Convergence of cutaneous, musculoskeletal, dural and visceral afferents onto nociceptive neurons in the first cervical dorsal horn
Abstract
The convergence of cutaneous, musculoskeletal, dural and visceral afferents onto nociceptive neurons in the first cervical dorsal horn was investigated in urethane/chloralose-anesthetized rats. Electrical stimulation was applied to facial, neck, shoulder and forepaw skin, cornea (COR), dura, second cervical (C2) nerve, hypoglossal nerve, temporomandibular joint, masseter (MAS) muscle and superior laryngeal nerve. In addition, acetic acid was injected intraperitoneally and microinjection of glutamate was applied to the tongue, MAS muscle, splenius cervicis muscle, dura and intrapericardial area. A total of 52 nociceptive neurons classified as wide dynamic range (n = 28) or nociceptive-specific (n = 24) was studied. All nociceptive neurons received afferent input from the skin and at least one COR, musculoskeletal, dural or visceral afferent source in the trigeminal (V) or cervical area but input from afferent sources caudal to the C2 innervation territory was sparse. The proportion of neurons responding to COR, dural, C2 nerve, hypoglossal nerve, temporomandibular joint, MAS muscle and superior laryngeal nerve stimulations was 87, 54, 85, 52, 73, 64 and 31%, respectively. Electrical stimulation of all tested sites showed a double logarithmic stimulus–response relation, and cluster analysis of the excitability to COR, musculoskeletal, dural and visceral stimulations revealed two groups of neurons, one mainly containing wide dynamic range neurons and one mainly containing nociceptive-specific neurons. These findings indicate that afferent convergence in first cervical dorsal horn nociceptive neurons may be limited to the craniofacial area and that they may play an important role in the integration of craniofacial and upper cervical nociceptive inputs.
Introduction
The upper cervical spinal cord includes the first cervical (C1) and second cervical (C2) spinal segments. Previous findings suggest that the dorsal horn (DH) of the upper cervical spinal cord represents a transition zone between the trigeminal spinal tract subnucleus caudalis (Vc) of the trigeminal brainstem complex, which has also been termed the medullary DH (Gobel et al., 1981; Dubner & Bennett, 1983; Sessle, 2000), and the rest of the spinal cord (Piovesan et al., 2003; Hu et al., 2005).
Several anatomical studies have reported that the C1 DH receives extensive primary afferent inputs from the lateral aspect of the face, cornea (COR), dura, major craniofacial muscle nerves and upper cervical afferents (Kerr, 1972; Shigenaga et al., 1986, 1988; Pfaller & Arvidsson, 1988; Kaube et al., 1993; Strassman et al., 1994; Hathaway et al., 1995; Goadsby et al., 1997; Zhou et al., 1999; Clement et al., 2000; Foreman, 2000; Bartsch & Goadsby, 2003b).
Furthermore, electrophysiological studies have reported that many C1 DH neurons have a mechanoreceptive field (RF) that includes the facial region and the C2 and third cervical (C3) dermatomes (Chudler et al., 1991) as well as areas distant from the cervical area, e.g. the hindlimbs (Smith et al., 1991). Many C1 DH neurons have also been shown to receive convergent input from other afferent sources such as the COR (Meng et al., 1997), hypoglossal (XII) nerve (Hu et al., 2005), temporomandibular joint (TMJ), masseter (MAS) muscle and neck muscles, vascular and meningeal tissues (Burstein et al., 1998; Yamamura et al., 1999; Malick et al., 2000; Bartsch & Goadsby, 2003b), phrenic nerve (Razook et al., 1995; Chandler et al., 1998, 1999), vagal and sympathetic fibers (Chandler et al., 1996), and the superior laryngeal nerve (SLN), which carries visceral afferents and is a branch of the vagal nerve (Chandler et al., 1996), and to respond to intrapericardial injections of algogenic chemicals (Qin et al., 2001). The afferent convergence patterns have been implicated as an important process underlying pain referral in conditions such as temporomandibular disorders, whiplash, headache and angina pectoris (Foreman, 1999, 2000; Sessle, 1999, 2000; Bogduk, 2001; Bartsch & Goadsby, 2003b; Piovesan et al., 2003).
Each of the aforementioned studies tested only for a limited number of convergent inputs so the present study was initiated with the aim of testing the hypothesis that nociceptive C1 DH neurons show extensive convergence of afferent inputs from cutaneous, musculoskeletal, dural and visceral tissues. Electrical stimulation was applied to six cutaneous sites in the trigeminal and cervical areas, as well as to the COR, dura, C2 nerve, XII nerve, TMJ, MAS muscle and SLN. Microinjection of the algesic chemical glutamate was applied to the tongue, MAS muscle, splenius cervicis muscle, intrapericardial area, and dura; furthermore, acetic acid was injected intraperitoneally.
Preliminary data have been reported in abstract form (Mørch et al., 2004).
Materials and methods
Animals and surgery
This study was carried out on 58 adult male Sprague-Dawley rats (290–420 g) that were anesthetized intraperitoneally with α-chloralose (50 mg/kg) and urethane (1 g/kg). Adequacy of anesthesia was determined periodically by noting a lack of autonomic responses (e.g. heart rate changes to pinching the paw and the presence of a constricted pupil); a supplementary anesthetic dose (5 mg/kg chloralose, 100 mg/kg urethane, intraperitoneally) was administered if necessary. The percentage expired CO2, heart rate and rectal temperature were continuously monitored, and maintained at 3.5–5%, 330–460 beats/ min and 37–38 °C, respectively. Tracheal and venous cannulae were inserted and, for C1 DH neuron recording, the animals were paralysed with panacronum bromide and artificially ventilated (3.0–3.5 mL/ stroke, 75–85 strokes/min) with an air/O2 mixture. Procedures for animal preparation, stimulation, neuronal recording and classification were similar to those previously described in detail (Hu, 1990; Hu et al., 1992, 2005). All surgeries and procedures were approved by the University of Toronto Animal Care Committee in accordance with the Ontario Animal Research Act (Canada).
Preparation of stimulation sites
The following surgical preparations were carried out to enable stimulation of musculoskeletal structures. The midline incision for the placement of the tracheal cannula was also used for approaching the XII nerve and SLN. The C2 nerve was approached from the dorsal side and exposed. A bipolar platinum (27-gauge) cuff electrode with its two poles separated by 1 mm was used for stimulation of these nerves. The cuff electrodes were coated with dental cement (2 mm wide) to provide insulation from the surrounding tissues and the nerve/electrode assembly was also further insulated with high-viscosity petroleum jelly. For stimulation of the TMJ, the joint was approached from the dorsal side and two 33-gauge insulated wire electrodes with 0.5-mm exposed tips were located just anterior and posterior to the joint. Two similar wire electrodes were inserted into the MAS muscle. Partial craniotomy, removing parts of the parietal and occipital bones, was performed for stimulation of the dura and middle sagittal sinus. A blunt bipolar electrode was placed on the exposed dura and a pool of mineral oil was made to avoid evaporation. A blunt bipolar electrode was also used for electrical stimulation of the COR. Electrical stimulation was applied with a set of bipolar needle electrodes to six ipsilateral cutaneous locations: the ophthalmic (V1) innervation area above the eye; maxillary (V2) innervation area below the eye; mandibular (V3) innervation area posterior to the corner of the mouth; neck (C2/C3); shoulder and forepaw.
Recording of first cervical neurons
The animal was placed in a stereotaxic frame, and a cervical laminectomy of the dorsal part of the C1 vertebrae and the rostral part of the C2 spinal process was carried out to allow the introduction of an epoxylite-coated tungsten microelectrode into the left C1 segment of the spinal cord for recording the extracellular activity of single units. As the microelectrode was advanced, mechanical stimuli were applied to the orofacial and neck skin and COR to search for neurons receiving sensory input. In most experiments, the electrode was lowered perpendicular to the spinal cord to record from the C1 DH. In other experiments the electrode was angled (43° off vertical, 60° off midline) to record from the lateral aspect of the C1 DH where units can be found 300–500 µm below the surface (Meng et al., 1997; Hirata et al., 1999). Slight tension was placed on the tail of the rat with tape to minimize the vibrations on the surface of the spinal cord. Neuronal activity or responses were amplified, filtered, discriminated and displayed on a digitizing storage oscilloscope and simultaneously stored for off-line analysis (spike2, CED, Cambridge, UK).
Characterization of neurons by cutaneous stimulation
All neurons were examined for responses to mechanical stimulation of the skin, which was shaved to aid in delineation of the RF. Mechanical innocuous stimulation consisted of hair movement, gentle strokes with a brush and sustained light pressure (∼2.5 g/mm2 for 5 s) to the RF. Mechanical noxious stimuli were applied and quantified by skin pinch with a blunt forceps (tip-contact area of 1 mm2) with electronic force monitoring (Chiang et al., 1998) and heavy pressure to the RF (> 6 g/mm2 for 5 s) by an electronic von Frey hair (Somedic, Sweden). The neurons were also tested for responses to noxious radiant heat. The heat stimulus, which was not used for a detailed temperature–response analysis, was an 8-V 50-W focused projector bulb, which when run at 4 V and held 3–5 cm from the skin produced a 5- to 10-mm focused spot of light on the skin. A sensation of pricking pain was evoked ∼3 s after the onset of the heat stimulus when it was similarly applied to the experimenters' skin. The heat stimulus lasted for a maximum of 3 s, which produced a cutaneous temperature of 49–51 °C (Chiang et al., 1994) and the mean firing rate was recorded. The RF characteristics and responses to the radiant heat and graded mechanical skin stimulation enabled us to classify units into primary afferents and three major types of cutaneous nociceptive or non-nociceptive neurons according to previously outlined criteria (Price et al., 1976; Hu et al., 1981; Hu, 1990). These types included low-threshold mechanoreceptive neurons that responded to hair movement or light tactile stimulation and showed no increase in firing rate to more intense stimulation. The other two major classes of neurons were nociceptive neurons. Wide dynamic range (WDR) neurons responded to non-noxious mechanical stimuli and increased their firing rate with increased pressure and pinch stimulation into the noxious range. Neurons not responding to low-intensity mechanical stimuli but responding to heavy pressure and pinch or only pinch were classified as high-threshold or nociceptive-specific (NS) neurons. Only nociceptive neurons were studied further. The WDR and NS neurons frequently also responded to noxious heating of the RF. Noxious mechanical stimuli were used sparingly so as to avoid excessive damage to the skin and sensitization, and noxious heat was only applied towards the end of the experiment.
Stimulation
Mechanical
Gentle (< 5 mg) mechanical stimulation was applied to the COR and exposed dura with a blunt glass rod. Noxious mechanical stimulation (∼100 g with a 1-mm diameter blunt probe) was applied to the posterior aspect of the TMJ. This stimulation has been shown to evoke responses in TMJ nociceptive primary afferents (Cairns et al., 2001c). The sizes of the cutaneous RF were compared before and after the electrical stimuli were applied to evaluate possible expansion of the RF.
Electrical
Electrical stimulation was applied to the COR, dura, C2 nerve, XII nerve, TMJ, MAS muscle, SLN and six cutaneous sites, as stated above. A train of five stimuli was applied at each tested site (1 Hz, 0.1–2.0 ms, 0.1–10.0 mA constant current pulses) to determine the threshold for A- and C-fiber input as the lowest intensity that evoked a response to at least three of the five electrical stimuli. A- and C-fiber-evoked responses to cutaneous and COR stimulation were separated by a silent period. Response latencies to dural stimulation less than 10 ms were considered as A-fiber-mediated and as C-fiber-mediated if the response latency was longer than 10 ms (Levy & Strassman, 2002). For other stimulation sites, responses with latencies greater than 20 ms were considered C-fiber-mediated (Hu, 1990; Meng et al., 1997). The close proximity of the stimulation sites to the recording site sometimes resulted in stimulation artifacts interfering with short-latency action potentials, hence the short-latency responses evoked from some sites were sometimes obscured and the latency values could not be ascertained in these cases.
Chemical
The tip of a 27-gauge cannula connected to a Hamilton syringe (50 µL) with polyethylene tubes was carefully inserted into the tongue, MAS muscle, neck muscle (splenius cervicis) or intrapericardially and was used for glutamate injection. Glutamate has been shown to have an algesic effect (Yu et al., 1996; Cairns et al., 2001b,c). The glutamate (0.5 m, 10 µL, pH 7.0) was injected slowly (∼5 s) into the tongue, MAS muscle, splenius cervicis muscle and intrapericardial area or dripped onto the dura. The neural activity was monitored 15 min after each application of glutamate. The cannula was removed after 30 min and reinjected. Towards the end of some experiments, 0.5 mL 3.5% acetic acid was slowly (∼5 s) applied by intraperitoneal injection to test for possible evoked responses from peritoneal tissue.
Data analysis
Stimulus–response functions
Stimulus–response functions were constructed for mechanical stimuli applied to the center of the RF and for electrical stimuli applied to all tested sites. The mean firing rates of responses evoked by the application of a 5-s constant force to the center of the cutaneous RF were determined. The electrical stimulus intensity was normalized to the response threshold and the number of spikes to five stimuli was counted and displayed in a double logarithmic plot.
Location of neurons
Electrolytic lesions (anodic current; 8–10 µA for 10 s) were made at selected recording loci so that verification of loci could be made. The animal was killed by T61 (i.v., Haechst) and perfused intracardially with saline followed by 10% formalin solution. The C1 part of the spinal cord was embedded in a paraffin block, cut in 10-µm serial sections and stained with cresyl violet, and the lesion indicating the recording site was determined (Hu, 1990; Hu et al., 1992). The reconstructed histological data were based upon camera lucida drawings (Hu, 1990; Hu et al., 1992) and transferred onto a diagram of the C1 spinal cord as outlined and defined by Molander et al. (1989). Iso-excitability maps were constructed to illustrate areas in the C1 DH where neurons responding to electrical stimulation were located. The neural excitability to stimulation of a specific site was set as the reciprocal of the logarithm of the electrical activation thresholds, in order to transform the data into a normal distribution. Sets of iso-excitability maps were then constructed for both the A- and C-fiber-evoked excitability. The histological reconstruction of the location provided the spatial position of the neuron and the excitability described the value at the location of the neurons. To obtain an estimate of the excitability throughout the C1 DH, a two-dimensional thin-plate spline interpolation of the excitability was constructed (matlab, MathWorks). The thin-plate interpolation was smoothed to avoid negative values of the interpolation (Boor, 1978). The mean point-to-point distances between pairs of iso-excitability maps were calculated to estimate differences and similarities between the pairs of iso-excitability maps. A large number of pairs of iso-excitability maps was randomly generated with a distribution of excitability similar to the measured excitability in order to obtain a statistical estimation of random differences between iso-excitability maps. The probability that the difference between two measured iso-excitability maps could belong to the distribution of differences between randomly generated iso-excitability maps was used to test for statistical significance (see below). If the difference between two iso-excitability maps was significantly larger than what could be expected from the randomly generated iso-excitability maps, the iso-excitability maps were considered significantly different and if the difference between two iso-excitability maps was significantly smaller than what could be expected from the randomly generated iso-excitability maps, the iso-excitability maps were considered significantly similar.
Correlation analysis
To test if the nociceptive C1 DH neurons that responded to stimulation of one afferent source also responded to stimulation of other specific afferent sources, the correlation matrix of the neural excitability to A- and C-fiber stimulations of the afferent sources was constructed. The correlation matrix consists of the correlation coefficients, which are normalized measures of a possible linear relationship between the excitability to stimulation of two afferent sources (Mardia et al., 1979). The correlation matrix was preferred over the covariance matrix, as the neural excitability may not vary over similar ranges for all of the afferent sources. The correlation coefficients may range from −1.00 to 1.00, where −1.00 indicates perfect negative correlation, 0.00 indicates no correlation and 1.00 indicates perfect positive correlation. A positive correlation between the neural excitability to stimulation of two afferent sources will indicate that these sources evoked responses in similar neurons and with similar activation thresholds.
Cluster analysis
To evaluate possible associations of neural excitability, principal component analysis was applied on the excitability related to A- and C-fiber inputs from COR, dura, C2 nerve, XII nerve, TMJ, MAS muscle and SLN (Hotelling, 1933; Mardia et al., 1979). The k-means cluster algorithm was used to search for clusters in the principal component transformed observables. The number of clusters was found iteratively by minimizing the sum of Euclidian distances between points and cluster centroids. To investigate if the grouping reflected the WDR/NS classification, a χ2 comparison between the number of WDR and NS neurons in the different clusters was performed. Furthermore, the centroids of the clusters were transformed back to the original parameter space to describe the excitability of neurons in each cluster.
Statistical analysis
Results are presented as median (interquarter range, 25%, 75%) values and were analysed by Kruskal–Wallis anova on ranks and Dunn's post-hoc analysis when normality could not be assumed. Normal data were represented as the mean ± SEM, and analysed by anova and Tukey post-hoc analysis. Analysis of covariance (ancova) was used to test for increasing stimulus–response functions and differences between slopes. Double logarithmic stimulus functions were used as a model for the electrical stimulus–response functions as they provided a better fit than the linear model. Tukey post-hoc analysis was used to account for multiple comparisons between the slopes. anova was used to compare the mean point-to-point distance between pairs of iso-excitability maps with the randomly generated distribution of distances between pairs of iso-excitability maps. Statistical significance was in general considered as P < 0.05 and P < 0.001 for the correlation coefficients in the correlation matrix and the differences between iso-excitability maps, to counteract possible type II statistical errors.
Results
Histological examination of the regions 3–4 mm behind the obex confirmed anatomical landmarks consistent with the descriptions given by Molander et al. (1989) as the C1 DH. A total of 55 neurons were recorded in histologically confirmed loci in the C1 DH. They were evenly distributed throughout laminae I–V of the C1 DH except for the most ventrolateral region (Fig. 1). The DH neurons were classified as low-threshold mechanoreceptive (3), WDR (28) or NS (24). Only WDR and NS neurons were included in further analysis. The WDR and NS neurons were found throughout the C1 DH in laminae I/II, III/IV and V. An additional two neurons were located in the ventral horn and two neurons in the lateral cervical nucleus and were excluded from further analysis.
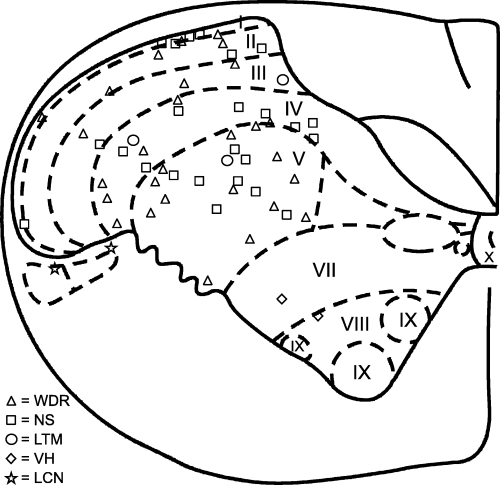
The location of first cervical spinal cord neurons histologically reconstructed from lesion sites; 28 wide dynamic range (WDR), 24 nociceptive-specific (NS) and three low-threshold mechanoreceptive (LTM) neurons were located in the dorsal horn. Two neurons were located in lateral cervical nucleus (LCN) that responded to gentle touch with a brush to large parts of the body; another two neurons were located in the ventral horn (VH) and responded only to noxious stimuli applied to all areas of the face.
Spontaneous discharge was observed in 18% of the WDR neurons (mean discharge rate 1.7 ± 1.0 Hz) and 17% of the NS neurons (mean discharge rate 1.3 ± 0.1 Hz).
Receptive field properties
All WDR and NS neurons could be activated by mechanical stimulation of their RF, as shown in the example of a WDR neuron in Fig. 2A and B and an NS neuron in Fig. 3A and B. Mechanical stimulation of the COR, TMJ (2, 3) and dura (2, 3) also evoked responses in several neurons (Table 1).

An example of a wide dynamic range (WDR) neuron that received convergent afferent inputs from several tested sources. The WDR neuron responded to brush, pressure and pinch (A), increasing pressure (B) stimulations of the cutaneous mechanoreceptive field (RF) (shown in G), pinch RF also include ipsilateral posterior tongue; stippled area (tactile) and outlined (pinch) area, pressure (∼100 mg force) applied to the skin overlying the temporomandibular joint (TMJ) (C) and gentle pressure applied to the dura with a glass rod (D). Responses to electrical stimuli of the skin innervated by the second branch of the trigeminal nerve (V2), cornea (COR), dura, second cervical nerve (C2), hypoglossal nerve (XII), TMJ, masseter muscle (MAS) and superior laryngeal nerve (SLN) displayed different response patterns and latencies (E). The histologically verified location of the neuron is shown in F (arrow).
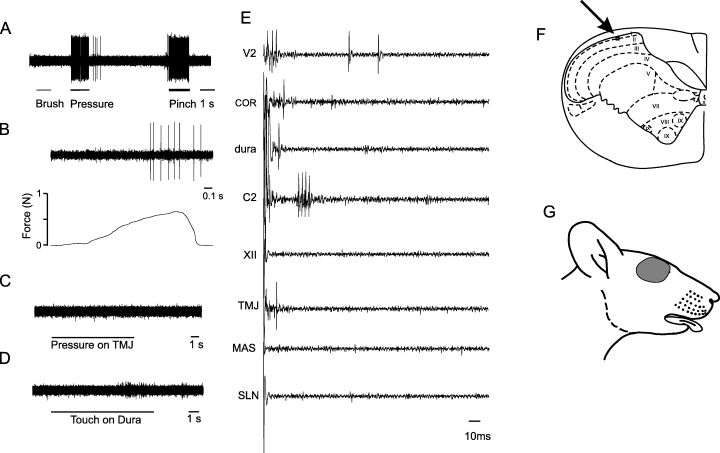
An example of a nociceptive-specific (NS) neuron that received convergent afferent inputs from several tested sources. The NS neuron responded to pressure and pinch (A), and increasing pressure (B) stimulations of the cutaneous mechanoreceptive field (shown in G) but not to brush pressure (∼100 mg force) applied to the skin overlying the temporomandibular joint (TMJ) (C) and gentle pressure applied to the dura with a glass rod (D). Responses to electrical stimuli of the maxillary (V2), cornea (COR), dura, second cervical nerve (C2) and TMJ displayed different response patterns and latencies, whereas stimulation of the hypoglossal nerve (XII), masseter muscle (MAS) and superior laryngeal nerve (SLN) did not evoke responses (E). The histologically verified location of the neuron is shown in F (arrow).
| Mechanical stimulation | Electrical stimulation | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| WDR | NS | WDR | NS | |||||||||||||
| (%) | (n) | (%) | (n) | (%) | (n) | A-fiber latency (ms) | A-fiber threshold (mA) | C-fiber latency (ms) | C-fiber threshold (mA) | (%) | (n) | A-fiber latency (ms) | A-fiber threshold (mA) | C-fiber latency (ms) | C-fiber threshold (mA) | |
| V3 | 82 | 28 | 63 | 24 | 50 | 28 | 6.1 ± 0.3 | 2.4 ± 0.7 | 77 ± 16 | 3.7 ± 1.1 | 38 | 24 | 7.0 ± 1.6 | 3.2 ± 1.0 | 44 ± 24 | 5.9 ± 0.9 |
| V2 | 79 | 28 | 46 | 24 | 100 | 28 | 5.5 ± 0.3 | 2.2 ± 0.5 | 62 ± 4 | 3.6 ± 0.7 | 88 | 24 | 6.4 ± 0.5 | 3.1 ± 0.8 | 55 ± 6 | 6.0 ± 0.9 |
| V1 | 64 | 28 | 46 | 24 | 75 | 28 | 5.5 ± 0.4 | 1.7 ± 0.5 | 54 ± 8 | 5.3 ± 1.2 | 83 | 24 | 5.4 ± 0.6 | 4.8 ± 1.7 | 43 ± 6 | 10.0 ± 0.1 |
| C2/C3 | 21 | 28 | 4 | 24 | 54 | 28 | 7.9 ± 1.0 | 5.4 ± 1.3 | 49 ± 12 | 6.4 ± 0.9 | 50 | 24 | 5.5 ± 1.2 | 6.1 ± 1.9 | 46 ± 14 | 7.7 ± 1.2 |
| COR | 32 | 28 | 21 | 24 | 89 | 28 | 6.5 ± 0.6 | 5.7 ± 0.8 | 38 ± 5 | 5.4 ± 0.9 | 83 | 24 | 5.4 ± 0.6 | 5.8 ± 0.9 | 33 ± 4 | 5.6 ± 0.9 |
| Dura | 38 | 8 | 25 | 12 | 71 | 17 | 8.6 ± 0.8 | 7.5 ± 1.4 | 32 ± 5 | 5.6 ± 1.5 | 40 | 20 | 7.2 ± 1.5 | 8.2 ± 1.2 | 31 ± 12 | 6.0 ± 1.9 |
| C2 | – | – | – | – | 93 | 28 | 5.7 ± 0.7 | 1.6 ± 0.6 | 38 ± 10 | 1.8 ± 0.9 | 75 | 24 | 5.8 ± 0.5 | 3.8 ± 1.4 | 26 ± 2 | 3.2 ± 0.9 |
| XII | – | – | – | – | 57 | 28 | 11.9 ± 2.9 | 2.3 ± 0.7 | 38 ± 6 | 4.9 ± 0.9 | 46 | 24 | 9.2 ± 0.5 | 6.5 ± 3.5 | 43 ± 7 | 3.7 ± 1.3 |
| TMJ | 71 | 28 | 50 | 24 | 85 | 27 | 6.8 ± 0.6 | 5.6 ± 0.7 | 53 ± 4 | 4.7 ± 0.8 | 58 | 24 | 6.7 ± 0.5 | 6.2 ± 0.9 | 37 ± 6 | 6.3 ± 1.0 |
| MAS | – | – | – | – | 78 | 27 | 5.1 ± 0.4 | 5.4 ± 0.8 | 62 ± 9 | 5.5 ± 1.0 | 48 | 23 | 5.4 ± 0.5 | 7.4 ± 1.1 | 44 ± 8 | 7.2 ± 1.4 |
| SLN | – | – | – | – | 30 | 27 | 6.1 ± 1.3 | 5.5 ± 0.5 | 34 ± 5 | 3.8 ± 0.9 | 33 | 24 | – | – | 51 ± 11 | 5.4 ± 1.5 |
| SHO | 0 | 28 | 0 | 24 | 4 | 28 | 5.6 | 8.0 | – | – | 13 | 24 | 7.2 | 6.0 | 33 ± 2 | 5.0 ± 2.0 |
| PAW | 0 | 28 | 0 | 24 | 4 | 28 | 5.9 | 3.0 | – | – | 4 | 24 | – | – | 130 | 10.0 |
- The latencies and thresholds to electrical stimulation are presented as the mean ± SEM. C2, second cervical nerve; C2/C3, neck; COR, cornea; MAS, masseter; PAW, forepaw; SHO, shoulder; SLN, superior laryngeal nerve; TMJ, temporomandibular joint; V1, ophthalmic; V2, maxillary; V3, mandibular; XII, hypoglossal nerve.
All WDR and NS neurons had a cutaneous RF located posterior to the whisker pad and generally anterior to the ear, although some (7% of WDR and 9% of NS) neurons responded to pinch applied to the anterior aspects of the ear (Fig. 4). No neurons in the C1 DH were found with an RF including parts of the shoulder, trunk, limbs or tail. The cutaneous pinch RFs of the WDR neurons (3.0 ± 0.4 cm2) were significantly larger than those of the NS neurons (1.2 ± 0.1 cm2) (one-way anova, P < 0.05). There was no difference between the sizes of the cutaneous RF tested at the beginning of the experiment compared with the cutaneous RF tested towards the end of the experiment (one-way anova, P > 0.05).
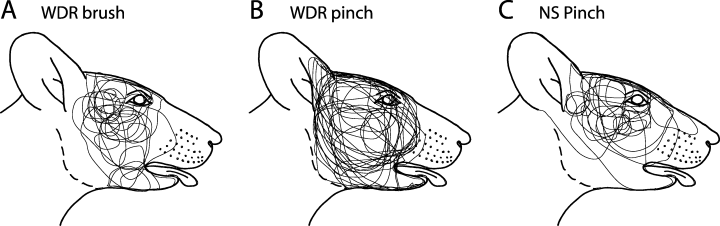
Outlines of the cutaneous mechanoreceptive field (RF) of the first cervical dorsal horn nociceptive neurons. The wide dynamic range (WDR) neurons had a cutaneous RF to brush (A) and pinch (B), and the nociceptive-specific (NS) neurons had an RF to pinch stimulation only (C). The cutaneous RFs were in general restricted to the area posterior to the whiskers and anterior to the ear.
Mechanical stimulus–response functions
The firing rate of both WDR (R = 0.4) and NS (R = 0.5) neurons increased as the force applied to the cutaneous RF increased (ancova, R = 0.52, P < 0.05; Fig. 5). The firing rates at all force levels and the slope for WDR neurons (0.17 ± 0.03 Hz/g) were significantly higher compared with the firing rates and slope for NS neurons (0.10 ± 0.02 Hz/g; ancova, P < 0.05).
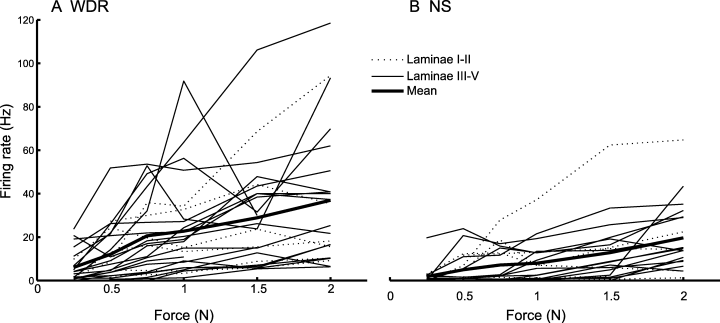
The stimulus–response functions for mechanical pinch applied to the center of the cutaneous mechanoreceptive field of (A) wide dynamic range (WDR) and (B) nociceptive-specific (NS) neurons in the first cervical dorsal horn. The stimulus–response functions of individual laminae I–II neurons are shown by thin dashed lines and the stimulus–response functions of laminae III–V neurons are shown by thin solid lines. The mean stimulus–response functions of WDR and NS neurons (thick solid lines) showed significant increases as the force increased (linear regression, P < 0.05, RWDR = 0.4, RNS = 0.5).
Response to heat stimulation
A total of 92% (24/26) WDR and 86% (18/21) NS neurons responded to heat stimulation applied to the cutaneous RF. The median firing rate of the WDR neurons (17 Hz) was significantly larger than the median firing rate of the NS neurons (3 Hz; Mann–Whitney ranked test, P < 0.001).
Response to chemical stimulation
Microinjection of glutamate to the MAS muscle, tongue or splenius cervicis muscle evoked responses in 20%, 7% and 20% of 15 WDR neurons tested, and in 27%, 7% and 13% of 15 NS neurons tested, respectively. Glutamate administration to the dura (n = 19) or heart (n = 10) did not activate any of the neurons tested.
Electrical stimulation
All neurons responded to electrical stimulation applied to the skin and at least one musculoskeletal, dural or visceral afferent source (see 2, 3; Table 1). There was no significant difference in the proportions of WDR and NS neurons that received COR, musculoskeletal, dural and SLN afferent input (one-way anova, P > 0.05; Fig. 6). Neurons located deeper in the DH received afferent input from more afferent sources than more superficially located neurons (linear regression, P < 0.05). In general, distinct volleys of A- and C-fiber-evoked responses were observed, except that stimulation of the SLN and XII nerve rarely evoked A-fiber responses (see Table 2). Responses in both WDR and NS C1 DH neurons evoked by shoulder and forepaw cutaneous stimulations were less frequent than responses evoked by stimulation of other sites (χ2-test, P < 0.05). Furthermore, responses to cutaneous, COR, musculoskeletal, dural and SLN stimulations were evoked more frequently in WDR than NS neurons (χ2-test, P < 0.05).
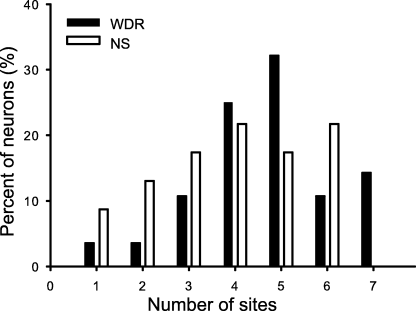
All of the wide dynamic range (WDR) and nociceptive-specific (NS) neurons in the first cervical dorsal horn received excitatory afferent inputs from one or more of the cornea, dura, second cervical nerve, hypoglossal nerve, temporomandibular joint, masseter muscle or superior laryngeal nerve tested sites. There was no significant difference between the number of the afferent sources that evoked responses in the WDR (4.4 ± 0.3) and NS (4.1 ± 0.3) neurons (one-way anova).
| Numbers of wide dynamic range (WDR) first cervical dorsal horn neurons | Numbers of nociceptive-specific (NS) first cervical dorsal horn neurons | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Total tested | Total activated | Only A-fiber activated | Only C-fiber activated | A- and C-fiber activated | Total tested | Total activated | Only A-fiber activated | Only C-fiber activated | A- and C-fiber activated | |
| V3 | 28 | 14 | 1 | 4 | 9 | 24 | 9 | 6 | 2 | 1 |
| V2 | 28 | 28 | 2 | 1 | 25 | 24 | 21 | 4 | 5 | 12 |
| V1 | 28 | 21 | 6 | 2 | 13 | 24 | 20 | 3 | 6 | 11 |
| C2/C3 | 28 | 15 | 5 | 4 | 6 | 24 | 12 | 5 | 5 | 2 |
| COR | 28 | 25 | 6 | 6 | 13 | 24 | 20 | 3 | 6 | 11 |
| Dura | 17 | 12 | 4 | 4 | 4 | 20 | 8 | 3 | 5 | 0 |
| C2 | 28 | 26 | 3 | 4 | 19 | 24 | 18 | 1 | 9 | 8 |
| XII | 28 | 16 | 0 | 12 | 4 | 24 | 11 | 0 | 9 | 2 |
| TMJ | 27 | 23 | 8 | 1 | 14 | 24 | 14 | 6 | 3 | 5 |
| MAS | 27 | 21 | 6 | 3 | 12 | 23 | 11 | 5 | 4 | 2 |
| SLN | 27 | 8 | 0 | 6 | 2 | 24 | 8 | 0 | 8 | 0 |
| SHO | 28 | 1 | 1 | 0 | 0 | 24 | 3 | 1 | 2 | 0 |
| PAW | 28 | 1 | 1 | 0 | 0 | 24 | 1 | 1 | 0 | 0 |
- C2, second cervical nerve; C2/C3, neck; COR, cornea; MAS, masseter; PAW, forepaw; SHO, shoulder; SLN, superior laryngeal nerve; TMJ, temporomandibular joint; V1, ophthalmic; V2, maxillary; V3, mandibular; XII, hypoglossal.
Latencies of electrically-evoked responses
The latencies of A-fiber-mediated responses evoked by C2 nerve and MAS muscle electrical stimulations were significantly shorter than those evoked by dural and TMJ stimulations (Kruskal–Wallis one-way anova on ranks, Dunn’s test, P < 0.05; see Table 1). The latencies of C-fiber-mediated responses evoked by C2 nerve stimulation were significantly shorter than those evoked by V2 cutaneous, V3 cutaneous and MAS muscle stimulations. Likewise, the latencies of C-fiber-mediated responses evoked by COR stimulation were significantly shorter than those evoked by V2 cutaneous stimulation (Kruskal–Wallis one-way anova on ranks and Dunn’s test, P < 0.05). In general, the WDR and NS neurons received afferent input through either A-fibers alone, C-fibers alone or both A- and C-fibers (Table 2), except for NS neurons receiving dural afferent input through either A- or C-fibers but not both A- and C-fiber inputs; furthermore, afferent input through XII nerve or SLN A-fibers alone was not observed.
Stimulus–response functions to electrical stimulation
The electrical stimulus–response functions of the normalized intensities were approximated with a double logarithmic function and were significantly increasing for all tested sites (one-way ancova, P < 0.001). The correlation coefficients of the double logarithmic relations, R, were 0.68, 0.65, 0.76, 0.68, 0.64, 0.50, 0.61, 0.79, 0.67, 0.62 and 0.62 for stimulation of the V1, V2, V3, C2/C3 cutaneous areas, COR, dura, C2 nerve, XII nerve, TMJ, MAS muscle and SLN, respectively. The slopes of the double logarithmic stimulus–response functions were not significantly different between the stimulation sites (ancova, P > 0.05) but the slopes of the stimulus–response functions of the NS neurons were significantly greater than those of the WDR neurons (ancova, P < 0.05).
Location of neurons responding to afferent input
There were significant differences in the location of neural excitability related to A-fiber inputs from V2 cutaneous and V1 cutaneous stimulations compared with other stimulation sites (see Fig. 7A). V2 cutaneous stimulation activated many more neurons in the intermediate part of the C1 DH compared with SLN and XII nerve stimulations; furthermore, V1 cutaneous stimulation activated neurons mainly in the lateral part of the C1 DH whereas V3 cutaneous stimulation activated neurons mainly in the medial part of the C1 DH (P < 0.001). The excitability for C2/C3 cutaneous, XII nerve and SLN stimulations was low compared with V2 cutaneous stimulations and the excitability evoked by C2/C3 cutaneous, XII nerve and SLN stimulations was more similar than could be expected by randomly distributed excitability (see Fig. 7A; P < 0.001).
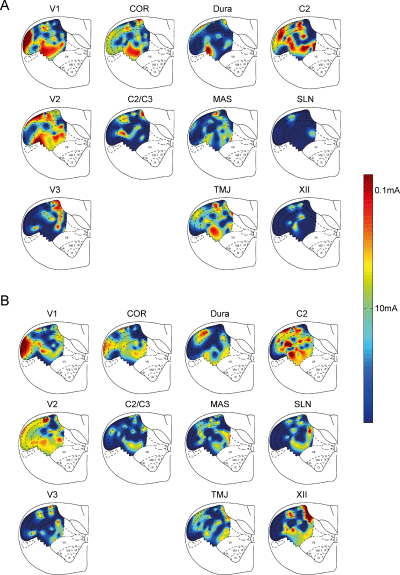
Spatial distribution of the neural excitability of first cervical dorsal horn neurons with various A-fiber (A) and C-fiber (B) inputs evoked by mandibular (V3), maxillary (V2), ophthalmic (V1), neck (C2/C3) cutaneous, cornea (COR), dural, second cervical nerve (C2), hypoglossal nerve (XII), temporomandibular joint (TMJ), masseter muscle (MAS) and superior laryngeal nerve (SLN) stimulations. Spline interpolation was used to estimate the excitability defined as the reciprocal of the logarithm of the thresholds to electrical stimulation. The color bar on the right indicates the threshold.
There were also significant differences in the location of neural excitability related to C-fiber inputs (see Fig. 7B). C2 nerve and V2 cutaneous stimulations activated many more neurons throughout most of the C1 DH compared with SLN, V3 cutaneous or C2/C3 cutaneous stimulations (P < 0.001). There were significant similarities in the excitability for certain pairs of stimulation sites (see Fig. 7B): SLN and V3 cutaneous; SLN and C2/C3 cutaneous; V3 cutaneous and MAS muscle; and TMJ and C2/C3 cutaneous sites (P < 0.001).
Correlation between paired afferent inputs
The correlation analysis between the neural excitability to stimulation of pairs of afferent sources revealed that the excitability to V1 cutaneous and V2 cutaneous stimulations, V3 cutaneous and MAS muscle stimulations, COR and V1 cutaneous stimulations, dural and V1 cutaneous stimulations, TMJ and V2 cutaneous stimulations, TMJ and C2 nerve stimulations as well as SLN and XII nerve stimulations was significantly correlated (see Table 3). The analysis indicated a high probability of co-occurrence of both inputs in a given nociceptive neuron. A negative correlation coefficient in neural excitability was found between V1 cutaneous and V3 cutaneous stimulations (P < 0.05), indicating that neurons rarely responded to both V1 cutaneous and V3 cutaneous stimulations.
| V3 | V2 | V1 | C2/C3 | COR | Dura | C2 | XII | TMJ | MAS | SLN | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| V3 | 0.04 | −0.24 | 0.17 | −0.14 | 0.12 | −0.05 | 0.10 | 0.02 | 0.34† | 0.16 | |
| V2 | 0.38† | 0.10 | 0.21 | 0.22 | 0.20 | 0.10 | 0.32† | 0.26 | −0.04 | ||
| V1 | * | *** | 0.10 | 0.41† | 0.32† | 0.25 | 0.05 | 0.17 | −0.04 | 0.08 | |
| C2/C3 | 0.10 | 0.14 | 0.28 | 0.31 | 0.34† | 0.12 | 0.24 | ||||
| COR | * | *** | 0.17 | 0.22 | 0.13 | 0.23 | −0.04 | 0.12 | |||
| Dura | * | *** | 0.26 | 0.26 | 0.24 | 0.06 | 0.26 | ||||
| C2 | * | ** | ** | * | ** | 0.18 | 0.39† | 0.20 | 0.04 | ||
| XII | ** | ** | 0.21 | 0.14 | 0.55† | ||||||
| TMJ | *** | *** | * | * | *** | * | 0.11 | 0.02 | |||
| MAS | *** | ** | * | 0.04 | |||||||
| SLN | * | ** | *** |
- † Significant correlation coefficients were considered as: P < 0.001. The significance levels are shown in the lower left half of the table as:
- * P < 0.05,
- ** P < 0.01 and
- *** P < 0.001. C2, second cervical nerve; C2/C3, neck; MAS, masseter; TMJ, temporomandibular joint; V1, ophthalmic; V2, maxillary; V3, mandibular; XII, hypoglossal nerve.
Cluster analysis
Clustering of the principal component transformation of the neural excitability disclosed two types of neurons that were different in their excitability to COR, musculoskeletal, dural and SLN stimulation (Fig. 8). The first group comprised nine WDR and 18 NS neurons, and the second group comprised 19 WDR and six NS neurons, and there was a significantly different distribution of WDR and NS neurons in the two groups (χ2, P < 0.05). Transformation of the centroids of the clusters showed that neurons of the first group had, on average, lower neural excitability related to A- and C-fiber inputs from COR, musculoskeletal, dural and SLN stimulation sites compared with the second group of neurons. In addition, neurons of the first group had, on average, lower excitability related to A-fiber, but not C-fiber, inputs from the dura compared with the second group of neurons.
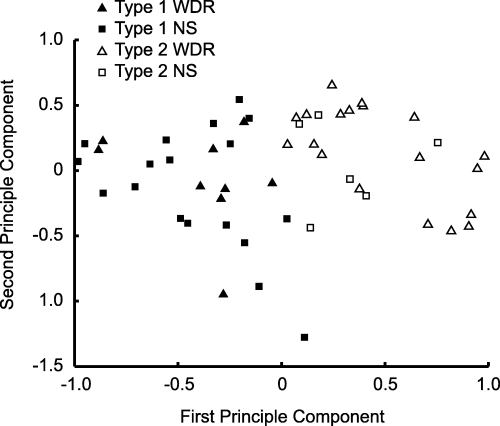
The neural excitability related to A- and C-fiber stimulation of cornea, dural, second cervical nerve, hypoglossal nerve, temporomandibular joint, masseter muscle and superior laryngeal nerve was transformed by principal component analysis and clustered by a k-means algorithm. Two types of neurons were identified (filled symbols) contained more nociceptive-specific (NS) (square) and fewer wide dynamic range (WDR) (triangle) neurons than type 2 (open symbols) (χ2, P < 0.05).
Discussion
The present study has provided novel findings that nociceptive neurons in the rat C1 DH receive excitatory afferent input from trigeminal and cervical cutaneous areas but only sparse afferent input from cutaneous areas caudal to the C2/C3 dermatome. In addition to cutaneous afferent inputs, the majority of the neurons also receive afferent input from COR, dura, C2 nerve, XII nerve, TMJ, MAS muscle and SLN sources. Furthermore, electrical stimulation of all tested sites has revealed a stimulus–response relationship indicating that these neurons may be capable of encoding sensory information from all tested afferent sources. Correlation and cluster analysis of the neural excitability showed a complex linkage between afferent sources. These findings indicate that the nociceptive C1 DH neurons may play an important role in nociceptive integration.
Technical considerations
A single experiment lasted several hours, and therefore the results may have been confounded as the firing rate (Ota et al., 1998) and RF size (Yanagidani et al., 1998) of DH neurons might be reduced by general anesthesia. Furthermore, multiple noxious stimuli were applied, which may result in sensitization of the neurons reflected in expansion of their cutaneous RFs (Hu et al., 1992; Chiang et al., 1998). However, the size of the cutaneous RF tested at the beginning of the experiment was not significantly different from the size assessed at the end of the experiment.
Response properties of first cervical dorsal horn neurons
Mechanoreceptive field properties and cutaneous afferent inputs
The reconstruction of the recording sites of the C1 DH neurons was made in accordance with the anatomical demarcations of the C1 DH defined by Molander et al. (1989). The cutaneous RF, response properties and laminar distribution of the WDR and NS neurons were in general similar to those previously delineated in the C1 (Hu et al., 2005). Neurons were located throughout laminae I–V of the C1 DH including laminae III/IV. Although nociceptive neurons are commonly reported to be located in laminae I/II and V, other studies have reported nociceptive neurons located in laminae III/IV of the C1 DH (Qin et al., 2001; Hu et al., 2005).
Neurons responding to V1, V2 or V3 cutaneous stimulations were located in the lateral, intermediate and medial part of the superficial laminae, respectively, and throughout the deep laminae of the C1 DH (see Fig. 7). Furthermore, the neural excitabilities to V1 and V2 cutaneous stimulations were correlated, which may be related to the frequent overlap of the V1 and V2 cutaneous areas in the cutaneous RF (Fig. 4). The correlation coefficient between the neural excitabilities to V1 and V3 cutaneous stimulations was negative, indicating that most neurons did not respond to both V1 and V3 cutaneous stimulations. This was also reflected in the large difference in the locations of neurons responding to V1 and V3 cutaneous stimulations, as V1 cutaneous stimulation evoked responses in the lateral part and V3 cutaneous stimulation in the medial part of the C1 DH. In studies using Fos-like immunoreactivity evoked by noxious mechanical stimulation (Strassman & Vos, 1993) and horseradish peroxidase labeling (Shigenaga et al., 1986), a more pronounced somatotopic organization of the superficial C1 DH laminae has been observed. In the present study, few neurons responded to electrical and none to mechanical shoulder or forepaw cutaneous stimulation (Table 1). Previous studies have reported neurons with a large cutaneous RF including the head and extending to the forelimb and, in some cases, the trunk and hindlimb but these studies included neurons outside laminae I–V of the C1 DH (Chandler et al., 1996, 1998; Clement et al., 2000). The present study and a previous study also reporting RFs restricted to facial and upper cervical areas (Hu et al., 2005) included neurons only in laminae I–V.
Corneal afferent input
Fos-like immunoreactivity studies have reported that DH neurons in the Vc/C1 transition zone (Lu et al., 1993) and in C1 (Strassman & Vos, 1993; Meng & Bereiter, 1996) that responded to COR stimulation were located in the lateral part of lamina I. Horseradish peroxidase tracing has shown termination sites of COR afferents in the dorsal aspects of lamina I as well as deeper parts of the C1 DH (van Ham & Yeo, 1996). The present study showed that neurons responding to COR A- and C-fiber stimulation were located in the lateral and intermediate part of laminae I and V (Fig. 7). The COR-responsive neurons all had a cutaneous RF, which is in agreement with previous studies showing that COR-sensitive neurons in the Vc/C1 transition zone also respond to cutaneous stimulation (Meng et al., 1997). Neurons responding to both COR and V1 cutaneous stimulations have been reported (Meng et al., 1997; Hirata et al., 1999, 2004; Malick et al., 2000) and this is consistent with the present findings of a significant correlation between the neural excitability to COR and V1 cutaneous stimulations.
Dural afferent input
Mechanical (Strassman et al., 1994) or electrical (Kaube et al., 1993) stimulation of the dural surface has been shown to produce Fos-like immunoreactivity primarily in the ventrolateral portion of lamina I and in lamina V of the C1 DH. Electrophysiological studies, including the present study, have reported dura-sensitive neurons located throughout the C1 DH (Davis & Dostrovsky, 1988; Burstein et al., 1998; Malick et al., 2000), although neurons in the Vc responding to dural stimulation were concentrated in the ventrolateral area (Burstein et al., 1998). Dura-sensitive neurons in Vc (Burstein et al., 1998), C1 (present study) and C2 (Bartsch & Goadsby, 2002) are considered to provide an important neural basis of pain in primary headache syndromes, such as migraine and cluster headache (Sessle, 2000; Burstein, 2001; Goadsby, 2003). Convergence of afferent inputs to the dura-sensitive neurons from facial skin (Burstein et al., 1998) or from cervical areas, e.g. innervated by the greater occipital nerve (Bartsch & Goadsby, 2002), was also seen in the present study, as there was a significant correlation between neural excitability to dural and V1 cutaneous stimulations, and common locations of neurons responding to dural and C2 nerve stimulations were observed. Such convergence may be related to the poor localization of head and neck pain, and the spread and referral of pain to facial and cervical areas that is often reported by headache patients (Anthony, 1992; Yamamura et al., 1999; Bogduk, 2001; Bartsch & Goadsby, 2003a).
Musculoskeletal afferent input
Noxious stimulation of muscles innervated by the C2 nerve (Kalezic et al., 2004), MAS muscle (Imbe et al., 1999), XII nerve (Bereiter et al., 2000) as well as the TMJ (Hathaway et al., 1995; Zhou et al., 1999) can evoke Fos-like immunoreactivity in the C1 DH, mainly in its laminae I and V. The present study has shown a more widespread distribution of nociceptive neurons responding to musculoskeletal afferent inputs. Neurons located throughout the DH may have extensive dendritic trees (Ralston, 1968; Renehan et al., 1986) and so receive input from primary afferents terminating in restricted areas of the DH, as well as receive non-primary afferent inputs from peripheral tissues. These features may explain the more widespread locations of nociceptive neurons receiving musculoskeletal afferent inputs described in electrophysiological studies compared with the anatomical description of the terminal areas of primary afferents supplying musculoskeletal tissue.
The present study showed a correlation between the neural excitability to TMJ and V2 cutaneous stimulations but not to TMJ and V3 cutaneous stimulations or to TMJ and MAS muscle stimulations, although the TMJ is principally innervated by the masseteric and auriculotemporal branches of the V3 nerve (Dubner et al., 1978). The excitabilities to V3 cutaneous and MAS muscle stimulations were correlated and the locations of neurons responding to V3 cutaneous and MAS muscle stimulations were generally similar. The excitability to XII nerve and SLN stimulations was significantly correlated and the locations of neurons responding to XII nerve and SLN stimulations were also similar. Although SLN afferents innervate the larynx and pharynx whereas the XII nerve afferents mainly innervate the tongue, the similar features of their effects in the C1 DH may be related to their common involvement in respiratory and alimentary functions (Dubner et al., 1978; Martin, 1996).
Application of glutamate to the splenius cervicis muscle, MAS muscle and tongue at a concentration that causes pain in humans (Cairns et al., 2001b) and evokes activity in primary afferents projecting to the Vc (Cairns et al., 2001c) activated fewer neurons than electrical stimulation of the C2 nerve, MAS muscle and XII nerve, respectively. This suggests that electrical stimulation activated more afferents than glutamate injection into a part of the innervation territory of each nerve (Bereiter et al., 2000).
Superior laryngeal nerve (visceral) input
In accordance with the present study, previous electrophysiological studies have shown neural responses evoked in the C1 DH by SLN stimulation (Chandler et al., 1996), a finding consistent with horseradish peroxidase studies showing SLN afferent terminations in the C1 DH (Nomura & Mizuno, 1983). Furthermore, SLN-evoked responses have been reported in Vc (Hu et al., 1981; Sessle et al., 1986). Stimulation of the vagal nerve or phrenic nerve fibers innervating the thoracic structures has been shown to activate C1 DH neurons, whereas stimulation of phrenic nerve fibers innervating the diaphragm or abdominal structures did not evoke responses in C1 DH neurons (Razook et al., 1995; Chandler et al., 1996, 1998). In the present study, intraperitoneal injection of acetic acid or intrapericardial injections of glutamate did not activate C1 DH neurons even though some of these neurons did respond to glutamate injection into the splenius cervicis, MAS muscle or tongue. Previous studies have shown that intrapericardial injections of other algogenic chemicals may activate neurons in the C1 DH (Qin et al., 2001). The present results may therefore indicate that glutamate receptors are either not present in the pericardial tissue or not sufficient for glutamate to evoke responses in nociceptive C1 DH neurons. However, glutamate receptors of vagal pre-ganglionic neurons supplying the heart have been shown but these afferents project to the dorsal vagal nucleus, intermediate reticular formation and nucleus ambiguus but not the C1 DH (Corbett et al., 2003).
Lack of responses to non-craniofacial stimulation
The present study found that none of the nociceptive neurons in the C1 DH responded to mechanical stimulation outside the cervical area and only a few responded to electrical (and none to mechanical) stimulation of the forepaw. This contrasts with some previous studies of the C1 DH or neighboring areas (Smith et al., 1991; Yezierski & Broton, 1991; Razook et al., 1995; Chandler et al., 1996, 1998, 1999; Villanueva et al., 1996; Ness et al., 1998; Qin et al., 2001, 2004). The likely explanation of this difference in findings is the use of different search paradigms, differences between species and the inclusion of only nociceptive neurons in laminae I–V of the C1 DH in the present study.
Afferent convergence
In accordance with previous anatomical (Pfaller & Arvidsson, 1988; Strassman & Vos, 1993) and electrophysiological (e.g. Piovesan et al., 2001; Bartsch & Goadsby, 2003a; Hu et al., 2005) studies, the present study has revealed a high degree of trigeminal and cervical afferent convergence from cutaneous, COR, musculoskeletal, dural and SLN sources onto C1 DH nociceptive neurons. Neurons in the deep C1 DH in particular received inputs from more afferent sources than neurons located in the superficial laminae, a finding consistent with a previous report of C1 DH neurons receiving pericardial afferent input (Qin et al., 2001). Several differences between deep and superficial nociceptive neurons have previously been discussed and reviewed extensively (see Dubner & Bennett, 1983; Sessle, 2000). As there were large differences in response latencies (2, 3, Table 1), the neurons may have been activated through either polysynaptic or direct primary afferent inputs. The cluster analysis indeed revealed two groups of neurons based on the neural excitability to COR, musculoskeletal, dural and SLN stimulations, one containing mainly NS neurons and the other containing mainly WDR neurons. The group containing mainly NS neurons generally had a lower excitability than the group of neurons containing mainly WDR neurons. The usual classification of the nociceptive neurons refers to the WDR neurons as neurons with higher excitability to cutaneous stimulation than the NS neurons (Price et al., 1976; Hu et al., 1981; Hu, 1990). Therefore, it appears that the greater excitability of WDR neurons to cutaneous stimulation may also be extended to a greater excitability of WDR neurons to COR, musculoskeletal, dural and SLN stimulations.
Double logarithmic regression of the stimulus–response functions to electrical stimulation of all tested sites showed similar and significant relations between stimulus intensity and responses. This suggests that the C1 DH nociceptive neurons may have similar encoding capacity for sensory information from a wide range of afferent sources. The extensive convergence demonstrated in the C1 DH (Piovesan et al., 2003; Hu et al., 2005; present study) as well as the Vc (Sessle et al., 1986; Hu, 1990; Burstein et al., 1998; Malick et al., 2000) supports other studies indicating that these structures may play a critical role in craniofacial nociceptive reflexes (Tsai et al., 1999; Cairns et al., 2001a) as well as craniofacial pain conditions, e.g. temporomandibular disorders (Sessle, 1999, 2000) and headache (Bogduk, 2001; Bartsch & Goadsby, 2003b; Piovesan et al., 2003). The afferent convergence from the trigeminal and cervical afferent sources in the C1 DH and adjacent Vc supports the view that the DH of the upper cervical spinal cord (including C1) and Vc form a functional continuum in processing sensory information (Piovesan et al., 2003; Hu et al., 2005).
Acknowledgements
The authors gratefully acknowledge the technical assistance of Mr K. Macleod and Ms S. Carter. This study was supported by Cell Signals CIHR strategic training grant, NIH grants DE-015420 and DE-04786 and the Danish National Research Foundation. B.J.S. holds a Canada Research Chair.
Abbreviations
-
- ancova
-
- analysis of covariance
-
- C1
-
- first cervical
-
- C2
-
- second cervical
-
- C2/C3
-
- neck
-
- C3
-
- third cervical
-
- COR
-
- cornea
-
- DH
-
- dorsal horn
-
- MAS
-
- masseter
-
- NS
-
- nociceptive-specific
-
- RF
-
- mechanoreceptive field
-
- SLN
-
- superior laryngeal nerve
-
- TMJ
-
- temporomandibular joint
-
- V1
-
- ophthalmic
-
- V2
-
- maxillary
-
- V3
-
- mandibular
-
- Vc
-
- subnucleus caudalis
-
- WDR
-
- wide dynamic range
-
- XII
-
- hypoglossal