the outer (retina) plexiform layers (Di Cosmo et al., 2000) come from these neurons. Other fibres to these layers could come from supraoesophageal mass, although the neuropil of the medulla does not show strong NOS positivity (Di Cosmo et al., 2000).
The basal lobes represent another set of lobes clearly containing NOS. It is interesting that Sepia NOS (both protein and mRNA) occurs in particular in the anterior basal lobe: in this lobe there are positive cells of varying sizes, but more importantly there are positively stained fibres in the neuropil, especially in the posterior anterior basal lobe (Di Cosmo et al., 2000).
This lobe is strongly involved in the control of movements: motor programmes, usually originating in the optic lobes, are executed via higher motor centres, where specific motor commands are generated and directed to the appropriate sets of motoneurons in the lower centres (Boycott, 1961; Messenger, 1983). Chichery & Chichery (1994) proposed a role for NO in the motor control in Sepia given that diaphorase staining was present in the spines of the anterior basal and peduncle lobes, two regions of the brain that are thought to constitute cerebellar analogues (Messenger, 1967a, b; Hobbs & Young, 1973; Woodhams & Messenger, 1974; Young, 1977, 1979; Budelmann & Young, 1984; Camm et al., 1985; Chichery & Chichery, 1987; Gleadall, 1990). Our present results, as well previous immunohystochemical study (Di Cosmo et al., 2000), corroborate this view.
The buccal system/inferior frontal system and the dorso-lateral/olfactory lobes constitute other systems characterized by the presence of NOS, both in numerous neurons (Di Cosmo et al., 2000; this paper) and in some positive fibres.
Conclusions and perspectives
Over the past few years, knowledge of the NOS from Sepia brain has advanced dramatically following continuing efforts from our laboratories, to the point that SoNOS stands today as one of the best characterized cephalopod NOSs. An important outcome of these studies is the recognition of Sepia as a convenient invertebrate model for investigating the glutamate/NO/cGMP signalling pathway in the nervous system. The glutamate/NO/cyclic GMP signalling pathway has been demonstrated in the brain of Sepia as well as in the ink gland, where NOS, as protein and now also as mRNA, and NMDA glutamate receptors immunoreactivity have been detected (Palumbo et al., 1997, 1999; Di Cosmo et al., 2004). Interesting perspectives in this field are also likely to derive from the elucidation of the role of NO in the post-translational modification of cytoskeletal proteins, such as α-tubulin, in Sepia CNS. We found that NOS stimulation with NMDA induces α-tubulin nitration (Palumbo et al., 2002) and its reversible degradation. This latter mechanism could be associated with the mechanism of cytoskeletal protein turnover and could be involved in microtubule assembly, which is critical for axonal transport, growth, degeneration and synaptic plasticity (Di Cosmo, 2003).
Acknowledgements
We thank the Services of the Zoological Station ‘Technology for the Study of Gene Expression’ for in situ hybridization and ‘Marine Resources for Research’ for assistance with living organisms.
Text abbreviations
-
- CNS
-
- central nervous system
-
- NO
-
- nitric oxide
-
- NOS
-
- nitric oxide synthase
-
- PBS
-
- phosphate-buffered saline.
Abbreviations used in the figures
-
- b.a.
-
- anterior basal lobe
-
- b.a.p.sp.
-
- spine of posterior anterior basal lobe
-
- b.dor.
-
- dorsal basal lobe
-
- b.l.
-
- lateral basal lobe
-
- b.med.
-
- median basal lobe
-
- br.
-
- brachial lobe
-
- buc.s.
-
- superior buccal lobe
-
- ch.p.
-
- posterior chromatophore lobe
-
- es.
-
- oesophagus
-
- fl.
-
- fin lobe
-
- fr.i.
-
- inferior frontal lobe
-
- fr.s.
-
- superior frontal lobe
-
- gr.in.
-
- inner granule cell layer
-
- med.
-
- medulla
-
- n.br.1,2,3,4
-
- brachial nerves
-
- n.opt.
-
- optic nerves
-
- n.pal.
-
- pallial nerve
-
- n.ten.
-
- tentacle nerve
-
- n.visc.
-
- visceral nerve
-
- opt.
-
- optic lobe
-
- pe.
-
- pedal lobe
-
- ped.
-
- peduncle lobe
-
- pil.
-
- neuropil
-
- plex.
-
- plexiform zone
-
- pv.cen.
-
- central palliovisceral lobe
-
- subv.
-
- subvertical lobe
-
- tr.opt.
-
- optic tract
-
- v.
-
- vertical lobe.