Photoperiod affects estrogen receptor α, estrogen receptor β and aggressive behavior
Abstract
Estrogens have important effects on male and female social behavior. Despite growing knowledge of the anatomy and behavioral effects of the two predominant estrogen receptor subtypes in mammals (ERα and ERβ), relatively little is known about how these receptors respond to salient environmental stimuli. Many seasonally breeding species respond to changing photoperiods that predict seasonal changes in resource availability. We characterized the effects of photoperiod on aggressive behavior in two species of Peromyscus that exhibit gonadal regression in short days. P. polionotus (old field mice) were more aggressive than P. maniculatus (deer mice) and both species were more aggressive in short days. We used immunocytochemistry and real-time polymerase chain reaction to characterize the effects of photoperiod on ERα and ERβ expression. In both species ERα-immunoreactive staining in the posterior bed nucleus of the stria terminalis (BNST) was increased in short vs. long days. Both species had reduced ERβ-immunoreactive expression in the posterior BNST in short days. In the medial amygdala ERβ immunoreactivity was increased in long days for both species. Using real-time polymerase chain reaction on punch samples that included the BNST, we observed that ERα mRNA was increased and ERβ mRNA was decreased in short days. These data suggest that the effects of photoperiod on ERα and ERβ expression may thus have important behavioral consequences.
Introduction
The effects of androgens (such as testosterone), on male behavior can occur via conversion to estrogens (such as estradiol), by aromatase within the brain. The discovery of multiple estrogen receptor (ER) subtypes resulted in many studies investigating the behavioral effects of ERα and ERβ. Generally, ERα is hypothesized to play a more important role than ERβ in regulating reproductive behaviors such as mating and parental behaviors (Ogawa et al., 1997; Champagne et al., 2006). Recent studies indicate that ERβ has a significant role in non-reproductive behaviors (Bodo & Rissman, 2006). In Mus musculus, selective deletion of ERα is associated with decreased male aggression (Ogawa et al., 1997; Scordalakes & Rissman, 2003), whereas selective deletion of ERβ is associated with increased aggression (Ogawa et al., 1999; Nomura et al., 2002, 2006). Few data exist describing how ERs are regulated by salient environmental stimuli. In many species, seasonal fluctuations in estrogen-dependent behaviors are mediated by changes in photoperiod. Thus, understanding how ER subtypes are affected by photoperiod could provide insights into mechanisms of behavioral plasticity.
The effect of photoperiod on the reproductive system has received extensive attention. Many seasonally breeding rodents that mate in spring and summer respond to short photoperiods by reducing the size and function of the reproductive system (Prendergast et al., 2001). In hamsters, short days increase male resident–intruder aggression (Phodopus sungorus, Demas et al., 2004; Wen et al., 2004; Mesocricetus auratus, Garrett & Campbell, 1980; Jasnow et al., 2000; Caldwell & Albers, 2004). This effect is paradoxical because increased aggression occurs when testosterone concentrations are at a nadir. Despite the lack of plasma androgens, estrogens may still be important. Adrenalectomy prevents increased aggression in short days in Siberian hamsters (Demas et al., 2004). Studies of zebra finches (Taeniopygia guttata) show that the adrenal hormone dehydroepiandrosterone can be indirectly converted into estrogens within the brain (Soma et al., 2004).
Immunocytochemistry studies show that ERα and ERβ have distinct but overlapping distributions in the brain (Shughrue & Merchenthaler, 2001; Greco et al., 2003; Mitra et al., 2003). Short-day housing decreases ERα-immunoreactive (ir) cell counts and mRNA in the medial pre-optic area (MPOA) and medial amygdala (MEA) of ovariectomized female hamsters housed in short days (Mangels et al., 1998). To our knowledge, no previous study has observed the effect of photoperiod on ER expression in intact male rodents.
We examined the effect of photoperiod on ER expression in two closely related species of Peromyscus that inhabit different climates. Individuals of both species respond to winter-like short photoperiods by decreasing testes mass (Trainor et al., 2006c). Using immunocytochemistry and real-time polymerase chain reaction (PCR) we comprehensively examined the effects of photoperiod on ERα and ERβ expression in Peromyscus. We focused our analyses on hypothalamic and limbic brain areas such as the lateral septum (LS) and bed nucleus of the stria terminalis (BNST) because these brain areas have been identified as important neural substrates for the control of social behaviors (Newman, 1999; Choi et al., 2005; Goodson, 2005).
Materials and methods
Animals
We examined the effects of photoperiod on behavior and ER expression in two species of Peromyscus purchased from the Peromyscus Stock Center (Columbia, SC, USA). Old field mice, Peromyscus polionotus, are found primarily in the south-eastern United States. Field studies suggest that this rodent is monogamous (Foltz, 1981) and that breeding activity occurs throughout the year (Blair, 1951; Caldwell & Gentry, 1965). Deer mice, Peromyscus maniculatus, are distributed broadly and can be found as far north as the North-West Territories of Canada and as far south as northern Mexico. Populations of this species exhibit differential sensitivity of reproductive activity to photoperiod and the breeding season duration varies depending on the population (Bronson, 1985). Field studies indicate that P. maniculatus have a polygynous mating system (Ribble & Millar, 1996). Despite inhabiting a relatively tropical habitat, P. polionotus exhibit testicular regression when housed in short days (Trainor et al., 2006c) as do P. maniculatus (Demas et al., 1996). All experimental procedures were approved by the Ohio State University Institutional Animal Care and Use Committee and animals were maintained in accordance with the recommendations of the National Institutes of Health Guide for the Care and Use of Laboratory Animals.
Experiments and behavioral testing
On arrival at our laboratory all males were individually housed and randomly assigned to be maintained in long (16 h light/8 h dark) or short (8 h light/16 h dark) days. In both long- and short-day treatments, lights were turned off at 14:00 h Eastern Standard Time. All males were between 4 and 8 months of age, sexually inexperienced and had not been tested in any behavioral tests. Animals were given access to food (Harlan Teklad 8640) and filtered tap water ad libitum. We used three different groups of animals to measure the effects of photoperiod on behavior, ER immunoreactivity and ER gene expression.
In Experiment I, males were tested in resident–intruder aggression tests after 8 weeks. For each test a group-housed sexually inexperienced male (conspecific) was introduced into each resident's home cage for 10 min under dim red light (between 15:00 and 18:00 h). Although it is possible that differences in phase angles between long- and short-day mice could contribute to differences in aggression, we took steps to minimize this possibility. Previously published studies on Peromyscus indicate that the effects of photoperiod on activity onset are typically less than 1 h (Johnston & Zucker, 1980; Majoy & Heideman, 2000) and we waited at least 1 h after lights out before testing mice to ensure that all mice had become active before testing. An individual who was unaware of treatment assignments scored videotapes and recorded the number of bites, bouts of boxing, bouts of allogrooming and attack latency. Boxing was defined as fighting with the forepaws. Allogrooming can be an antecedent to more intense aggression but can also function in a more pro-social context (Pellis & Pellis, 1997). The morning after behavioral tests (08:00–10:00 h), males were anesthetized with sodium pentobarbital (40 mg/kg; Nembutal, Sigma, St Louis, MO, USA) and both testes were removed with a sterile cautery for sperm measurements. Males were then immediately perfused through the heart with saline followed by 10% neutral buffered formalin. Brains were removed and post-fixed in formalin overnight at 4 °C. Each brain was then transferred to 30% sucrose in phosphate-buffered saline (PBS) for 24 h, frozen on dry ice and stored at −80 °C. For P. maniculatus, 10 brains (long days, n = 5; short days, n = 5) were processed for ERα immunocytochemistry and 11 brains were processed for P. polionotus (long days, n = 6; short days, n = 5). Testes were removed from the tunica, minced with scissors, and ground in a saline solution containing 0.05% Triton-X and 0.025 mm thimerosal for 25 s. Spermatid nuclei in the resulting homogenate were then counted on a hemacytometer (Weil et al., 2006).
In Experiment II we collected brains from P. polionotus and P. maniculatus housed in either long or short days that had not been tested in behavioral tests. Between 13:00 and 15:00 h males were anesthetized with isoflurane and decapitated. Brains were quickly removed and transferred to 5% acrolein in PBS overnight at 4 °C. We used acrolein fixation for these animals because we determined in pilot studies that acrolein fixation resulted in improved ER staining compared with formalin. Each brain was then transferred to 30% sucrose in PBS for 24 h, frozen on dry ice and stored at −80 °C for ERα and ERβ immunocytochemistry. For P. maniculatus, eight brains (long days, n = 4; short days, n = 4) were processed for ERα and ERβ immunocytochemistry and 10 brains were processed for P. polionotus (long days, n = 5; short days, n = 5).
In Experiment III, we collected micropunch samples from P. polionotus and P. maniculatus males that had been housed in long or short days. Males were anesthetized with isoflurane and decapitated between 08:00 and 10:00 h. Brains were quickly dissected with the use of a brain matrix to generate coronal slices. A slice starting at the optic chiasm and ending 2 mm anterior was collected, immediately transferred to RNAlater (Ambion, Austin, TX, USA) and kept at 4 °C overnight. Bilateral samples containing the LS/BNST (these brain areas are contained in the same punch sample), MPOA and ventromedial hypothalamus (VMH) were collected the next day with 1000-µm punches. Punch samples were kept in RNAlater at −20 °C for RNA extraction. For P. maniculatus, punch samples from eight brains (long days, n = 4; short days, n = 4) were processed for ERα and ERβ gene expression and 12 brains were processed for P. polionotus (long days, n = 6; short days, n = 6).
Immunocytochemistry
In Experiment I, formalin-fixed brains were sectioned at 40 µm on a cryostat and free-floating sections were processed for ERα immunocytochemistry. Sections were washed three times in PBS and then incubated in 1% sodium borohydride in PBS for 10 min. Sections were then rinsed in 20% normal goat serum and 0.3% hydrogen peroxide in PBS for 20 min. Sections were incubated in primary ERα antibody (1 : 50 000, C1355, Upstate Biotechnology, Chicago, IL, USA) in 1% normal goat serum at 4 °C for 48 h. The ERα antibody is well characterized (Friend et al., 1997; Greco et al., 2001) and has been previously used in Peromyscus (Kramer et al., 2005). Titration experiments indicated that the 1 : 50 000 dilution was optimal for the lot of primary antibody used in this study. Sections were rinsed in PBS and incubated for 2 h with biotinylated goat anti-rabbit antibody (Vector Laboratories, Burlingame, CA, USA) in PBS + Triton X (TX). The sections were rinsed in PBS and then incubated for 30 min in avidin–biotin complex (ABC Elite kit, Vector Laboratories). After rinses in PBS, the sections were developed in hydrogen peroxide and diaminobenzidine with nickel for 2 min. Sections were mounted on gel-coated slides, dehydrated and coverslipped.
In Experiment II, sections of acrolein-fixed brains were processed as described above except that alternate sections were incubated in either primary ERα (1 : 20 000, C1355, Upstate Biotechnology) or primary ERβ (1 : 400, D7N, Invitrogen, Carlsbad, CA, USA) antibody in 1% normal goat serum in 0.5% Triton-X PBS (PBS + TX) for 48 h at 4 °C. Although previously used in studies of human breast tissue (Skliris et al., 2001), to our knowledge the D7N antibody has not been previously used in brain tissue. In control experiments on Peromyscus brain tissue, the omission of primary antibody resulted in no positive staining and pre-incubation with ERβ peptide (1 : 500) completely abolished positive staining (Fig. 1). The dilutions used in Experiment II were chosen based on titration experiments using the lots of ERα and ERβ primary antibody available for this experiment.
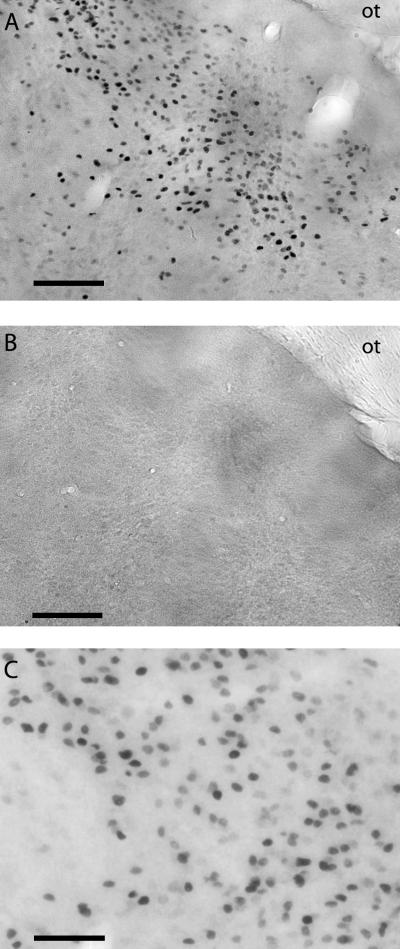
Estrogen receptor β-immunoreactive staining in the medial amygdala without (A) and with (B) pre-incubation with immunizing peptide. The optic tract (ot) is visible in the top right corner of each panel. The high-power photomicrograph in C shows the nuclear localization of the immunoreactivity. Scale bars: 100 µm, A and B; 50 µm, C.
Image analysis
In Experiment I, we used a Nikon E800 microscope to capture representative photomicrographs of each of the following brain areas using a mouse brain atlas (Paxinos & Franklin, 2002): ventral LS (bregma 0.26 mm), MPOA (bregma 0.02 mm) and VMH (bregma −1.70 mm). In these areas the number of ERα-ir cells within a 305 × 365 µm box was counted with the aid of neurolucida software (Microbrightfield, Williston, VT, USA) by an observer unaware of treatment assignments. We used a less conservative strategy to ensure that all cells in a given nucleus were counted, although this may have resulted in the inclusion of cells outside the regions of interest. When using brains fixed with acrolein, we detected ERα immunoreactivity in some areas that could not be observed in formalin fixed brains. In Experiment II we sampled the number of ERα-ir and ERβ-ir cells in the ventral LS, MPOA and VMH, and also in the posterior BNST (bregma 0.02 mm), paraventricular nucleus (PVN) (bregma −1.22 mm) and MEA (bregma −1.82 mm). For Experiment II we used a more conservative strategy for quantification. We used a 140 × 170 µm box, which ensured that our quantification was strictly limited to the regions of interest (Fig. 2). This approach has been used numerous times to quantify the expression of steroid receptors in hypothalamic and limbic brain areas (Lonstein et al., 2000; Scordalakes et al., 2002; Chung et al., 2006) and has also been used extensively to quantify immediate early gene expression in the brain (Gammie & Nelson, 2001; Kollack-Walker & Newman, 1995). We also quantified these same regions using a larger box (305 × 365 µm) and the results were essentially identical to the results presented below (data not shown).
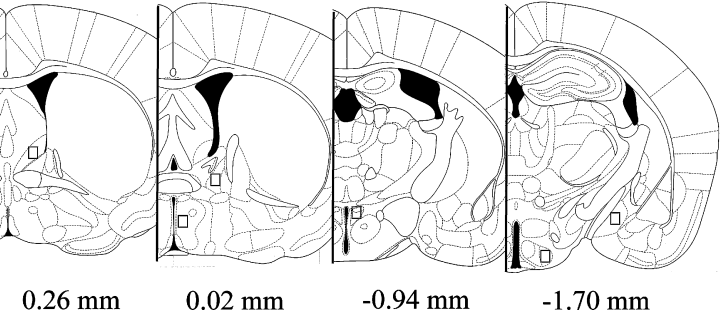
Representation of the quantification areas used for microscopic analyses in Experiment II. Reproduced from Paxinos & Franklin (2002), with permission from Academic Press. Figures 29 (ventral lateral septum), 31 (bed nucleus of stria terminalis and medial pre-optic area), 39 (paraventricular nucleus) and 45 (ventromedial hypothalamus and medial amygdala).
Quantitative real-time polymerase chain reaction
RNA was extracted from punch samples using RNaqueous (Ambion) kits. RNA samples were precipitated with lithium chloride and reconstituted in 20 µL of elution solution before spectrographic analysis. For each sample, 1 µg of RNA was reverse transcribed with Superscript (Invitrogen). Using cDNA pools of ovary tissue, we obtained partial sequences of the Peromyscus ERα and ERβ cDNAs via PCRs with degenerate primers based on sequences from mouse, rat and human. We visualized bands of approximately 400 bp for ERα and 450 bp for ERβ on 2% TAE-agarose gels containing ethidium bromide. The PCR products were purified and directly sequenced, revealing partial cDNA sequences for ERα (GenBank accession no. DQ357060) and ERβ (GenBank accession no. DQ357061), respectively.
Based on these sequences we designed the following primers and probes for ERα and ERβ:
ERα forward, 5′-GAACAGCCCCGCCTTGT-3′;
ERα reverse, 5′-GCATCCAGCAAGGCACTGA-3′;
ERα probe, 5′-TGACAGCTGACCAGATG-3′;
ERβ forward, 5′-GCTGATGTGGCGCTCGAT-3′;
ERβ reverse, 5′-CCCTCATCCCTGTCCAGAAC-3′ and
ERβ probe, 5′-ACCACCCTGGCAAGCTCATCTTT-3′.
The ERβ gene has multiple splice variants (Price et al., 2000) and we designed our ERβ primers to exclude the ER-β2 isoform that contains a 117 bp insertion between exons 5 and 6. Probes were labeled with the 6-FAM dye and MGB (non-fluorescent quencher) at the 5′ and 3′ ends, respectively. A TaqMan 18S ribosomal RNA primer and probe set (labeled with VIC dye; Applied Biosystems, Foster City, CA, USA) was used as a control gene for relative quantification. Amplification was performed on an ABI 7000 Sequencing Detection System with the Taqman® System. The universal two-step PCR cycling conditions used were: 50 °C for 2 min, 95 °C for 10 min, followed by 40 cycles of 95 °C for 15 s and 60 °C for 1 min. Relative gene expression of duplicate individual samples was calculated by comparison to standard curves consisting of serial dilutions of pooled P. polionotus ovary cDNA (1 : 102, 1 : 103, 1 : 104 and 1 : 105) followed by normalization to 18S rRNA gene expression.
Statistical analyses
Aggressive and mating behaviors were square root transformed for parametric statistical analyses to minimize any effects of outliers and to achieve homogeneity of variances between treatment groups (Zar, 1996). We used two-way anova to examine species differences and test for effects of photoperiod on aggression, ER-ir cell number and ER gene expression. We used planned comparisons to test for effects of photoperiod within each species. In Experiment I, for each species we used a principle component analysis on aggressive behavior to facilitate correlations with ER expression (see Results).
Results
Species differences and effects of photoperiod on behavior
In male–male resident–intruder aggression tests, P. polionotus were more aggressive than P. maniculatus (Table 1). Male P. polionotus exhibited higher levels of biting (F1,26 = 7.9, P < 0.01) and boxing (F1,26 = 43.1, P < 0.001), and had shorter attack latencies (F1,26 = 22.3, P < 0.001) than P. maniculatus. In general, aggression was increased in short compared with long days. Male P. polionotus showed increased biting and boxing behavior in short compared with long days (Table 1). Male P. polionotus housed in long days engaged intruders primarily via increased allogrooming (Table 1). We often observed that bouts of boxing could be initiated by the intruder following extended periods of the resident grooming the intruder, which occurred primarily in long days. In male P. maniculatus, boxing was increased in short-day males and there was a non-significant increase in biting in short-day males (Table 1). In P. polionotus, a principle component analysis identified one component that explained 67% of the variance in behavior. This component consisted of biting (component score = 0.68), boxing (0.79) and attack latency (−0.89). A similar component explaining 73% of the variance in behavior was identified in P. maniculatus. This component consisted of biting (component score = 0.82), boxing (0.77) and attack latency (−0.96). We refer to these variables below as the aggression composite score.
| Peromyscus maniculatus | Peromyscus polionotus | |||
|---|---|---|---|---|
| Long day | Short day | Long day | Short day | |
| Bites (per 10 min) | 0.1 ± 0.1 | 2.0 ± 1.0 | 4.7 ± 2.4† | 13.5 ± 7.0* |
| Boxing (bouts per 10 min) | 0.3 ± 0.3 | 5.7 ± 2.4* | 8.33 ± 2.38† | 27.12 ± 5.0* |
| Allogrooming (bouts per 10 min) | 0 ± 0 | 1.28 ± 1.0 | 26.2 ± 5.0† | 11.4 ± 3.5* |
| Attack latency (s) | 544.3 ± 55.5 | 423.1 ± 75.6 | 191 ± 73.3† | 112.2 ± 61 |
| Sperm count (sperm/mg testes) | 1.52 ± 0.10 × 105 | 0.7 ± 0.08 × 105* | 1.12 ± 0.13 × 105 | 0.88 ± 0.12 × 105 |
| (n) | (7) | (7) | (9) | (8) |
- * Effect of photoperiod, P < 0.05;
- † overall species difference, P < 0.05.
Effects of photoperiod and species differences on estrogen receptor α immunoreactivity
In Experiment I, observations were conducted on formalin-fixed brains from animals that had been tested in aggression tests. In the ventral LS P. polionotus had significantly more ERα-ir cells than P. maniculatus (F1,17 = 27.3, P < 0.001) and both species had significantly more ERα-ir cells in short compared with long days (Fig. 3A). There was no significant interaction between photoperiod and species. In the MPOA, the effect of photoperiod on ERα differed between the two species (interaction, F1,17 = 4.48, P < 0.05). In P. polionotus, ERα immunoreactivity was increased in short compared with long days, whereas in P. maniculatus, ERα immunoreactivity was increased in long compared with short days (Fig. 3B). In the VMH there were no apparent species differences, effect of photoperiod or interaction on ERα immunoreactivity (Fig. 3C). In P. polionotus, ERα immunoreactivity in the ventral LS was positively correlated with the aggression composite score (Spearman ρ = 0.60, P < 0.05, Fig. 3D). In neither the MPOA nor VMH was ERα immunoreactivity correlated with the aggression composite score in P. polionotus. In P. maniculatus, there were no significant correlations between ERα immunoreactivity and the aggression composite score.
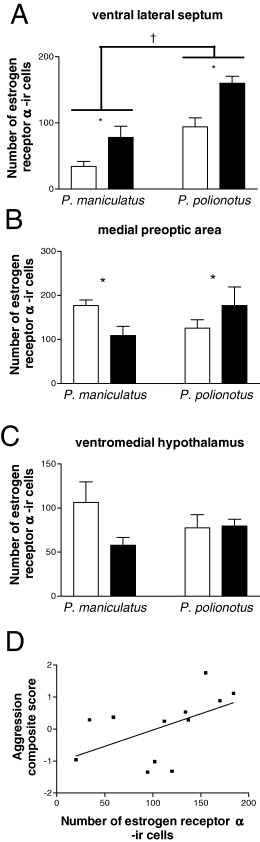
Estrogen receptor (ER)α-immunoreactive (ir) cell counts in ventral lateral septum (A), medial pre-optic area (B) and ventromedial hypothalamus (C) from formalin-fixed brains. Peromyscus maniculatus: long days (n = 5) and short days (n = 5). P. polionotus: long days (n = 6) and short days (n = 5). Open bars, long days; filled bars, short days. *Photoperiod effect, P < 0.05; †species difference, P < 0.05. In P. polionotus ERα-ir in the ventral lateral septum was positively correlated with the aggression composite score (D) (Spearman ρ = 0.60, P < 0.05).
In Experiment II, observations were conducted on acrolein-fixed brains from animals that had not been tested in behavioral tests. Acrolein fixation allowed the detection of ERα immunoreactivity in several regions that were undetectable in formalin-fixed brains (4, 6, Table 2). In the ventral LS, P. polionotus had significantly more ERα-ir cells than P. maniculatus (Table 2, F1,14 = 7.42, P < 0.05) and both species had significantly more ERα-ir cells in short days (Fig. 4). In the posterior BNST, P. polionotus had significantly more ERα-ir cells than P. maniculatus (Table 2, F1,14 = 5.25, P < 0.05). Additionally, in P. polionotus there were more ERα-ir cells in short-day mice compared with long-day mice, whereas this difference was not significant in P. maniculatus (Fig. 4, Table 2). In the MPOA, P. polionotus had more ERα-ir cells than P. maniculatus (Fig. 5, F1,14 = 11.3, P < 0.01) but there was no effect of photoperiod (Table 2). In the PVN, there was no effect of photoperiod on ERα immunoreactivity but P. polionotus had more ERα-ir cells than P. maniculatus (Fig. 6, Table 2, F1,14 = 13.0, P < 0.01). In the VMH there was no species difference in ERα immunoreactivity (Table 2) and both species had reduced ERα immunoreactivity in short days (Table 2, F1,14 = 5.30, P < 0.05). In the MEA there was no species difference or effect of photoperiod on ERα immunoreactivity (Table 2). There were no significant species by photoperiod interactions in any of the brain areas examined.

Photomicrographs of estrogen receptor (ER)α- and ERβ-immunoreactive staining in Peromyscus polionotus in long (left column) and short (right column) days. There were more ERα-immunoreactive cells in the ventral lateral septum (A and B) and bed nucleus of the stria terminalis (C and D) in short compared with long days. There were more ERβ-immunoreactive cells in the bed nucleus of the strial terminalis (E and F) and medial amygdala (G and H) in long compared with short days. ac, anterior commissure; lv, lateral ventricle; ot, optic tract. Scale bars, 170 µm.
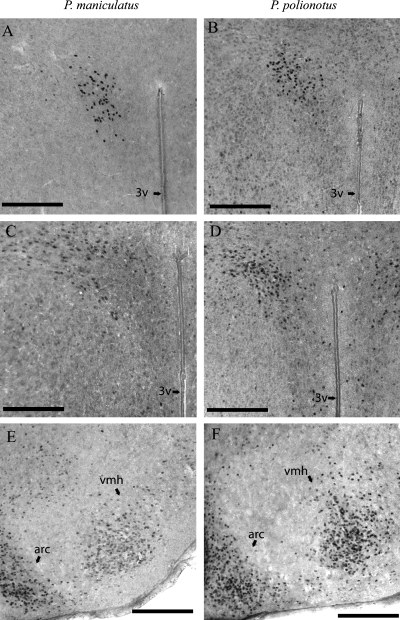
Photomicrographs of estrogen receptor (ER)α-immunoreactive staining in Peromyscus maniculatus (left column) and P. polionotus (right column) in the paraventricular nucleus (A and B) and ventromedial hypothalamus (E and F). Photomicrographs of ERβ-immunoreactive staining in P. maniculatus and P. polionotus in the paraventricular nucleus (C and D). arc, arcuate nucleus; 3v, third ventricle; vmh, ventromedial hypothalamus. Scale bars, 200 µm.
| ERα | ERβ | |||||||
|---|---|---|---|---|---|---|---|---|
| Peromyscus maniculatus | Peromyscus polionotus | Peromyscus maniculatus | Peromyscus polionotus | |||||
| Long day | Short day | Long day | Short day | Long day | Short day | Long day | Short day | |
| vLS | 2668 ± 87 | 3015 ± 213 | 2454 ± 250 | 3375 ± 323* | 832 ± 301 | 210 ± 210 | 1143 ± 187 | 269 ± 212* |
| MPOA | 2910 ± 259 | 3256 ± 479 | 3897 ± 229† | 4521 ± 309 | 2889 ± 255 | 3036 ± 443 | 3504 ± 209 | 3550 ± 220 |
| BNST | 2437 ± 45 | 2878 ± 175* | 3521 ± 256† | 3924 ± 142* | 4034 ± 349 | 2941 ± 375* | 4218 ± 95† | 3429 ± 161* |
| PVN | 1681 ± 212 | 1345 ± 357 | 2387 ± 224† | 2361 ± 153 | 1387 ± 250 | 1345 ± 280 | 1992 ± 191 | 1555 ± 351 |
| VMH | 2952 ± 150 | 2300 ± 203* | 2697 ± 244 | 2420 ± 169 | 1922 ± 405 | 742 ± 137* | 3235 ± 210† | 2153 ± 267* |
| MEA | 2342 ± 206 | 2423 ± 303 | 2218 ± 344 | 2294 ± 206 | 3831 ± 241 | 2489 ± 638* | 4579 ± 774 | 3134 ± 151* |
- * Effect of photoperiod within species, P < 0.05;
- † overall species difference, P < 0.05. BNST, bed nucleus of the stria terminalis; MEA, medial amygdala; MPOA, medial pre-optic area; PVN, paraventricular nucleus; LS, ventral lateral septum; VMH, ventromedial hypothalamus.
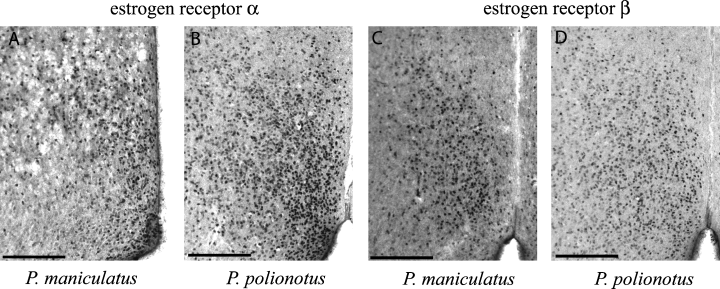
Estrogen receptor (ER) immunoreactivity in medial pre-optic area. ERα immunoreactivity in Peromyscus maniculatus (A) and P. polionotus (B). ERβ immunoreactivity in P. maniculatus (C) and P. polionotus (D). The third ventricle is visible in the lower right corner of each panel. Scale bars, 200 µm.
The physiological and endocrine data for the animals used in Experiment II have been published elsewhere (Trainor et al., 2006c). In both species, testes mass and testosterone were reduced in short days and there were no significant species differences in testosterone or testes mass (P > 0.05 in each case).
Effects of photoperiod and species differences on estrogen receptor β immunoreactivity
Few ERβ-ir cells were detected in the ventral LS and P. polionotus had more ERβ-ir cells when housed in long vs. short days (Table 2). In contrast, large numbers of ERβ-ir cells were observed in the posterior BNST of both species. Both species had increased ERβ immunoreactivity in long compared with short days (Fig. 4, F1,14 = 13.0, P < 0.01). There was no effect of photoperiod or any species differences in ERβ immunoreactivity in the MPOA (Fig. 5, Table 2). There was no species difference or effect of photoperiod on ERβ immunoreactivity in the PVN (Fig. 6, Table 2). In the VMH, ERβ immunoreactivity was increased in P. polionotus vs. P. maniculatus (Table 2, F1,14 = 21.7, P < 0.001). Also in the VMH, both species had significantly more in ERβ-ir cells in long days (Table 2, F1,14 = 15.0, P < 0.01). In the MEA, both species had increased ERβ immunoreactivity in long compared with short days (Fig. 4, Table 2, F1,14 = 8.8, P < 0.01). There were no significant species by photoperiod interactions in any of the brain areas examined.
Species differences and effects of photoperiod on estrogen receptor mRNA
In Experiment III, ER mRNA was measured with quantitative real-time PCR from punch samples that included both the ventral LS and posterior BNST. In these punch samples, both species had increased ERα mRNA in short compared with long days (Fig. 7A, F1,16 = 14.62, P < 0.01). No significant species differences in ERα mRNA were detected. In short days, both species had significantly lower ERβ gene expression (Fig. 7B). Again, no significant species differences were observed. In the MPOA, there was a marginal effect of photoperiod (F1,16 = 4.44, P = 0.05) and non-significant interaction on ERα mRNA (F1,16 = 3.65, P = 0.07). In P. polionotus, ERα mRNA was significantly increased in short as compared with long days (Fig. 7C) but this photoperiod effect was not significant in P. maniculatus. Also in the MPOA, there was a marginal species by photoperiod interaction (F1,16 = 4.5, P = 0.05) for ERβ expression. This reflected a significant up-regulation of ERβ gene expression in short days of P. maniculatus and no significant effect of photoperiod in P. polionotus. In the VMH, no species differences or effects of photoperiod on ERα or ERβ mRNA expression were observed. No significant species differences, effects of photoperiod or interactions on the cycle thresholds of 18s RNA samples were detected, suggesting that this gene was not differentially regulated across species or photoperiods.
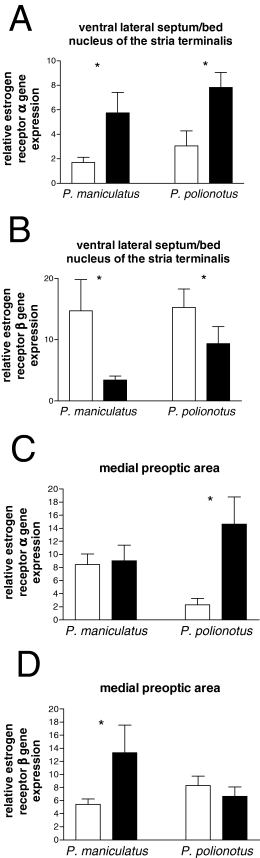
Estrogen receptor (ER)α and ERβ mRNA as measured by quantitative real-time polymerase chain reaction (PCR) in mice housed in long (open bars) and short (filled bars) days. Peromyscus maniculatus: long days (n = 4) and short days (n = 4). P. polionotus: long days (n = 6) and short days (n = 6). ERα (A) and ERβ (B) gene expression was measured in punch samples that included the ventral lateral septum and posterior bed nucleus of the stria terminalis (BNST). ERα (C) and ERβ (D) were measured in punch samples that included the medial pre-optic area (MPOA). Gene expression is normalized relative to 18s mRNA expression. *Effect of photoperiod, P < 0.05.
Discussion
We demonstrated by using immunocytochemistry and real-time PCR that photoperiod has differential effects on ER expression and that these effects are anatomically specific. The ability to measure both ER subtypes separately proved to be important because ERα and ERβ were often (but not always) inversely expressed. In short-day-housed animals we observed a consistent increase in ERα expression in the ventral LS and a corresponding decrease in ERβ expression in the posterior BNST. Increased ERα immunoreactivity in short days was observed in both naive animals and animals that were tested in aggression tests, indicating that the effects of photoperiod on ERα and ERβ was not mediated by experience in aggression tests. Additionally, these observations are supported by a real-time PCR experiment that demonstrated that short-day mice had increased ERα mRNA and decreased ERβ mRNA in LS/BNST punch samples. The effects of photoperiod in the MPOA were less consistent. In general, ERα expression in the MPOA of P. polionotus was up-regulated in short days. In contrast, both species had fewer ERβ-ir cells in the posterior BNST, MEA and VMH when housed in short days. These data suggest that photoperiod regulation of receptor expression in the brain could have important consequences for estrogen-sensitive behaviors.
Effects of photoperiod on estrogen receptor α and social behavior
We observed that, in the ventral LS, short days increased both ERα mRNA and the number of ERα-ir cells. In both P. maniculatus and P. polionotus we observed increased aggressive behavior in short days, although this effect was stronger in P. polionotus. In addition, the number of ERα-ir cells in the ventral LS (but not MPOA or VMH) was positively correlated with aggression in P. polionotus. In CD-1 mice (M. musculus), the number of ERα-ir cells in the LS is positively correlated with male aggression in resident–intruder tests (Trainor et al., 2006b) and numerous studies have demonstrated increased c-fos in the LS following male–male aggression tests (Kollack-Walker & Newman, 1995; Delville et al., 2000). Most studies in rodents have observed that estrogens increase aggression (Hilakivi-Clarke, 1999; Simon, 2002; Trainor et al., 2006b), presumably through activation of ERα.
There was some suggestion that male P. polionotus had increased ERα in MPOA when housed in short days, although this difference was not consistently observed in all experiments. There were no consistent effects of photoperiod on ERα immunoreactivity or mRNA in P. maniculatus. The MPOA is a critical brain area regulating male reproductive behavior (Hull et al., 2002). In particular, estrogens appear to promote mating behavior by binding to ERα (Ogawa et al., 1997; Wersinger et al., 1997). Thus, it seems counterintuitive that ERα should be increased in short days when testes are regressed. Although these changes may simply reflect effects of negative feedback on receptor expression, field observations on P. polionotus indicate that this species breeds throughout the year (Blair, 1951). Consistent with these observations, the relative decrease in testicular sperm production of short-day P. polionotus was much smaller than the observed decrease in sperm of short-day P. maniculatus.
Possible mechanisms of estrogen receptor α regulation
An obvious possible factor influencing ERα expression in short-day mice is reduced testosterone. Both P. maniculatus (Demas et al., 1996) and P. polionotus (Trainor et al., 2006b) have reduced testosterone concentrations in short compared with long days. Reduced testosterone almost certainly reduces estrogens in the brain by decreasing available substrate and also aromatase activity in areas of the brain such as the MPOA (Roselli et al., 1996). Previous studies suggest that the effect of castration on receptor immunoreactivity may depend on the antibody used. For example, castration in male rats increased the number of observed ERα-ir cells when antibodies raised to the ligand-binding domain of ERα were used but not when antibodies raised outside the ligand-binding domain were used (Clancy & Michael, 1994). Similar results have been reported in female rats (Weiland et al., 1997). The C1355 ERα antibody used in this study binds outside the ligand-binding domain (Friend et al., 1997), which suggests that the increased number of ERα-ir cells observed in the ventral LS of short-day mice is not due to competitive binding with endogenous estrogen. Thus, any possible effects of testosterone on ERα immunoreactivity in this study should have occurred at the transcriptional or translational levels. A role for testosterone is supported by observations in male P. californicus, in which photoperiod does not affect ERα or ERβ immunoreactivity in hypothalamic and limbic brain areas (B. C. Trainor, M. S. Finy & R. J. Nelson, unpublished). Males of this species do not decrease testes mass (Nelson et al., 1995) or testosterone concentrations (B. C. Trainor, M. S. Finy & R. J. Nelson, unpublished) when housed in short days.
Recent research suggests anatomical specificity in the regulation of ERα. When researchers created transgenic rats that expressed green fluorescent protein under the control of the ERα O/B promoter, they observed green fluorescent protein in forebrain regions (including BNST and MPOA) but not the VMH (Hamada et al., 2005). In P. polionotus we observed that ERα immunoreactivity and mRNA were increased in short days in ventral LS, posterior BNST and MPOA but not in VMH, PVN or MEA. These data suggest that the O/B promoter may mediate the effects of testosterone in the ventral forebrain but not other hypothalamic and limbic areas such as the VMH and MEA. Tissue-specific regulation of aromatase activity has also been observed in P. californicus. Reproductive experience altered aromatase activity in MPOA and ventral LS/BNST punch samples but not in VMH or MEA punch samples (Trainor et al., 2003). These findings suggest that similar mechanisms may regulate ERα expression and aromatase activity in an anatomically specific manner.
Photoperiod regulation of estrogen receptor β
This study is the first to report the effects of photoperiod on ERβ immunoreactivity and mRNA in the brain. In both P. polionotus and P. maniculatus ERβ immunoreactivity in the posterior BNST, MEA and VMH ERβ was decreased in short days. In M. musculus, castration increases ERβ immunoreactivity in the MPOA, BNST, VMH and PVN (Nomura et al., 2003) and in male Rattus norvegicus castration increases ERβ immunoreactivity in the VMH but not the MEA (Orikasa & Sakuma, 2004). In contrast to these previous observations, Peromyscus mice exhibited up-regulated ERβ in the posterior BNST in long days when testosterone is elevated. These data suggest that, if testosterone does have an effect on ERβ expression, it is positive as in prostate tissue (Asano et al., 2003). However, ERβ was also increased in the MEA in long-day mice, a tissue in which ERs typically do not respond to castration. This suggests that there may be some non-androgen-based mechanisms that may mediate the effect of photoperiod on ERβ in the MEA.
Several recent studies have demonstrated that ERβ activation can reduce anxiety-like behavior in female rats and mice (Imwalle et al., 2005; Lund et al., 2005; Walf & Frye, 2005). Recent studies on male Siberian hamsters (Ph. sungorus) have demonstrated that anxiety-like and depressive-like behaviors are increased in short days (Prendergast & Nelson, 2005; Pyter & Nelson, 2006). The amygdala and its projections to the BNST are thought to play an important role in modulating affective states (Phelps & LeDoux, 2005), so a decrease in ERβ activity in these regions during short days could contribute to increased anxiety-like behavior.
Species differences in estrogen receptor expression
In immunocytochemistry experiments, P. polionotus had significantly more ERα-ir and ERβ-ir cells than P. maniculatus in several brain areas. A previous study also reported increased ERα immunoreactivity in the PVN of P. polionotus compared with other species of Peromyscus (Kramer et al., 2005). At present the mechanistic bases and functional consequences of these differences are unclear. Our real-time PCR measurements did not detect any species differences in either ERα or ERβ mRNA. This could be due to species differences in post-translational processes or species differences in antibody–receptor binding. It is tempting to speculate that the increased ERα immunoreactivity expression in numerous brain regions in P. polionotus may contribute to the increased aggressive behavior relative to P. maniculatus or may be related to species differences in mating systems. It is unlikely that species differences in body size could account for increased ER immunoreactivity because the smaller species (P. polionotus) consistently exhibited more ERα-ir and ERβ-ir cells than P. maniculatus. Further study of ER function and regulation in Peromyscus is needed.
Conclusions
We have demonstrated that photoperiod has differential effects on ERα and ERβ expression, and that these effects are anatomically specific. For P. polionotus, animals that were tested in aggression tests and naive animals had increased ERα immunoreactivity in the ventral LS when housed in short days. Additionally, ERα mRNA in ventral LS/BNST punch samples was increased in short days, whereas ERβ mRNA was increased in long days. Photoperiod regulation of ERs in the ventral LS and BNST reflected a general pattern of increased ERα in short days and increased ERβ in long days, although not every brain area responded to photoperiod in this way. These changes in receptor expression may have important consequences for the control of estrogen-dependent processes including aggressive, mating and affective behaviors. Hormone manipulation experiments are needed to examine the behavioral consequences of these differences in ER expression.
Acknowledgements
We thank G. A. Bishop, J. D. Blaustein, L.B. Martin II, N. S. Hasen, L.M. Pyter and Z.M. Weil for helpful discussions, K.M. Greiwe, K. M. Kassouf, S.L. Kidder, J. R. Kuhlman, A.G. Trainor and J. E. West for technical assistance, and Invitrogen for generously donating ERβ blocking peptide. This work was supported by NIH MH076313 (B.C.T.) and NIH MH57535 (R.J.N.).
Abbreviations
-
- BNST
-
- bed nucleus of the stria terminalis
-
- ER
-
- estrogen receptor
-
- ir
-
- immunoreactive
-
- LS
-
- lateral septum
-
- MEA
-
- medial amygdala
-
- MPOA
-
- medial pre-optic area
-
- PBS
-
- phosphate-buffered saline
-
- PCR
-
- polymerase chain reaction
-
- PVN
-
- paraventricular nucleus
-
- TX
-
- Triton X
-
- VMH
-
- ventromedial hypothalamus