Effects of pilocarpine on the cortical and hippocampal theta rhythm in different vigilance states in rats
Abstract
It has been suggested that theta rhythm gates the flow of information between the hippocampus and cortex during memory processes. The cholinergic system plays an important role in regulating vigilance states and in generating theta rhythm. This study aims to analyse the effects of the muscarinic agonist pilocarpine (120 and 360 µg, i.c.v.) on hippocampal and frontal cortical theta rhythm during several vigilance states in rats. Pilocarpine injection increased the duration and number of episodes with theta activity, particularly when theta rhythm appeared during waking states in the cortex and hippocampus simultaneously. It seems that the effects of pilocarpine are related to the appearance of cortical theta activity in waking states, and suggest that pilocarpine could modify the transference rate of information from the hippocampus to cortex in rats during wakefulness states, in relation to the postulated effect of cholinergic system modulating memory consolidation.
Introduction
Cholinergic mechanisms play an important role in regulating a variety of behavioural functions, including alertness and some cortical and hippocampal electroencephalographic (EEG) patterns as well as rapid eye movement (REM) sleep (Crouzier et al., 2006). Spontaneous release of acetylcholine in the pontine reticular formation has been observed to be greater during REM sleep and waking when compared with slow wave sleep (SWS; Datta & Siwek, 1997). Two cholinergic systems are involved in the control of the vigilance states; one is located in the rhombencephalon where an important part of the control of REM sleep is performed (Velazquez-Moctezuma et al., 1989; Xi et al., 2004), the second depends on the cholinergic innervation of the neocortex and arises primarily from cell groups of the basal forebrain, which can also be activated by other neural systems such as the amygdala (see Dringenberg & Vanderwolf, 1998).
Cholinergic mechanisms also play an important role in generating theta rhythm in the EEG (Lee et al., 2005). Theta rhythm is defined as a sinusoidal-like waveform, with a peak frequency of 4–9 Hz and a small bandwidth (Oddie et al., 1997), and is mainly generated in the pyramidal neurons of the hippocampal formation (van Luijtelaar & Coenen, 1984; Kahana et al., 2001). Rhythmic oscillatory activities at theta frequency in the hippocampus have been found to be involved in several brain functions, including cognition, memory and learning (Kahana et al., 2001; Pedemonte et al., 2001; McKinney & Jacksonville, 2005). Theta rhythm is prominent during REM sleep and active waking (AW; Coenen, 1975; Pedemonte et al., 2001; Xi et al., 2004; Shin et al., 2005), and its function is currently accepted to be similar in both states (Lerma & Garcia-Austt, 1985).
A number of important physiological functions, such as behavioural arousal and motor control, are regulated by cholinergic muscarinic receptors (Tayebati et al., 2006). To date, five muscarinic receptor subtypes have been functionally described and cloned with a differential expression in different brain areas (Bonner, 1989; Velazquez-Moctezuma et al., 1989; Caulfield, 1993; Levey et al., 1995; Bymaster et al., 2003). Pilocarpine is a muscarinic agonist that acts on the central receptors by activating neural pathways (Takakura et al., 2003), and shows a low affinity for M1 and M2 receptors and a higher affinity for the M5 receptor subtype (Dong et al., 1995; Seifritz et al., 1998). Recent clinical studies have shown that a selective M1 agonist reduced REM latency sleep and SWS duration with no effects on memory consolidation (Nissen et al., 2006b). Orally administered pilocarpine has also been found to shorten the latency of REM sleep and to increase total REM time, the percentage of REM sleep and the duration of the first REM sleep period in humans (Berkowitz et al., 1990). An important role for pilocarpine in preventing the impairment of memory associated with ageing has also been reported (De-Mello et al., 2005).
As theta activity may support information transmission and storage within and between the hippocampus and cortex during performance of learned tasks (Muir & Bilkey, 1998), the aim of this study was to analyse the effects of pilocarpine on sleep–wake architecture and EEG characteristics, and on behavioural states with particular attention to the theta rhythm in the hippocampus as the main generator, and the frontal cortex as the main hippocampus target.
Materials and methods
Animals
Six adult male Wistar rats, 12 months old, weighing 350–375 g were used. Animals were housed individually and maintained during all experiments on a 12 : 12 light : dark (LD) cycle (lights on from 15.00 h to 03.00 h). Rats were kept under these light conditions for 1 month to fully adapt to the LD scheme. Standard laboratory animal food and water were available ad libitum. All procedures performed during the dark period were carried out under dim red light (< 0.5 Lux).
Experiments were performed following the ‘Principles of Laboratory Animal Care’ (NIH Publication no. 85-23, revised 1996) and according to the guidelines of the Local Ethics Committee of the Radboud University Nijmegen (The Netherlands).
Surgery
Under isoflurane (Abbot®, The Netherlands) inhalation anaesthesia all animals were submitted to aseptic surgery for implantation of electrodes and a cannula. Isoflurane anaesthesia was administered using a ventilated chamber coupled to a mask. Anaesthetic depth was checked by physiological parameters (immobility, absence of stimulus response, body temperature, heart and respiratory rate) of the animal. Atropine (Braun®, The Netherlands) was injected to avoid a rise in salivary secretion (0.05 mg i.m.). Stainless steel tripolar EEG electrodes (Plastics One Inc., The Netherlands) were placed into the frontal cortex (AP +2.0, ML +2.5), in the CA4 hippocampal region (AP −4.0, ML +2.0, DV −3.0), both relative to the bregma (Paxinos et al., 1980), with a reference in the cerebellum. Furthermore, one stainless steel cannula guide was placed in the lateral ventricle (AP −0.8, ML −2.0, DV −3.3, relative to the bregma) in order to inject the drugs. Two additional electrodes were placed over the dorsal neck muscles for bipolar EMG recording. Electrodes and cannula were attached to the skull with dental acrylic cement.
Experimental procedure
Animals were allowed to recover from surgery for at least 10 days before the beginning of the experiments. The animals were placed into the EEG recording boxes the day before the experiment to provide habituation to the experimental conditions. Boxes measuring 25 × 24 × 40 cm had walls made of clear Plexiglas, and the top was open to facilitate drug administration and the recordings in the freely moving rats. Rats were connected to the experimental setup through a rotating connector, which also prevented twisting of EEG wires. Six hours after lights off, the period in which theta activity is the lowest (van Luijtelaar & Coenen, 1984), rats were infused intracerebroventricularly inside the box without being unhooked from the recording cable. Before infusion, the presence of cortical and hippocampal EEG and electromyogram (EMG) patterns was observed and the recording was begun immediately. Each animal was submitted to EEG, EMG and behavioural recordings for 2 h each, in basal conditions (untreated animals), immediately after saline serum injection (1 µL i.c.v.) and after pilocarpine (Sigma-Aldrich Chemie®, Steinheim, Germany) injections (120 and 360 µg in 1 µL of saline serum, i.c.v.). At least 3 days elapsed between the different treatments to allow a complete washout of the administered substances. Basal recordings were performed in operated but untreated animals, whereas the saline control was made up of saline-injected, operated animals. All animals were submitted to the four treatments on separate days according to a 4 × 4 Latin square design experiment. Intracerebroventricular administrations were made using a Hamilton syringe coupled to a syringe pump (Razel Scientific Instruments®, Stanford, USA) through a polyethylene tube inserted into the cannula guide, with an injection rate of 1 µL/5 min. The doses of pilocarpine used in the present work were selected on the basis that a lack of neuronal damage in rat brain has been previously demonstrated after the same treatments with pilocarpine as described in the present study (Tejada et al., 2006a,b). As the maximal effect of muscarinic agonists occurs within 2 h (Timofeeva & Gordon, 2001), the recordings were restricted to this lapse after injection.
At the end of the experiments, animals were killed with an overdose of sodium pentobarbital (Nembutal®, The Netherlands) and successively submitted to intracardiac perfusion with saline and 10% formaldehyde. The brain was quickly removed for histological assessment of the cannula and electrode placement.
Electrographic recording and animal behaviour classification
EEG and EMG recordings were carried out using WINDAQ® (v. 2.29 for Windows®, USA). EEGs were filtered with a 1–100 Hz bandpass filter and sampled at a frequency of 512 Hz with a notch filter at 50 Hz (see Fig. 1 for a representative record). EMG was filtered with a 10–500 Hz bandpass filter.
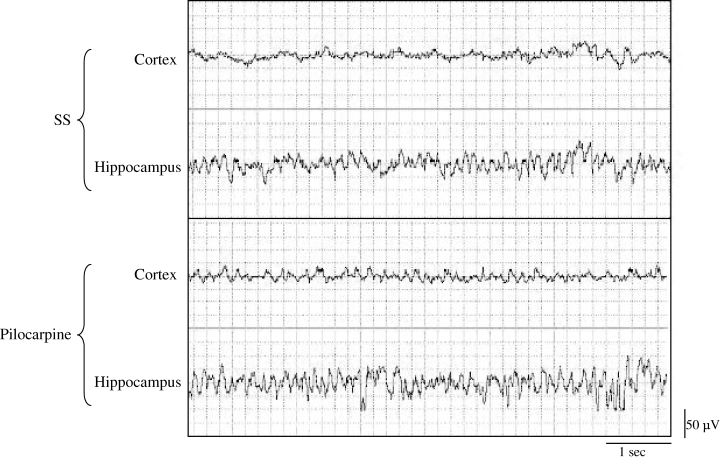
EEG recording examples in cortical and hippocampal regions after saline and pilocarpine treatment (360 µg i.c.v.) recorded during passive wake. Hippocampal theta is always evident in the hippocampus where it is further enhanced after pilocarpine injection. The drug also provoked the appearance of theta rhythm in the cortex. Similar results were found with 120 µg i.c.v. pilocarpine treatment.
Behavioural state was recorded by direct observation of the animals through a glass window at a distance of < 1 m. A keyboard was used to include the behavioural data into the polygraphic recordings and, in this way, animal behaviour and EEG recordings could later be correlated by visual inspection. Theta EEG rhythm was recognized as a sinusoidal-like waveform with a peak frequency of 4–9 Hz (Oddie et al., 1997; Kahana et al., 2001), and fragments of the recordings were submitted to a spectral analysis to evidence the appearance of a peak in the theta range. The behavioural states were classified according to Gottesman's (1992) criteria as follows. (1) Passive waking (PW) or waking without motor activity, which was further divided into three different substates: PW without theta rhythm (PW without theta); PW with theta rhythm only in the hippocampus (PW with hippocampal theta); and PW with theta rhythm in both the frontal cortex and hippocampus (PW with cortical and hippocampal theta). (2) AW or psychomotor AW, which was also divided into three different substates: AW without theta rhythm (AW without theta); AW with theta rhythm only in the hippocampus (AW with hippocampal theta); and AW with theta rhythm in both the frontal cortex and hippocampus (AW with cortical and hippocampal theta). (3) SWS. (4) REM sleep.
Additionally, behavioural variables such as number of peripheral cholinergic signs, e.g. tremors, sniffing and clonic movements of forelimbs, were annotated during the 2 h after the treatments.
Data analysis and statistics
Accumulated time spent in theta activity and number of episodes for each state and treatment, total time spent in each vigilance state and latencies (defined as the time from the beginning of the recording to the first appearance of the considered state) were obtained on the basis of EEG, EMG and behavioural parameters. Statistical analysis was carried out using SPSS® (v. 12.0 for Windows®, Madrid, Spain) using one-way analysis of variance (anova). Post hoc LSD paired comparisons were further made to recognize deviant groups. Results are expressed as mean ± SEM, and P < 0.05 was considered statistically significant.
Results
Behavioural observations
The animals were visually observed during the time period comprised between pilocarpine administration and the end of the recording session. The animals that received pilocarpine i.c.v. (120 or 360 µg) or saline did not show any behavioural signs characteristic of the epilepticus-like status or seizures.
Effects of pilocarpine on theta EEG rhythm
Figure 1 shows examples of cortical and hippocampal recordings after the treatments (1 µL i.c.v.) of serum saline or pilocarpine (360 µg). Independently of the treatment (saline or pilocarpine), theta activity was more evident in the hippocampus than in the frontal cortex. Cortical theta activity was never shown in the absence of hippocampal theta activity in all experimental conditions (basal, saline or both pilocarpine treatments) throughout the 2 h of the recorded EEG.
There was no significant difference between basal and saline control in any parameter studied, evidencing the absence of effects attributable to the i.c.v. injections (Fig. 2). On the other hand, the effects of the two administrations of pilocarpine were similar when both were compared (Fig. 2). After pilocarpine treatments, total theta rhythm time (without differentiating between cerebral regions) showed a clear increase throughout the 2 h of EEG recording (180%, P < 0.01 when compared with saline; F3,19 = 4.283; Fig. 2A). The number of episodes with theta activity also rose after both pilocarpine treatments (128–130%, P < 0.05 compared with saline; F3,17 = 3.765; Fig. 2B), and the duration of each theta episode changed from 5.6 to 7.6 and 6.7 s per episode for 120 and 360 µg of pilocarpine, respectively (P < 0.001 when compared with saline; F3,7391 = 4.299; Fig. 2C).
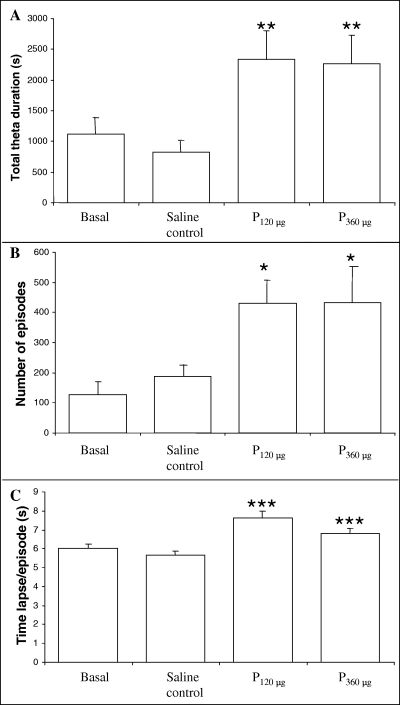
Variation in the amount of theta rhythm recorded in EEG (without differentiation between cerebral regions) during 2 h in basal, saline control (1 µL i.c.v.), pilocarpine 120 µg (P120, contained in 1 µL i.c.v.) and pilocarpine 360 µg (P360, contained in 1 µL i.c.v.) groups. (A) Time spent in theta rhythm (s). (B) Number of episodes presenting theta rhythm. (C) Average duration of the episodes presenting theta rhythm. Bars represent mean ± SEM (n = 6). *P < 0.05, **P < 0.01 and ***P < 0.001 when compared with the saline control group (one-way anova analysis).
Effects of pilocarpine on vigilance states
There was no significant difference between basal and saline control groups in any sleep–wake parameter (3, 4). Additionally, the two administrations of pilocarpine did not differ from each other (3, 4, Tables 1 and 2).
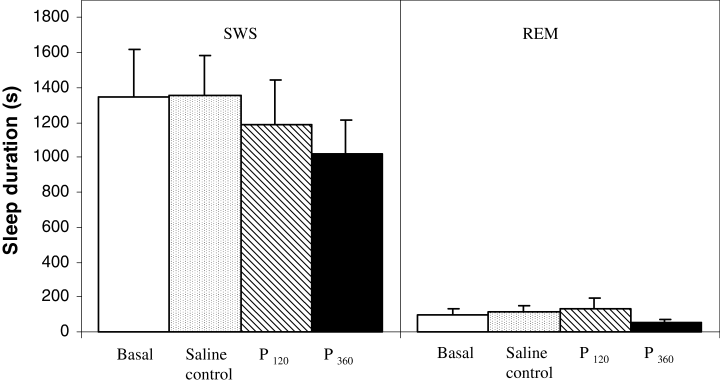
Duration of slow wave sleep (SWS) and rapid eye movement (REM) sleep during 2 h of EEG recording (s). Bars represent mean ± SEM (n = 6) in basal, saline (1 µL i.c.v.), pilocarpine 120 µg (P120, contained in 1 µL i.c.v.) and pilocarpine 360 µg (P360, contained in 1 µL i.c.v.) groups. No differences were found when one-way anova was used to comparisons.
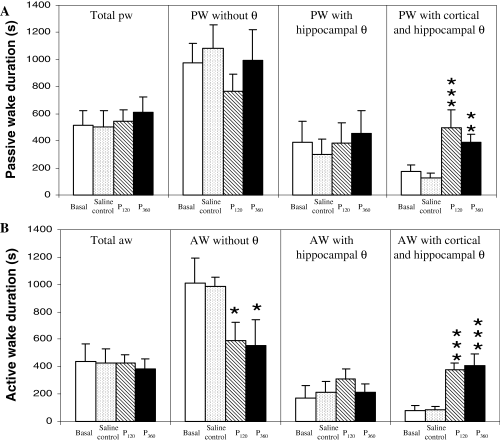
Duration of waking substates during 2 h of EEG recording (s). (A) Passive wake (PW) duration: total duration (Total PW), PW without theta rhythm (PW without θ), PW with theta only in hippocampus (PW with hippocampal θ), PW with theta in both frontal cortex and hippocampus (PW with cortical and hippocampal θ). (B) Active wake (AW) duration: total duration (Total AW), AW without theta rhythm (AW without θ), AW with theta only in hippocampus (AW with hippocampal θ), and AW with theta in both frontal cortex and hippocampus (AW with cortical and hippocampal θ). Bars represent mean ± SEM (n = 6) in basal group, saline control (1 µL i.c.v.), pilocarpine 120 µg (P120, contained in 1 µL i.c.v.) and pilocarpine 360 µg (P360, contained in 1 µL i.c.v.). *P < 0.05, **P < 0.01 and ***P < 0.001 when compared with the saline control group (one-way anova analysis).
| Number of episodes in 2 h | |||||
|---|---|---|---|---|---|
| Basal | Saline control | Pilocarpine 120 µg | Pilocarpine 360 µg | F-values | |
| SWS | 40.8 ± 6.8 | 41.7 ± 4.7 | 40.2 ± 5.4 | 46.3 ± 9.4 | F 3,20 = 0.167 |
| REM | 3.2 ± 2.2 | 4.5 ± 1 | 2.5 ± 1.1 | 2.3 ± 1.1 | F 3,20 = 0.471 |
| Total PW | 68.9 ± 10.8 | 75.7 ± 14.5 | 94.6 ± 9.4 | 115.2 ± 15.3** | F 15,176 = 6.54 |
| PW without θ | 111 ± 19.2 | 146.2 ± 17.2 | 114.5 ± 17.3 | 152.3 ± 30 | F 3,20 = 0.969 |
| PW hippocampal θ | 56 ± 12.2 | 54.8 ± 15.6 | 77.3 ± 18.2 | 95 ± 22.5 | F 3,20 = 1.192 |
| PW with cortical and hippocampal θ | 39.7 ± 10.7 | 26 ± 4.8 | 92 ± 11.36** | 98.2 ± 23.7** | F 3,20 = 6.401 |
| Total AW | 44 ± 8.4 | 55.1 ± 9.9 | 64.6 ± 8.8 | 62.1 ± 10 | F 15,176 = 6.54 |
| AW without θ | 75.3 ± 10.8 | 103.5 ± 8.6 | 75.8 ± 20.2 | 74.7 ± 19.7 | F 3,20 = 0.808 |
| AW hippocampal θ | 30.3 ± 13 | 41.5 ± 12.5 | 53.2 ± 13.5 | 48.7 ± 13.8 | F 3,20 = 0.567 |
| AW with cortical and hippocampal θ | 26.3 ± 12 | 20.3 ± 5.1 | 64.8 ± 12.2* | 63 ± 19.1* | F 3,20 = 3.244 |
- Data represent mean number of episodes in 2 h ± SEM (n = 6) in basal group, saline control (1 µL i.c.v.), pilocarpine 120 µg (contained in 1 µL i.c.v.) and pilocarpine 360 µg (contained in 1 µL i.c.v.). *P < 0.05 and **P < 0.01 when compared with the saline control group (one-way anova analysis). AW, active wake; PW passive wake; REM, rapid eye movement; SWS, slow wave sleep.
| Latency of onset of each state after treatment (min) | |||||
|---|---|---|---|---|---|
| Basal | Saline control | Pilocarpine 120 µg | Pilocarpine 360 µg | F-values | |
| SWS | 43.69 ± 6.28 | 38.04 ± 3.43 | 37.69 ± 2.85 | 35.37 ± 2.17 | F 3,20 = 0.779 |
| REM | 88.91 ± 15.27 | 70.62 ± 11.56 | 76.70 ± 14.65 | 70.58 ± 16.64 | F 3,20 = 0.348 |
| PW without θ | 37.14 ± 2.48 | 31.87 ± 0.46 | 33.83 ± 1.29 | 34.75 ± 2.20 | F 3,20 = 1.491 |
| PW with hippocampal θ | 52.69 ± 13.86 | 41.36 ± 8.08 | 42.70 ± 5.63 | 35.31 ± 2.28 | F 3,20 = 0.707 |
| PW with cortical and hippocampal θ | 46.98 ± 9.89 | 64.23 ± 12.65 | 33.72 ± 1.29* | 34.64 ± 2.03* | F 3,20 = 3.077 |
| AW without θ | 34.08 ± 1.26 | 33.18 ± 0.99 | 38.13 ± 3.46 | 51.79 ± 9.02* | F 3,20 = 3.089 |
| AW hippocampal θ | 68.14 ± 10.70 | 61.53 ± 13.37 | 52.90 ± 13.83 | 53.62 ± 13.50 | F 3,20 = 0.312 |
| AW with cortical and hippocampal θ | 70.00 ± 15.96 | 60.38 ± 7.82 | 36.32 ± 1.93* | 36.65 ± 2.69* | F 3,20 = 3.55 |
- Data represent mean latency ± SEM (n = 6) in basal group, saline control (1 µL i.c.v.), pilocarpine 120 µg (contained in 1 µL i.c.v.) and pilocarpine 360 µg (contained in 1 µL i.c.v.). *P < 0.05 when compared with the saline control group (one-way anova analysis). AW, active wake; PW, passive wake; REM, rapid eye movement; SWS, slow wave sleep.
Figure 3 shows SWS and REM sleep durations during the interval studied. After both pilocarpine treatments, no changes were found either in SWS or in REM sleep (F35,177 = 9.126). Similarly, the number of SWS and REM sleep episodes did not change after pilocarpine injections (Table 1). However, a tendency to diminish the SWS duration was observed, although statistical differences were not found. A greater dose of pilocarpine and/or number of animals could perhaps reveal an effect on SWS duration.
Although the total duration of PW and AW did not change after pilocarpine administration, significant changes did appear between substates (for details, see ‘Electrographic recording and animal behaviour classification’; Fig. 4, F35,177 = 9.126). PW without theta rhythm and PW with hippocampal theta rhythm durations did not change after pilocarpine treatments, although a rise in PW with cortical and hippocampal theta rhythm was observed after both pilocarpine injections (Fig. 4A). With respect to AW, the duration of AW without theta rhythm diminished after pilocarpine treatments; on the other hand, AW with hippocampal theta rhythm duration did not change; and, finally, AW with cortical and hippocampal theta rhythm duration increased after both pilocarpine treatments (Fig. 4B). All in all, pilocarpine injections increased theta rhythm in the cortex. Although the duration of total PW did not change (Fig. 4), the number of episodes (Table 1) rose at the high administration of pilocarpine (F15,176 = 6.54, P < 0.000). In addition, the number of episodes with simultaneous theta in the cortex and hippocampus was also increased (F3,20 = 6.401, P < 0.01 for PW and F3,20 = 3.244, P = 0.05 for AW).
The effects of pilocarpine on the latencies of the different vigilance states are shown in Table 2. No differences were found in sleep latencies, either in SWS (F3,20 = 0.779, P = 0.519) or in REM sleep (F3,20 = 0.348, P = 0.791) after pilocarpine treatments. Moreover, pilocarpine treatments did not change the latency of the PW without theta rhythm (F3,20 = 1.491, P = 0.247) or the PW with theta rhythm only in the hippocampus (F3,20 = 0.707, P = 0.559), but it did shorten the latency of PW when theta rhythm was present in both cortical and hippocampal regions (F3,20 = 3.077, P = 0.051). On the other hand, latency of AW without theta rhythm increased after pilocarpine administration (360 µg, i.c.v.; F3,20 = 3.089, P < 0.05), while latency was reduced for AW simultaneously showing theta in the cortex and hippocampus after both pilocarpine treatments (F3,20 = 3.550, P < 0.05).
Discussion
EEG analysis in freely moving animals is considered a useful method in order to assess drug effects on behaviour. In the present study, the pharmacological action of pilocarpine on hippocampal and cortical EEG, with particular attention on the occurrence of the theta rhythm, was studied. The results show that the concentrations of pilocarpine used in the present work (120 µg and 360 µg i.c.v., 2 h) induced similar effects: increasing the duration and number of episodes in which the theta rhythm appeared in the EEG. More particularly, the administration of pilocarpine increased the duration and number of episodes, with a shortened latency of the waking states with theta rhythm appearing simultaneously in both the cortex and hippocampus.
Hippocampal theta activity is a characteristic of REM sleep and also of some waking behavioural states (Bland, 1986; Leung, 1998; Pedemonte et al., 1999; Kahana et al., 2001; Gambini et al., 2002; Bouwman et al., 2005; Lee et al., 2005; Vyazovskiy & Tobler, 2005), and increased hippocampal theta activity has been shown after the administration of muscarinic agonists (Lawson & Bland, 1993; Yamamoto, 1998; Tai et al., 2006). The rise in the production of theta EEG observed in the present report was due to an increase in the episodes presenting theta rhythm and also to the increase in the duration of each episode. This was observed in both PW and AW, but was most important in the cortex, while the amount of exclusive hippocampal theta remained unchanged. Taking into account that the theta rhythm is generated in the hippocampus, and that the increase in cortical theta was not accompanied by an increase in hippocampal theta, these results could suggest a pilocarpine-induced increase in the theta wave transfer from the hippocampus to the cortex. This possibility is consistent with the hypothesis of Buzsaki (1996) who proposes that a sharp-wave burst initiated in the hippocampus during SWS and associated with theta oscillation provides the mechanism by which information may be relayed back to the cortex during memory consolidation. This is also consistent with the role of acetylcholine inhibiting the information flow from the hippocampus to the cortex postulated by Hasselmo (1999) and supported by Gais & Born (2004). The effects of pilocarpine observed in the present work could be related with the activation of muscarinic autoreceptors mediating the inhibition of acetylcholine release (Kilbinger et al., 1993), as muscarinic autoreceptors may belong to the five subtypes cloned to date (Vilaro et al., 1994).
There is evidence that the cholinergic system and also the theta rhythm are related to different aspects of learning and memory consolidation. Theta activity may ‘gate’ the flow and storage of information within the hippocampus and neighbouring cortical regions during various stages of mnemonic processing (Muir & Bilkey, 1998). Cholinergic antagonist treatments and disruption of cholinergic inputs have been observed to impair memory consolidation (Stemmelin et al., 1999; Power et al., 2004; Nissen et al., 2006b), whereas cholinergic agonists were found to improve it (Smith et al., 1996). Furthermore, a single administration of pilocarpine prevented and reversed age-related learning impairment in rats conducting a spatial task in a water maze (De-Mello et al., 2005). In addition, theta-patterned stimulation improved memory retention and influenced the induction of long-term potentiation, a putative memory mechanism (see Muir & Bilkey, 1998). In fact, theta and gamma oscillations initiated in the hippocampus may provide the mechanism by which information is sent to the neocortex during memory consolidation (Buzsaki, 1996; Power, 2004). A significant deficit in hippocampus-dependent spatial and non-spatial memory tasks has been observed in mice lacking the muscarinic M5 receptor subtype (Araya et al., 2006), and M3 and M5 muscarinic agonists have been suggested to alleviate amnesia and the decrease in theta power related with ageing (Markowska et al., 1995; De-Mello et al., 2005). As pilocarpine displays a high affinity for the M5 receptor subtype, which is abundant in the hippocampus and cortex (Bymaster et al., 2003), the pilocarpine-induced increase of the theta rhythm appearing simultaneously in the cortex and hippocampus observed in the present work could be mediated by the M5 receptor subtype.
Besides these effects, it is well known that the cholinergic system is involved in the generation and maintenance of REM sleep, but the contribution of the individual muscarinic receptors has not been determined. Several authors have reported that muscarinic M2-agonists (oxometrine-M, cisdioxo, carbachol) elicited a significant rise in REM sleep with a shortened latency (Velazquez-Moctezuma et al., 1989; Bueno et al., 2000; Crouzier et al., 2006). Moreover, the muscarinic M1 receptor subtype has been found to be involved in REM processes (Bueno et al., 2000; Nissen et al., 2006a,b). Pilocarpine in the present work did not produce significant differences in the SWS and REM sleep parameters, and as pilocarpine has a low affinity for M1 and M2 receptors and a higher affinity for the M5 receptor subtype (Dong et al., 1995; Seifritz et al., 1998), it could be inferred that the current effects of pilocarpine were not mediated by the M1 and M2 receptor subtypes. Human studies, on the other hand, have shown a shortened latency and an increase in the duration of REM sleep after the oral administration of pilocarpine (Berkowitz et al., 1990), but these discrepancies could be due to a number of methodological variables. That is, the effects of a low dose of pilocarpine might be mediated by M5 receptors, while a higher dose could be required to activate the M1 and M2 receptor subtypes. There is scarce information relating the M5 subtype with the wake–sleep cycle − partly due to a lack of selective ligands to block or activate M5 receptors (Eglen, 2006; Nissen et al., 2006a) − but m5 mRNA levels have been reported not to change in a line of rats with an increased REM sleep pattern (Greco et al., 1998).
In conclusion, our results show that the increased theta rhythm induced by the cholinergic agonist pilocarpine does not bear a direct relation on the generation and maintenance of REM sleep. Instead, the main effect of pilocarpine is produced on the amount of theta activity, a result probably produced in the telencephalic regions (such as the hippocampus) regulating cortical arousal. Moreover, the results suggest pilocarpine may increase the transfer of the hippocampal theta rhythm to the frontal cortex during vigilance in rats, and this could be mediated by the muscarinic M5 receptor subtype.
Acknowledgements
This work was supported by grant BFI2002-04583-C02-029 and 36AA/04 of Conselleria de Salut i Consum (Govern de les Illes Balears, Spain). Silvia Tejada was supported by a FPI grant (Govern de les Illes Balears, Spain). We also thank Ma Antonia Comas Soberats for her ideas and help.
Abbreviations
-
- AW
-
- active wake
-
- EEG
-
- electroencephalogram
-
- EMG
-
- electromyogram
-
- LD
-
- light : dark
-
- PW
-
- passive wake
-
- REM sleep
-
- rapid eye movement sleep
-
- SWS
-
- slow wave sleep