Inducible and neuronal nitric oxide synthases (NOS) have complementary roles in recovery sleep induction
Abstract
Sleep homeostasis is the process by which recovery sleep is generated by prolonged wakefulness. The molecular mechanisms underlying this important phenomenon are poorly understood. We have previously shown that nitric oxide (NO) generation increases in the basal forebrain (BF) during sleep deprivation (SD). Moreover, both NO synthase (NOS) inhibition and a NO scavenger prevented recovery sleep induction, while administration of a NO donor during the spontaneous sleep–wake cycle increased sleep, indicating that NO is necessary and sufficient for the induction of recovery sleep. Next we wanted to know which NOS isoform is involved in the production of recovery sleep. Using in vivo microdialysis we infused specific inhibitors of NOS into the BF of rats during SD, and found that an inhibitor of inducible NOS (iNOS), 1400W, prevented non-rapid eye movement (NREM) recovery, while an inhibitor of neuronal NOS (nNOS), L-N-propyl-arginine, decreased REM recovery but did not affect NREM recovery. Using immunoblot analysis we found that iNOS was not expressed during the spontaneous sleep–wake cycle, but was induced by prolonged wakefulness (increased by 278%). A known iNOS inducer, lipopolysaccharide, evoked an increase in sleep that closely resembled recovery sleep, and its effects were abolished by 1400W. These results suggest that the elevation of NO produced by induction of iNOS in the BF during prolonged wakefulness is a specific mechanism for producing NREM recovery sleep and that the two NOS isoforms have a complementary role in NREM and REM recovery induction.
Introduction
Sleep loss, induced by prolonged wakefulness, produces a decline in cognitive and motor performance (Dinges et al., 1997), mood disturbances, memory deficits (Chee & Choo, 2004) and affects immune function (Bryant et al., 2004). These effects are restored by recovery sleep, characterized by prolongation and intensification of both the non-rapid eye movement (NREM) and the REM component of sleep. Although the two-process model of sleep regulation (Borbély, 1982) accurately describes the expected duration of recovery sleep, the molecular mechanisms that underlie this regulation remain less clear.
Nitric oxide (NO) is an intercellular signalling molecule that regulates both physiological and pathophysiological processes in the CNS (Garthwaite & Boulton, 1995; Gross & Wolin, 1995; Keynes & Garthwaite, 2004). It has a pronounced effect on mitochondrial energy metabolism, where it acts as inhibitor of the electron transport chain (Clementi et al., 1998; Sarti et al., 2003). Of the three NO synthases (NOS) (Alderton et al., 2001), neural NOS (nNOS) and endothelial NOS (eNOS) are constitutively expressed, while inducible NOS (iNOS) is normally absent from the brain, but is induced by different types of stressors, such as immunological challenge (Murphy et al., 1993) and immobilization stress (Madrigal et al., 2001). In the brain iNOS expression is mostly limited to activated microglia and astrocytes (Murphy et al., 1993; Wong et al., 1996), but some reports indicate also neuronal iNOS synthesis (Moro et al., 1998). nNOS is co-localized with acetylcholine (ACh) in most of the basal forebrain (BF) nuclei, as well as in the laterodorsal tegmental (LDT) and pedunculopontine tegmental (PPT) nuclei that project to the BF (Vincent & Kimura, 1992), and is involved in the regulation of the sleep–wake cycle (Leonard & Lydic, 1997; Hars, 1999).
Several previous studies have suggested that NO may have a role as a sleep-facilitating agent (Kapás et al., 1994; Dzoljic et al., 1996; Kapás & Krueger, 1996; Burlet et al., 1999; Hars, 1999; Ribeiro et al., 2000; Monti et al., 2001; Gautier-Sauvigne et al., 2005; Ribeiro & Kapás, 2005); other studies have suggested that NO has a pro-arousal (Pape & Mager, 1992; Marino & Cudeiro, 2003) or pro-REM (Leonard & Lydic, 1997) effect. We have previously shown that NO production increases in the BF during sleep deprivation (SD). Further, both NOS inhibition and a NO scavenger prevented recovery sleep induction after 3 h SD, while administration of a NO donor during the spontaneous sleep–wake cycle increased sleep that qualitatively and quantitatively resembled recovery sleep (Kalinchuk et al., 2006). These data demonstrated a causal role of NO production in the BF in recovery sleep.
Our general hypothesis was that the activity of the waking-active neurons in and projections to the BF induce the increase in NO during SD, and that this increase induces the increase in sleep, described as recovery sleep. In the present study we addressed the question of which NOS isoform is responsible for the increase in NO during SD, and whether the NREM and REM sleep recoveries are induced by the same or different NOS. For this purpose we studied the effects of specific inhibitors for nNOS and iNOS on recovery sleep by infusing them locally into the BF during SD and measuring the subsequent amounts of NREM and REM recovery sleep.
Materials and methods
Animals and surgery
Male Wistar rats (300–400 g) were kept under constant temperature (23.5–24 °C) conditions on a 12 h light : dark cycle, with lights on at 08.30 h. Water and food were provided ad libitum. Under general anaesthesia (0.1 mg/kg s.c. medetomidine + 30 mg/kg i.p. pentobarbital), rats were implanted with electrodes for recording electroencephalogram (EEG) and electromyogram (EMG), and with a unilateral guide cannula for microdialysis probes (CMA/11 Guide, CMA/Microdialysis, Stockholm, Sweden) targeting the BF cholinergic area (AP = −0.3; ML = 2.0; V = 5; Paxinos & Watson, 1998) (Fig. 1A and B). After a recovery and adaptation period of 2 weeks, before microdialysis probe insertion, a 30 h baseline EEG recording was obtained for each rat. The experimental protocol was accepted by the Ethical Committee for Animal Experiments at the University of Helsinki and the provincial government of Uusimaa, Finland, and was in accordance with the laws of Finland and the European Union.
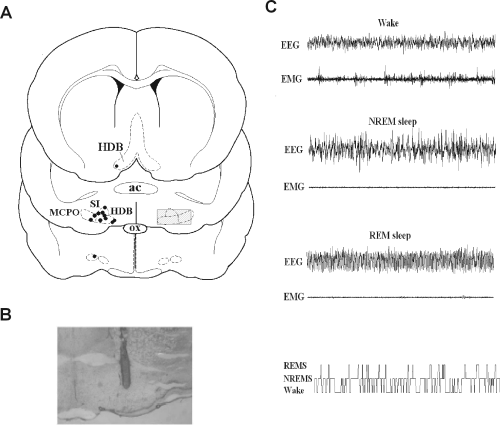
Location of microdialysis probe tips and electroencephalography (EEG) recording. (A) Camera lucida drawing of the microdialysis probe tips in the BF (n = 12). ac, anterior commissure; HDB, horizontal limb of diagonal band; MCPO, magnocellular preoptic area; ox, optic chiasm; SI, substantia innominata. The position of the cut lines in a 2-mm brain slice used for collection of the tissue sample for immunoblotting is presented as the hatched area of the right side of the middle section. (B) Photograph of the track of the representative probe tip located in the BF. (C) Fragments of EEG/electromyogram (EMG) recordings characteristic of states of wakefulness, non-rapid eye movement (NREM) sleep and REM sleep, and a hypnogram prepared of scoring of a 6-h light phase recording, showing the distributions of wakefulness, NREM and REM sleep (lowest row in C).
Microdialysis experiments
Microdialysis probes (CMA/11, membrane length and diameter 2 mm and 0.24 mm, respectively; CMA/Microdialysis) were inserted into the target area at least 20 h before the start of the experiments (Porkka-Heiskanen et al., 1997) and stayed in place permanently during the 2-week experimental period. In case a probe became clogged or dried out it was replaced by a new one. For experiments, animals were connected to the microdialysis leads for 6 h starting after lights on at 09.00 −09.30 h. Artificial cerebrospinal fluid (aCSF; in mm: NaCl, 147; KCl, 3; CaCl2,1.2; MgCl2, 1.0) or solutions of the studied drugs (dissolved in aCSF) were pumped through the microdialysis probe at 1 µL/min. The microdialysis leads were disconnected after 1 h of recovery sleep after the deprivation at 15.30–16.00 h.
Each rat participated in two to four experiments with 24–48 h breaks between the experiments. EEG was recorded also during the non-experimental days to ensure that there was no carry-over effect of the drugs on sleep. The first experiment was always a baseline aCSF infusion for 6 h starting at 09.30 h. All subsequent experiments were preceded by a daily pretreatment baseline period of CSF infusion for 2–3 h, during which samples were collected for metabolite analysis. For each rat, the average of values obtained during the pretreatment period was used for normalization of concentrations of metabolites. As probe insertion per se may affect the levels of cytokines (Woodroofe et al., 1991) and thus, theoretically, also iNOS levels, we performed control measurements of iNOS levels in the tissue surrounding the probe tip after 20 h, 1 week and 2 weeks of the probe insertion. Comparison of iNOS levels on the probe side to the levels on the contralateral side did not reveal upregulation at any of the time points (data not shown).
To test the effect of the NOS inhibitors on spontaneous sleep, the drug was infused for 3 h during the spontaneous sleep–wake cycle (11.30–14.30 h). SD was performed between 11.30 h and 14.30 h. To test the effect of NOS inhibitors on recovery sleep the drug was infused during SD. Drug infusions combined with SD were started 1 h before the SD at 10.30 h, and continued through the deprivation until 14.30 h. In all experiments microdialysis samples for adenosine and nitrite plus nitrate (NO–x) analysis were collected at 30 min intervals from 09.30 to 15.30 h, and EEG was recorded for 30 h (24 h after the treatment). Baseline recordings and SD were performed for all animals (n = 12). In the other groups n = 4–6 (for details, see the figure legends).
Sleep deprivation
For the SD experiments, the animals are trained to human presence for at least 1 week prior to the experiment. During the daily training sessions the animals were handled, by taking them out of the cage and letting them play with the researcher and then returned back to the cage. The daily sessions last up to 10 min, and the animals are regarded as trained when they do not show fear reaction when the researcher enters the room and approaches the cage. For the procedures of in vivo microdialysis, the animals were trained by immobilizing them to the position that is used for probe insertion and microdialysis lead attachment to the probe, starting from a few seconds at the first time and lasting up to 1 min before the start of the experiments. The baseline (Baseline EEG 1) was recorded before the probe insertion and scored. The probe was inserted and experiments started only when the proportion of REM sleep was normal in the baseline recording.
Rats were sleep-deprived for 3 h between 11.30 and 15.30 h using a gentle handling procedure (Franken et al., 1991), which included introduction of new objects into the cages in order to keep the animals occupied and replacement of them by new ones when animals appeared to become sleepy. Any physical contact with animals was excluded. Monitoring of food consumption did not show significant differences between spontaneous wakefulness and SD periods. Continuous monitoring of EEG/EMG during the deprivation period was used to assess the behavioural state of the animals.
EEG recording and analysis
EEG/EMGs were recorded as described previously (Kalinchuk et al., 2003). EEG recordings were scored using the Spike 2 program (Cambridge Electronic Devices, Cambridge, UK) in 30-s bins semi-automatically for NREM sleep and manually for REM sleep and wakefulness. Semi-automatic scoring of NREM sleep was performed based on quantification of power in the delta band (0.5–4.5 Hz), sigma band (11–15 Hz) and gamma band (30–45 Hz) using custom scripts for power spectral analysis as described previously (Stenberg et al., 2003). Scoring of NREM sleep was validated by correlating the results of the semi-automatic scoring with results of manual scoring for 16 records (30 h each); the correlation was 93.7 ± 0.8% (mean ± SEM). Manual analysis of wakefulness, NREM sleep (for validation of results of semi-automatic scoring) and REM sleep were performed in accordance with classical criteria: wakefulness was identified by the presence of low-amplitude desynchronized activity in the EEG and high-amplitude activity in the EMG; NREM sleep was identified by the presence of high-amplitude slow-waves in the EEG and decreased activity in the EMG as compared with wakefulness; REM sleep was distinguished as a state with regular theta activity (5–8 Hz) in the EEG and decreased muscle tone as compared with wakefulness (Fig. 1C). The 30 h recordings were divided into 3-h and 6-h bins; the amounts of NREM sleep, REM sleep and delta power intensity during NREM episodes (‘delta power’) in each bin during the experimental day were compared with the corresponding time bin on the baseline day, and percentage differences were calculated. A period of 18 h after the treatments (14.30–08.30 h, marked as shaded area in the figures) was used for the final quantitative analysis as this was the period of maximal change in sleep/delta power.
Definitions of the baselines
Three baselines were defined as follows.
- (i)
Baseline EEG 1 (30 h) was recorded before probe insertion to ensure the animal had recovered completely from the operation.
- (ii)
Baseline EEG 2 (30 h) was recorded before the start of the experiments to confirm that CSF infusion per se does not affect the sleep–waking cycle. The probe was inserted at least 20 h before the start of the recording. Microdialysis lines were attached and CSF was infused for 6 h at 09.30–15.30 h. As there was no difference in sleep between the two baselines, baseline EEG 2 was used when normalizing the sleep data.
- (iii)
Daily pretreatment baseline was determined for adenosine and NO–x measurements. On each experimental day 30-min microdialysis samples were collected for 2–3 h before treatment (starting at 09.30 h) during CSF infusion, and the average of the concentrations of these samples was used as the baseline to which the effect of the treatment was compared. The daily baseline values of each animal were monitored through the 2-week experimental period in order to ascertain the stability of the preparation.
High-performance liquid chromatography (HPLC) analysis
Adenosine was measured using HPLC coupled to a UV detector (Waters 486, Waters, MA, USA). Details of the adenosine assays have been published previously (Porkka-Heiskanen et al., 1997). The amounts of lactate and pyruvate were measured as described by Hallström et al. (1989). The detection limits of the assays were for adenosine 0.8 nm (signal to noise ratio 2 : 1), pyruvate 0.6 µm (3 : 1) and lactate 10 µm (3 : 1) (Grob, 1985). Concentrations of the samples collected during SD or drug infusions were normalized to the mean concentration of samples collected during the baseline pretreatment period when aCSF was infused (100%).
NO–x measurements
Because no endogenous source other than NO is known for NO–2 and NO–3 (NO–x), this metabolite has generally been used as indicative of NO production (Mackenzie et al., 1996). NO–x concentrations were measured using the Nitrate/Nitrite Fluorometric Assay Kit (Cayman Chemical Company, Michigan, USA) according to the manufacturer's instructions. The detection limit of the assay was 0.06 µm in the final reaction mixture. The results are expressed as NO–x.
Drug infusions
According to the manufacturer's instructions for CMA probes and our previous measurements (Porkka-Heiskanen et al., 1997), the probe recoveries for most substances are 10–15%. Thus, the effective BF concentrations of drugs are estimated to have been about one-tenth of those in the infusion solutions. The concentrations indicated below are concentrations in the infusion solution.
For inhibition of nNOS, Nω-propyl-L-arginine [(L-NPA), Tocris; Ki = 57 8500 and 1.8 × 105 nm for nNOS, eNOS and iNOS, respectively,] (Zhang et al., 1997) at 30 µm was used. In order to find the optimal concentration for selective nNOS inhibition, we also tested L-NPA at 3 µm, 300 µm and 1 mm. The higher doses did not further modulate any of the measured parameters (data not shown). The selective iNOS inhibitor 1400W [(N-3-aminomethylphenylmethyl-ethanimidamide dihydrochloride), Tocris; IC50 = 14 428 and 1000 µm for iNOS, nNOS and eNOS, respectively] (Garvey et al., 1997; Starkey et al., 2001) was infused at 250 µm. The concentration was extrapolated from those shown to be effective in vitro (Starkey et al., 2001); in addition, 25 µm was also tested, with similar results as obtained with the higher dose (data not shown).
Microinjections
To further evaluate the role of iNOS expression in sleep induction, we injected lipopolysaccharide (LPS; from Escherichia coli, serotype 055:B5, Sigma, Lot L2637), a well-known iNOS inducer (Wong et al., 1996; Harada et al., 1999; Cheepsunthorn et al., 2001) locally into the BF and measured subsequent changes in sleep. At 11.30 h, 5 µg of LPS in 2 µL sterile phosphate-buffered saline was injected over a period of 2 min into the BF through a microdialysis probe that was modified by removing the microdialysis membrane. The probe was kept in place for an additional 2 min. After injection animals were connected to recording leads and EEG/EMG were recorded continuously for 30 h. Metabolite levels could not be measured in microinjection studies as this method did not allow sample collection.
To test the effect of 1400W on recovery sleep after the LPS microinjection, 1400W was infused through an unmodified microdialysis probe into the BF starting at 10.30 h and continuing through the deprivation till 14.30 h. At 11.30 h the LPS was microinjected to the BF as described above. Infusion of 1400W was continued for 3 h after LPS injection.
Immunoblotting
Using immunoblot, we assayed iNOS protein expression in brain tissue (BF and cortex) collected after a spontaneous sleep period (3 h with 90% sleep), after a spontaneous waking period (25 min), and after a 3-h SD period. Samples after 3 h SD and after 3 h spontaneous sleep period were taken at 15.00–15.30 h. Samples after a spontaneous waking period were taken at the beginning of the dark period at 21.00–21.30 h. To obtain the tissue samples, the brains were rapidly removed after decapitation and a 2-mm slice was prepared of the region of the BF. The slices were partly frozen and the BF and cortex were cut out using a scalpel (see the cut lines in the Fig. 1A). The tissues were then completely frozen and stored for the assay. When assayed, tissues (40–60 mg) were lysed in Tris buffer and centrifuged for 15 min. Protein in the tissue extract was determined by the Lowry method using bovine serum albumin as a standard. Equal amounts of protein (50 µg) in a final volume of 20 µL per lane were loaded with Laemmli buffer and separated by 10% sodium dodecyl sulphate–polyacrylamide gel electrophoresis. The gels were blotted to nitrocellulose membranes and incubated with a specific polyclonal anti-iNOS antibody (Santa Cruz, 1 : 1000 dilution) overnight. After incubation with secondary FITC-conjugated antibody (Chemicon, 1 : 200 dilution), iNOS protein was measured by fluorescence detection. β-Tubulin was used as an internal standard. For a positive iNOS control, brain tissue was taken from a rat injected with LPS (20 mg/kg) i.p. and killed 6 h after injection (Iwase et al., 2000). Band optical density was quantified using an Image QuantTM program. The protein expression levels were determined by calculating the ratio of densitometric values in samples collected after SD and spontaneous wakefulness in relation to spontaneous sleep when expression was minimal, and expressed as a percentage.
Histological verification of the probe locations
After the experiments, the probe tip locations were verified histologically, as described previously (Kalinchuk et al., 2003) (Fig. 1A).Figure 1B shows the location of a representative probe tip in the BF.
Data presentation
To emphasize the homeostatic component of sleep regulation (Borbély, 1982), we normalized the sleep data from experimental days to corresponding time bins from the baseline day − a procedure that eliminated the circadian variation in the data presentation. The effects of this procedure on the original data are exemplified in Fig. 2. Because data presented either in 3- or 6-h bins equally described the results, 6-h bins were chosen for the presentations. The data analysis is intraindividual so that each animal serves as its own control. For statistics, three 6 h mean values obtained during the first 18 h after the treatment were averaged and normalized to the respective three 6 h mean values obtained during the baseline day.
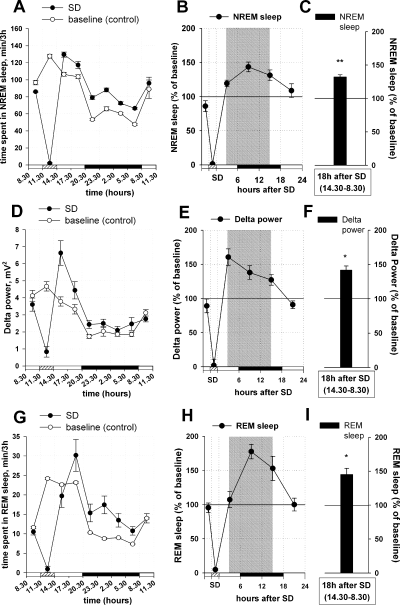
Effects of sleep deprivation (SD) on non-rapid eye movement (NREM) sleep, REM sleep and delta power. The left panels (A, D and G) show the distribution of NREM/REM sleep (min/3h) and EEG power density (mV2) in the delta range (0.5–4 Hz) at different time points during a baseline day (baseline, empty circles) and during a SD day (SD, filled circles). Recordings were divided into 3-h bins and mean 3-h values were calculated for each individual rat (n = 12), and the individual means were averaged for each bin. (B, E and H) For presentation of the data in 6-h bins, two 3-h mean values were averaged. The data shown in the middle panels (B, E and H) were obtained by normalizing for each rat the SD day values to the baseline day values (100%) in 6-h bins. (C, F and I) For the final quantitative analysis (shown in the right panels), three 6-h bins (18 h after the treatment, corresponding to the time points between 14.30 and 08.30 h, shown by the shaded area in the central panel) were averaged. The bars show quantitative changes in sleep/delta power during the first 18 h of recovery sleep after the SD period. The horizontal axis shows light periods (open horizontal bars), SD during the light period (hatched bar) and the dark period (black bar). (A and B) SD induced an increase in NREM recovery sleep, with a maximum at 9–12 h after SD. (C and D) Maximal increase in delta power intensity during NREM sleep (= delta power) observed at 3–6 h after SD. (E and F) REM sleep was not changed during the first 6 h after SD, but was increased at 9–12 h after SD. **P < 0.001; *P < 0.05 vs. control (baseline EEG recording).
For the analysis of adenosine and/or NO–x, four samples were collected before the SD/drug infusion. Concentrations detected in these samples were averaged and used as pretreatment baseline (100%). In the case when SD was combined with drug infusions, the average of two samples, collected before drug infusion, served as the pretreatment baseline. Six samples were collected during the 3 h SD/drug infusion and three of them were averaged for adenosine and three for NO–x calculation. The change between the baseline pretreatment period (average) and the experimental period (average) was compared, and the change was expressed as percentage increase over baseline.
Statistics
Data are expressed as mean ± SEM. Statistical analysis was performed using SigmaStat 3.0 Statistical software (SPSS, Chicago, IL, USA). To evaluate the statistical significance of the effects of SD and drug infusions on NREM/REM sleep and delta power intensity we used the Mann–Whitney Rank Sum Test (when two groups were compared) and Kruskal–Wallis One-Way Analysis of Variance on Ranks, followed by Dunn's post hoc test (for comparison of three or more groups). The effect of SD on iNOS protein was evaluated using Kruskal–Wallis One-Way Analysis of Variance on Ranks, followed by Dunn's post hoc test. To evaluate the effects of different treatments on concentrations of NO–x and adenosine before and after treatment for the same animals we used a paired t-test or Wilcoxon Signed Test (for non-normally distributed data).
Results
Effects of SD on subsequent sleep (= recovery sleep), delta power and metabolite concentrations
Sleep
The effect of SD on sleep was evaluated by comparison between the EEG recordings obtained on the SD day and on the baseline day (100%). SD significantly increased subsequent NREM sleep, which was elevated by 32.2 ± 3% as compared with baseline (n = 12, Mann–Whitney Rank Sum Test, T = 222.000, P < 0.001) (Fig. 2A–C) and led to a 41.9 ± 6% increase in delta power intensity (‘Delta Power’) during NREM sleep that was also significant (Mann–Whitney Rank Sum Test, T = 210.000, P < 0.05) (Fig. 2D–F). SD also increased REM sleep by 45.2 ± 9%, as compared with baseline (Mann–Whitney Rank Sum Test, T = 153.000, P < 0.001) (Fig. 2G–I).
NO–x, adenosine, lactate and pyruvate
The basal concentration of NO–x measured in samples collected before SD was 0.8 ± 0.1 µm. NO–x concentrations significantly increased during the 3 h of SD by 70.5 ± 32% as compared with baseline pre-deprivation values (n = 6, Wilcoxon Signed Rank Test, W = 21.000, P < 0.05) (Fig. 3). This elevation in NO–x concentration was accompanied by an increase in adenosine concentration, as also shown previously (Porkka-Heiskanen et al., 1997). The basal pre-deprivation concentration of adenosine was 2.2 ± 0.7 nmol/L. During the 3 h SD the adenosine concentration increased by 200.2 ± 59% as compared to the baseline pre-deprivation values (paired t-test, t = 3.635, P < 0.05) (Fig. 3). In agreement with our recently published data (Kalinchuk et al., 2003), levels of lactate and pyruvate during SD were increased by 48.9 ± 6% (paired t-test, t = 3.026, P < 0.05) and by 34.4. ± 5% (paired t-test, t = 3.827, P < 0.05), respectively (Fig. 3).
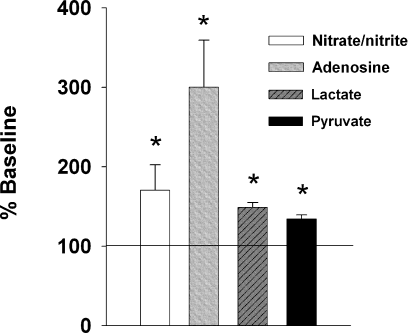
Changes in NO–x, adenosine, lactate and pyruvate concentrations in the BF during SD. For each metabolite, three measurements during SD and two measurements during the pre-deprivation baseline period were averaged, and the SD values were normalized to the pre-deprivation value (100%). Concentrations of NO–x (A), adenosine (B), lactate (C) and pyruvate (D) during SD (n = 7) were significantly higher than before SD. *P < 0.05 vs. control (pre-deprivation baseline level).
The effect of specific nNOS inhibitor L-NPA on NO–x and adenosine concentrations, and recovery sleep
NO–x concentration
We originally hypothesized that the source of NO is either local NOS containing neurons or projecting neurons, for example, from the LDT/PPT. Surprisingly, infusion of L-NPA into the BF during SD did not prevent the increase in NO–x concentrations characteristic of SD (increase by 40.0 ± 8% as compared with the pre-treatment baseline level; n = 6, paired t-test, t = 4.649, P < 0.01) (Fig. 4A).
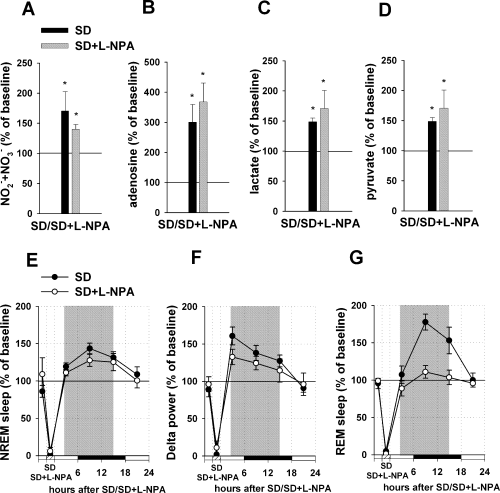
Effect of L-NPA infusion into the BF during sleep deprivation (SD) on metabolite concentrations, non-rapid eye movement (NREM)/REM recovery sleep and delta power. (A–D) Upper panels demonstrate changes in metabolite concentrations during the SD combined with L-NPA infusion (black bars, SD; grey bars, SD + L-NPA). For each metabolite, three measurements during the SD + L-NPA infusion period and two measurements during the pre-deprivation baseline period were averaged, and the SD + L-NPA infusion values were normalized to the pre-deprivation value (100%). Infusion of L-NPA into the BF during SD (n = 6) did not suppress increases in NO–x (A), adenosine (B), lactate (C) and pyruvate (D) levels, which were quantitatively similar to the effects of sole SD. (E–G) the bottom panels demonstrate changes in sleep/delta power and REM sleep after the SD combined with L-NPA infusion (black dots, SD ; white dots, SD + L-NPA). The values were obtained by normalizing SD + L-NPA infusion day values to the baseline day values (100%) in 6-h bins for each rat. Three 6-h time bins (representing the first 18 h after the treatment, shown by shaded areas) were averaged and taken for the final quantitative analysis. Infusion of L-NPA during SD did not prevent increases in NREM sleep (E) and delta power (F), which were similar to increases observed in recovery sleep after sole SD. (G) Infusion of L-NPA during SD significantly blocked subsequent recovery REM sleep. *P < 0.05 vs. control (control, pre-treatment baseline level for A–D and baseline EEG recording for E–G).
Adenosine, lactate and pyruvate
Infusion of L-NPA into the BF during SD did not prevent the adenosine increase, which was 267.7 ± 63% compared with baseline (Wilcoxon Signed Rank Test, W = 21.000, P < 0.05) (Fig. 4B). Also, increases in lactate and pyruvate were not blocked: lactate was increased by 70.7 ± 30% as compared with the pre-deprivation level (paired t-test, t = 2.573, P < 0.05) and pyruvate by 65.9 ± 30% (paired t-test, t = 2.753, P < 0.05) (Fig. 4C and D).
Sleep and delta power
Most importantly, L-NPA infusion into the BF during SD did not significantly decrease NREM recovery. Sleep was increased by 20.9 ± 5% as compared with baseline (n = 6, Kruskal–Wallis One-Way Analysis of Variance on Ranks, H2 = 23.991, P < 0.001; Dunn's post hoc test, Q = 2.575, P < 0.05) (Fig. 4E) and did not differ from the SD effect (Dunn's post hoc test, Q = 1.250, P > 0.05). An increase in the concentration of L-NPA to 300 µm and 1 mm as well as its decrease to 3 µm did not decrease the NREM recovery sleep (data not shown).
L-NPA infusion into the BF did not block the increase in delta power produced by SD (as compared with the SD effect, Kruskal–Wallis One-Way Analysis of Variance on Ranks, H2 = 17.859, P < 0.001; Dunn's post hoc test, Q = 0.454, P > 0.05); the increase that occurred (25.6 ± 10%) was significantly different from control (Dunn's post hoc test, Q = 2.726, P < 0.05) (Fig. 4F). Infusions of different doses of L-NPA (3 µm, 300 µm and 1 mm) also did not change delta power during NREM sleep (data not shown).
In contrast to the lack of effect on NREM sleep, infusion of L-NPA inhibited REM sleep recovery produced by SD (increase by 0.1 ± 6% as compared with control, Kruskal–Wallis One-Way Analysis of Variance on Ranks, H2 = 19.017, P < 0.001; Dunn's post hoc test, Q = 0.000, P > 0.05) (Fig. 4G). The amount of REM sleep was significantly different from the effect observed after SD alone (Dunn's post hoc test, Q = 3.227, P < 0.05). A smaller dose of L-NPA (3 µm) induced a similar effect, while using higher doses (300 µm and 1 mm) led to attenuation of the effect observed probably because of non-specific inhibition of other NOSs (data not shown).
In summary, treatment that specifically inhibited nNOS activity in the BF during SD did not block adenosine accumulation in the BF and did not prevent development of recovery NREM sleep and increase in delta power, while REM sleep recovery was inhibited.
The effect of the specific iNOS inhibitor 1400W on NO–x and adenosine concentrations, and recovery sleep
NO–x concentration
Infusion of 1400W into the BF during SD totally prevented the increase in NO–x concentration characteristic for SD (decrease by 8.2 ± 12% as compared with pretreatment baseline level, n = 6, paired t-test, t = 1.577, P > 0.05) (Fig. 5A).
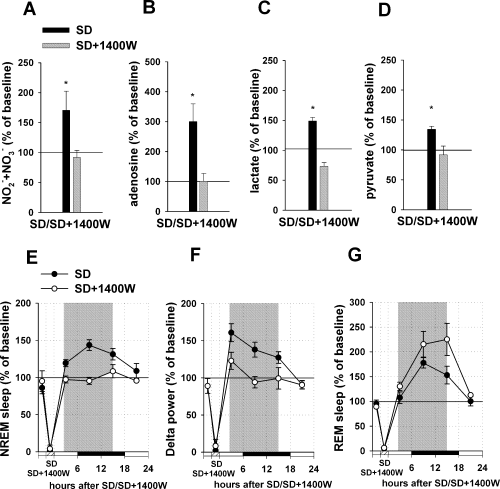
Effect of 1400W infusion into the BF during sleep deprivation (SD) on metabolite concentrations, non-rapid eye movement (NREM)/REM recovery sleep and delta power. (A–D) The upper panels demonstrate changes in metabolite concentrations during the SD combined with 1400W infusion (black bars, SD; grey bars, SD + 1400W). For each metabolite, three measurements during the SD and SD + 1400W infusion period, and two measurements during the pre-deprivation baseline period were averaged, and SD + 1400W infusion values were normalized to the pre-deprivation value (100%). 1400W infusion totally blocked increases in NO–x (A), adenosine (B), lactate (C) and pyruvate (D) levels during SD (n = 6). (E–G) The bottom panels demonstrate changes in sleep/delta power and REM sleep after the SD combined with 1400W infusion (black dots, SD; white dots, SD + 1400W). The values were obtained by normalizing SD + 1400W infusion day values to the baseline day values (100%) in 6-h bins for each rat. Three 6-h time bins (representing the first 18 h after the treatment, shown by shaded areas) were averaged and taken for the final quantitative analysis. After infusion of 1400W during SD, NREM recovery sleep (D) and increase in delta power (E) were totally blocked. (E) Selective inhibition of iNOS with 1400W during SD did not prevent an increase in REM sleep after SD. *P < 0.05 vs. control (baseline EEG recording).
Adenosine, lactate and pyruvate
Infusion of 1400W into the BF during SD completely abolished the adenosine increase characteristic of SD (increase by 0.7 ± 27%, paired t-test, t = 0.324, P > 0.05) (Fig. 5B). Also, infusion of 1400W during SD blocked increases in lactate (actually a decrease of 26.6 ± 6% was observed, paired t-test, t = 2.238, P > 0.05) and in pyruvate (decrease by 8.5 ± 15%, paired t-test, t = 0.871, P > 0.05) (Fig. 5C and D).
Sleep and delta power
Infusion of 1400W into the BF during SD abolished NREM sleep recovery (n = 6, Kruskal–Wallis One-Way Analysis of Variance on Ranks, H2 = 22.324, P < 0.001; Dunn's post hoc test, Q = 3.408, P < 0.05 compared with SD); NREM sleep did not under these circumstances differ from control (increase by 0.3 ± 6%, Dunn's post hoc test, Q = 0.000, P > 0.05) (Fig. 5E).
Infusion of 1400W into the BF during SD prevented an increase in delta power after the treatment (Kruskal–Wallis One-Way Analysis of Variance on Ranks, H2 = 16.291, P < 0.001; Dunn's post hoc test, Q = 2.442, P < 0.05 compared with SD); after this treatment delta power was not different from control (increase by 5.3 ± 8%, Dunn's post hoc test, Q = 0.644, P > 0.05) (Fig. 5F).
REM sleep recovery was not affected by infusion of 1400W during SD: sleep was increased by 90.2 ± 15% as compared with control (Kruskal–Wallis One-Way Analysis of Variance on Ranks, H2 = 22.527, P < 0.001; Dunn's post hoc test, Q = 4.116, P < 0.05) (Fig. 5G) and did not differ from the SD effect (Dunn's post hoc test, Q = 1.129, P > 0.05).
In summary, treatment that specifically inhibited iNOS activity in the BF during SD blocked adenosine accumulation in the BF and prevented the development of NREM recovery and increase in delta power.
The effect of SD on iNOS induction
The pharmacological data suggested that expression of iNOS is induced in or near the BF as a consequence of SD. iNOS is normally expressed at very low levels in the brain (Harada et al., 1999; Heneka et al., 2000; Madrigal et al., 2001), so induction of expression of this enzyme as a consequence of SD should be detectable on immunoblot. Tissue was taken from the cortex and the BF of animals that were spontaneously awake or asleep, or subjected to 3 h of SD, and assayed for iNOS expression by immunoblot (Fig. 6). After SD, expression of iNOS was significantly increased by 278 ± 121% in the BF as compared with spontaneous sleep (P < 0.05) and spontaneous wakefulness periods. The effect was site-specific: SD did not increase iNOS expression in the cortex (P > 0.05).
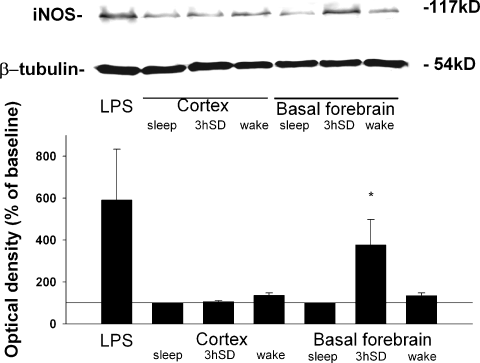
iNOS level in the BF and cortex after sleep deprivation (SD). Immunoblotting of iNOS after spontaneous sleep (sleep, n = 4), SD (n = 4) and spontaneous wakefulness (wake, n = 4) in the cortex and the BF. After SD (11.30–14.20 h) and during the spontaneous sleep period samples were taken at 15.00–15.30 h; and during the spontaneous waking period, at 21.00–21.30 h. SD elevated the iNOS level in the BF as compared with spontaneous sleep and waking, but did not change the iNOS level in the cortex. The upper panel shows a representative immunoblot. Mouse macrophage lysate (not shown) and the brain of a lipopolysaccharide (LPS)-treated rat (20 mg/kg, i.p.) were used as positive controls for iNOS. β-Tubulin was used as an internal standard. *P < 0.05 vs. control (iNOS level after spontaneous sleep).
The effect of LPS injection on subsequent sleep
LPS is a bacterially derived agent frequently used to activate iNOS, which it does through interaction with Toll-like receptor 4 (TLR4) (Lehnardt et al., 2002). We reasoned that if production of NO by iNOS in the BF is necessary and sufficient to produce recovery sleep, then instilling a non-physiological inducer of iNOS into the BF should produce sleep, provided that cells were present that possess the TLR4 receptor. In this circumstance, if LPS produced an increase in NREM sleep, then this would be consistent with the hypothesis. Failure to induce sleep would kill the hypothesis, unless it was shown that TLR4 was not present.
Microinjection of LPS into the BF increased NREM sleep by 37.8 ± 6% as compared with control (n = 6, Kruskal–Wallis One-Way Analysis of Variance on Ranks, H3 = 26.765, P < 0.001; Dunn's post hoc test, Q = 3.649, P < 0.05) (Fig. 7A); the effect was not different from the effects of SD (Dunn's post hoc test, Q = 0.653, P > 0.05). The effect lasted 18 h after the injection and it resembled previously described effects of a NO donor (Kalinchuk et al., 2006).
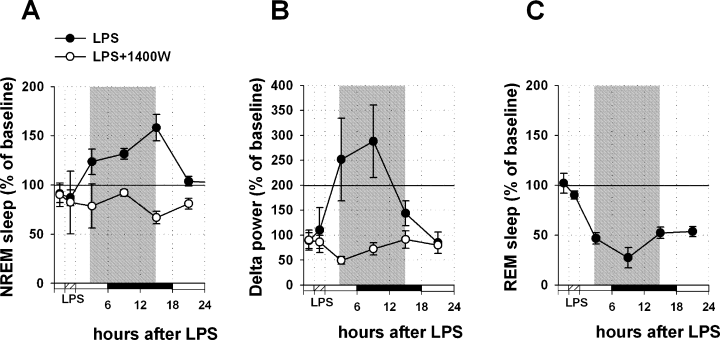
Effects of lipopolysaccharide (LPS) microinjection into the basal forebrain on non-rapid eye movement (NREM)/REM sleep and delta power. Data were obtained by normalizing LPS injection day (filled circles) or LPS injection combined with 1400W infusion day (empty cycles) values to the baseline day values (100%) in 6-h bins for each rat. Three 6-h mean values from the period of 18 h after the treatment (shown by shaded area) were averaged and taken for the final quantitative analysis. LPS induced an increase in NREM sleep (A) and delta power (B) during 18 h after injection into the BF (n = 6). Pretreatment with the specific iNOS inhibitor 1400W prevented the increase in NREM sleep and in delta power (n = 4). (C) LPS during 18 h after the injection significantly decreased REM sleep.
Pretreatment with the iNOS-specific inhibitor, 1400W, which was continuously infused into the BF for 4 h starting 1 h before LPS injection, prevented the NREM sleep increase induced by LPS alone (as compared with effect of LPS injection; n = 4, Dunn's post hoc test, Q = 3.448, P < 0.05). In this case, NREM sleep was not changed as compared with control (non-significant decrease by 23.1 ± 13%, Dunn's post hoc test, Q = 0.696, P > 0.05) (Fig. 7A) and significantly differed from the effect of SD (Dunn's post hoc test, Q = 3.290, P < 0.05).
Microinjection of LPS into the BF increased delta power by 103.5 ± 38% (Kruskal–Wallis One-Way Analysis of Variance on Ranks, H3 = 23.863, P < 0.001; Dunn's post hoc test, Q = 2.778, P < 0.05) (Fig. 7B). The effect was not different from that of SD (Dunn's post hoc test, Q = 0.414, P > 0.05). Pretreatment with 1400W prevented the increase induced by LPS (as compared with sole LPS injection, Dunn's post hoc test, Q = 3.592, P < 0.05); delta power did not change as compared with control (non-significant decrease by 43.3 ± 9%, Dunn's post hoc test, Q = 1.612, P > 0.05) (Fig. 7B). The effect was significantly different from the effect induced by SD (Dunn's post hoc test, Q = 3.791, P < 0.05).
Interestingly, microinjection of LPS decreased REM sleep by 55.5 ± 3% (Kruskal–Wallis One-Way Analysis of Variance on Ranks, H1 = 10.735, P < 0.01; Dunn's post hoc test, Q = 2.469, P < 0.05) (Fig. 7C). This effect was quantitatively similar to that previously observed after DETA/NO infusion (Kalinchuk et al., 2006).
In summary, microinjection of LPS into the BF during the spontaneous sleep–wake cycle, a treatment that stimulates iNOS induction and leads to an increase in NO synthesis, increased subsequent NREM sleep and delta power, producing a state similar to NREM recovery after SD and resembling the effects of a NO donor infusion. The LPS-induced decrease in REM sleep was similar to that induced by DETA/NO infusion. These data are consistent with the hypothesis that induction of iNOS into or close to the BF is necessary and sufficient for the generation of NREM recovery sleep.
The effects of L-NPA on spontaneous sleep
NO–x, adenosine, lactate and pyruvate concentrations
Infusion of L-NPA during the spontaneous sleep–waking cycle decreased NO–x level by 66.5 ± 5.6% (n = 6, paired t-test, t = 2.991, P < 0.05). Levels of adenosine, lactate and pyruvate were moderately and non-significantly decreased by 21.9 ± 17.8% (Wilcoxon Signed Rank Test, W = 6.000, P > 0.05), 12.5 ± 5.9% (paired t-test, t = 1.790, P > 0.05) and 17.4 ± 13.0% (paired t-test, t = 1.607, P > 0.05), respectively (Fig. 8A).
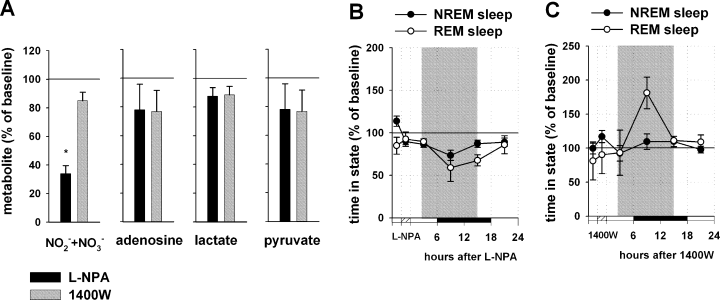
Effect of L-NPA and 1400W infusions into the BF on spontaneous sleep. (A) Changes in metabolite concentrations during the 3 h of L-NPA (black columns) and 1400W (grey columns) infusion. For each metabolite, three measurements during the drug infusion period, and two measurements during the pre-treatment baseline period were averaged and the drug infusion values were normalized to pre-treatment value (100%). L-NPA infusion decreased NO–x level, while the levels of adenosine, lactate and pyruvate (n = 6) were not significantly affected. 1400W infusion did not change significantly any of the metabolites. Changes in non-rapid eye movement (NREM) and REM sleep after the L-NPA (B) and 1400W (C) infusion. The values were obtained by normalizing drug infusion day values for NREM sleep (filled circles) and REM sleep (empty circles) to the baseline day values (100%) in 6-h bins for each rat. The three 6-h time bins (representing the first 8 h after the treatment, shown by shaded areas) were averaged and taken for the final quantitative analysis. L-NPA infusion (n = 6) induced a significant decrease in NREM sleep and in REM sleep (P < 0.05 vs. baseline EEG recording), while the 1400W infusion (n = 4) did not effect NREM sleep but induced a significant increase in REM sleep (P < 0.05 vs. baseline EEG recording). *P < 0.05 vs. baseline.
Sleep and delta power
Infusion of L-NPA into the BF during the spontaneous sleep–wake cycle decreased NREM sleep by 15.2 ± 6.0% as compared with baseline (n = 6, Mann–Whitney, T = 57.000, P < 0.01) and delta power was decreased by 32.8 ± 4.7% (Mann–Whitney, T = 57.000, P < 0.01). REM sleep was also significantly decreased by 24.2 ± 6.7% (Mann–Whitney, T = 57.000, P < 0.01) (Fig. 8B).
The effects of 1400W on spontaneous sleep
NO–x, adenosine, lactate and pyruvate concentrations
Infusion of 1400W during the spontaneous sleep–waking cycle induced a moderate but significant decrease in NO–x level by 15.6 ± 5.9% (n = 6, paired t-test, t = 2.581, P < 0.05); adenosine was non-significantly decreased by 23.2 ± 14.8% (Wilcoxon Signed Rank Test, W = 3.000, P > 0.05), lactate by 11.6 ± 6.0% (paired t-test, t = 2.205, P > 0.05) and pyruvate by 23.2 ± 11.3% (paired t-test, t = 2.283, P > 0.05) (Fig. 8A).
Sleep and delta power
Infusion of 1400W had no effect on NREM sleep (n = 6, increase by 1.9 ± 5%, Mann–Whitney, T = 22.000, P > 0.05) and on delta power (increase by 2.3 ± 13%, Mann–Whitney, T = 14.000, P > 0.05), while REM sleep was increased by 28 ± 11% as compared with baseline (Mann–Whitney, T = 10.000, P < 0.05) (Fig. 8C).
Discussion
The main finding of the present study is that iNOS was induced during prolonged wakefulness in the BF, and that its inhibition during the wakefulness period prevented the induction of NREM recovery sleep. Moreover, inhibition of nNOS prevented REM sleep recovery, suggesting that the two synthases play a complementary role in the induction of recovery sleep.
Infusion of LPS, a completely independent method to increase iNOS expression, also increased NREM sleep, and this elevation could be blocked by the specific iNOS inhibitor 1400W, further confirming that the induction of NREM sleep was dependent upon production of NO by iNOS. The increase in NREM sleep through iNOS was specifically associated with prolonged wakefulness as inhibition of iNOS during the spontaneous sleep–wake cycle failed to significantly modulate sleep, and the very low basal level of iNOS did not vary during the sleep–wake cycle. The specific inhibitor of nNOS failed to modulate NREM sleep recovery. These data suggest that iNOS-induced increase in NO in the BF is a specific mechanism activated by SD to produce recovery sleep. The generation of recovery sleep has most often been interpreted as an extension of the regulation of the spontaneous sleep–wake cycle. The present results introduce a different view: that NREM recovery sleep may be induced by a specific mechanism, by which prolonged wakefulness activates expression of iNOS, which is otherwise present only at low levels during the spontaneous sleep–wake cycle.
As sleep regulation is thought to be associated with neuronal activity, the finding that prolonged wakefulness led to the expression of iNOS, which is induced in response to immunological challenge and stress (Murphy et al., 1993; Madrigal et al., 2001), predominantly in the glia (Murphy et al., 1993), is somewhat surprising. Previous studies have established a bridge between the immune system and sleep regulation: several molecules that mediate immune responses also induce sleep (reviewed in Krueger & Obal, 2003). Cytokines are activated in response to immunological challenge and stress, and they are effective inducers of iNOS (Nathan & Xie, 1994). Cytokines are present also in the normal brain regulating synaptic plasticity and sleep (Vitkovic et al., 2000). Of the multiplicity of cytokines, particularly IL-1 and TNFα have been implicated in the regulation of sleep − their concentrations undergo diurnal variation and increase during SD (Mackiewicz et al., 1996; Bredow et al., 1997). Cytokines also interact with other hormones and other endogenous sleep factors (Krueger & Obal, 2003), including NO and adenosine, in the regulation of sleep. TNFα-induced increase in NREM was decreased in nNOS but not iNOS KO mice (Chen et al., 2004b), and influenza virus-induced NREM was decreased in both iNOS and nNOS KO mice (Chen et al., 2004a). The exact relationship between cytokine responses and iNOS activation during SD remains to be established by further experiments. We propose that cytokine production is upstream of induction of iNOS, and that iNOS production of NO evokes an increase in extracellular adenosine that acts as the endogenous somnogen in recovery NREM sleep (Porkka-Heiskanen et al., 1997, 2000; Basheer et al., 2001).
The basic cause for iNOS expression during prolonged wakefulness remains to be resolved by further research. As it has been shown that iNOS is expressed in response to stress (Madrigal et al., 2001), the easiest explanation would be that SD constitutes a stress to the brain, and iNOS is expressed in response to that stress. However, the explanation is not compatible with the localization of the iNOS expression exclusively in the BF, which is not central in the physiology of the stress response (De Kloet et al., 2005). An alternative explanation is that iNOS is expressed in response to energy depletion (Moro et al., 1998), which develops locally in the BF during prolonged wakefulness (Benington & Heller, 1995; Porkka-Heiskanen et al., 1997; Kalinchuk et al., 2003). In one previous study iNOS activity was increased in the laterobasal forebrain and posterolateral hypothalamus after long-term exposure of mice to intermittent hypoxia (Zhan et al., 2005). The amounts of NO produced by iNOS are considerably higher than those produced by the constitutively expressed NOS, nNOS and eNOS, ranging up to 100–1000-fold increases in the NO concentration (Licinio et al., 1999). These high NO concentrations have profound effects on mitochondrial energy production (Almeida & Bolanos, 2001; Moncada & Erusalimsky, 2002).
The expression of iNOS was found in the BF but not in the cortex, suggesting a local induction. The site-specificity is in agreement with our earlier studies, where the increase in adenosine levels during prolonged wakefulness was found to be restricted to the BF (Porkka-Heiskanen et al., 2000; Kalinchuk et al., 2003). We have further shown that in addition to an adenosine increase, also NO–x, lactate and pyruvate increase during prolonged wakefulness only in the BF, and that experimental increase of NO using DETA/NO induces sleep only when administered to the BF (Kalinchuk et al., 2006). The sites where we find the effects correspond closely to the area where the cholinergic cells of the BF are situated − horizontal limb of the diagonal band of Broca, substantia innominata and magnocellular preoptic area. The present results suggest that recovery sleep may be regulated through mechanisms that involve cholinergic cells. Another possibility is that adenosine could work through activation of adenosine receptors in the ventrolateral preoptic nucleus, which is located in the immediate vicinity of the BF area (Chamberlin et al., 2003; Saper et al., 2005). Though in some cases iNOS expression has been found also in neurons (Minc-Golomb et al., 1996; Moro et al., 1998), the predominant expression of iNOS takes place in glia, suggesting a role for glia in the regulation of sleep homeostasis.
We further show that NREM and REM sleep recovery are regulated by different NOS types: inhibition of nNOS in the BF abolished REM sleep recovery, while iNOS inhibition did not, suggesting that REM sleep recovery was induced through NO production by nNOS. Several previous studies have shown that changes of NO concentrations occur in the brainstem LDT/PPT regions (Leonard & Lydic, 1997; Hars, 1999) and dorsal raphe nucleus (Burlet et al., 1999) and regulate REM sleep. Although REM sleep is predominantly regulated by the LDT/PPT area through modulation of ACh secretion (Jones, 2004), other structures in the BF are also able to modulate it (Baghdoyan et al., 1993; Cape et al., 2000). The BF contains cholinergic cells maximally active during REM sleep (REM-on neurons) and NO might regulate REM sleep by directly modulating the activity of these neurons (Lee et al., 2005). It is possible that the BF neurons have a more central role in the regulation of REM sleep recovery than spontaneous REM sleep; the mechanism(s) of this regulation remain to be established. One possibility for the regulation are the direct projections from BF to the LDT/PPT (Swanson et al., 1984, 1987; Parent et al., 1988; Koliatsos et al., 1990), which are predominantly non-cholinergic, and at least partly g-aminobutyric acid (GABA)ergic (Gritti et al., 1998). NO facilitates the release of ACh at moderate concentrations (Prast & Philippu, 2001). However, high concentrations of NO, produced by iNOS, appear to promote GABA release (Casamenti et al., 1999), which may inhibit ACh release (Prast et al., 1998; Vazquez et al., 2002). We propose that large increases in NO release in the BF after iNOS activation can increase GABA release from terminals in the brainstem, thus leading to inhibition of cholinergic REM sleep-promoting neurons located in this area and decreasing REM sleep. Conversely, pharmacological inhibition of iNOS will lead to disinhibition of cholinergic brainstem neurons and an increase in REM sleep. In accordance with the present study, a previous study reported that iNOS activity in several brain areas remained unchanged during REM SD, while nNOS activity in the frontal cortex was affected (Clement et al., 2004).
A recent study in mice with targeted disruption of nNOS and iNOS genes showed that in the nNOS KO mice REM sleep was decreased, while in the iNOS KO mice it was increased (Chen et al., 2003), and the authors suggested that nNOS and iNOS play opposite roles in REM sleep regulation. Both studies, as well as the present study, suggest a role for nNOS in the regulation of REM sleep and REM sleep recovery. It may further be suggested that different cells regulate NREM and REM recovery sleep, as iNOS would be expected to be expressed in non-neuronal cells such as microglia or astrocytes, and nNOS would be expected to be expressed in neurons.
NO production undergoes state-dependent modulation during the sleep–wake cycle both in the thalamus and the cortex, probably due to state-dependent changes in the activity of nNOS-containing projection neurons (Burlet & Cespuglio, 1997; Williams et al., 1997). Only a few previous studies have assessed the role of locally administered, specific NOS inhibitors in the regulation of the spontaneous sleep–wake cycle regulation, and none in the regulation of recovery sleep. In one study a systemic administration of a specific nNOS inhibitor decreased slow wave sleep (Cavas & Navarro, 2005). As inhibition of iNOS in the present study did not change the amount of NREM sleep during the spontaneous sleep–wake cycle, these data suggest that NO production by nNOS, but not by iNOS, modulates the amount of spontaneous NREM sleep. However, iNOS KO mice had less NREM sleep in the end of the dark period, while there was no difference during the light period (Chen et al., 2003), suggesting that iNOS may also have some role in the regulation of normal sleep homeostasis. Spontaneous REM sleep was decreased with the nNOS inhibitor, in accordance with the idea that REM sleep is regulated with NO produced by nNOS activity. It is also possible that the drug-induced decrease in REM sleep contributed to the decrease in REM recovery after the SD. The increase in spontaneous REM sleep after inhibition of iNOS may be explained by disinhibition of the cholinergic cells, as discussed above. However, the low level of iNOS expression during the spontaneous sleep–wake cycle leaves this explanation somewhat controversial.
The present results, in addition to confirming NO as a powerful sleep-promoting agent, provide strong evidence that NO, through expression of iNOS locally in the BF, is a critical part of the homeostatic sleep control mechanism responding to prolonged wakefulness. Further, iNOS and nNOS appear to serve complementary roles in the production of NREM and REM recovery sleep. The mechanism underlying induction of recovery sleep appears to exploit a component of the innate immune system in the brain (Krueger & Karnovsky, 1990). This apparent recruitment of iNOS for homeostatic sleep regulation encourages a re-evaluation of the health consequences of prolonged wakefulness, and may provide a mechanistic basis for pharmacological intervention.
Acknowledgements
We thank Dr rer. Nat. Ernst Mecke, Mrs Pirjo Saarelainen and Mrs Sari Levo-Siitari for excellent technical assistance. This work was funded by NIH grant P50 HL60292, the Academy of Finland, European Union grant LSHM-CT-2005-518189, Finska Läkaresällskapet, the Sigrid Juselius Foundation and the ESRS Sanofi-Synthelabo Research Award to A.K.
Abbreviations
-
- ACh
-
- acetylcholine
-
- aCSF
-
- artificial cerebrospinal fluid
-
- BF
-
- basal forebrain
-
- DETA/NO
-
- NO donor (Z)-1-[N-(2-aminoethyl)-N-(2-ammonioethyl)amino]diazen-1-ium-1,2-diolate
-
- EEG
-
- electroencephalography
-
- EMG
-
- electromyogram
-
- GABA
-
- γ-aminobutyric acid
-
- HPLC
-
- high-performance liquid chromatography
-
- iNOS
-
- inducible nitric oxide synthase
-
- LDT/PPT
-
- laterodorsal/pedunculopontine tegmental nuclei
-
- LPS
-
- lipopolysaccharide
-
- NO
-
- nitric oxide
-
- NO–x
-
- nitrate + nitrite (NO–2 + NO–3)
-
- nNOS
-
- neural nitric oxide synthase
-
- NOS
-
- nitric oxide synthase
-
- NREM
-
- non-rapid eye movment sleep
-
- REM
-
- rapid eye movment sleep
-
- SD
-
- sleep deprivation
-
- TLR4
-
- Toll-like receptor 4