Unbiased cell quantification reveals a continued increase in the number of neocortical neurones during early post-natal development in mice
Abstract
The post-natal growth spurt of the mammalian neocortex has been attributed to maturation of dendritic arborizations, growth and myelination of axons, and addition of glia. It is unclear whether this growth may also involve recruitment of additional neurones. Using stereological methods, we analysed the number of neurones and glia in the neocortex during post-natal development in two separate strains of mice. Cell counting by the optical fractionator revealed that the number of neurones increased 80–100% from the time of birth to post-natal day (P)16, followed by a reduction by approximately 25% in the young adult mouse at P50–55. Unexpectedly, at the time of birth less than half of the neurones and at P8 only 65% of the neurones expressed neuronal nuclear antigen (NeuN), a marker of mature post-migratory neurones. In accordance with these observations, NeuN acquisition by neurones in layer VIa was delayed until P16. The number of glia reached its maximum at P16, whereas the number of oligodendroglia, identified using a transgenic marker, increased until P55, the latest time of observation. Neurones continued to accumulate in the developing neocortex during the first 2 weeks of post-natal development, underscoring fundamental differences in brain development in the mouse compared with human and non-human primates. Further, delayed acquisition of NeuN by neurones in the deepest neocortical layers and continued addition of oligodendroglia to the neocortex suggested that neocortical maturation should be regarded as an ongoing process continuing into the young adult mouse.
Introduction
Corticogenesis is a complex process involving cellular division, cellular migration and differentiation as well as apoptosis (Angevine & Sidman, 1961; Bayer & Altman, 1991; Caviness & Sidman, 1973; Takahashi et al., 1999). Both in the human and in rodents, the post-natal development of the brain is characterized by a massive increase in weight and size, termed the post-natal growth spurt (Dobbing & Sands, 1979; Duffell et al., 2000; Samuelsen et al., 2003; Molnar et al., 2006). This has been ascribed to branching and pruning of the dendritic trees (Petit et al., 1988; Larsen & Callaway, 2006), growth of intracortical axonal connections (Del Rio et al., 2000; Miller et al., 2001; Portera-Cailliau et al., 2005; Price et al., 2006) and addition of glia and myelin (Fish & Winick, 1969; Baumann & Pham-Dinh, 2001; Dalmau et al., 2003; Goldman, 2003).
In the mouse the neurones destined for the neocortex are formed through cycles of asymmetric cell divisions of the pseudostratified epithelium in the ventricular zone prior to embryonic day (E)17 (Takahashi et al., 1999; Levers et al., 2001; Caviness et al., 1995, 2003), whereas migration of post-mitotic neurones into the cortical plate is reported to continue until post-natal day (P)3 (Takahashi et al., 1999; Noctor et al., 2001, 2004). However, other studies (Nadarajah et al., 2002; Wichterle et al., 2002; Noctor et al., 2004), including a recent study by Adle-Biassette et al. (2007), have revealed several origins and patterns of migration of post-mitotic neurones that, in combination with the post-natal growth spurt, raise the question of whether neuronal migration might extend further into post-natal development than P3. Using the optical fractionator estimator of cell numbers (West et al., 1991), the number of neurones in the murine neocortex has been established to be 6.4 million at P10 (Bonthius et al., 2004), 10.2 million at P12 (Hodge et al., 2005), 10.6 million at 8 months (Bondolfi et al., 2002) and 12.6 million at 14–16 months (Calhoun et al., 1998). The advantage of this stereological method is that it is insensitive to volume changes of the neocortex, cell sizes and cell density (West, 1993; Dorph-Petersen et al., 2001). Although limited to a single age per study, the observations indicate that the number of neocortical neurones may increase during early post-natal development.
To resolve whether neuronal migration into the murine neocortex continues into post-natal development, the number of neurones was estimated at different developmental stages using the optical fractionator. As the first results were obtained in a transgenic (tg) line, to allow for simultaneous estimation of the number of oligodendroglia visualized using a tg marker (Forghani et al., 2001), they were confirmed in C57BL/6J mice. In addition, neuronal migration and differentiation were analysed by estimating the number of neurones expressing neuronal nuclear antigen (NeuN), a marker of mature post-migratory neurones (Mullen et al., 1992; Sarnat et al., 1998), and by analysing neuronal expression of β-tubulin III (Geisert & Frankfurter, 1989; Menezes & Luskin, 1994) and microtubule-associated protein-2 (MAP-2) (Bernhardt & Matus, 1984; Honig et al., 1996).
Materials and methods
Animals and tissue processing
The study was performed in C57BL/6J and myelin basic protein (MBP)-LacZ tg mice (Forghani et al., 2001) that express β-galactosidase (β-gal) in oligodendrocytes (Drojdahl et al., 2004; Nielsen et al., 2006). Mice of the following ages were used: P0, P8, P16, P24, P50 and P55. The LacZ gene was under the control of a 9 kb upstream region of the MBP promoter terminating at the SacII site (Forghani et al., 2001). MBP-LacZ tg mice were provided by Dr Alan C. Peterson (McGill University, Montreal, Canada) and bred on a mixed genetic background derived from B6C3F2 mice (Forghani et al., 2001). The C57BL/6J mice were bred from mice purchased from Taconic M and B (Ry, Denmark). Mice were housed at the Laboratory of Biomedicine, University of Southern Denmark under a constant light/dark cycle and provided with mouse chow and water ad libitum. Mice were deeply anaesthetized with intraperitoneal administration of pentobarbital (P0-P8:20 mg, P16:40 mg, P24–P55:60 mg. Stock 200 mg/mL, 7278631, Den kgl. Veterinær-og Landbohøjskoles Apotek, Copenhagen, Denmark) and perfused transcardially with 5 mL of 0.15 m Sørensen's phosphate buffer (SPB) (3.71 g KH2PO4, 21.84 g Na2HPO4.2H2O in 1000 mL H2O, pH 7.4) followed by 10–20 mL of 4% paraformaldehyde (PFA) (Sigma-Aldrich Denmark A/S, Brøndby, Denmark) in 0.15 m SPB, pH 7.4. The volume of perfusate depended on the age of the animal. Procedures were approved by the Danish Ethical Animal Health Care Committee (permission no. 192000/561-272).
Brains from MBP-LacZ tg mice were post-fixed for 2 h at 4 °C in 4% PFA, immersed in 20% sucrose (VWR International, Albertslund, Denmark) in 0.15 m SPB for 24 h at 4 °C, embedded in Cryo-M-Bed (AX-Laboratory A/A, VWR International) and frozen with CO2 snow. Brains were sectioned into parallel series of 35 µm coronal sections that were mounted on Superfrost glass slides coated with a solution containing 0.5% gelatine A (Sigma-Aldrich, Denmark) and 0.05% KCr(SO4)2.12H2O (Merck, Darmstadt, Germany). Sections were stored at −20 °C until further processing. Brains from C57BL/6J mice were post-fixed for 24 h at 4 °C in 4% PFA, embedded in 5% agar (1.01614.1000, Merck) and fixed for an additional 24 h in 4% PFA. Brains were serially cut into 80 µm vibratomic sections (VT 1000 S, Leica Instruments GmbH, Nussloch, Germany) in the sagittal plane, as this was favourable for stability during sectioning. Free-floating sections for toluidine blue (TB) staining were mounted from Tris buffer (0.05 m Trizma base, pH 7.4) onto gelatine-coated glass slides and stained within 30 min after mounting, whereas those for immunohistochemistry were stored in a cryoprotective solution (West et al., 1996) at −14 °C until staining. Finally, brains from two MBP-LacZ tg mice at P8, P16 and P55, and from two C57BL/6J mice at P8 were processed into 7 µm thick cryostat sections and stored at −20 °C until used for double staining experiments.
Histochemistry
Toluidine blue and β-galactosidase staining
Sections were immersed for 10 min in a solution containing 0.01% TB O (Merck), 0.08 m Na2HPO4.2H2O (Merck) and 0.07 m citric acid (Merck), pH 4. Sections were thereafter rinsed in three changes of distilled water for 10, 20 and 30 s (cryosections only), dehydrated in ethanol, cleared in xylene and coverslipped using Depex (Gurr, VWR International). For β-gal staining, sections were dried for 30 min at room temperature (RT; 21°C) and incubated for 1 h at 37 °C in a solution containing 102 mg ferricyanide (C6FeK3N6, 4973, Merck), 130 mg ferrocyanide (C6FeK4N6.3H2O, 4984, Merck), 0.2 mmol MgCl2 (1.05833.0250, Merck) and 40 mg 5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside (98% pure, B4252, Sigma-Aldrich) diluted in 100 mL 0.1 m SPB, pH 7.4. Thereafter, sections were rinsed in 0.1 m SPB, pH 7.4, at RT, post-fixed in 4% PFA in 0.15 m SPB, pH 7.4, for 2 h at RT, rinsed in distilled water and coverslipped using Aquatex (Merck).
Immunohistochemistry
Antibodies
Biotinylated monoclonal mouse anti-NeuN antibody (MAB377B; working dilution 1 : 300) (Mullen et al., 1992; Wolf et al., 1996; Sarnat et al., 1998), monoclonal mouse anti-β-tubulin III antibody (MAB1637; 1 : 1000) (Geisert & Frankfurter, 1989; Menezes & Luskin, 1994), polyclonal rabbit anti-MAP-2 antibody (AB5622; 1 : 1000) (Bernhardt & Matus, 1984; Honig et al., 1996) and polyclonal rabbit anti-NG2 chondroitin sulphate proteoglycan antibody (AB5320; 1 : 1000) (Ong & Levine, 1999; Chang et al., 2000) were all from Chemicon International (purchased from AH Diagnostics, Aarhus, Denmark). Polyclonal rabbit anti-MBP antibody (A0623; 1 : 500) (Johnson et al., 1988; Hardy et al., 1996) was from Dako Cytomation (Dako Nordic a/s, Glostrup, Denmark) and biotinylated mouse anti-oligodendroglia (Rip) antibody (clone Rip, Developmental Studies Hybridoma Bank, University of Iowa, USA; 1 : 200) (Friedman et al., 1989; Watanabe et al., 2006) was kindly donated by Dr Trevor Owens (Montreal Neurological Institute, McGill University, Montreal, Canada).
The control antibodies consisted of biotinylated inert mouse IgG (ab18425-200) (Abcam plc, Cambridge, UK), inert mouse IgG1 (X0931), normal rabbit serum (X0902) and inert rabbit IgG fraction (X0903, 20 mg/mL) (Dako Cytomation).
Neuronal nuclear antigen, β-tubulin III and microtubule-associated protein-2
Free-floating 80 µm vibratomic sections were rinsed in Tris-buffered saline (TBS) (0.05 m Tris, 0.15 m NaCl, pH 7.4) at 4 °C overnight. Endogenous peroxidase activity was blocked by 15 min incubation in a solution of 10% methanol and 10% H2O2 in TBS. Sections were then rinsed for 2 × 10 min in TBS containing 1% Triton X-100 (TBS + T) (X-100, Sigma-Aldrich) and pre-incubated for 45 min in TBS containing 10% fetal calf serum (TBS + FCS) (Gibco BRL, Glasgow, Scotland). Sections were incubated with primary antibodies in TBS + FCS for 2 days at 4 °C, followed by rinsing for 3 × 15 min in TBS + T and incubation overnight at 4 °C with the secondary reagent diluted 1 : 200 in TBS + FCS. For the biotinylated antibody against NeuN, the secondary reagent was horseradish peroxidase-conjugated streptavidin (P0397, Dako Cytomation), which was developed in TBS containing 0.05% 3,3′-diaminobenzidine (Sigma) and 0.01% H2O2. Development was arrested by rinsing in Tris buffer (0.05 m Tris, pH 7.4). The antibodies against β-tubulin III and MAP-2 were visualized using biotinylated anti-mouse IgG (X0931; Dako Cytomation) or biotinylated goat anti-rabbit antibody (RPN 480; Amersham Biosciences, UK), respectively, followed by rinsing for 3 × 15 min in TBS + T, incubation for 2 h with horseradish peroxidase-conjugated streptavidin diluted 1 : 200 in TBS + FCS and development with 3,3′-diaminobenzidine. Sections were then mounted on gelatine-coated glass slides, dried and dehydrated in a graded series of alcohol, cleared in xylene and coverslipped with Depex.
Staining for NeuN and β-tubulin III in 7 µm cryostat sections from C57BL/6J mice at P8 was performed according to the same protocol but with overnight incubation with the primary and secondary antibodies. A few sections from P8 brains were counterstained with TB.
Control stainings consisted of substitution of the primary antibody with the same concentration of inert antibody or substitution with inert mouse or rabbit IgG and/or rabbit serum. Controls were all devoid of specific staining.
Oligodendroglial cell lineage and myelin markers
The 35 µm cryostat sections were rinsed in TBS for 15 min followed by 3 × 15 min in TBS + T, pre-incubation in TBS + FCS and incubation with primary antibody diluted in TBS + FCS overnight at 4 °C. The next day, sections were rinsed for 3 × 15 min in TBS + T. Sections labelled with biotinylated anti-Rip antibody were incubated with horseradish peroxidase-conjugated streptavidin diluted 1 : 200 in TBS + FCS, rinsed and developed with 3,3′-diaminobenzidine. Sections labelled with rabbit polyclonal antibodies were incubated with biotinylated goat anti-rabbit IgG diluted 1 : 200 in TBS + FCS for 1 h at RT, rinsed for 3 × 15 min in TBS + T and incubated for 1 h at RT with horseradish peroxidase-conjugated streptavidin diluted 1 : 200 in TBS + FCS. Sections stained for Rip were incubated in 5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside staining solution at 37 °C for 25 min, fixed in 4% PFA in 0.15 m SPB for 2 h prior to rinsing and coverslipped using Aquatex.
The thin 7 µm cryostat sections from brains of P8, P16 and P55 MBP-LacZ tg mice were stained for MBP or Rip in combination with β-gal or as immunohistochemical double stainings. Staining with the first primary antibody was performed as outlined above, after which some sections were counterstained in Mayers acidic haematoxylin solution (Apoteket, Odense University Hospital, Odense, Denmark), whereas other sections were double stained with β-gal. For immunohistochemical double staining, sections were boiled in a microwave oven for 5 min in a 10 mm Tris buffer containing 0.5 mm EGTA, pH 9.0 (Apoteket, Odense University Hospital), to coagulate and remove cross-linked antibodies. The second immunohistochemical staining was then performed as described above using alkaline phosphatase-conjugated streptavidin diluted 1 : 200 in TBS + FCS for visualization of biotinylated primary or secondary antibodies. After rinsing for 3 × 5 min in TBS + 0.5% T, sections were rinsed for 3 × 5 min in 0.05 m TB, pH 8.2, and developed with the substrate Vector Alkaline Phosphatase Substrate Kit 1 (Vector Red; SK-5100, Vector Laboratories Inc., purchased from VWR International) according to the recommendations of the manufacturer. Development was arrested by rinsing first in Tris buffer, pH 8.2, and then in distilled water, and coverslips were mounted using Aquatex.
Stereological estimation of cell numbers
Equipment
Counting of the MBP-LacZ tg and C57BL/6J mice was performed in separate experiments but, due to the nature of the results obtained by stereology, the data are easily compared (Schmitz & Hof, 2005). In the MBP-LacZ tg mice, cell counting was performed at a final magnification of × 3193 using a × 100 oil lens with a numerical aperture of 1.35 and immersion oil, a BH-2 microscope (Olympus, Germany) fitted with a microcator (Heidenhain MT12), a Sony DXC-151P analogue colour video camera and a computerized specimen stage (Multicontrol 2000, Märzhäuser Wetzlar GmbH, Steindorf, Germany). This set-up was connected to a PC and handled using CAST-GRID software (Olympus). In the C57BL/6 mice cell counting was performed using a BX50 microscope (Olympus) fitted with a Colorview II digital camera (Olympus), a Proscan Prior motorized specimen stage and a microcator connected to a PC with the CAST-2 software (Visiopharm, Denmark). In this case cell counting was performed at a final magnification of × 3067 using a 100 × oil-immersion lens with a numerical aperture of 1.30 (Olympus).
Delineation
The neocortex was delineated on the basis of macroscopic and microscopic anatomical landmarks. Based on the terminology of Franklin & Paxinos (1997), the following regions were included: anterior part of the agranular insular cortex, orbital cortex, cingulate cortex, frontal cortex, pre-limbic cortex, granular insular cortex, retrosplenial agranular cortex, anterior parts of retrosplenial granular cortex, ectorhinal cortex, parietal cortex, temporal cortex and all visual areas of the occipital cortex (Paxinos & Watson, 2004). For delineation, different clues were used in different experiments as the brains of the MBP-LacZ tg mice were processed into coronal sections and the brains from the C57BL/6 mice were cut into sagittal sections. In the coronal sections from the MBP-LacZ tg mice (Fig. 1) the pial surface formed a natural border, as did the border between the neocortical layer VIb and the white matter of the corpus callosum and external capsule. Laterally, the rhinal fissure and the cytoarchitectonic differences between neocortex and piriform cortex (anteriorly) or entorhinal cortex (posteriorly) were used to draw the border from the pial surface to the external capsule. Finally, the medial border was drawn based on: (i) the corpus callosum and subcortical white matter, and (ii) the cytoarchitectonic differences between proisocortical regions and isocortical regions. The latter could be distinguished by drawing a line from the dorsal hippocampal commissure to the pial surface. In the sagittal sections from the C57BL/6 mice, the surface of the brain formed the dorsal border, and the corpus callosum and external capsule formed the ventral border. Rostrally and caudally, neocortex merged into other cortical areas (Paxinos & Watson, 2004). At the rostral end, the rhinal fissure and the histoarchitectonic difference between the six-layered neocortex and the three-layered piriform cortex marked the boundary of the palaeocortex. The retrosplenial agranular cortex provided a border to the neocortex caudally.
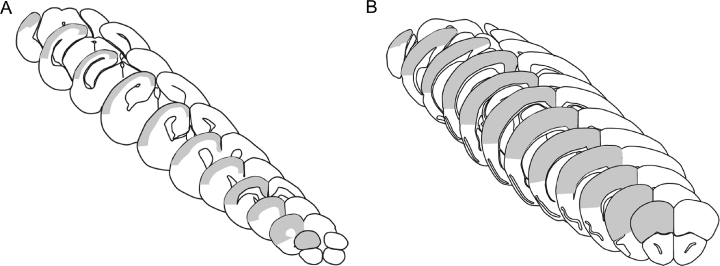
Schematic illustration of the delineation of the neocortex marked in grey in coronal sections from newborn (A) and young adult (B) mouse.
Counting criteria
In all analyses the nucleus was the counting item determined as the largest focal plane on the nucleus relative to the disector. In sections stained by TB, cells were classified as neurones or glia based on morphological characteristics (Korbo et al., 1990; Bondolfi et al., 2002; Bonthius et al., 2004; Davanlou & Smith, 2004). Neurones had an ovoid to round, pale nucleus containing a single nucleolus. The cytoplasm contained Nissl substance and showed a polygonal shape (Fig. 2A and B). Glial cells had smaller, round to elongated nuclei without clearly defined nucleoli and no or very small amounts of cytoplasm were visible (2, 3) (Ling et al., 1973; Davanlou & Smith, 2004). The nuclei of endothelial cells and perivascular cells were not counted. In the NeuN stain the neurones were distinguished by a densely stained nucleus and a more lightly stained cytoplasm (Fig. 2C and D) (Lind et al., 2005). The NeuN-immunopositive (NeuN+) neurones were evenly dispersed throughout the depth of the entire section, as shown by performing an analysis of the z-axis distribution in section no. 8 of all animals (data not shown). The β-gal-immunopositive (β-gal+) cells were recognized by the clear blue colouration of their cell bodies and counted when a thin but distinct blue rim around the oval cell body was observed in the focal plane (Fig. 2E and F). Two different cell types were counted: lightly stained small cells most frequently observed in young animals (Fig. 2E) and darkly stained, large cells becoming more numerous with age (Fig. 2F).
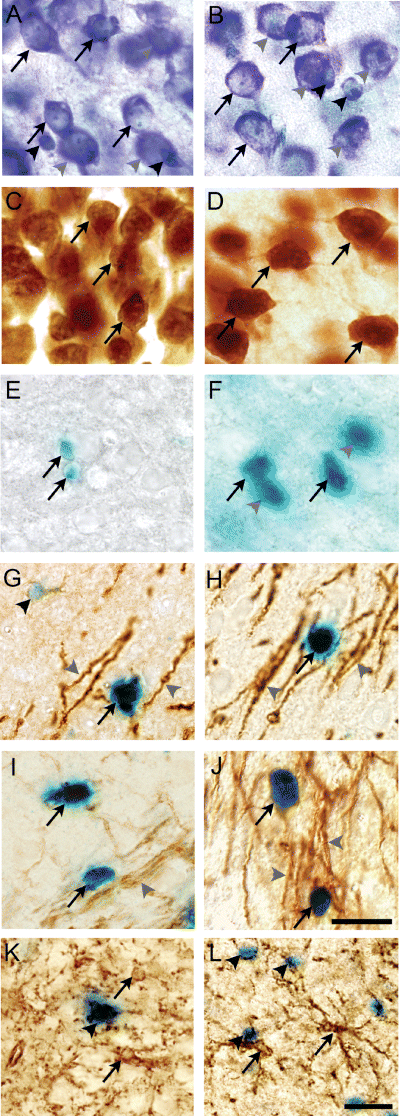
Classification of neurones and glia. Morphology of toluidine blue-stained neurones (arrows) and glia (black arrowheads) in layer V of C57BL/6J mice at post-natal day (P)8 (A) and P50 (B). Photomicrographs were obtained from vibratome sections cut at 80 µm. Neurones were identified by their large euchromatic nucleus containing a nucleolus and the Nissl substance in the cytoplasm. Neuronal cell profiles out of the optimal focus plane are indicated by grey arrowheads. (C and D) Neuronal nuclear antigen (NeuN)-stained neurones in layer V of P8 and P50 C57BL/6J mice. The nucleus inside the NeuN-stained neurones was identified as it stained more densely. β-galactosidase-positive (β-gal+) cells situated in the callosal radiation in myelin basic protein (MBP)-LacZ transgenic mice at P8 (E) and P55 (F). The β-gal+ cells occurred as lightly stained β-gal+ cells (arrows in E) and intensely labelled β-gal+ cells (arrows in F). β-gal+ cell profiles outside the focal plane are indicated by grey arrowheads (F). (G–L) Double stainings showing that the myelin markers MBP (G and H) and Rip (I and J), in addition to the myelinated fibres (grey arrowheads), are associated with the β-gal+ cells (arrows). Note the brown rim of immunoreactivity surrounding the β-gal+ cells. This is in contrast to the NG2+ cells (arrows) that are distinctly different from the β-gal+ cells (arrowheads) (K and L). Photomicrographs in E–L were obtained in 35 µm cryostat sections. Bars: 10 µm (A–J) and 20 µm (K and L).
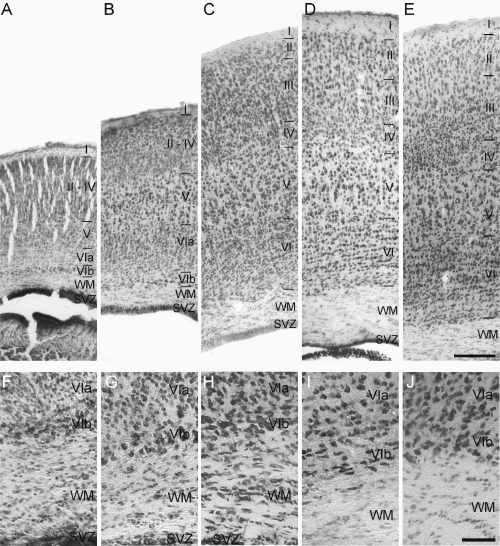
Post-natal maturation of the telencephalic wall of the mouse brain. The upper row shows all neocortical layers (A–E) and the bottom row shows layer VI and the subcortical white matter (WM) (F–J). Neocortical layers I–VIb are indicated by roman numbers. The photomicrographs were obtained from toluidine blue-stained 35 µm thick cryostat sections obtained from post-natal day (P)0 (A and F), P8 (B and G), P16 (C and H), P24 (D and I) and P55 (E and J) myelin basic protein-LacZ transgenic mice. (A–E) The maturation and decondensation of the neocortical layers start at P0, when only layers I, V, VIa and VIb can be identified, through P8 (B), when lamination becomes more developed, to P16 (C), P24 (D) and P55 (E), when the neocortex has developed into a six-layered structure and has reached its final height. (F–J) The developing corpus callosum and WM are more cell rich at early than late developmental stages. SVZ, subventricular zone. Bars: 300 µm (A–E) and 50 µm (F–J).
Estimation of cell numbers and neocortical volume
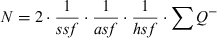
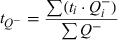
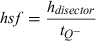
Details of the stereological designs used for estimation of the different types of neurones and glia are given in Table 1A for the analysis of the MBP-LacZ tg mice and in Table 1B for the analysis of C7BL/6J mice. The total neocortical volume was estimated by point counting using the principle of Cavalieri (Gundersen et al., 1988). Neuronal density was calculated as the ratio of the total number of TB-stained neurones to the volume. Data are presented in dot diagrams with a horizontal line representing the mean.
| MBP-LacZ transgenic mice | |||||
|---|---|---|---|---|---|
| P0 | P8 | P16 | P24 | P55 | |
| No. of animals | 6 | 5 | 6 | 6 | 6 |
| TB-stained sections | |||||
| Section sampling fraction | 1/12 | 1/12 | 1/18 | 1/18 | 1/24 |
| No. of sections sampled | 9–11 | 12–14 | 10–12 | 11–13 | 9–10 |
| Disector area (µm2) | 250.5 | 221.0 | 318.5 | 318.5 | 250.5 |
| Area per step (µm2) | 250 000 | 250 000 | 490 000 | 490 000 | 360 000 |
| Final section thickness (µm) | 21.8 ± 1.7 | 21.5 ± 1.7 | 21.2 ± 1.9 | 20.9 ± 2.0 | 20.9 ± 1.6 |
| No. of disectors | 51 ± 4.5 | 128 ± 13 | 81 ± 5.5 | 98 ± 3.5 | 91 ± 11 |
| ΣQ –Total | 231 ± 22 | 273 ± 36 | 343 ± 65 | 291 ± 39 | 199 ± 33 |
| ΣQ –Neurones | – | 187 ± 21 | 182 ± 33 | 147 ± 18 | 103 ± 14 |
| ΣQ –Glia | – | 76 ± 9 | 161 ± 33 | 144 ± 25 | 96 ± 21 |
| β-gal-stained sections | |||||
| Section sampling fraction | 1/12 | 1/12 | 1/12 | 1/12 | 1/12 |
| No. of sections sampled | 9–11 | 13–14 | 15–17 | 15–18 | 18–20 |
| Area per step (µm2) | 62 500 | 62 500 | 160 000 | 160 000 | 160 000 |
| Disector area (µm2) | 626.3 | 551.0 | 626.3 | 638.2 | 626.3 |
| Final section thickness (µm) | 22.0 ± 2.4 | 22.6 ± 1.7 | 21.9 ± 2.9 | 22.4 ± 2.8 | 23.3 ± 4.3 |
| No. of disectors | 213 ± 14 | 493 ± 21 | 395 ± 23 | 492 ± 51 | 426 ± 36 |
| ΣQ –β-gal | 47 ± 19 | 174 ± 38 | 92 ± 13 | 117 ± 17 | 137 ± 29 |
- The number of disectors analysed, number of cells counted (ΣQ–) and final section thickness varied between animals in the groups, and are therefore given as the mean ± SD. See text for details on calculation of the estimated cell numbers. β-gal, β-galactosidase; MBP, myelin basic protein; NeuN, neuronal nuclear antigen; P, post-natal day; TB, toluidine blue.
| C57BL/6J mice | ||||
|---|---|---|---|---|
| P0 | P8 | P16 | P50 | |
| N (animals) | 6 | 6 | 6 | 6 |
| Section sampling fraction | 1/3 | 1/5 | 1/5 | 1/5 |
| No. of sections sampled | 11–12 | 9–10 | 11–12 | 11–12 |
| Disector area (µm2) | 193 | 193 | 193 | 193 |
| Area per step (µm2) | 250 000 | 250 000 | 490 000 | 490 000 |
| TB-stained sections | ||||
| Final section thickness (µm) | 17.7 ± 0.6 | 23.8 ± 2.8 | 24.0 ± 2.1 | 23.7 ± 1.6 |
| No. of disectors | 180 ± 9 | 296 ± 18 | 231 ± 14 | 227 ± 19 |
| ΣQ–Neurones | 286 ± 25 | 241 ± 25 | 142 ± 11 | 147 ± 12 |
| NeuN-stained sections | ||||
| Final section thickness (µm) | 17.4 ± 0.5 | 18.7 ± 0.8 | 22.8 ± 3.6 | 27.4 ± 1.1 |
| No. of disectors | 142 ± 14 | 205 ± 11 | 196 ± 34 | 210 ± 13 |
| ΣQ–NeuN+ neurones | 124 ± 19 | 164 ± 22 | 150 ± 13 | 113 ± 9 |
- See the Footnote to Table 1A.
Precision of the estimation procedure
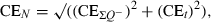
| No. of cells (106) | |||||
|---|---|---|---|---|---|
| P0Mean (CE) CV | P8Mean (CE) CV | P16Mean (CE) CV | P24Mean (CE) CV | P50/P55 Mean (CE) CV | |
| MBP-LacZ tg | |||||
| Total cell | 5.8 (0.07) 0.11 | 7.5 (0.06) 0.10 | 19.7 (0.06) 0.17 | 17.0 (0.06) 0.15 | 14.4 (0.07) 0.17 |
| Neurone | n.d. | 5.1 (0.07) 0.12 | 10.5 (0.08) 0.17 | 8.5 (0.08) 0.12 | 7.4 (0.09) 0.12 |
| Glia | n.d. | 2.2 (0.11) 0.16 | 9.2 (0.08) 0.13 | 8.2 (0.09) 0.16 | 7.0 (0.10) 0.22 |
| Oligodendroglia | 0.13 (0.15) 0.41 | 0.49 (0.08) 0.18 | 0.62 (0.10) 0.20 | 0.81 (0.09) 0.15 | 0.89 (0.08) 0.25 |
| %β-gal+ oligodendroglia | n.d. | 22 | 6.7 | 9.8 | 13 |
| C57BL/6J | |||||
| Neurone | 4.7 (0.06) 0.11 | 7.2 (0.06) 0.05 | 8.6 (0.08) 0.07 | n.d. | 6.8 (0.08) 0.09 |
| NeuN+ neurone | 2.2 (0.09) 0.14 | 4.7 (0.08) 0.10 | 8.5 (0.08) 0.06 | n.d. | 6.7 (0.09) 0.08 |
| % NeuN+ neurones | 45 | 65 | 99 | n.d | 99 |
- Percentages of β-galactosidase-positive (β-gal+) oligodendroglia compared with the total number of glia and neuronal nuclear antigen-immunopositive (NeuN+) neurones compared with the total number of neurones. CE, coefficient of error; CV, coefficient of variation; MBP, myelin basic protein; n.d., not done; P, post-natal day; tg, transgenic.
Evaluation of neuronal nuclear antigen, myelin basic protein and Rip expression
Neuronal expression of NeuN at P8 was evaluated by estimating the percentage of NeuN+ TB-stained neurones in layers II–VIa using a × 100 oil lens. Delineation of individual layers was performed at a lower magnification. Within each layer approx. 100 cells were counted in ∼ 50 fields of view per section selected in the fronto-parietal region to represent each of the two P8 animals analysed. In a similar way the relative numbers of β-gal+ cells coexpressing MBP or Rip were analysed at P8, P16 and P55 in two animals per age, analysing at least four sections per animals and counting at least 100 cells in at least 120 fields of view.
Statistical analysis
The distribution of the stereological data within each age group was analysed for normal distribution using the Kolmogorov-Smirnov test, showing that the data were likely to be normally distributed (P-values > 0.1). Variation between age groups was examined by one-way anova followed by Bonferroni's multiple comparisons test as a follow-up using GraphPad Prism 4 for Mac OS X. Comparison of different sets of data from the same animals was performed by a paired t-test. Differences were considered significant for P < 0.05.
Photodocumentation
All micrographs were obtained with an Olympus DP 70 digital camera mounted on a BX51 Olympus microscope. The photomicrographs were arranged in figures using Adobe Photoshop CS.
Results
Maturation of neocortex during early post-natal development
At P0 the cytoarchitectonical arrangement of the cells visualized by TB made it possible to distinguish the rather cell-sparse layer I, the pyramidal neurones of layer V and the neurones of layers VIa and VIb in both MBP-LacZ tg and C57BL/6J mice (Fig. 3A, shown for MBP-LacZ tg mice only). At P8 the thickness of neocortex had increased (Fig. 3B), although layers II, III and IV had not fully developed until P16 (Fig. 3C). At this age and at P24, the neocortex had additionally increased in thickness and was almost indiscernible from the characteristic six-layered neocortex in the young adult MBP-LacZ tg and C57BL/6J mice (Fig. 3C–E). Similar to the neocortex, the future subcortical white matter underwent major changes during early post-natal development in both strains. From being thin and densely populated with cells at P0 (Fig. 3F), it had developed into two distinct regions, an outer layer containing radially and tangentially orientated chromaphilic cells and an inner layer with tangentially orientated cells of mainly glial morphology at P8 (Fig. 3G). At P16 the thickness of the subcortical white matter had increased (Fig. 3H) and the cells were organized in interfascicular rows resembling adult white matter but still more cell-rich than in P24 and P55 MBP-LacZ tg mice (Fig. 3I and J), and in P50 C57BL/6J mice (data not shown).
The maturation of the neocortex was reflected in a massive growth that was estimated in both strains of mice using the Cavalieri principle for volume estimation. The processing of the brains from the C57BL/6J mice into vibratome sections resulted in better neuronal morphology, and only modest tissue deformation and shrinkage. Thus, the most reliable volumetric data could be obtained in these mice. In the C57BL/6J mice, the volume of neocortex increased significantly from 21.9 ± 1.8 mm3 at P0 to 62.8 ± 6.4 mm3 at P8 (Fig. 4A). Volume expansion continued during the second week of life to reach 89.8 ± 3.8 mm3 at P16 (P < 0.001), whereas the neocortical volume at P16 was similar to the volume at P50 (86.3 ± 6.5 mm3) (Fig. 4A). The neocortical volumes were consistently smaller in the MBP-LacZ tg mice than in the C57BL/6J mice. The volume in MBP-LacZ tg mice was estimated to be 9.9 ± 0.6 mm3 at P0, 26.5 ± 2.8 mm3 at P8 and to reach 47.8 ± 2.1 mm3 at P16. The volume reached 59.3 ± 2.6 mm3 at P24 and was reduced to 50.6 ± 5.8 mm3 at P55 (data not shown). Despite the shrinkage, the age-related changes in neocortical volume were similar overall in MBP-LacZ tg and C57BL/6J mice.
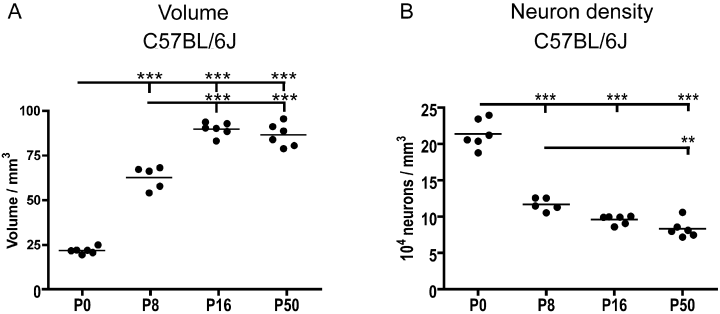
Developmental changes in neocortical volume and neuronal density as a function of post-natal age. Graphs are based on data obtained from C57BL/6J mice. (A) Neocortical volume estimated using the Cavalieri method in toluidine blue (TB)-stained sections. The volume peaked at post-natal day (P)16 and has decreased in the young adult mouse at P50. (B) Developmental changes in neuronal density of the neocortex calculated from the number of TB-stained neurones (shown in Fig. 5B) and the neocortical volume (shown in Fig. 4A). Filled circles show estimated cell number or volume in individual animals. Mean values of the age groups are indicated by horizontal bars. Estimated cell numbers within age groups were tested for normal distribution using the Kolmogorov-Smirnov test. Differences between age groups were analysed using one-way anova combined with Bartlett's test for equal variances and Bonferroni's multiple comparison test. **P < 0.01 and ***P < 0.001.
Post-natal increase in the number of neocortical neurones
Numerical changes of toluidine blue-stained neurones and glia in myelin basic protein-LacZ transgenic mice
Due to the high cell density in the cryostat sections at P0, neuronal and glial morphologies were first clearly distinguishable at P8. The number of neurones at P8 was estimated to be 5.1 × 106 increasing to 10.5 × 106 at P16 (P < 0.001; Fig. 5A, Table 2). Furthermore, it appeared that the number of neurones peaked around P16, as it had declined to 8.5 × 106 at P24 and 7.4 × 106 at P55 (Fig. 5A). The difference between P16 and P55 corresponded to a 30% reduction and was statistically significant (P < 0.01).
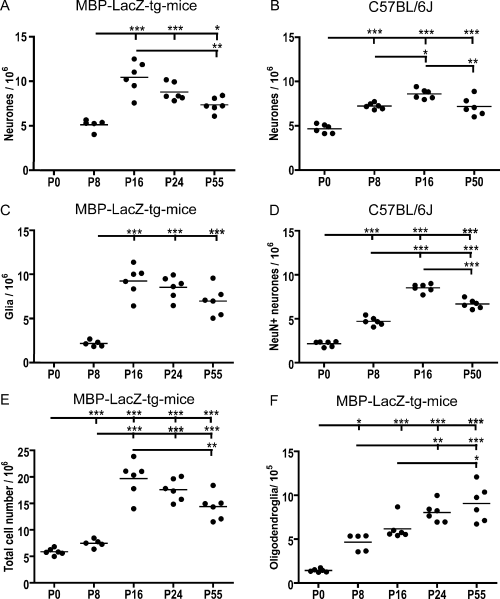
Developmental changes in the number of neocortical neurones, glia and oligodendroglia in the mouse. The estimated numbers are based on data obtained by optical fractionator counting of cells in myelin basic protein (MBP)-LacZ transgenic (tg) (A, C, E and F) and C57BL/6J (B and D) mice. The observation of an increased number of toluidine blue (TB)-stained neurones at post-natal day (P)16 in MBP-LacZ tg mice (A) was reproduced in C57BL/6J mice (B). (C) Estimation of the number of glia shows a marked increase in cell numbers during early development. (D) Estimation of the number of neuronal nuclear antigen-immunopositive neurones in sections parallel to the TB-stained sections subjected to quantification in B. Comparison is suggestive of delayed neuronal acquisition of adult levels of neuronal nuclear antigen expression. Estimation of the number of total cells (neurones + glia) (E) and the number of β-galactosidase-positive oligodendroglia (F) in MBP-LacZ tg mice. Note the continued increase in the number of oligodendroglia. Filled circles show estimated cell number in individual animals. Mean values of the age groups are indicated by horizontal bars. Estimated cell numbers within age groups were tested for normal distribution using the Kolmogorov-Smirnov test. Differences between age groups were analysed using one-way anova combined with Bartlett's test for equal variances and Bonferroni's multiple comparison test. Statistically significant differences between the groups are indicated by *P < 0.05, **P < 0.01 and ***P < 0.001.
In accordance with previous reports (Levers et al., 2001; Peretto et al., 2005), we also observed a significant increase in the number of glia post-natally. The total number of glial cells at P8 was 2.2 × 106 (Fig. 5C) and during the second week of life the glial cell number increased to 9.2 × 106 at P16 (P < 0.001; Fig. 5C). Thereafter the number of glia decreased to 8.2 × 106 at P24 and 7.0 × 106 at P55 (Fig. 5C) but this decline did not reach statistical significance. As predicted from the estimates of the numbers of neurones and glia, the total cell population reached a peak of 19.7 × 106 cells at P16 (Fig. 5E). The total number of cells at P0 was estimated to 5.8 × 106 (Fig. 5E, Table 2).
Numerical changes of toluidine blue-stained neurones in C57BL/6J mice
Neuronal development in the neocortex of the widely used C57BL/6J mouse was analysed to validate the observation of a post-natal increase in the number of neurones in the MBP-LacZ tg mouse. Again we used the optical fractionator method but brains were sampled in the sagittal plane by vibratome sectioning, resulting in a sampling scheme different from that used in the MBP-LacZ tg mice. In the C57BL/6J mice the number of TB-stained neurones was estimated to be 4.7 × 106 at P0 increasing to 7.2 × 106 at P8 (P < 0.001) and 8.6 × 106 at P16 (P0–P16, P < 0.001; P8–P16, P < 0.05) (Fig. 5B, Table 2). As observed for the MBP-LacZ tg mice the number of neurones decreased from P16 to P50 (P < 0.01; Fig. 5B). However, we still observed the number of neurones at P50 (6.8 × 106) to be significantly higher than at P0 (P < 0.001; Fig. 5B, Table 2). Based on the individual estimates of neuronal numbers and neocortical volume, we also calculated the density of neocortical neurones at the different developmental ages. Neuronal density decreased 44% during the first post-natal week (P < 0.001; Fig. 4B) and continued to decrease from P8 through P16 to P50 (Fig. 4B).
Delayed neuronal acquisition of neuronal nuclear antigen during neocortical development
Discrepancy in the number of neuronal nuclear antigen- immunoreactive and toluidine blue-stained neurones
Having shown that the number of neocortical neurones also underwent a major increase during early post-natal development in the C57BL/6J mouse, we next analysed the number of NeuN+ neurones in the same mice (Fig. 5D). The rationale for this was that NeuN, with the exception of Cajal-Retzius cells, should be expressed in the nucleus and cell soma of most post-migratory mature neocortical neurones (Mullen et al., 1992; Sarnat et al., 1998), making it a marker for neurones of a certain stage of differentiation useful for cell counting. Unexpectedly, at the time of birth the number of NeuN+ neurones was only 2.2 × 106, corresponding to 45% of the number of TB-stained neurones, increasing during the first week to 4.7 × 106 neurones at P8, corresponding to 65% of the number of TB-stained neurones (Fig. 5D, Table 2). In comparison, the number of NeuN+ and TB-stained neurones was similar at P16 and P50 (Fig. 5B and D, Table 2). Similar to the results obtained in the TB-stained sections, the number of NeuN+ neurones underwent a significant reduction from 8.5 × 106 at P16 to 6.7 × 106 at P50 (P < 0.001; Fig. 5D, Table 2).
Acquisition of neuronal nuclear antigen immunoreactivity is delayed in layer VIa neurones
In line with the quantitative data, the cytoarchitectonic arrangement of the NeuN+ neurones was markedly different from that of the TB-stained neurones at P0 and P8 (compare Fig. 6A, B, E and F with Fig. 3A, B, F and G). Whereas the neurones located in layers II–V and VIb were NeuN+, as were a few neurones in layer I, the majority of neurones in layer VIa were NeuN-immunonegative or expressed NeuN at a very low level at P0 and P8 (Fig. 6A, B, E and F). To additionally document this observation, the proportion of NeuN+ neurones in layers II–V and VIa at P8 was determined in the thin 7 µm sections stained for NeuN and counterstained with TB (data not shown). In layers II–V 96% of the neurones were NeuN+, whereas in layer VIa the proportion of NeuN+ neurones was only 68%. At P16 virtually all neurones counterstained with TB, including the neurones in layer VIa, were NeuN+ (compare Fig. 6C and G with Fig. 3C and H). Similarly, at P50 the arrangement of NeuN+ neurones was identical to the neuronal cytoarchitecture visualized in the TB-stained sections (compare Fig. 6D and H with Fig. 3E and J). The neuronal phenotype of NeuN-immunonegative neurones in layer VIa was additionally verified by staining parallel sections for the neurone-specific antigens β-tubulin III (Fig. 6I–L) and MAP-2 (Fig. 6M–P). The observation of a strong, confluent staining of all neocortical layers, including layer VIa, at all ages (Fig. 6I–P) emphasized the neuronal phenotype of the NeuN-immunonegative neurones in layer VIa at P0 and P8 (Fig. 6A, B, E and F).
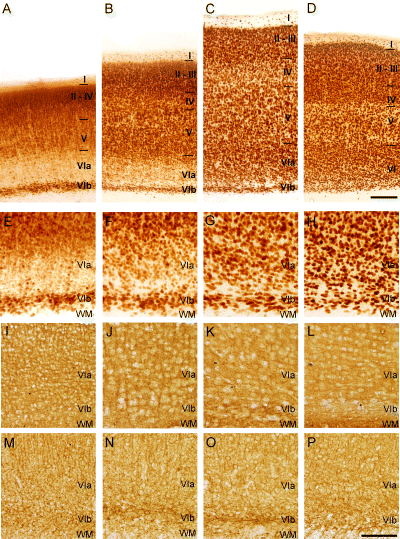
Delayed neuronal acquisition of NeuN by neurons in neocortical layer VIa during postnatal development of murine neocortex. Photomicrographs of vibratome sections stained with NeuN (A–H), β-tubulin III (I–P) or MAP-2 (M–P) from P0 (A, E, I and M), P8 (B, F, J and N), P16 (C, G, K and O) and P50 (D, H, L and P) C57BL/6J mice. The top row (A–D) provides an overview of neocortex at different ages, while the rows below (E–P) show higher magnifications of layer VI and the subcortical white matter. (A–H) NeuN staining reveal an uneven distribution of NeuN immunopositive neurons at P0 and P8 with superficial layers II–V and layer VIb begin intensely stained, whereas layer VIa contains few faintly stained neurons (A, B, E and F). From P16 all neocortical layers contain NeuN immunopositive neurons and lamination appears to be complete (C, D, G and H). (I–P) In contrast stainings for β-tubulin-III (I–L) and MAP-2 (M–P) show high levels of β-tubulin-III and MAP-2 expression in neuronal cytoplasm and processes, but not nuclei, through all neocortical layers at all ages, including P0 and P8. WM, subcortical white matter. Bars: 200 μm (A–D), 100 μm (E–P).
Post-natal increase in the number of oligodendroglia correlates with myelination
Analysis of β-gal-stained and MBP-immunostained parallel sections from the MBP-LacZ tg mice confirmed previous observations of overlapping expression patterns at all developmental ages (Fig. 7A and C, shown for P8 and P55). The staining for β-gal was combined with immunohistochemical staining for the oligodendroglial markers MBP and Rip, and the precursor cell marker NG2 in 7 µm cryostat sections from P8, P16 and P55 brains to validate that the β-gal+ cells were oligodendroglia expressing MBP and not oligodendroglial precursor cells. Double-labelled cells showed round blue β-gal+ cell bodies that were typically surrounded by a scant rim of MBP or Rip immunoreactivity (Fig. 2G–J) and by relatively straight MBP-immunopositive (MBP+) (2, 7) or Rip+ (2, 7) fibres. Cell counting showed that > 90% of stained cells coexpressed MBP and β-gal at the investigated ages, whereas 75% of stained cells at P8 coexpressed MBP and β-gal. More than 91% of stained cells at P16 and P55 coexpressed Rip and β-gal. The pale β-gal+ cells (Fig. 2E and G) were the major single-labelled cell type. Finally, using the fluorescent properties of the Vector Red precipitate for analysis of double-stained sections, we confirmed that MBP and Rip were coexpressed at all ages (data not shown). As expected (Nishiyama et al., 1996, 1997; Nielsen et al., 2006), there was no evidence of NG2 expression by β-gal+ cells (Fig. 2K and L).
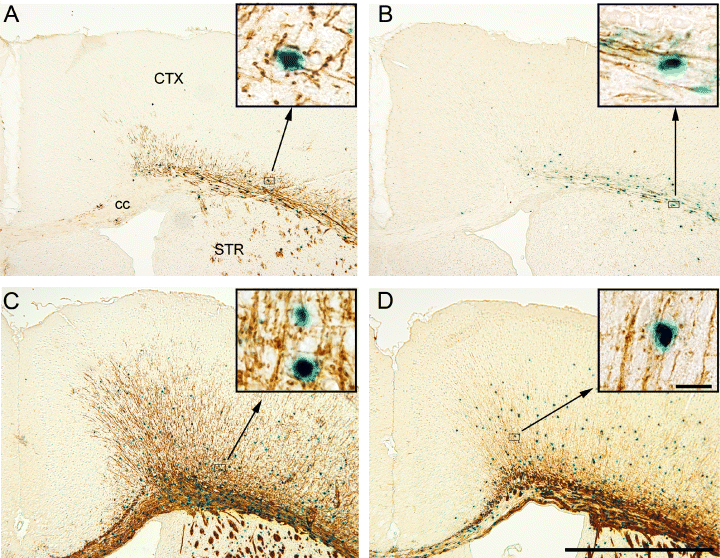
Myelination of the dorsal neocortex (CTX) occurs according to a lateral-to-medial gradient. Combined stainings for β-galactosidase (blue) and myelin basic protein (MBP) (A and C) or Rip (B and D) from post-natal day (P)8 (A and B) or P16 (C and D) performed on 10 µm cryostat sections from MBP-LacZ transgenic mice. (A and B) At P8 the callosal commissure is almost devoid of MBP-immunopositive (MBP+) β-gal-immunopositive (β-gal+) and Rip+β-gal+ cells or fibres, whereas the external capsule is intensely stained. (C and D) At P16 the callosal commissure is packed with MBP+β-gal+ cells and MBP+ fibres. Inserts show high magnifications of double-labelled cells. The Rip+/β-gal+ staining indicates a lower Rip labelling of fibres but a similar frequency of labelled cells. cc, corpus callosum; STR, striatum. Bars: 1000 µm, 20 µm (inserts).
As the double stainings confirmed that the vast majority of β-gal+ cells were MBP+ or Rip+ oligodendroglia, and not NG2+ precursor cells, we next estimated the total number of β-gal+ oligodendroglia during post-natal development. The analysis showed a large increase in the number of β-gal+ oligodendroglia during the first post-natal week, from 1.3 × 105 at P0 to 4.9 × 105 at P8 (P < 0.01; Fig. 5E, Table 2). A continuing increase in the number of β-gal+ cells was observed from P8, through 6.2 × 105 at P16 and 8.1 × 105 at P24, reaching 8.9 × 105 cells at P55. The differences from P8 to P24, P8 to P55 and P16 to P55 were statistically significant (Fig. 5F, Table 2).
Discussion
The results showed that the populations of neocortical neurones and glia underwent a significant increase during early post-natal development. This result was obtained using stereological methods for the unbiased quantification of cells in two separate strains of mice. Neurones and glia continued to accumulate in the neocortex during the first 2 weeks of post-natal life, followed by a minor reduction of both cell populations in the young adult mouse. In contrast, the number of oligodendroglia continued to increase from P0 into the young adult mouse. NeuN, a marker of mature post-migratory neurones, was expressed by virtually all neocortical neurones at P16 and P50 but in only a fraction of neurones at P0 and P8, reflecting a delayed acquisition of NeuN immunoreactivity by the neurones in neocortical layer VIa.
During development the neocortex is subject to massive growth (Dobbing & Sands, 1979; Duffell et al., 2000; Samuelsen et al., 2003), along with changes in the molecular composition (Martínez, 1982; Geschwind & Hockfield, 1989). The optical fractionator was applied to overcome bias from these sources, as this stereological method is regarded as insensitive to the changes in brain volume or differential shrinkage of the tissue sections at different developmental stages (West et al., 1991, 1996; Dorph-Petersen et al., 2001; Schmitz & Hof, 2005). We estimated the number of TB-stained neurones identified by morphological criteria, as described earlier (Korbo et al., 1990; Bondolfi et al., 2002; Bonthius et al., 2004; Davanlou & Smith, 2004). In cryostat sections glial cells could be distinguished from P8, in accordance with the time schedule for gliogenesis and glial migration (Levers et al., 2001; Peretto et al., 2005). On the basis of current literature, the majority of the cells identified at P0 in the MBP-LacZ tg mice were probably neurones. With the better morphology in the vibratome sections, we counted neurones in the C57BL/6J mice at P0, reaching a result that was not statistically different from the total cell number in the MBP-LacZ tg mice (Table 2). Taken together, this suggests that the majority of neocortical cells at P0 in the C57BL/6J mouse are neurones and that the neuronal population increases 80–100% from the time of birth to P16 in the mouse.
For each developmental stage, sampling was designed to ensure that the CE of the estimate of the total number of TB-stained neurones in individual mice, and thus the mean CE of the estimated neuronal numbers in the different groups of mice, did not exceed 0.10 (range 0.06–0.09) (Table 2). The CV is a measure of the total variation within a group, i.e. the biological variation and the variation related to the sampling and given by the mean CE (West et al., 1996; Gundersen et al., 1999). The CV was higher for MBP-LacZ tg (range 0.12–0.17) than for C57BL/6J (range 0.07–0.09) mice (Table 1) but within the range for CVs obtained by other groups using stereology to estimate neuronal numbers in mice (Bonthius et al., 2004; Hodge et al., 2005) and other species (Pakkenberg & Gundersen, 1997; Samuelsen et al., 2003; Jelsing et al., 2006a; Abitz et al., 2007). The consistency of the estimates of the number of TB-stained neurones in the MBP-LacZ tg and C57BL/6J mice with the number of neurones reported in other stereologic studies [6.4 × 106 neurones at P10 (Bonthius et al., 2004), 10.2 × 106 at P12 (Hodge et al., 2005) and 10.8 × 106 in the adult (Bondolfi et al., 2002)] supports the credibility of our counting criteria, taking into consideration that the entorhinal cortex was included in the analysis in the study by Bondolfi et al. (2002). Furthermore, we were encouraged by observing that the mean CE of the estimates of the total number of NeuN+ neurones did not exceed 0.10 (range 0.08–0.10), whereas the CVs ranged from 0.06 to 0.13 (Table 2).
The considerably larger CV (range 0.09–0.19) associated with the estimates of the numbers of oligodendroglia indicated a variation in the numbers of cells expressing β-gal, especially in the youngest animals. This might be due to the mixed genetic background of the MBP-LacZ tg mice resulting in different onset of expression of the transgene. However, the β-gal+ cells consistently coexpressed MBP, indicating that the difference in timing might also apply to the endogenous MBP gene, reflecting a true biological variation between mice of mixed genetic background. Interestingly, in human neocortex the oligodendroglia should account for 75% of the total number of glia, based on morphological classification of the cells (Pelvig et al., 2003). Here, only 14% of neocortical glia were β-gal+ oligodendroglia. Possibly our criteria for identification were more stringent, identifying myelinating oligodendroglia only, or the difference might be attributed to differences between mouse and man.
For practical reasons sampling was performed in the right hemisphere in both strains of mice. This might introduce a bias to the bilateral estimates, due to biological differences between the left and right hemisphere. We observed differences in the shrinkage in the z-axis of cryostat and vibratome sections. The frozen sections shrank to ∼70% of the original thickness for all ages. This probably reflected the fact that the frozen tissue was dehydrated by the cryoprotective treatment making the tissue shrink before cryostat sectioning, unlike the vibratome sections that shrank after mounting onto the glass slides. Thus, in the P0 brains the thickness of vibratome sections shrank from an original 80 µm to a final thickness of 17 µm, which meant that the height of the disector had to be reduced to 8 µm. Although this thickness was at the lower limit for the use of the optical fractionator, we regarded this to be the best result achievable in developing brains, as shrinkage has been reported to occur even in fully developed brain tissue (West et al., 1996; Dorph-Petersen et al., 2001; Gardella et al., 2003; Wirenfeldt et al., 2003). Despite this shrinkage, we observed no difficulty in distinguishing the position of cells when scanning through the sections using a high-magnification oil-immersion lens with high numerical aperture. The local differences in final section thickness were taken into account by calculation of the Q–-weighted height sampling fraction (Dorph-Petersen et al., 2001; Jelsing et al., 2006b; Larsen et al., 2006; Witgen et al., 2006).
The neurones accumulating in the neocortex after birth can in principle originate from cellular proliferation or immigration, or a combination of the two. As the neurogenesis of neocortical neurones is classically described as a pre-natal event in rodents (Angevine & Sidman, 1961; Bayer & Altman, 1991; Caviness et al., 1995, 2003; Takahashi et al., 1999), the doubling in neuronal numbers during early post-natal development might be explained by late arrival of migrating neurones. Two sources of neocortical neurones have been identified in the mouse (Parnavelas et al., 1991; Tan et al., 1998; Anderson et al., 2002). The pyramidal projection neurones derive from the proliferative zones in the telencephalic wall (Noctor et al., 2004; Molyneaux et al., 2005) and neocortical interneurones derive from the proliferative zones in the medial ganglionic eminence (Anderson et al., 1997; Wichterle et al., 2002). For both classes of neurones it has been reported that neurones generated at E11 settle in the deeper neocortical layers, whereas neurones generated at E17 settle in the superficial layers of the neocortex (Angevine & Sidman, 1961; Caviness & Sidman, 1973; Anderson et al., 1997; Takahashi et al., 1999; Noctor et al., 2004; Adle-Biassette et al., 2007). The study by Takahashi et al. (1999) pointed out one exception to this general pattern of neuronal formation and migration in that the neurones generated at E11 and settling in layer VI only constituted a fraction of newly formed cells. This observation raised the interesting possibility that the early generated neurones destined for layer VI might sojourn in the ventricular or subventricular zone and migrate into the neocortex at a later developmental stage. Indeed, recent studies have described complex patterns of migration (Nadarajah et al., 2002), with pausing of radially migrating neurones in the subventricular zone of the E16 mouse for up to 24–114 h and a speed of tangential migration of 6.4 ± 0.7 µm/h (Noctor et al., 2004). Considering the thickness of the telencephalic wall at P0, these observations are compatible with the view that neuronal migration continues beyond P3, as previously assumed (Bayer & Altman, 1991; Del Rio et al., 2000). Additional evidence that late arrival could be the explanation for the increased neuronal number at P8 and P16 was recently provided by a study by Adle-Biassette et al. (2007), showing that the migration of neurones destined for layer IV can be inhibited between P1 and P3. Finally, we observed that the subcortical white matter at P0 and P8 was rich in cells not showing the interfascicular organization of glia in the white matter at later ages. These cells might represent migrating cells, possibly being both neurones and glia.
Recent stereological studies in the human brain reported similar numbers of neocortical neurones at birth and in the adult (Samuelsen et al., 2003; Larsen et al., 2006). A similar study comparing the number of neocortical neurones in newborn and adult pigs reported no differences in Danish Landrace pigs, whereas the number of neurones increased by 28% during post-natal development of the Göttingen minipig (Jelsing et al., 2006a). On this basis, Jelsing et al. (2006a) concluded that the Göttingen minipig should be used only cautiously as a model of human brain development. We think that the same caution should be taken when using the mouse as a model in developmental studies. However, as none of these studies performed detailed investigations of post-natal development, it is difficult to draw direct comparisons to our study. It still remains to be elucidated whether the observed peak in neuronal cell number also occurs in other mammals, including humans. Meanwhile, it should be emphasized that the occurrence of a post-natal increase in neuronal numbers in the mouse is compatible with the general view that mouse pups are born at an earlier developmental stage than humans (Rice & Barone, 2000; Back et al., 2001).
The number of NeuN+ neurones and the number of TB-stained neurones correlated well in the adult brain as was previously reported for the human cortex (Gittins & Harrison, 2004). This was in contrast to the early post-natal period where the number of neurones identified in the TB-stained sections far exceeded the number of NeuN+ neurones. The discrepancy between the two staining patterns was most obvious in layer VIa, in which the majority of neurones did not express NeuN at P0 and P8 but acquired mature levels of NeuN expression at P16. The difference in neuronal NeuN expression could possibly reflect different levels of phosphorylation of NeuN rather than neuronal expression of NeuN protein, as recognition of the epitope by the anti-NeuN antibody depends on NeuN phosphorylation (Lind et al., 2005). The neuronal phenotype of the cells in layer VIa was assured by staining for MAP-2 and β-tubulin III. In agreement with previous reports on a cytoskeletal localization of these proteins (Bernhardt & Matus, 1984; Draberova et al., 1998), staining also resulted in a confluent staining for MAP-2 and β-tubulin III of neuronal bodies and processes through all cortical layers in the P0 and P8 brain. Of additional interest, in P7 rats the dense band of neurones in the deep part of the future layer VI has been identified as Martinotti cells (Kristt, 1979), an interneurone known to secrete the regulatory protein reelin (Pesold et al., 1998). This band of neurones was later recognized as the primordial neuronal plexus known as the subplate (Del Rio et al., 2000). In this study the neurones in layer VIb expressed NeuN, indicating that Martinotti cells, in contrast to Cajal-Retzius cells, do express NeuN or that the majority of neurones in layer VIb were post-migratory mature neurones.
The post-natal growth spurt of the brain has previously only been described quantitatively in terms of an increase in weight (Fish & Winick, 1969; Dobbing & Sands, 1979; Duffell et al., 2000). In this study, a four-fold increase in neocortical volume was observed during post-natal development, increasing constantly from birth to P16 in C57BL/6J mice and until P24 in MBP-LacZ tg mice, accompanied by a concomitant reduction in neuronal density. The volume expansion has traditionally been ascribed to the maturation of dendritic arborizations (Miller, 1981; Petit et al., 1988; Larsen & Callaway, 2006), the growth and myelination of efferent and afferent axonal connections (Suzuki & Raisman, 1994; Del Rio et al., 2000; Price et al., 2006), and the addition of glia (Levers et al., 2001; Peretto et al., 2005). Additional maturation of the neuronal network has been described to take place during the third week of post-natal development (Miller et al., 2001; Portera-Cailliau et al., 2005; Larsen & Callaway, 2006; Price et al., 2006). Our study adds the observation of large increments in the number of glial cells from P0 to P16, with a continuous increment in the number of oligodendroglia and staining for myelin. In the mouse the myelination of the cortex starts at the time of birth with a peak in myelin production from P15 to P30 (Sikes et al., 1981), continuing for 45–60 days post-natally (Baumann & Pham-Dinh, 2001; Kessaris et al., 2006). Thus, our observation of a continuous increase in the number of oligodendroglia from P0 to P55 is in accordance with the literature. From P16 through P24 to P55 we observed a significant reduction in the numbers of neurones and glia other than oligodendroglia. Selective apoptosis has been described during neocortical development as a means of eliminating redundant cells (Haydar et al., 1999; Ibanez, 2000; Kuan et al., 2000; Gohlke et al., 2004). It is likely that this process remains active during the maturation and consolidation of the neuronal network.
In the mouse the oligodendroglial progenitor cells destined for the neocortex have been reported to be generated from different parts of the telencephalic ventricular zone through three sequential waves of cells that compete and successively extinct each other. The first wave starts at E12.5 from the medial ganglionic eminence and enteropedunduncular area. The second wave starts at E15.5 from the medial ganglionic eminence and the third wave starts around the time of birth from NG2- and platelet-derived growth factor receptor α-expressing precursor cells situated within the cortex, and proceeds until P10 (Kessaris et al., 2006). This corresponds well with our observation of lightly stained β-gal+ oligodendroglia being distributed throughout the neocortex at P0, with a few β-gal+ cells coexpressing MBP and Rip at P8. The observation of the delayed expression of endogenous MBP, compared with the very early expression of the reporter gene in the MBP-LacZ tg mouse, is in agreement with reports of a stepwise myelination process (Foran & Peterson, 1992; Back et al., 2001; Craig et al., 2003; Jakovcevski & Zecevic, 2005). It is of note that at P0 the first MBP+ cells were observed in the developing white matter at the corner of the lateral ventricle, which was 2 days earlier than previously reported (Ivanova et al., 2003; Craig et al., 2003). Further, the β-gal+, Rip+ and MBP+ fibres and cells created a gradient from the lateral parts of the corpus callosum towards the callosal commissure at P8, suggesting that myelination of the subcortical white matter and the corpus callosum started from the corner of the lateral ventricle at the place where the projection fibres enter to traverse the striatum. The results show resemblance to observations of a similar developmental distribution of cells expressing myelin-associated glycoprotein (Nakahara et al., 2003) and observations by Hardy & Friedrich (1996) pointing to oligodendroglial precursor cells and radial glia in establishing the initial contact with sprouting axons.
In conclusion, the observation of an increment in the number of neocortical neurones during the first two post-natal weeks and a delayed acquisition of neuronal NeuN expression in layer VIa raise questions about the origin of excess neurones and the apparently protracted maturation of the neocortex. Answering these questions will require additional studies on neuronal birth dating, migration and differentiation in the neocortex. Overall, our results suggest that neocortical maturation may take a more protracted course in the mouse than previously anticipated. This should be taken into consideration when using the mouse as an experimental model for human neurological diseases affecting the cerebral cortex.
Acknowledgements
We acknowledge the excellent technical assistance of Lene Jørgensen and Susanne Petersen. The study received financial support from The Augustinus Foundation, The Beckett-Foundation, The Carlsberg Foundation, The Danish Multiple Sclerosis Society, Else Poulsens Mindelegat, Fonden til Lægevidenskabens Fremme, The Gangsted Family Foundation, The Hede Nielsen Family Foundation, The Lundbeck Foundation, The Velux Foundation of 1981 and The Danish Medical Research Council.
Abbreviations
-
- β-gal
-
- β-galactosidase
-
- β-gal+
-
- β-galactosidase-positive
-
- CE
-
- coefficient of error
-
- CV
-
- coefficient of variation
-
- E
-
- embryonic day
-
- MAP-2
-
- microtubule-associated protein-2
-
- MBP
-
- myelin basic protein
-
- MBP+
-
- myelin basic protein-immunopositive
-
- NeuN
-
- neuronal nuclear antigen
-
- NeuN+
-
- neuronal nuclear antigen-immunopositive
-
- P
-
- post-natal day
-
- PFA
-
- paraformaldehyde
-
- RT
-
- room temperature
-
- SPB
-
- Sørensen's phosphate buffer
-
- TB
-
- toluidine blue
-
- TBS
-
- Tris-buffered saline
-
- TBS + FCS
-
- Tris-buffered saline containing 10% fetal calf serum
-
- TBS + T
-
- Tris-buffered saline containing 1% Triton X-100
-
- tg
-
- transgenic.