Exercise affects energy metabolism and neural plasticity-related proteins in the hippocampus as revealed by proteomic analysis
Abstract
Studies were conducted to evaluate the effect of a brief voluntary exercise period on the expression pattern and post-translational modification of multiple protein classes in the rat hippocampus using proteomics. An analysis of 80 protein spots of relative high abundance on two-dimensional gels revealed that approximately 90% of the proteins identified were associated with energy metabolism and synaptic plasticity. Exercise up-regulated proteins involved in four aspects of energy metabolism, i.e. glycolysis, ATP synthesis, ATP transduction and glutamate turnover. Specifically, we found increases in fructose-bisphosphate aldolase C, phosphoglycerate kinase 1, mitochondrial ATP synthase, ubiquitous mitochondrial creatine kinase and glutamate dehydrogenase 1. Exercise also up-regulated specific synaptic-plasticity-related proteins, the cytoskeletal protein α-internexin and molecular chaperones (chaperonin-containing TCP-1, neuronal protein 22, heat shock 60-kDa protein 1 and heat shock protein 8). Western blot was used to confirm the direction and magnitude of change in ubiquitous mitochondrial creatine kinase, an enzyme essential for transducing mitochondrial-derived ATP to sites of high-energy demand such as the synapse. Protein phosphorylation visualized by Pro-Q Diamond fluorescent staining showed that neurofilament light polypeptide, glial fibrillary acidic protein, heat shock protein 8 and transcriptional activator protein pur-alpha were more intensely phosphorylated with exercise as compared with sedentary control levels. Our results, together with the fact that most of the proteins that we found to be up-regulated have been implicated in cognitive function, support a mechanism by which exercise uses processes of energy metabolism and synaptic plasticity to promote brain health.
Introduction
The beneficial effects of exercise on the function of the brain and spinal cord are becoming well recognized. A plethora of studies have provided evidence that exercise enhances cognitive function in both humans and animals (Fordyce & Wehner, 1993; Kramer et al., 1999; Laurin et al., 2001). Substantial progress has been made in the last few years in defining the mechanisms underlying the action of exercise on the central nervous system. However, given the complexity of biological functions activated by exercise, there remain major gaps in our knowledge. In particular, it is not well understood how exercise orchestrates the action of multiple factors at the protein level. The development of proteomic technology to scrutinize multiple proteins in the same preparation offers the unique opportunity for studies of complex biological functions, which inherently involve a large number and network of proteins. Proteomics enables us to evaluate protein complexes, signalling pathways and protein changes (Grant & Blackstock, 2001; Phizicky et al., 2003; Tyers & Mann, 2003) to further our understanding of the protein interactions involved in the cellular machinery of exercise.
The goal of the present study was to use a proteomic approach to examine the molecular diversity in the expression pattern and post-translational modifications of protein classes in the hippocampus of rats exposed to exercise. We chose to examine the hippocampus as it is a critical brain region involved in supporting learning and memory processes. Our results showed that exercise up-regulates multiple proteins that have a defined role in energy metabolism, comprising enzymes involved in glucose catabolism, ATP synthesis and glutamate turnover. Moreover, our results showed that exercise increases specific classes of proteins related to synaptic plasticity, i.e. proteins involved in cytoskeletal structure and protein-folding dynamics. Studies have shown that exercise influences molecular systems associated with synaptic function and plasticity (Tong et al., 2001; Molteni et al., 2002; Vaynman et al., 2003) that may ultimately serve higher-order functions such as learning and memory. The fact that brain energy expenditure accounts for about 80% of total body energy consumption, when the brain itself constitutes only about 2% of body weight, emphasizes the importance of energy metabolism in supporting neuronal function and plasticity. Although exercise is intrinsically related to energy metabolism (Maddaiah, 1984; Constable et al., 1987; Westerterp & Plasqui, 2004), the possibility that exercise can affect energy-related proteins has been poorly studied in the brain. Proteomics has enabled us to show that exercise up-regulates maps of proteins related to neurometabolism (Lubec et al., 1999). A unifying theme in our findings is that all proteins affected by exercise have been implicated in cognitive function. These results seem to indicate that the impact of exercise on the brain is achieved by coordination with mechanisms that modulate energy metabolism and synaptic plasticity.
Materials and methods
Exercise paradigm
Adult male Sprague-Dawley rats (approximately 220 g) were purchased from Charles River Laboratories (Wilmington, MA, USA). Rats were randomly assigned to two groups, exercise and sedentary (n = 5 per group), and individually housed in standard polyethylene cages in a 12-h light/dark cycle at 20–22 °C with food and water ad libitum. The exercise rats were given access to a wheel (diameter 31.8 cm, width 10 cm) that freely rotated against a resistance of 100 g and was connected to a computer system (vitalviewer data acquisition system, Minimitter Company, Inc., Sunriver, OR, USA), which monitored their revolutions. We chose to use a voluntary exercise model as our paradigm because it simulates aspects of human behaviour by enabling animals to choose how much to run. The sedentary control rats were confined to the cages with no access to a running wheel. Exercise rats were exposed to five consecutive nights of the running wheel. On the morning immediately following the last night of running, rats were killed by decapitation. Hippocampi were quickly removed, frozen on dry ice and stored at −70 °C until used. All animal protocols were approved by the UCLA Animal Research Committee and followed the guidelines of the American Physiological Society concerning animal care.
Materials
Immobiline DryStrips, CHAPS, acrylamide/Bis solution, dithiothreitol, TEMED, ammonium persulphate and the Reagent Compatible and Detergent Compatible (RCDC) protein assay kit were from Bio-Rad (Hercules, CA, USA). Pepstatin A, leupepsin, α-cyano-4-hydroxycinnamic acid and ProteoProfile™ PTM marker were from Sigma (St Louis, MO, USA). Sequencing grade modified trypsin was obtained from Promega (Madison, WI, USA). The Pro-Q® Diamond phosphoprotein gel stain kit and SYPRO® Ruby protein gel stain kit were from Molecular Probes (Eugene, OR, USA).
Sample preparation
The rat hippocampus was homogenized in the lysis buffer containing 8 m urea, 4% w/v CHAPS and 0.8% Biolytes 100 mm dithiothreitol. Protease inhibitors, 10 µg/mL aprotinin, 1 µg/mL leupeptin and 1 mm phenylmethylsulphonyl fluoride, were contained in the lysis buffer. The protein concentration was determined with the RCDC method with bovine serum albumin as the protein standard.
Two-dimensional gel electrophoresis
The two-dimensional gel electrophoresis gels for the exercise and sedentary groups were run in parallel, such that each rat had a corresponding gel. The two-dimensional gel electrophoresis gels for isoelectric focusing were run using the following presettings (24-cm immobilized ph gradient strips, pH 3–10, 500 mg protein loaded): 200 V for 1 h, 500 V for 1 h, 1000 V for 1 h, 8000 V (gradient) for 0.5 h, maximizing at 8000 V when the total gel running sequence (200–8000 V) reached 30 kV.h (kV × hours). For the second dimension, sodium dodecyl sulphate–polyacrylamide gel electrophoresis gels for the exercise and sedentary groups were run in parallel (260 × 200 × 1.5 mm, 12.5% total monomer, 2.6% crosslinker). No samples were pooled.
Gel staining
The two-dimensional gels were first stained with Pro-Q Diamond dye for visualizing phosphoprotein followed by SYPRO Ruby for visualizing total proteins. Gel imaging was performed on a Molecular Imager FX (Bio-Rad) using excitation and emission wavelengths for both protein dyes. Pro-Q Diamond has an excitation maximum at ∼555 nm and emission at ∼580 nm, and SYPRO Ruby has two excitation maxima, one at ∼280 nm and one at ∼450 nm, and the emission maximum is near 610 nm. ProteoProfile™ PTM marker (Sigma) was used as the positive phosphoprotein standard. Imaging analysis and comparison (between sedentary and exercise groups) were fulfilled with pdquest software (Bio-Rad).
In-gel digestion
Selected protein spots in two-dimensional gels were automatically excised by ProteomeWorks Spot Cutter (Bio-Rad). Gel plugs were dried on a Savant speedvac evaporator (Thermo Electron Corporation, Waltham, MA), rehydrated with 5 µL of 10 ng/µL trypsin solution in 50 mm NH4HCO3 and incubated overnight at 37 °C. The digested peptide fragments were extracted twice with 50 µL of 5% trifluoroacetic acid in 50% acetonitrile.
Mass spectrometry
Peptide mass spectra were recorded in positive ion mode on a Voyager DE-STR MALDI time-of-flight mass spectrometer (Applied Biosystems, Foster City, CA, USA). Mass spectra were obtained by averaging 500 individual laser shots. Two trypsin autohydrolytic peptide isotopic peaks (m/z 842.5094 and 2211.1046) were used for internal calibration standards. When m/z 2211.1046 failed to be observed, an external standard of 0.65 pmol of the synthetic peptide A19L (C9H150N25O29) was mixed with samples, thereby employing m/z 2025.1133 as an internal calibration standard. Selected proteins were identified by MALDI time-of-flight mass spectrometry. Methodologically, four to five matched peptide fragments with mass accuracy < 50 parts per million (p.p.m.) can unambiguously identify a known protein from the database (Berndt et al., 1999). Here, we used more stringent criteria for accepting a database hit. First, due to double internal calibration, the mean mass accuracy of matched proteins used was 11 p.p.m., far < 50 p.p.m.. In addition, most of the identified proteins had more than five matched masses (on average 10 masses matched for each protein) and the average sequence coverage for all identified proteins was 30%(Table 1 shows the average sequence coverage for each protein spot). We also took the relative mass intensity into consideration; for example, for the most intensive masses the three to five strongest signals had to be covered in the matched protein. Such an additional criterion efficiently distinguished the target protein from the interference background. In most cases, the experimental molecular weights and isoelectric points (pIs) matched protein theoretical data (Table 1). To confirm the reliability of the MALDI time-of-flight mass spectrometry, a second method, tandem mass spectrometric analysis, was used to identify two random proteins, glutamate dehydrogenase (GDH)1 and ATP synthase. Tandem mass spectrometric analysis was performed using a QSTAR XL QqTOF MS (Applied Biosystems/MDS Sciex, Applied Biosystems) with the NanoSpray™ ion sources.
| Spots | Protein name | Swiss-proteinentry | Mw/pI*,Experimental | Mw/pI*,Theoretical | Sequence coverage | Matched experimental masses | Mass error (mean, p.p.m.) |
|---|---|---|---|---|---|---|---|
| 1 | Aconitase, mitochondrial | ACON_RAT | 85/7.9 | 85/7.9 | 24% | 935.5, 985.51, 1067.57, 1170.69, 1463.75, 1500.77, 1581.72, 1601.8, 1667.77, 1861.79, 1868.91, 2129.16, 2158.03, 2780.44 | 8 |
| 2 | Actin beta | ACTB_RAT | 45/5.6 | 42/5.23 | 36% | 795.47, 976.45, 1132.53, 1198.71, 1515.77, 1790.92, 1954.04, 2231.05, 2359.13,3199.57 | 8 |
| 3 | Actin beta | ACTB_RAT | 40/5.3 | 42/5.3 | 39% | 795.46, 923.57, 976.46, 1132.53, 1198.7, 1515.76, 1516.72, 1790.9, 1954.06, 2231.07, 3199.7 | 9 |
| 4 | Actin beta | ACTB_RAT | 35/5.6 | 42/5.3 | 39% | 795.47, 976.45, 1132.53, 1198.71, 1515.76, 1516.73, 1790.91, 1954.05, 2231.06, 3199.6 | 6 |
| 5 | Aldo-keto reductase | AK1A1_RAT | 38/7.0 | 36/6.8 | 33% | 897.56, 928.5, 1285.67, 1375.76, 1505.65, 1551.79, 1928.02, 2098.1, 2202.07 | 14 |
| 6 | Aldolase A, | ALDOA_RAT | 36/8.4 | 39/8.7 | 30% | 1044.58, 1342.73, 1646.83, 1668.83, 1824.94, 2123.12, 2139.11, 2272.18, 3039.54 | 11 |
| 7 | Aldolase A | ALDOA_RAT | 35/8.9 | 39/8.7 | 37% | 1107.59, 1342.71, 1646.81, 1646.81, 1668.82, 1808.93, 1808.93, 1824.93, 2088.1, 2091.12, 2258.04, 2272.16 | 4 |
| 8 | Aldolase C | ALDOC_RAT | 40/6.4 | 39/6.7 | 44% | 1018.51, 1050.54, 1173.67, 1421.71, 1644.83, 1652.94, 1667.82, 1810.9, 1811.9, 2299.19, 3067.59 | 9 |
| 9 | Aldolase C | ALDOC_RAT | 40/6.9 | 39/6.7 | 23% | 1644.87, 1652.95, 1667.81, 2299.18, 3067.58 | 10 |
| 10 | Aldolase C | ALDOC_RAT | 39/7.4 | 39/6.7 | 38% | 1018.51, 1173.65, 1421.72, 1488.8, 1644.83, 1652.94, 1667.82, 2100.94, 2299.18, 3067.56 | 11 |
| 11 | ATP synthase alpha chain | ATPA_RAT | 48/8.7 | 59/9.3 | 33% | 892.49, 1026.6, 1438.84, 1483.81, 1553.75, 1575.82, 1610.87, 1683.76, 2325.15, 2338.18, 2338.18, 2369.26 | 7 |
| 12 | ATP synthase alpha chain | ATPA_RAT | 49/8.7 | 59/9.3 55/8.3 | 29% | 892.51, 1000.6, 1026.6, 1438.86, 1553.76, 1575.8, 1610.89, 1911.02, 2325.14, 2338.19, 2369.24 | 11 |
| 13 | ATP synthase alpha chain | ATPA_RAT | 49/8.6 | 55/8.3 | 31% | 1000.58, 1026.58, 1438.85, 1483.84, 1553.74, 1575.8, 1610.88, 1683.8, 2325.14, 2338.18, 2369.25 | 7 |
| 14 | ATP synthase beta chain | ATPB_RAT | 50/5.6 | 56/5.2 | 40% | 1038.6, 1401.7, 1406.69, 1435.76, 1439.79, 1617.8, 1650.93, 1831.86, 1919.11, 1921.99, 1988.03, 3842.95 | 5 |
| 15 | ATP synthase beta subunit | ATPB_RAT | 49/5.3 | 51/4.9 | 52% | 975.57, 1038.6, 1401.69, 1406.7, 1435.77, 1439.8, 1617.8, 1650.94, 1831.86, 1919.13, 1922.00, 1988.06, 2038.98, 2298.07, 2334.16,2691.39, 3714.9, 3714.9, 3843.03 (IGLFGGAGVGK;IPVGPETLgr;VVDLLAPYAK)† | 10 |
| 16 | Chaperonin, (CCT)beta subunit | TCPQ_MOUSE | 49/5.8 | 59/5.4 | 22% | 1130.56£1330.68£1582.94£1956.1£2097.12£2288.18£2363.18 | 9 |
| 17 | Cofilin 1 | COF1_RAT | 16/8.5 | 18/8.5 | 36% | 769.34, 1309.68, 1337.62, 1790.86 | 11 |
| 18 | Creatine kinase, mito-chondrial/(uMtCK) | KCRU_RAT | 42/8.1 | 47/8.7 | 28% | 873.39, 1071.6, 1270.54, 1420.74, 1548.83, 1576.84, 1645.82, 1677.83, 1734.96, 2463.3, 2960.33 | 21 |
| 19 | Creatine kinase, brain B chain | KCRB_RAT | 45/5.7 | 43/5.3 | 36% | 1230.56, 1303.73, 1557.79, 1602.85, 1671.86, 1864.95, 1964.95, 2121.03, 2471.11, 2518.19 | 7 |
| 20 | Cytochrome c oxidase, subunit Va | COX5A_RAT | 16/4.8 | 12/5.0 | 38% | 992.56, 1148.6, 2053.05, 3428.8 | 20 |
| 21 | acetyltransferase | ODP2_RAT | 68/5.7 | 59/5.7 | 10% | 884.53, 1188.66, 1754.94, 1880.07 | 7 |
| 22 | Dihydropyrimidinase-related protein-2 | DPYL2_RAT | 65/5.8 | 62/5.9 | 30% | 766.45, 795.44, 908.49, 1015.55, 1140.61, 1310.68, 1682.89, 1792.83, 1911.03, 2169.08, 2182.1, 2377.18, 2900.51 | 7 |
| 23 | Enolase 2, gamma, neuron specific, NSE | ENOG_RAT | 45/5.3 | 47/5.0 | 22% | 1130.59, 1620.73, 1620.73, 1804.94, 1855.89, 1970.97, 2102.09 | 12 |
| 24 | Enolase 2, gamma, neuron specific, NSE | ENOG_RAT | 44/5.3 | 47/5.0 | 34% | 776.43, 848.42, 1174.58, 1599.73, 1620.71, 1804.95, 1855.89, 1970.93, 2102.1, 2220.19, 2353.1 | 14 |
| 25 | Enolase 2, gamma, neuron specific, NSE | ENOG_RAT | 45/5.1 | 47/5.0 | 29% | 776.41, 840.37, 848.41, 1130.62, 1174.56, 1620.72, 1804.95, 1855.91, 1970.94, 2102.06 | 14 |
| 26 | Enolase , non-neural | ENOA_RAT | 48/5.8 | 47/6.2 | 20% | 1439.76, 1804.95, 1928.95, 1960.92, 2208.01 | 6 |
| 27 | Enolase, non-neural, | ENOA_RAT | 47/5.8 | 47/6.2 | 30% | 704.41, 766.36, 806.46, 1143.65, 1439.74, 1557.74, 1804.94, 1928.93, 2047.04, 2207.98 | 13 |
| 28 | Enolase, non-neural, NNE1 | ENOA_RAT | 47/5.8 | 47/6.2 | 24% | 704.39, 766.36, 806.45, 1439.73, 1804.95, 1928.96, 2047.04, 2208.01 | 10 |
| 29 | Ferritin light chain | FRIL1_RAT | 28/7.5 | 35/6.4 | 15% | 1173.59, 1213.62, 1309.62, 1586.80 | 14 |
| 30 | Glial fibrillary acidicprotein delta (GFAP) | Q9Z2S0_RAT | 49/5.6 | 49/5.7 | 61% | 715.41, 793.47, 898.49, 905.44, 959.5, 988.52, 1039.57, 1060.51, 1094.56, 1098.64, 1177.63, 1224.6, 1245.66, 1277.72, 1291.68, 1387.77, 1409.74, 1499.81, 1546.8, 1619.89, 1629.95, 1664.8, 1795.93, 1871.99,3076.54 | 10 |
| 31 | Glial fibrillary acidicprotein delta (GFAP) | Q9Z2S0_RAT | 59/5.7 | 49/5.7 | 41% | 959.49, 988.51, 1094.55, 1098.63, 1177.64, 1224.59, 1245.65, 1277.72, 1291.68, 1387.77, 1409.74, 1499.77, 1546.8, 1619.89, 1629.94, 1872.02, 1872.02, 3076.55 | 9 |
| 32 | GAPDH | G3P_RAT | 36/7.9 | 36/8.4 | 13% | 1556.82, 1627.97, 1779.82 | 10 |
| 33 | GAPDH | G3P_RAT | 36/8.7 | 36/8.7 | 27% | 665.36, 805.43, 811.39, 1179.61, 1556.83, 1627.96, 1779.82, 2611.33 | 11 |
| 34 | GAPDH | G3P_RAT | 36/8.5 | 36/8.7 | 22% | 811.41, 1556.83, 1627.96, 1779.86, 2611.32 | 14 |
| 35 | GAPDH | G3P_RAT | 36/8.2 | 36/8.4 | 22% | 811.39, 1556.82, 1627.98, 1779.81, 2611.32 | 14 |
| 36 | GAPDH | G3P_RAT | 36/7.9 | 36/8.7 | 20% | 1556.87, 1627.96, 1779.8, 2611.34 | 12 |
| 37 | Guanine deaminase | GUAD_RAT | 46/5.6 | 51/5.5 | 25% | 998.52, 1058.63, 1173.63, 1371.8, 1544.75, 1700.87, 2074.99, 2087.07, 2147.13,2511.26 | 14 |
| 38 | H+-ATP synthaseatpase, V1 subunit A | Q6IRF8_RAT | 74/5.6 | 68/5.4 | 32% | 885.44,1145.61, 1230.64, 1262.63, 1308.73, 1316.74, 1487.73, 1731.82, 1731.82, 1749.88, 1797.81, 1883.78, 2042, 2086.05, 2143.06, 2186.12, 2681.26 | 16 |
| 39 | H+-ATP synthase, | AT5G2_RAT | 22/5.8 | 19/6.2 | 32% | 1177.55, 1338.64, 1417.72, 1980.03 | 9 |
| 40 | HSP 60kD (HSP60) | CH60_RAT | 58/5.7 | 61/5.9 | 17% | 1684.89, 1905.07, 1938.9, 2033.15, 2365.32, 2560.25 | 8 |
| 41 | HSP 70Kd | Q9DC41_MOUSE | 79/5.6 | 72/5.1 | 36% | 986.53,997.51, 1074.56, 1191.64,1210.6,1224.59, 1228.64,1233.62, 1316.65, 1329.62, 1460.78, 1528.75, 1566.79, 1588.88,1590.79, 1590.79, 1604.83, 1816.01, 1887.98, 1934.04, 1999.11, 2016.07, 2149.02, 2178.02 | 11 |
| 42 | Heat shock protein 8 (Hsc71) | HSP7C_RAT | 73/5.6 | 71/5.4 | 22% | 1081.55, 1199.68, 1228.63, 1253.63, 1480.76, 1487.73, 1691.74, 1982.01, 2774.3, 2997.44 | 9 |
| 43 | Hsp70/Hsp90-organizing protein | STIP1_RAT | 64/6.3 | 63/6.4 | 16% | 1004.54, 1065.56, 1116.64, 1132.64, 1242.66, 1298.64, 1455.74, 1488.8,1812.98 | 10 |
| 44 | Internexin alpha | AINX_RAT | 63/5.6 | 56/5.2 | 24% | 1056.62, 1133.57, 1518.87, 1544.79, 2143.08, 2175.06, 2527.39 | 12 |
| 45 | Isovaleryl-coa dehydrogenase | IVD_RAT | 43/8.6 | 46/8.5 | 14% | 840.41, 1012.54, 1280.68, 1467.76, 2301.18 | 13 |
| 46 | LDH, B isoform | LDHB_RAT | 38/5.7 | 36/5.7 | 24% | 742.46, 913.59, 957.6, 1186.64,1248.6, 1629.84, 2312.15 | 6 |
| 47 | Memory related protein-2 (GDH-1) | DHE3_RAT | 51/7.2 | 56/6.7 | 48% | 963.54, 1016.45, 1059.55, 1196.68, 1425.64, 1507.72, 1723.89, 1764.88, 1894.09, 1931.91, 1936.92, 2242.17 (YNLGLDLR;ALASLMTYK;YSTDVSVDEVK)† | 6 |
| 48 | Myelin basic protein | MBP_RAT | 17/8.5 | 21/11.2 | 45% | 726.38, 728.33, 1110.53, 1339.7, 1352.61, 1460.71,1800.85 | 11 |
| 49 | Neurofilament, light polypeptide | NFL_RAT | 69/4.8 | 61/4.6 | 20% | 869.47, 1021.54, 1021.54, 1072.56, 1154.73, 1281.63, 1723.8, 1747.88, 2295.13 | 14 |
| 50 | PGP9.5 | UCHL1_RAT | 26/5.5 | 25/5.1 | 37% | 886.47, 1863.83, 1983.95, 2230.04, 2519.33 | 25 |
| 51 | PGP9.5 | UCHL1_RAT | 26/5.4 | 25/5.1 | 36% | 886.5, 980.51, 1983.91, 2230.05, 2519.26, 2976.51 | 10 |
| 52 | Neuronal protein 22(NP22) | Q8VHH3_RAT | 22/6.6 | 23/6.8 | 31% | 965.46, 1015.47, 1121.58, 1267.67, 1665.93, 2358.21 | 11 |
| 53 | Nucleoside diphos-phate kinase B | NDKB_RAT | 17/6.9 | 17/6.9 | 32% | 1051.5, 1175.67, 1344.77, 1801.88 | 9 |
| 54 | 3-Oxoacid coA transferase | Q5XIJ9_RAT | 51/7.4 | 56/7.1 | 14% | 776.42, 1647.76, 2159.15, 2217.15, 2407.11 | 14 |
| 55 | Peroxiredoxin 2 | PRDX2_RAT | 22/5.5 | 22/5.3 | 28% | 789.42, 937.45, 1108.59, 1211.69, 1706.91, 1835.07, 2699.36, 2827.39 | 15 |
| 56 | Phosphatidylethanol-amine binding protein | PEBP2_MOUSE | 22/5.6 | 21/5.5 | 29% | 912.49, 1560.83, 1688.93, 1758.85, 1963.94 | 9 |
| 57 | Phosphoglyce-rate kinase 1 (PGK-1) | PGK1_RAT | 39/8.7 | 44/8.3 | 23% | 1101.55, 1634.81, 1769.02, 2067.07, 2772.35 | 12 |
| 58 | Phosphoglycerate mutase B chain | Q6P0K6_RAT | 32/6.3 | 29/6.7 | 32% | 1150.69, 1312.63, 2131.12, 2425.19, 2433.05 | 15 |
| 59 | Prohibitin | PHB_RAT | 32/5.7 | 30/5.6 | 48% | 1058.54, 1149.6, 1185.67, 1396.85, 1460.66, 1606.86, 1998.1, 2119.12, 2371.28 | 9 |
| 60 | Proteindisulfide-isomerase A3 | PDIA3_RAT | 59/5.7 | 57/6.0 | 15% | 1188.52, 1191.61, 1397.72, 1529.79, 1587.83, 2302.24 | 15 |
| 61 | Pyruvate kinase | Q6P7S0_RAT | 52/7.0 | 53/8.4 | 13% | 990.56, 1359.71, 1473.82, 1875.9, 2435.3 | 12 |
| 62 | Serum albumin precursor | ALBU_RAT | 71/5.7 | 69/6.1 | 26% | 1266.62, 1439.8, 1455.8, 1465.8, 1479.8, 1609.81, 1662.86, 1882.94, 1948.93, 2060.04, 2139.01 | 10 |
| 63 | Serum albumin precursor | ALBU_RAT | 71/5.7 | 69/6.1 | 34% | 1134.47,1149.58, 1266.6, 1299.71, 1439.78, 1455.8, 1465.78, 1479.81, 1563.74, 1609.8, 1662.86, 1684.82, 1714.8, 1882.94, 1948.95, 2060.04, 2139.00 | 13 |
| 64 | Synapsin II | SYN2_RAT | 55/6.3 | 52/8.4 | 8% | 1249.70, 1567.75, 1677.83 | 22 |
| 65 | Synaptosomal-assoc-iatedprotein 25 kda | SNP25_RAT | 25/4.7 | 21/4.7 | 43% | 1669.81, 1669.81, 1676.81, 1776.74, 1822.91, 2175.99 | 10 |
| 66 | Transcriptional activator | PUR_MOUSE | 40/5.9 | 35/6.1 | 37% | 750.43, 788.37, 1060.5, 1078.61, 1216.61, 1368.7, 2311.25, 2889.39, 3270.53 | 9 |
| 67 | Tubulin alpha 1 | TBA1_RAT | 57/4.7 | 50/4.9 | 22% | 1023.45, 1085.64, 1265.55, 1410.79,1487.9,1701.94, 1718.9, 1756.96 | 10 |
| 68 | Tubulin alpha 1 | TBA1_RAT | 58/4.9 | 50/4.9 | 14% | 1487.84 1593.87 1701.91 1718.86 2409.22 | 10 |
| 69 | Tubulin alpha 1 | Q52L87_MOUSE | 57/5.0 | 50/4.9 | 18% | 1085.62, 1410.76, 1487.88, 1701.92, 1718.9, 1756.96, 2409.19 | 7 |
| 70 | Tubulin alpha 6 | Q52L87_MOUSE | 57/5.8 | 63/5.9 | 14% | 1487.9, 1701.91, 1718.88, 2409.22 | 6 |
| 71 | Tubulin beta 15 | TBB1_RAT | 50/4.7 | 48/4.7 | 29% | 1039.6, 1077.52, 1130.6, 1159.62, 1245.58, 1258.72, 1287.72, 1401.68, 1401.68, 1631.83, 1636.82, 1659.89,1959.01, 2798.37 | 9 |
| 72 | Tubulin beta 15 | TBB1_RAT | 50/4.5 | 48/4.7 | 41% | 1039.59, 1077.52, 1130.59, 1159.61, 1245.58, 1258.71, 1287.69, 1401.78, 1631.82, 1636.82, 1659.82, 1696.79, 1822.86, 1959.01, 2724.31, 2798.39 | 19 |
| 73 | Tubulin beta 15 | TBB1_RAT | 58/5.5 | 50/4.8 | 41% | 1028.52, 1039.6, 1077.53, 1130.6, 1159.62, 1245.59, 1258.69, 1287.71, 1620.84, 1631.83, 1636.83, 1958.99, 2724.33, 2798.37, 3102.43 | 4 |
| 74 | Tubulin beta 15 | TBB1_RAT | 58/5.6 | 50/4.8 | 40% | 738.35, 1028.53, 1039.6, 1077.52, 1130.6, 1159.61, 1245.58, 1258.69, 1287.72, 1631.84, 1636.81, 1822.84, 1958.98, 2724.33, 2798.34 | 9 |
| 75 | Tubulin beta 15 | TBB1-RAT | 58/5.7 | 50/4.8 | 52% | 534.3, 738.36, 1028.52, 1053.61, 1077.52, 1130.6, 1143.63, 1159.62, 1229.61, 1245.59, 1258.7, 1287.71, 1620.84, 1631.83, 1636.82, 1696.81, 1822.92, 1873.94, 1958.99, 2724.32, 3102.42 | 6 |
| 76 | Tubulin beta 15 | TBB1_RAT | 50/4.5 | 48/4.7 | 23% | 1077.52, 1130.6, 1159.61, 1258.74, 1287.71, 1631.81, 1636.81, 1959.00 , 2798.30 | 15 |
| 77 | Tubulin beta 15 | TBB1_RAT | 35/5.6 | 50/4.5 | 35% | 534.31, 738.34, 1077.53, 1130.61, 1159.62, 1258.71, 1287.72, 1355.67, 1631.82, 1636.81, 1723.9, 1822.93, 1958.99, 2798.35 | 10 |
| 78 | Ubiquitin | UBIQ_RAT | 11/6.6 | 9/6.6 | 47% | 1039.51, 1067.62, 1523.78, 1787.92, 2130.16 | 3 |
| 79 | Valosin-containing protein | TERA_RAT | 95/5.6 | 89/5.1 | 21% | 1067.53, 1329.72, 1645.83, 1823.91, 1823.91, 2185.94, 2498.33, 2518.41, 3672.84 | 14 |
| 80 | Voltage-dependent anion channel 1 | VDAC1_RAT | 31/8.2 | 32/8.3 | 33% | 1946.03, 2103.17, 2128.05, 2176.06, 2600.18 | 8 |
- A stringent criterion for protein identification was used, employing a mean mass accuracy of 11 p.p.m. with more than five matched masses for most of the identified proteins. *Molecular weight/isoelectric point. †Matched peptide fragment sequences from tandem mass spectrometric analysis.
Database search
Protein identification with peptide mass data was accomplished using the ProFound searching tool against the latest version of non-redundant database (NCBInr) (http://prowl.rockefeller.edu/profound_bin/WebProFound.exe?FORM=1). Interference mass signals from matrix, keratins and trypsin were manually picked out before database searching (Ding et al., 2003). Searching parameter presettings were as follows: maximum cleavage missing, 1; mass tolerance, ± 0.1 Da; taxonomic category, Rattus; cysteine and methionine residues partially alkylated and oxidized, respectively. The ProFound search engine was used to calculate the Bayesian probability of each candidate sequence being the protein analysed by comparison of search results against the estimated random match population (Zhang & Chait, 1995.). Z-scores were obtained based on the probability value of each protein candidate. Z-scores > 1.65 were considered significant (P < 0.05). Only proteins with an expression fold change ≥ 1.6 (previously established in Ding et al. 2006) with a Z-score of > 1.65 were considered as biological differences differentially expressed by exercise.
Western blot
Protein changes of creatine kinases in the hippocampus were checked by western blot, using the tissue from the same animals used for proteomic analysis (n = 5 animals per group). Briefly, equal amounts of protein samples (25 µg) were separated on a 12.5% polyacrylamide gel and electrotransferred to a polyvinylidene difluoride membrane. Non-specific binding sites were blocked for 1 h at room temperature (25 °C) with 5% non-fat milk in Tris-buffered saline with tween buffer, pH 7.5, and then incubated overnight at 4 °C with the primary antibody of either brain-type creatine kinase (CKBB) (1 : 1000, rabbit IgG, Biogenesis) or ubiquitous mitochondrial creatine kinase (uMtCK) (1 : 1000, goat IgG, Santa Cruz Biotechnology) followed by 1 h incubation at room temperature with secondary anti-rabbit (or goat) horseradish peroxidase conjugate (1 : 10 000). As previously described, actin (primary antibody 1 : 2000; Santa Cruz Biotechnology, followed by the secondary anti-goat horseradish peroxidase conjugate) was used as the standard internal control (Molteni et al., 2004; Vaynman et al., 2006a, b) in the CKBB and uMtCK western blots. Immuno-complexes were visualized by chemiluminescence using the ECL kit (Amersham Pharmacia Biotech Inc., Piscataway, NJ, USA). Statistical differences were considered significant when P < 0.05. We used the mean value of the sedentary control group to calculate the percentage for each animal for the sedentary and exercise groups. A t-test was used for between-group comparisons. Values represent the mean ± SEM.
Results
Representative two-dimensional maps of overall protein expression from the hippocampus of sedentary and exercise rats are shown in Fig. 1. The majority of proteins were distributed on the left and central regions of the map in the Cartesian coordinate system. What this means is that the major proteins displayed here are acidic and neutral proteins. This is consistent with the fact that nervous system proteins are mainly composed of acidic and neutral proteins (Yang et al., 2004; Witzmann et al., 2005). In terms of protein size, the majority of proteins were between 30 and 100 kDa. Differentially displayed proteins induced by exercise were found by map matching. Enlarged areas from boxes in Fig. 1A and B are shown in the panels underneath (Fig. 1C and D) and demonstrate representative protein expression changes.
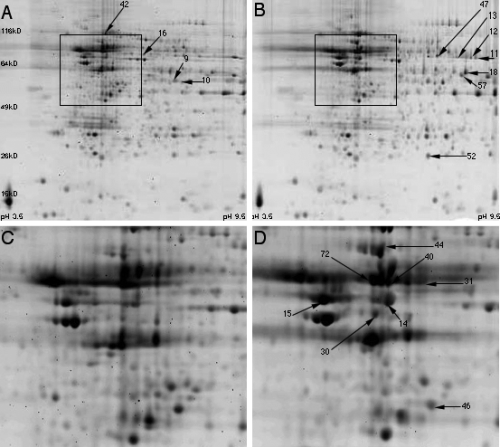
Representative two-dimensional gel electrophoresis maps of the hippocampus from sedentary (A) and exercise (B) rats. The boxes in A and B represent the areas enlarged in C and D showing the position of protein spots. All protein spots differentially expressed by exercise in Tables 2 and 3 have been labelled to be consistent with the number designation set out in Table 1.
A total of 80 protein spots of relatively high abundance were identified by peptide mass mapping (Table 1). Several proteins had multiple protein spots, possibly due to post-translational modifications. Protein expression changes of two creatine kinases, i.e. cytosolic CKBB and mitochondrial uMtCK, were confirmed by western blot (Fig. 3).
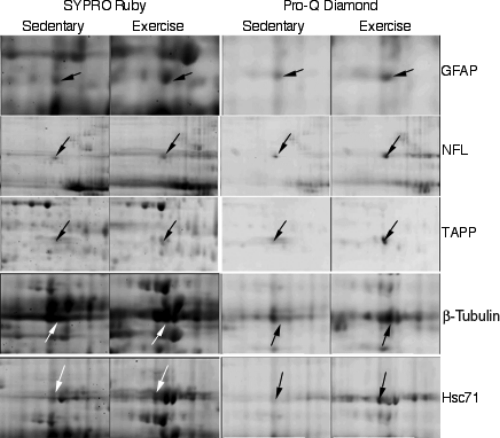
Differential change in the pattern of total (SYPRO Ruby-stained) and phosphorylated (Pro-Q Diamond-stained) hippocampal proteins induced by voluntary exercise. GFAP, glial fibrillary acidic protein; NFL, neurofilament light polypeptide; TAPP, transcriptional activator protein pur-alpha; Hsc71, heat shock protein 8.
The Pro-Q Diamond phosphoprotein gel stain provided a method for selectively staining phospho-proteins in polyacrylamide gels (Martin et al., 2003). This fluorescent stain allowed the direct in-gel detection of phosphate groups attached to tyrosine, serine or threonine residues. Representative Pro-Q Diamond-stained spots are shown in Fig. 3. Phosphorylation intensity fold changes and possible phosphate sites are listed in Table 4, from which the phosphorylated proteins fall into the category of plasticity-related proteins. We found that the phosphorylated proteins glial fibrillary acidic protein (GFAP), neurofilament light polypeptide (NFL), transcriptional activator protein pur-alpha (TAPP), β-tubulin and heat shock protein 8 exhibited changed fold increases with exercise (Table 4). For illustrative purposes, all exercise-modified proteins are grouped in Tables 2–4according to their functions and are summarized below.
| Protein name | Protein (fold change) | Possible phosphosites* | |
|---|---|---|---|
| Phospho- protein | Total protein | ||
| Beta tubulin | 2.0 | 1.9 | GIDPTGSY36HGDSDLQ |
| Neurofilament light polypeptide | 2.7 | 1.1 | SSLSVRRS55YSSSSGSSSGSLMPS66LENLDLSEAKDEPPS472EGEAEEE |
| Glial fibrillary acidic protein delta | 1.7 | 1.7 | MERRRIT7SARRSYAMERRRITS8ARRSYASRITSARRS12YASETVVLGTIPRLS36LSRMTPP |
| Heat shock protein 8 | 4.8 | 3.4 | GIDLGTTY15SCVGVFQGVPQIEVT477FDIDANG |
| Transcriptional activator protein pur-alpha (TAPP) | 2.5 | 1.0 | SRLTLSMS104†VAVEFRDQEETTAAT309†LLLQGEE |
- *All possible phosphosites are cited from the PhosphoSite ™ website unless otherwise indicated (http://www.phosphosite.org). †These two possible phosphosites are predicted by Scansite motif scan (http://scansite.mit.edu/motifscan_id.phtml).
| Protein name | Spots | Total protein (fold change) | Function | Z-score |
|---|---|---|---|---|
| Energy generation | ||||
| Fructose-bisphosphate aldolase C | 9 | 2.8 | Brain isoform, glycolysis | 2.43 |
| 10 | 1.7 | 2.43 | ||
| Phosphoglycerate kinase 1 (PGK-1) | 57 | 1.9 | glycolysis | 2.16 |
| Lactate dehydrogenase B (LDH) | 46 | 1.8* | Turnover of pyruvate to lactose | 2.25 |
| ATP synthase alpha chain, mitochondrial precursor | 11 | 1.9 | Mitochondrial, F1 unit | 2.43 |
| 12 | 3.0 | 2.37 | ||
| 13 | 3.0 | 2.43 | ||
| ATP synthase beta chain, mitochondrial precursor | 14 | 1.9 | Mitochondrial, F1 unit | 2.43 |
| 15 | 1.8 | 2.43 | ||
| Energy transduction | ||||
| Ubiquitous mitochondrial creatine kinase (uMtCK) | 18 | 1.7 | Mitochondrial | 2.30 |
| Glutamate turnover | ||||
| Glutamate dehydrogenase-1 (GDH-1, memory related protein 2) | 47 | 1.7 | Glutamate breakdown | 2.14 |
- Proteins have been organized according to their functional and sequential roles in energy metabolism, to comprise the categories of energy generation, energy transduction and glutamate turnover. A protein fold change of ≥ 1.6 and a Z-score of > 1.65 were considered to reflect a significant effect of exercise on protein expression, i.e. the proteins were considered to be differentially expressed by exercise. *LDH protein decreased.
| Protein name | Spots | Total protein (fold change) | Z-score |
|---|---|---|---|
| Cytoskeletal proteins | |||
| Beta tubulin | 72 | 1.9 | 1.89 |
| Glial fibrillary acidic protein delta (GFAP) | 30 | 1.7 | 2.43 |
| 31 | 1.7 | 2.43 | |
| α-internexin | 44 | 2.0 | 2.03 |
| Chaperones | |||
| Heat shock protein 8 (Hsc71) | 42 | 3.4 | 1.72 |
| Heat shock 60Kd protein 1 (HSP60) | 40 | 2.0 | 2.43 |
| Chaperonin containing TCP-1 beta subunit (CCT) | 16 | 1.9 | 2.38 |
| Neuronal protein 22 (NP22) | 52 | 2.2 | 2.43 |
- Proteins have been organized according to their functional roles in synaptic plasticity, comprising the categories of cytoskeletal proteins and chaperones. A protein fold change of ≥ 1.6 and a Z-score of > 1.65 were considered to reflect a significant effect of exercise on protein expression, i.e. the proteins were considered to be differentially expressed by exercise. TCP-1, t-complex peptide 1.
Proteins involved in energy metabolism
Enzymes involved in glucose catabolism
We found that many enzymes pertaining to glycolysis were affected by exercise (Table 2). Exercise up-regulated the brain isoform of aldolase C and phosphoglycerate kinase 1 (PGK-1). We found two protein spots for aldolase C with change fold increases of 1.7 and 2.8 (Table 2). PGK-1 exhibited a fold change increase of 1.9 (Table 2). In contrast, enzymes involved in the subsequent steps of energy metabolism related to the tricarboxylic acid cycle, such as dihydrolipoyllysine-residue acetyltransferase and aconitase, remained unchanged. Mitochondrial isovaleryl-coA dehydrogenase, an enzyme involved in amino-acid catabolism, remained unchanged during exercise. We found that the memory-related GDH-1, an enzyme facilitating the turnover of the excitatory neurotransmitter glutamate to 2-oxoglutarate was differentially expressed by exercise, exhibiting a protein fold change of 1.7 (Table 2).
Mitochondrial ATP synthases
The mitochondrial ATP synthase complex is composed of F1 and F0 units. The F1 mitochondrial ATPase forms the catalytic component whereas the F0 unit functions as the membrane proton channel. Exercise significantly affected the expression of the mitochondrial ATP synthase by increasing both the alpha and beta chains of the F1 unit. We found three protein spots for the alpha form, with increased fold changes from 1.9 to 3.0 (Table 2). Two protein spots for the beta form expressed increased fold changes of 1.8 and 1.9 (Table 2).
Enzymes of energy transduction
Creatine kinases reversibly catalyse the transfer of phosphate between ATP and creatine as well as various other phosphogens. We found that the increase of uMtCK was pronounced following exercise, showing a fold change of 1.7 (Table 2). However, the brain cytosolic isoform, CKBB, was not significantly changed with exercise, with a protein fold change of 1.5. Western blot analysis confirmed that exercise significantly increased the levels of uMtCK (129 ± 7.2%) above sedentary control levels (100 ± 6.2%) without significantly altering the levels of CKBB (110 ± 16.5%) from those of sedentary control animals (100 ± 18.2%; Fig. 2).
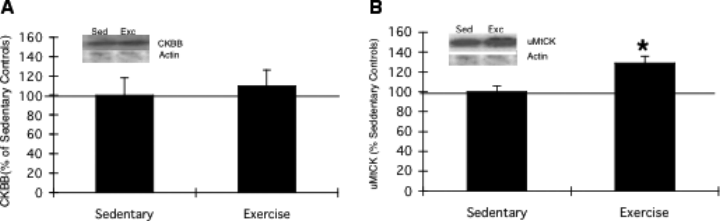
Western blot of brain-type (CKBB) (A) and ubiquitous mitochondrial (uMtCK) (B) creatine kinases. Consistent with two-dimensional gel electrophoresis analysis, exercise significantly increased uMtCK expression without significantly affecting the cytosolic isoform CKBB. Representative immunoblots for CKBB and uMtCK, with actin as the control, are shown for the exercise (Exc) and sedentary (Sed) control groups. Results are displayed as percentages of sedentary levels (represented by the 100% line). Data are expressed as the mean ± SEM (n = 5 per group, t-test, *P < 0.05).
Proteins involved in synaptic plasticity
Cytoskeletal proteins
Cytoskeletal proteins form the structural integrity necessary for synaptic plasticity. Our results show that exercise up-regulated cytoskeletal proteins in the hippocampus. We found a protein fold increase in β-tubulin of 1.9 and α-internexin of 1.7 (Table 3). Moreover, the phosphorylated form of β-tubulin was differentially expressed after exercise with a fold change of 2.0 (Table 4). We found two spots for glial fibrillary acidic protein (GFAP), both exhibiting a fold increase of 1.7 (Table 3). Additionally, Pro-Q Diamond fluorescent staining showed that the phosphorylation of GFAP was also significantly enhanced with exercise, with an intensity fold change of 1.7 (Table 4, Fig. 3). Interestingly, Pro-Q Diamond fluorescent staining showed that the phosphorylated form of NFL was increased with exercise, exhibiting a fold change of 2.7, even though its total protein levels were not differentially increased by exercise (protein fold 1.1; Table 4). We also found an increased fold change in the phosphorylated form of the TAPP following exercise, exhibiting a protein fold increase of 2.5 (Table 4; Fig. 3).
Chaperones
Chaperones are a category of molecules that assist in protein folding and oligomeric assembly in which many heat shock proteins have been categorized as neural plasticity-related molecules (Nedivi et al., 1993; Hong et al., 2004). In this experiment, we found that voluntary exercise increased the expression of heat shock protein 8, heat shock 60-kDa protein 1 and chaperonin-containing t-complex peptide 1 (TCP-1) (CCT) (beta subunit) in the hippocampus (Table 3). Neuronal protein 22 (NP22), a newly identified chaperone-like protein, was also found to be significantly up-regulated by exercise with a fold change of 2.2 (Table 3). Heat shock 60-kDa protein 1 exhibited a fold change of 2.0. CCT (beta subunit) displayed a fold change of 1.9 (Table 3). Of the chaperones that we found to be increased with exercise, hippocampal heat shock protein 8 exhibited the largest fold change of 3.4 (Table 3) and was significantly phosphorylated following exercise, exhibiting a fold change of 4.8 (Table 4).
Discussion
Studies have shown that exercise is capable of inducing molecular mechanisms that support cognitive function. However, it is not well understood how exercise orchestrates the action of multiple factors at the protein level, in particular those related to energy metabolism and synaptic plasticity. Proteomics provided us with a powerful tool to profile protein expression and modification on a global level. After analysing many protein spots of relatively high abundance on two-dimensional gel electrophoresis gels from the hippocampus, it was particularly evident that the proteins differentially expressed by exercise were associated with energy metabolism and neuronal plasticity.
Exercise up-regulates proteins involved in energy metabolism
Proteins involved in glucose catabolism
Short-term exercise up-regulates enzymes in the energy-consuming and energy-generating steps of glycolysis, fructose-bisphosphate aldolase C and PGK-1, respectively. Aldolase C catalyses fructose 1,6-bisphosphate to glycerone phosphate and glyceraldehyde 3-phosphate (Penhoet & Rutter, 1975). PGK-1 is involved in the second step of the second phase of glycolysis, to convert 1,3-diphosphoglycerate to 3-phosphoglycerate while forming one molecule of ATP. ATP from PGK-1 can be used to ensure maximum glutamate accumulation into synaptic vesicles (Fig. 4). A reduction in PGK-1 is associated with age-related impaired cellular processes (Oliver et al., 1987). It has been found that caloric restriction, which has been shown to have a beneficial effect on synaptic plasticity and cognitive function, increases PGK-1 levels in the hippocampus (Poon et al., 2006).
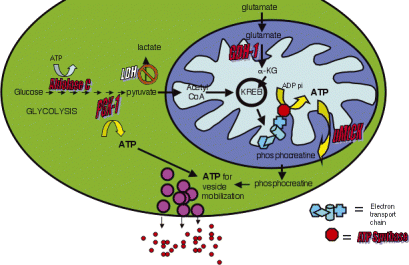
Potential mechanism by which exercise modulates proteins involved in energy metabolism and synaptic plasticity in the hippocampus. Exercise up-regulates proteins involved in four stages of energy metabolism: glucose catabolism, ATP synthesis, energy transduction and glutamate turnover. The increase in the enzymes involved in the two stages of glycolysis, fructose-bisphosphate aldolase C and phosphoglycerate kinase 1 (PGK-1), provides more substrate for the Kreb cycle. Likewise, the breakdown of glutamate to α-ketoglutarate by glutamate dehydrogenase (GDH)1 not only increases the turnover of glutamate but also provides another entry point to the Kreb cycle. An increase in the alpha and beta forms of ATP synthase, which form the complex for ATP synthesis, may constitute a mechanism by which exercise increases energy production. Any increased mitochondrial ATP supply can be transferred to areas in need of energy in the cytoplasm by phosphocreatine produced through ubiquitous mitochondrial creatine kinase (uMtCK). Increased ATP generated from the mitochondrion and from glycolysis may be used to advance vesicle mobilization necessary for high synaptic transmission. Excess glutamate can then be recycled by GDH-1 into the Kreb cycle to perpetuate ATP production. Proteins increased by exercise are shown in red. Lactate dehydrogenase (LDH) is shown in white to indicate the possible decline in the reliance on the pyruvate to lactate conversion for energy production during exercise. α-KG, α-ketoglutarate.
Proteins involved in ATP synthesis
Proteomics revealed that exercise increased ATP synthase alpha and beta forms, members of the F1 synthase enzymatic complex that synthesizes ATP in oxidative phosphorylation (Poon et al., 2006). ATP synthase may contribute to the metabolic malfunction (consisting of mitochondrial dysfunction and glutamate dysregulation) characterizing age-related cognitive impairment (Poon et al., 2006).
Our results showed that exercise decreased lactate dehydrogenase, which exhibited a protein fold decrease of 1.8 (Table 2). Lactate dehydrogenase provides another pathway to energy production, an alternative to the Kreb cycle, by catalysing the interconversion of pyruvate to lactate with the concomitant interconversion of NADH and NAD+. Our finding that exercise produced a decrease in lactate dehydrogenase may indicate that the lactate is not a primary source of energy production (Fig. 4). Given that lactate dehydrogenase levels have been used as a measure of neuronal plasma membrane damage for the effects of oxidative stress (Laev et al., 1993; Cimarosti et al., 2005), our findings may also indicate that exercise has a beneficial affect on neuronal integrity.
Proteins involved in energy transduction
Exercise up-regulated uMtCK, a finding concordant with evidence that uMtCK is modulated by neuronal activity (Boero et al., 2003). uMtCK is functionally coupled to oxidative phosphorylation, as mitochondrial-derived ATP is preferentially used by uMtCK to transfer high-energy phosphates to creatine (Jacobus & Lehninger, 1973; Rojo et al., 1991). The resultant phosphocreatine acts as the cytosolic transport and storage form of energy; it is used to regenerate ATP from cytosolic ADP (Rango et al., 1997), which is essential for maintaining the ATP supply during high synaptic transmission (Whittingham & Lipton, 1981; Fig. 4). Increased energy demands modify uMtCK distribution and expression, thus providing a mechanism of energy transduction in which ATP synthesized in the mitochondrion is transferred to sites of ATP utilization.
Proteins involved in glutamate turnover
Exercise increased the inner mitochondrial enzyme GDH-1. GDH-1 eliminates excitotoxic glutamate in the brain by oxidizing glutamate to α-ketoglutarate (Cooper & Meister, 1985). GDH activity is prominent in synaptic terminals (Lai et al., 1977, 1986; Lai & Clark, 1979; Erecinska & Nelson, 1990; McKenna et al., 1993), where much of the endogenous glutamate enters into the Kreb cycle primarily through GDH (McKenna et al., 2000; Fig. 4). Widely expressed throughout the hippocampus, GDH may support learning and memory by increasing glutamate turnover (Sakimura et al., 1995; McHugh et al., 1996; Cavallaro et al., 1997; Fig. 4). In fact, GDH mRNA levels increase in the hippocampus after spatial learning (Cavallaro et al., 1997).
Exercise up-regulates proteins involved in synaptic plasticity
Expanding the cellular network: cytoskeletal proteins
Studies have shown that exercise induces neurogenesis in the adult brain (van Praag et al., 1999, 2005). In support of this, our results indicate that exercise increases a diverse set of proteins related to cytoskeletal function and neuronal development, i.e. α-internexin, phosphorylated NFL, phosphorylated TAPP, β-tubulin, GFAP (total and phosphorylated) and NP22 (Fig. 3, Table 4).
α-internexin, another neurone-specific protein (Evans et al., 2002), is critical for neuronal development (Kaplan et al., 1990; Fliegner et al., 1994) as it stabilizes neurones and their processes by providing a scaffold for the assembly of other intermediate filament proteins (Fliegner et al., 1994). It is notable that we found increases in both α-internexin and phosphorylated NFL after exercise, as both may indicate the presence of new neurones. In the developing brain, α-internexin and NFL are sequentially expressed, with the presence of NFL and other intermediate filaments designating different states of neuronal maturation (Shaw & Weber, 1982; Carden et al., 1987; Kaplan et al., 1990). α-internexin may also be important for energy homeostasis, as it is significantly correlated with glutamate transporter levels in the energetically compromised postinjury brain (Yi et al., 2005). The contribution of α-internexin to cytoskeletal integrity is important for axonal calibre and nerve conduction speed, intimating that a dysfunction in this and other intermediate filaments may contribute to the memory deficits seen in diseases characterized by cognitive impairment (Lalonde & Strazielle, 2003).
Exercise increased the phosphorylated form of the TAPP. TAPP regulates the transcription of several genes including myelin basic protein (Tretiakova et al., 1999) and the nicotinic acetylcholine receptor β4 gene (Du et al., 1997; Melnikova et al., 2000). Knockout of pur-alpha in mice has been found to produce neural-specific abnormalities associated with abnormal cellular proliferation (Khalili et al., 2003).
Increases in GFAP may indicate the action of exercise on astrocyte proliferation or neurogenesis. GFAP is generally used as a hallmark of mature astrocytes (Raju et al., 1981), although in the hippocampal subventricular zone, GFAP-positive cells can generate both neurones and glia (Doetsch et al., 1999). GFAP is associated with cognitive function and synaptic plasticity, as both inhibitory avoidance and habituation alter the in vitro phosphorylation of NFL and GFAP (Schroder et al., 1997).
Neuronal protein 22 is a novel neurone-specific protein that has putative cytoarchitectural functions as it holds an actin-binding domain and shares sequence homology with proteins, calponin and smooth muscle 22α (SM22α), that bind the cytoskeleton (Ito et al., 2005). NP22 colocalizes with all three major components of neuronal microtubules, tau, α-tubulin and MAP2 (Depaz et al., 2005), and with F-actin (Depaz et al., 2005), which is highly expressed in dendrites, synapses, dendritic spines and postsynaptic densities (Markham & Fifkova, 1986; Fifkova & Morales, 1992; Adam & Matus, 1996; Halpain, 2000). These qualifications, coupled with the finding that NP22 has a protein kinase C binding domain for extracellular-induced activation (Niki et al., 1996), suggest that NP22 may be involved in mediating changes in synaptic plasticity.
Molecular chaperones
Molecular chaperones are a category of proteins that are involved in protein import, folding and assembly (Beckmann et al., 1990). Their functions extend to combating oxidative stress and apoptosis and facilitating synaptic activity, as illustrated by the chaperones that we found to be up-regulated by exercise, i.e. CCT, heat shock 60-kDa protein 1 and heat shock protein 8.
CCT belongs to a subclass of the chaperonin family that facilitates the folding of large non-native proteins (Kubota et al., 1995; Hartl & Hayer-Hartl, 2002). CCT maintains cytoarchitecture by correcting the three-dimensional structure of cytoskeletal proteins such as actin, tubulin and other polypeptides in the cytosol (Sternlicht et al., 1993). CCT may also help the cell withstand oxidative stress by promoting cellular homeostasis (Kane et al., 2004). In fact, the neurodegenerative diseases Alzheimer's and Parkinson's, with an aetiology of oxidative stress (Mariani et al., 2005), have been linked to CCT down-regulation (Lopez Salon et al., 2000; McNaught & Jenner, 2001).
Heat shock 60-kDa protein 1 is a mitochondrial chaperone that may protect mitochondria from oxidative stress by facilitating the proper assembly of mitochondrial proteins (Bozner et al., 2002; Boyd-Kimball et al., 2005). Heat shock 60-kDa protein 1 has anti-apoptotic functions (Lin et al., 2001) and its expression is decreased in Alzheimer's disease (Yoo et al., 2001).
Heat shock protein 8, otherwise known as heat shock cognate 71-kDa protein, belongs to the heat shock protein 70 family and has anti-apoptotic functions. It is strongly expressed in neurones under homeostatic conditions but is up-regulated in astrocytes during brain injury (Kanninen et al., 2004). Heat shock protein 8 has been reported to be oxidatively modified in Alzheimer's disease (Castegna et al., 2002).
Conclusions
By using a proteomics approach, this study has demonstrated that exercise affects a diverse network of proteins related to energy metabolism and synaptic plasticity in the hippocampus, a brain region central to cognitive function. Exercise increased four different aspects of energy metabolism (Fig. 4). An up-regulation of glycolytic enzymes may indicate increased glucose catabolism. An increase in GDH-1 increases glutamate turnover and provides another entry point into the Kreb cycle. Oxidative phosphorylation also seems to be affected by exercise as the ATP synthase machinery was increased. Finally, by up-regulating uMtCK, exercise may increase the cellular bio-availability of mitochondrial-produced ATP.
An obvious question arising from these findings, to be explored by future research, is how exercise enables energy metabolism to interact with synaptic plasticity to affect brain functions, i.e. spatial learning and memory in the hippocampus. Glucose catabolism may support brain function as glucose utilization is directly correlated with cognitive function (McNay et al., 2000). Furthermore, the increased production and transduction of mitochondrial ATP may be used for vesicle mobilization, especially under high synaptic transmission (Verstreken et al., 2005; Fig. 4). Possibly, the binding of cytoskeletal proteins to enzymes of energy metabolism may modulate cellular functions (Durrieu et al., 1987; Shearwin et al., 1990; Knull & Walsh, 1992). Finally, as mitochondrial function may significantly increase during exercise, chaperones such as heat shock 60-kDa protein 1 and CCT may be essential for protecting the mitochondrion and cell from oxidative stress. Notwithstanding this, it is notable that most of the proteins up-regulated by exercise, whether metabolic, cytoskeletal or chaperone related, have been implicated in supporting cognitive function.
Acknowledgements
This study was supported by NIH awards NS 045804 and NS 39522.
Abbreviations
-
- CCT
-
- chaperonin-containing TCP-1
-
- CKBB
-
- brain-type creatine kinase
-
- GDH
-
- glutamate dehydrogenase
-
- GFAP
-
- glial fibrillary acidic protein
-
- NFL
-
- neurofilament light polypeptide
-
- NP22
-
- neuronal protein 22
-
- PGK-1
-
- phosphoglycerate kinase 1
-
- TAPP
-
- transcriptional activator protein pur-alpha
-
- TCP-1
-
- t-complex peptide 1
-
- uMtCK
-
- ubiquitous mitochondrial creatine kinase