Rapid pH and PO2 changes in the tissue recording chamber during stoppage of a gas-equilibrated perfusate: effects on calcium currents in ventral horn neurons
Abstract
In vitro studies often use bicarbonate-buffered saline solutions to mimic the normal extracellular environment of tissues. These solutions are typically equilibrated with gaseous O2 and CO2, the latter interacting with bicarbonate ions to maintain a physiological pH. In vitro tissue chambers, like those used for electrophysiology, are usually continually perfused with the gassed buffer, but stopping the perfusion to add expensive chemicals or acquire imaging data is a common practice. The present study demonstrates that this procedure leads to rapid (< 30 s) increases in pH and decreases in PO2 of the detained solution in the tissue chamber. During the first 200 s, pH increased by 0.4 units and resulted in a 25% PO2 reduction of the detained solution. The rates of these changes were dependent on the volume of solution in the chamber. In experiments using acute transverse slices from the lumbar spinal cord of neonatal (postnatal day 0–10) mice, perfusion stoppage of the same duration was accompanied by a 34.7% enhancement of the peak voltage-gated calcium current recorded from ventral horn neurons. In these cells both low voltage-activated and high voltage-activated currents were affected. These currents were unaffected by decreasing PO2 when a CO2-independent buffer was used, suggesting that changes in pH were responsible for the observed effects. It is concluded that the procedure of stopping a bicarbonate/CO2-buffered perfusate results in rapid changes in pH and PO2 of the solution detained in the tissue chamber, and that these changes have the potential to covertly influence experimental results.
Introduction
A major goal of researchers using in vitro preparations of the nervous system is to maintain conditions as close as possible to those found in normal in vivo physiology. For many in vitro experimentalists, this constitutes the use of the naturally occurring bicarbonate/CO2 buffer system as opposed to solution buffers that utilize organic acids such as HEPES (N-2-hydroxyethylpiperazine-N-2-ethanesulfonic acid) or PIPES (piperazine-N,N′-bis-(2-ethanesulfonic acid)). A major drawback to the use of bicarbonate-buffered solutions with standard electrophysiological recording chambers is the need for a constant supply of CO2 gas. Unlike buffers utilizing organic acids, which have their pH set by titration with a strong base, bicarbonate-buffered solutions require this gas to maintain their pH. Typically, a 95% O2/5% CO2 gas mixture is bubbled into a large volume of solution that is continually perfused through the recording chamber. Both the large volume and the constant flow of solution puts the use of bicarbonate/CO2 buffered solutions at odds with the use of expensive chemicals and imaging studies, respectively. One often-reported experimental solution to this problem is to stop the perfusion and add small amounts of chemicals to the stationary solution in the tissue chamber or to acquire imaging data while the flow is stopped. Even though one might expect this procedure to result in a pH change of the detained solution, the magnitude and time course of this change are surprisingly not well defined. Furthermore, regardless of the buffer used, the PO2 of the detained O2-gassed solution also likely decreases, but again this is not well described at present. Because changes in pH and PO2 can independently have both short- and long-term effects on the tissue of interest (Bickler & Hansen, 1994; Krishek et al., 1996; Hainsworth et al., 2001; Honma et al., 2004; Cummins & Taylor, 2005), this procedure has the potential to complicate the interpretation of experimental results.
The present study provides objective measures of two sequelae resulting from stopping the perfusion of a tissue chamber while utilizing a bicarbonate/CO2 buffer system. Rapid alterations in voltage-gated calcium currents recorded from CNS neurons in a slice preparation demonstrate that these changes in the bulk solution are of sufficient magnitude and time course to be relevant for in vitro studies.
Materials and methods
Both pH and PO2 measurements were obtained from the detained solution in a standard tissue slice recording chamber (model RC-22C; Warner Instruments, Hamden, CT, USA). This chamber was perfused at ∼ 5 mL/min using a gravity-driven system in which solutions were gassed in 60-mL syringes. Chamber perfusion was stopped by simultaneously clamping off the input and suction lines of the tissue chamber. The volume of the solution in the tissue chamber was controlled indirectly by adjusting the height of the suction tube. Using the standard suction tube height, the approximate volume of buffer in the tissue chamber was 500 µL.
The pH of the small volume of solution in the tissue chamber was measured with a flat-surface pH electrode (model # 913600; Orion Research, Beverly, MA, USA) connected to an Accumet Basic pH meter (Fisher Scientific, Hampton, NH, USA). The pH of the HEPES-buffered solutions was adjusted prior to the experiment and rechecked after the experiment (n = 3), ensuring the pH did not change.
An ISO2 dissolved oxygen meter (World Precision Instruments, Sarasota, FL, USA) was used to measure the partial pressure of dissolved oxygen (PO2) in the buffered solutions. The meter output was run through an Axon 1200 A/D and the signal recorded with either AxoScope or pClamp software. The electrode was calibrated as per the manufacturer's instructions. Data were recorded at 100 Hz, and re-sampled and plotted at 0.1 Hz (the 0–90% response time of the O2 electrode). During experiments in which both calcium current amplitude and oxygen measurements were made, the two values were sampled consecutively at 0.05 Hz.
The isolation of the spinal cord and preparation of slices were as described in detail previously (Carlin et al., 2000; Carlin, 2005). Briefly, after anesthetizing the postnatal (P0–P10) Balb/C mice with an intraperitoneal injection of ketamine (100 mg/kg), the spinal cords were harvested and 150–200-µm-thick transverse slices were prepared from the lumbar enlargement. Whole-cell voltage-clamp recordings were obtained from large ventral horn cells, presumed to be motoneurons. These cells were located within the first two cell layers of the tissue slice. Isolated calcium currents were elicited from a holding potential (Vh) of either −60 mV or −80 mV with linear leak correction made offline.
The pipette solution contained (in mm): Cs-methane-sulfonate, 100; TEA-Cl, 30; MgCl2, 1; EGTA, 10; HEPES, 10; CaCl2, 0.5; NaCl, 5; ATP-Mg, 3; GTP, 0.3; leupeptin, 0.1. In some experiments 10–20 mm sucrose was added to the intracellular solution to stabilize the series resistance. The bicarbonate-buffered recording solution contained (in mm): NaCl, 86; TEA-Cl, 30; KCl, 1.9; NaH2PO4, 1.2; NaHCO3, 26; MgCl2, 5; glucose, 10; 4-AP, 4; CsCl, 2; CaCl2, 1–2; tetrodotoxin (TTX), 1 µm (pH ∼ 7.4 when bubbled with 95% O2/5% CO2). The HEPES-buffered recording solution contained (in mm): NaCl, 105; TEA-Cl, 30; KCl, 1.9; HEPES, 10; MgCl2, 2; glucose, 10; 4-AP, 4; CsCl, 2; CaCl2, 1–2; TTX, 1 µm (pH = 7.4; bubbled with 100% O2).
All experiments were performed at room temperature (∼ 22 °C). It should be noted that changes in PO2 may have greater acidification effects within the tissue slice at elevated temperatures compared with those used in the present experiments (Krnjevic & Walz, 1990). Experimental procedures were approved by the University of Manitoba Animal Care Committee and conformed to the standards of the Canadian Council of Animal Care.
Low-voltage-activated (LVA) current amplitude was defined as the peak current elicited by a voltage step from the holding potential to either −30 or −40 mV. The sustained high-voltage-activated (HVAS) current was defined as the current remaining at the end of a 150-ms step depolarization to 0 mV. The transient HVA (HVAT) current was defined as the difference between the peak current and sustained current (Carlin et al., 2000).
Results
pH and PO2 changes in a bicarbonate buffer
Measurement of pH in the tissue chamber during periods when the perfusion was stopped revealed a rapid alkalinization of the solution (Fig. 1A). This increase in pH was observable in less than 60 s and continued to increase, nearing a change of 0.8 pH units at approximately 10 min. During the first 200 s after the perfusion was stopped, a timeframe used in the following electrophysiological experiments, the pH of the detained solution increased by 0.4 pH units (Fig. 1A, dotted lines). This increase was completely reversible upon resuming perfusion of the tissue chamber with bicarbonate-buffered solution bubbled with 95% O2/5% CO2. The rate of the increase in pH was inversely related to the volume of solution detained in the tissue chamber (Fig. 1B). This relationship was best described by an exponential function suggesting that the rate of change stabilizes with higher volumes of buffer. As noted in the Materials and methods section, the volume of solution in the tissue chamber was estimated to be 500 µL when using the standard height adjustment of the suction tube. The rate of change in pH from the experiment presented in Fig. 1A using an estimated volume of 500 µL is also plotted on the graph in Fig. 1B(open circle), but was not used for curve fitting.
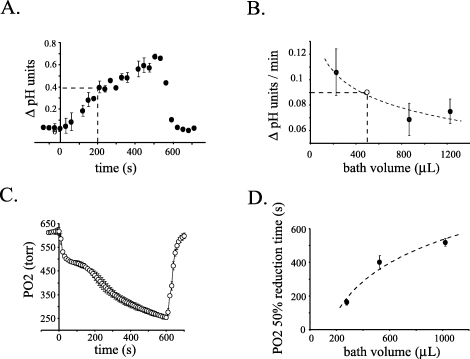
Both pH and PO2 of a bicarbonate-buffered solution rapidly change while detained in the tissue chamber. (A) Reversible alkalinization of the bicarbonate/CO2-buffered solution in the tissue chamber when the perfusion was stopped. The perfusion was stopped at time zero and restarted 540 s later (n = 2 or 3 measurements/point; error bars, ± SD). The dotted line indicates the pH change occurring over first 200 s. (B) The rate of alkalinization of the detained bicarbonate-buffered solution is inversely related to the volume of solution. The rate of change of the pH was determined for three known volumes of solution over a 5-min interval with five measurements taken during this time period. The rate was approximated by the slope of the linear regression fit of the data. Rate measurements were repeated six times for each volume and averaged (filled circles). Error bars are the 95% confidence intervals. Data were fit (dotted line) by an exponential equation; R2 = 0.88. The open circle represents the rate of pH change over a 5-min period determined from the data presented in (A) and the estimated volume of 500 µL. (C) Time course of the reversible decrease in PO2 of the bicarbonate-buffered solution in the tissue chamber during perfusion stoppage (n = 3; error bars are ± 2SD). Measurements were taken with standard suction tube height (volume was 525 µL). (D) As the volume of the detained solution was increased, the rate of PO2 change decreased. Data were fit (dotted line) by an exponential equation; R2 = 0.96. Each point represents n = 3 (error bars, 95% confidence intervals). Solution volumes in B–D were determined using a weight–volume calibration curve after experimental procedures.
Concomitant with this increase in pH, there was a rapid decrease in the PO2 of the detained bicarbonate-buffered solution in the tissue chamber. As demonstrated in Fig. 1C, this reduction in PO2 could be detected in as little as 10 s after stopping the perfusion of the tissue chamber. The decrease in PO2 was biphasic over the measured time period. There was a rapid initial exponential-like decrease followed by a second phase with a much slower rate of decay. Over the 10-min period of perfusion stoppage shown in Fig. 1C, the PO2 decreased by 59% (n = 3; 364 torr ± 13 SD). Over the first 200 s, the PO2 of the solution decreased by 25%. Unlike the biphasic PO2 decreases seen with the standard volume (and 1020 µL volume), when the volume of detained solution was decreased to 275 µL, a single rapid exponential-like decay of the PO2 was observed (n = 3; data not shown).
As seen with pH, the volume of the detained solution affected the rate of PO2 decrease (Fig. 1D). As the solution volume in the chamber was increased, the time required for the PO2 to decrease to 50% of its initial value also increased. This relation was again fit best with an exponential function, suggesting the rate of PO2 decrease levels off with increased volumes of detained solution in the tissue chamber.
Effects of perfusion stoppage on neuronal calcium currents
The potential for these rapid changes in pH and PO2 of the detained bicarbonate-buffered solution to have physiologically relevant effects on the functioning of neuronal tissue was explored using transverse slices of neonatal mouse spinal cord. For these experiments, physiological function was assayed using voltage-gated calcium currents elicited from presumed motoneurons. As described previously (Mynlieff & Beam, 1992; Carlin et al., 2000), the HVA calcium current in these cells consisted of varying amounts of both inactivating and sustained components. As well, a transient LVA current is seen in a portion of these cells. In the present study, stopping the perfusion of the 95% O2/5% CO2-bubbled solution led to an enhancement of the calcium current amplitude in 85% of cells tested (12/14 cells; Fig. 2A). Restarting the perfusion readily reversed the enhancement in eight of these cells. Assuming linear current rundown (Randall & Tsien, 1995), the current was enhanced 34.7% (± 12 SD) by stopping the perfusion for a 200-s period (calculated from cells with Vh = −80 mV only). The enhancement of the current was accompanied by a modulation of the rate of current inactivation as demonstrated by the fact that this time constant was reversibly decreased during perfusion stoppage (Fig. 2B). The non-specific calcium channel blocker, cadmium, completely blocked the inward current in cells during stoppage of the perfusion, indicating that the enhancement was mediated by voltage-gated calcium channels and not by the activation of other ionic conductances (n = 2; data not shown).
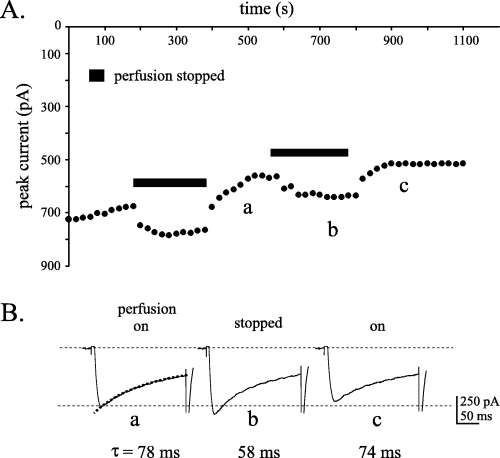
Isolated calcium currents from ventral horn neurons are enhanced when the perfusion of a bicarbonate/CO2-buffered solution is stopped. (A) Time plot demonstrating the reversible potentiation of the peak calcium current during periods when the perfusion was stopped. Upon restarting the perfusion, the peak current returned to the previous rate of current rundown. Voltage steps to 0 mV from a Vh of −80 mV. (B) Raw current traces taken from the time points indicated by the letters (a) to (c) in the time plot demonstrate the enhancement of the peak current. A single exponential fit of the current inactivation demonstrates a reversible increase in the rate of current decay with stoppage of the perfusion.
Examination of the difference currents, obtained by subtracting the corresponding current traces during periods when the perfusion was flowing from those recorded when the perfusion was stopped, demonstrated that both LVA and HVA currents are enhanced during this procedure (Fig. 3A). Furthermore, within the HVA class both transient and sustained current components can be seen in the difference currents. To determine the relative contributions of these current components to the 34.7% enhancement observed with voltage steps to 0 mV, correlations were studied between the various current components and the current enhancement. As voltage steps to 0 mV would have a contribution from LVA channels as well as HVA (McCobb et al., 1989; Umemiya & Berger, 1994), the LVA component of the current was also included in the analysis. In a subset of seven cells, the peak LVA and transient and sustained HVA components of the current were quantified (Fig. 3A; see Materials and methods for details) and plotted against the peak current enhancement observed during 200 s of perfusion stoppage normalized to pre-stoppage current amplitude. To varying degrees, the three current components all demonstrated a positive relationship with the degree of current enhancement. This supports the observation that more than one current component, and therefore calcium channel subtype, is affected during the perfusion stoppage. Although there was no significant correlation between the non-inactivating HVA current component and the degree of current enhancement, both of the inactivating current components covaried to a significant degree with the current enhancement (Fig. 3B; for LVA, R = 0.78, P-value = 0.038; and for transient HVA, R = 0.82, P-value = 0.024; Pearson product moment correlation). This suggests a greater contribution of these two current components to the enhancement observed during perfusion stoppage. Visual inspection of the raw current traces, as well as the stoppage-dependent modulation of the current inactivation time constant (Fig. 2B), also support the suggestion that the inactivating components of the current are mainly responsible for the observed enhancement.
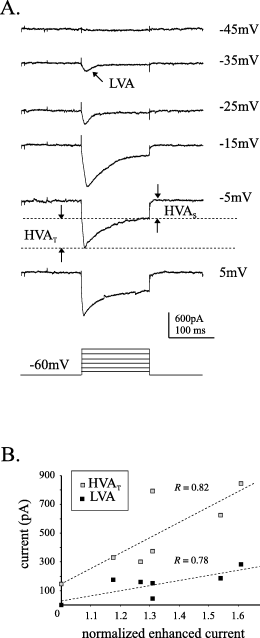
Various calcium current components are enhanced during perfusion stoppage. (A) A family of voltage-gated currents derived by subtracting the currents recorded when the perfusion was ON from those recorded when the perfusion was stopped (i.e. difference currents). To generate the difference currents the perfusion was stopped to potentiate the current then quickly reversed by restarting the perfusion. This permitted the smallest time period between recordings in the two conditions. Components of the ensemble calcium current are identified (HVAS, sustained high-voltage-activated; LVA, low-voltage-activated; HVAT, transient high-voltage-activated). Currents were elicited by voltage steps from a holding potential of −60 mV to the various potentials illustrated. (B) Correlations between the enhancement of the current during perfusion stoppage and the LVA and HVAT components on the current. Normalized current values were determined from time plots similar to the example illustrated in Fig. 2A.
PO2 reduction and calcium currents
As decreased PO2 levels have been shown to potentiate voltage-gated calcium currents in other cell types (see Discussion), this was investigated in the present set of experiments. To examine the effects of changing PO2 on the calcium current without the confounding effects of the changing pH, the bicarbonate-buffered solution was replaced with a HEPES-buffered solution. This allowed the PO2 of the extracellular solution to be manipulated while maintaining a constant pH (7.4).
Both the biphasic pattern and volume-dependence of the rate of PO2 decrease seen with a bicarbonate-buffered solution was also seen when using a HEPES-buffered solution (data not shown). With the standard chamber volume (∼ 500 µL), the PO2 of the detained HEPES-buffered solution decreased by 79 torr (± 29 SD) in 200 s (Fig. 4A). In this set of experiments, larger changes in PO2 were obtained by switching between a non-oxygenated perfusate and one that was bubbled with 100% O2. As illustrated in Fig. 4A, this method produced changes in PO2 that were threefold greater than with perfusion stoppage; 266 torr (± 114 SD) vs. 79 torr (± 29 SD). However, in contrast to the situation in the bicarbonate-buffered solution when PO2 was decreasing simultaneously with a change in pH (Fig. 2A), in the HEPES-buffered solution neither the decrease in PO2 occurring during perfusion stoppage nor the exaggerated changes in PO2 had discernable modulatory effects on the peak amplitude of the calcium currents (Fig. 4B) or on the rate of current inactivation (Fig. 4C and D). This was true for currents elicited from a holding potential of both −60 mV (n = 2) and −80 mV (n = 2). A gradual decrease in the current inactivation time constant over the course of the experiment was noted (Fig. 4C), and was possibly the result of a differential rundown of the current components (Ryu & Randic, 1990; Tombaugh & Somjen, 1997).
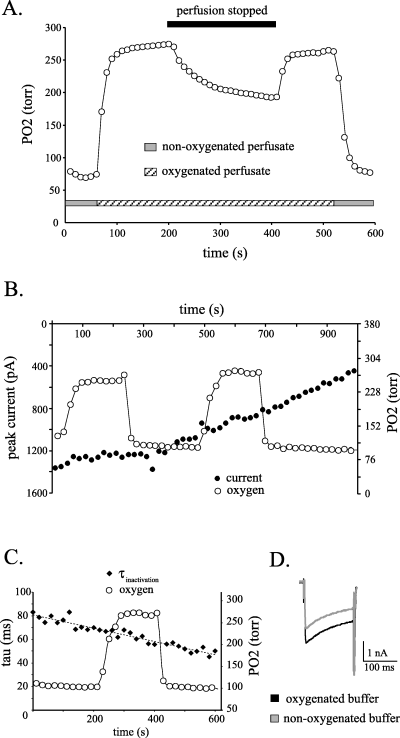
The amount of dissolved oxygen in the tissue chamber can be controlled independent of pH changes, by using an oxygenated HEPES-buffered solution. (A) The time course of the decrease in PO2 in a HEPES-buffered solution is similar to that recorded in a bicarbonate-buffered solution during perfusion stoppages. Switching between oxygenated and non-oxygenated HEPES-buffered solutions produced larger changes in PO2 in the recording chamber than occurred during periods of perfusion stoppage. An initial rapid rise in the amount of dissolved oxygen can be seen upon switching the perfusate from a non-oxygenated solution (grey bar) to one that was bubbled with oxygen (hatched bar). When the perfusion is subsequently stopped and then restarted, the PO2 level is reversibly decreased. Once again switching to the non-oxygenated solution leads to a much larger decrease in the oxygen level (second grey bar). (B) Simultaneous measurements of PO2 and the calcium current demonstrate that large changes in PO2 do not modulate the calcium current amplitude. The oxygen content was manipulated by switching between oxygenated and non-oxygenated HEPES-buffered solutions. Voltage steps to 0 mV from a Vh of −60 mV. (C) These large changes in the solution PO2 also do not modulate the rate of current inactivation. Time constants were derived from the single exponential fit of the current inactivation and demonstrate a gradual decrease over time rather than a reversible modulation by the change in PO2. The dotted line is a linear regression fit of the data. Data from the same experiment as in (B). (D) Current traces recorded while the tissue chamber was being perfused with an oxygenated (black trace) and non-oxygenated solution (grey trace) demonstrating an absence of effect on the current kinetics. The two recording periods were 3 min apart, with each current trace being the average of three consecutive recordings. Currents were elicited with a voltage step to 0 mV from a Vh of −80 mV.
These data demonstrate that the stoppage-dependent modulation of the calcium current seen in a bicarbonate-buffered solution equilibrated with 95% O2/5% CO2 is unlikely due to the rapid PO2 decrease. Rather, the enhancement of the calcium current can be attributed to the increase in solution pH, a result consistent with our previous experiments (Carlin, 2005).
Discussion
Tissue slice experiments involving the use of expensive pharmacological agents or high-resolution image analysis often preclude the possibility of continuous perfusion of the tissue chamber. This study assessed two consequences of stopping the perfusion of a tissue recording chamber when using a bicarbonate/CO2 buffer system. During this procedure there is a rapid alkalinization of the detained solution as well as a rapid drop in the PO2. Given a tissue chamber of fixed dimensions, the rate and magnitude of the changes in these two parameters are dependent on the volume of solution − with smaller volumes providing less stability of both parameters. Using the standard chamber volume, a change in pH was detected in as little as 30 s and a change in PO2 was detected within the first 10 s (first time points sampled for each parameter).
The present set of experiments quantified the changes in the stationary bulk solution of the tissue chamber. Even though changes in both pH (Okada et al., 1993) and PO2 (Bingmann & Kolde, 1982; Fujii et al., 1982; Ballanyi et al., 1996) of the bulk solution can be rapidly detected in the extracellular space of an in vitro tissue preparation, measurements from the detained solution in the tissue chamber may not necessarily reflect the absolute value of these parameters in the extracellular fluid. Hence, it was unclear if the magnitude of the changes in the bulk solution resulted in physiologically relevant changes within the tissue. It was possible that the increase in pH of the detained bicarbonate-buffered solution would be offset by: (i) the fact that tissue slices retain CO2 at levels higher than the bulk solution (Voipio & Kaila, 1993); or (ii) by an extracellular acidification caused by the concomitant PO2 reduction (Hansen, 1985). Therefore, a test of the physiological relevance of the magnitude of the changes in pH and PO2 measured in the bulk solution was conducted. For this we used voltage-gated calcium currents elicited from ventral horn neurons of the neonatal mouse spinal cord, as we have previously demonstrated these currents to be sensitive to changes in extracellular pH (Carlin, 2005). Stopping the CO2-bubbled bicarbonate-buffered solution resulted in an obvious enhancement of the current mediated by these channels over the 200-s time period tested − suggesting that this procedure resulted in changes within the tissue that were physiologically relevant. The similarity in the degree of current enhancement seen in the present study (35%) and our previous study using a 0.6 pH unit alkalinization (27%) suggests that the 0.4 pH unit increase recorded in the bulk solution was, if at all, a slight underestimation of the intratissular pH change.
The current enhancement appears to result from the rapid increase in pH occurring in the detained solution, as a decrease in PO2, even larger than that occurring during perfusion stoppage, was an ineffective modulator of the calcium current. The effects of an increase in extracellular pH on voltage-gated calcium channels presented here are consistent with results from previous studies with respect to the magnitude of current enhancement and the current components affected (Carlin, 2005).
In the present study, an increase in extracellular pH led to an increase in the rate of current inactivation measured at 0 mV. This is different than our previous observations in this same cell type when recorded in HEPES buffer (Carlin, 2005). In that study, the rate of inactivation significantly decreased with an increase in pH when currents were elicited with voltage steps to potentials more positive than −10 mV. The reason for this difference is unclear at present, but may result from differential effects of HEPES and bicarbonate ions on the various calcium channel subtypes present in ventral horn neurons (Bruehl et al., 2000; Gu et al., 2000; Bruehl & Witte, 2003).
Some studies evaluating the effect of decreases in PO2 on voltage-gated calcium currents have reported potentiating effects of hypoxia on central neurons (Krnjevic et al., 1989; Sun & Reis, 1994; Mironov & Richter, 1998; Lukyanetz et al., 2003). In these studies, the ‘normoxic’ values of the recording solution ranged from 148 to 200 torr, while the ‘hypoxic’ values used were between 5 and 50 torr. The fact that a reduction in PO2 did not have an observable effect on the calcium current in the present experiments may, in part, be due to the fact that the drop in PO2 during perfusion stoppage resulted in values that would be considered greater than normoxic in these previous studies (see Fig. 4A). Even the PO2 of the non-oxygenated solution would be considered only slightly hypoxic in those studies, and even ‘normoxic’ compared with in vivo measurements in the rat brain (reviewed in Erecinska & Silver, 2001).
Even though the present work used voltage-gated calcium channels to assay the effects of changes in pH and PO2, effects would be expected on any cellular process affected by alterations in these parameters. Besides effects on voltage-gated ion channels, changes in extracellular pH can also alter numerous processes, including: ligand-gated conductances (Tang et al., 1990; Krishek et al., 1996), transmitter release (Pittaluga et al., 2005) and pharmacological binding efficacies (Hainsworth et al., 2001). Even though the partial pressure of O2 in the bulk solution did not drop to levels that would be considered hypoxic in the present experiments, the use of thick tissue sections or en bloc preparations could potentially create such conditions in the inner cell layers during perfusion stoppage, as these tissues would already be at reduced PO2 levels (Wilson et al., 2003). Apart from the aforementioned effects on voltage-gated calcium currents, the cellular effects of hypoxia are also numerous and can include: activation of other ion channels (reviewed in Lopez-Barneo, 1996), induction of apoptosis (Honma et al., 2004), changes in enzyme activity (Packianathan et al., 1995; Liu et al., 2005), increases in cytosolic calcium (Bickler & Hansen, 1994) and activation of transcription factors (Cummins & Taylor, 2005). Stopping a bicarbonate/CO2-buffered perfusate produces rapid changes in the tissue chamber environment that have the capacity to elicit a myriad of unintended cellular responses. Therefore, the use of this experimental procedure has the potential to covertly influence experimental results.
Acknowledgements
The authors wish to acknowledge Drs L.M. Jordan, Z. Jiang and B. Fedirchuk for their helpful comments on a previous version of the manuscript, and D. Manchur and C. Gibbs for their technical assistance. This work was supported by grants to R.M. Brownstone from the Canadian Institute of Health Research (CIHR), Amyotrophic Lateral Sclerosis Society of Canada, Manitoba Health Research Council and Nova Scotia Health Research Council, and grants to L.M. Jordan from CIHR. K.P. Carlin was supported by a salary award from the Manitoba Neurotrauma Initiative.
Abbreviations
-
- HEPES
-
- N-2-hydroxyethylpiperazine-N-2-ethanesulfonic acid
-
- HVA
-
- high-voltage-activated
-
- LVA
-
- low-voltage-activated
-
- TTX
-
- tetrodotoxin