Is the CCK2 receptor essential for normal regulation of body weight and adiposity?
Abstract
Cholecystokinin (CCK) is a gastrointestinal satiety signal released from the duodenum to terminate feeding, via CCK1 receptors. CCK2 receptors are considered to be involved in anxiety. CCK2 receptor knockout mice have increased body weight and food intake. Little is known regarding the effects of CCK2 receptor deficiency on adipose distribution and hypothalamic feeding regulators such as neuropeptide Y (NPY), a powerful stimulator of feeding. Adult (10 week) CCK2 receptor knockout and wild-type mice were anaesthetized and killed by decapitation. Brain sections, organs and fat tissue were dissected. Plasma leptin, insulin and brain NPY content were measured by radioimmunoassay. Female CCK2 receptor knockout mice weighed more than control mice (22.0 ± 0.2 vs. 19.9 ± 0.4 g, P < 0.05), with this difference being less marked in male mice (26.4 ± 0.4 vs. 25.6 ± 0.6 g). Fat masses in all locations sampled were significantly smaller in CCK2 receptor knockout mice of both genders (P < 0.05), resulting in lower plasma leptin and insulin levels. NPY concentrations were significantly increased in arcuate nucleus and anterior hypothalamus in both male and female CCK2 receptor knockout mice, and total hypothalamic NPY content was increased by 7 and 9% in males and females, respectively (P < 0.05). CCK2 receptor deletion was associated with increased body weight and hypothalamic NPY content, but reduced fat masses and plasma leptin and insulin. Increased NPY might contribute to increased food intake in CCK2 receptor knockout mice. Further work needs to focus on the metabolic changes.
Introduction
Cholecystokinin (CCK) was first discovered in 1928 and purified from porcine duodenum in 1966 (Jvy & Oldberg, 1928; Jorpes & Mutt, 1966; Moran & Schwartz, 1994). CCK was subsequently shown to be present in the CNS. In the human brain, the most abundant form is CCK8, whereas in the periphery CCK8, CCK22, CCK33 and CCK58 are present (Little et al., 2005). CCK is widely distributed throughout the gastrointestinal tract, but is most concentrated in the duodenum and the jejunum, and its functions include the stimulation of pancreatic secretion, gallbladder contraction, intestinal motility and the inhibition of gastric emptying.
CCK functions as a satiety signal. CCK is secreted from duodenal cells in response to nutrients in the lumen, and CCK levels are markedly increased in plasma within minutes of ingestion of mixed meals, casein, amino acid or glucose solutions, or lipid emulsions (Geary, 2004). Circulating CCK stimulates the pancreas and liver to secrete appropriate enzymes into the duodenum to facilitate digestion. Food deprivation has been shown to decrease intestinal CCK mRNA (Crawley & Corwin, 1994). Administration of CCK can inhibit food intake in most mammalian species, including rodents, pigs, rhesus monkeys and humans (Canova & Geary, 1991; Parrott et al., 1991; Asin & Bednarz, 1992; Geary et al., 1992; Moran et al., 1993; Crawley & Corwin, 1994). However, tolerance develops to the anorexigenic effects of CCK after repeated injection, and CCK is short-acting and thus only reduces meal size without affecting meal frequency; as a result, animals compensated by eating more meals within a day (Crawley & Beinfeld, 1983; West et al., 1984, 1987). Therefore, total daily food intake and body weight are not changed by repeated CCK administration.
The biological actions of CCK are mediated by cell-surface membrane receptors on its target cells (Liddle, 1994, 1997). Two subtypes of CCK receptors have been identified with different pharmacological characteristics and different tissue distributions. Both the CCK1 and the CCK2 receptors consist of seven transmembrane-spanning protein domains, and are members of the G-protein-coupled superfamily of receptor molecules (Kopin et al., 1992). The CCK1 receptor is abundant in peripheral organs (gall bladder, pancreas, pylorus, intestine, spinal cord, vagus nerve) and in a few discrete brain regions (nucleus tractus solitarius, the area postrema and the hypothalamus), whereas the CCK2 receptor is present in the cerebral cortex, the hypothalamus, vagal nerve, spinal cord and gastric mucosa (Smith et al., 1984; Moran et al., 1986; Hill et al., 1987). Early studies suggested the ability of CCK to terminate feeding was mediated through CCK1 receptors (Gibbs et al., 1973; Lorenz et al., 1979). The CCK1 receptor-selective tetrapeptide agonist, A71623, caused a dose-dependent suppression of food intake, whereas peripheral administration of a range of doses of a selective, high-affinity CCK2 receptor-specific agonist failed to produce satiety (Asin & Bednarz, 1992). Otsuka Long Evans Tokushima Fatty (OLETF) rats, bearing a null mutation of the CCK1 receptor gene, display chronically increased meal size, increased food intake, obesity and diabetes (Moran et al., 1998). However, CCK1 receptor knockout mice do not display hyperphagia, obesity or diabetes, suggesting the possibility of species differences (Kopin et al., 1999; Bi et al., 2001, 2004).
Current thinking is that hormonal CCK released from the small intestine when nutrients enter the duodenum stimulates CCK1 receptor on sensory fibres of vagal afferents (Woods, 2004) that transfer signals to the nucleus tractus solitarius in the brainstem. This satiety signal can be reduced by vagotomy, vagal transection, deactivation of vagal afferents or lesion of the nucleus tractus solitarius (Konturek et al., 2004).
CCK also interacts with other central appetite regulators and this may be important in its satiety-inducing effects. Neuropeptide Y (NPY) is a powerful neurochemical stimulator of feeding in many species (Williams et al., 2000). An increase in dorsomedial hypothalamus (DMH) NPY mRNA was found in pre-obese OLETF rats. CCK injection was also shown to regulate DMH NPY expression. However, no obvious relationship between NPY changes and hyperphagia was observed in CCK1 receptor knockout mice (Bi et al., 2004). Previously, increased body weight and food intake have been observed in CCK2 receptor knockout (CCK2R KO) mice (Weiland et al., 2004). However, the role of hypothalamic NPY in food intake regulation in CCK2 receptor-deficient mice has not been investigated. In the current study, the effects of CCK2 receptor deficiency on growth, fat distribution and brain NPY content were investigated.
Materials and methods
Animals and sample collection
Wild-type and homozygous CCK2R KO mice were sourced from La Trobe University (Melbourne, Australia), from a colony developed through gene targeting as described previously (Nagata et al., 1996; Weiland et al., 2004). Male and female CCK2R KO mice and age-matched wild-type mice (C57BL/6) (total n = 75, age: male wild-type 10.0 ± 0.2 vs. CCK2R KO 9.8 ± 0.0 weeks, female wild-type 10.1 ± 0.1 vs. CCK2R KO 9.9 ± 0.0 weeks) were bred and housed in the La Trobe University Central Animal House. Briefly, mice were housed at 23 ± 1 °C, and maintained on a 12 : 12 h light : dark cycle (lights on at 06.00 h) with their littermates. They were allowed ad libitum access to standard rodent chow and water.
Mice were killed for blood, brain and tissue collection. Animals were anaesthetized [ketamine/xylazine 180/32 mg/kg, intraperitoneal, Parnell Laboratories (AUST), NSW, Australia, and Bayer Australia, NSW, Australia, respectively] and tissue harvested between 09.30 and 14.00 h. Body weight, naso-anal (N-A) length, tibia length and girth (rib and groin circumferences) were recorded. Approximately 0.5 mL of cardiac blood was collected by heart puncture into heparinized (5000 IU/mL; Fisons, QLD, Australia) tubes and centrifuged. Separated plasma was stored at −80 °C for subsequent assays. Animals were killed by decapitation and the brain was rapidly dissected on ice into regions containing paraventricular nucleus (PVN), arcuate nucleus (Arc), anterior hypothalamus (AH), posterior hypothalamus (PH), medulla and cerebral cortex. Brain regions were weighed, frozen on dry ice, and later stored at −80 °C for determination of NPY peptide content. Brown adipose tissue (BAT), retroperitoneal (Rp) white adipose tissue (WAT), testicular/ovarian WAT, leg subcutaneous WAT, visceral WAT, liver, spleen, heart, thymus and kidney were dissected and weighed.
NPY, leptin, insulin and corticosterone assays
Endogenous NPY from the various brain regions was extracted by boiling the tissues in 0.5 m acetic acid, homogenization by hand with a glass homogenizer, and centrifugation at 7500 g for 30 min at 4 °C. Samples of supernatant (50 µL) were lyophilized and reconstituted with assay buffer (0.04 m sodium phosphate buffer containing 0.01 m EDTA, 0.1 m NaCl, 0.02% NaN3, 0.25% bovine serum albumin, pH 7.3). NPY-like immunoreactivity was measured by a specific radioimmunoassay developed in our laboratory (Morris et al., 1986) using synthetic NPY as standard (10–1280 pg/tube, Auspep, VIC, Australia). Samples were incubated with NPY antibody (Professor Bevyn Jarrott, Monash University, VIC, Australia) overnight at 4 °C. 125I-NPY labelled with Bolton and Hunter reagent (3000 Ci/mmol, Amersham, NSW, Australia) was added and the incubation continued overnight. Bound and free radioligand were separated by the addition of non-immunized rabbit serum and sheep anti-rabbit second antibody (Silenus, VIC, Australia) followed by centrifugation at 3000 g (RT7, Sorvall instruments, MA, USA) at 4 °C for 35 min. The detection limit for the radioimmunoassay was routinely 2 pg NPY per tube, and the intra- and interassay coefficients of variation were 6% and 13%, respectively (gamma counter, Cobra ÉÉ series Autogamma, Packard Counting System, CT, USA). NPY in each brain region was calculated as ng NPY per mg tissue.
Plasma leptin, insulin and corticosterone concentrations were measured using commercially available radioimmunoassays (Linco, Missouri, USA, and MP Biomedicals Europe, Belgium, respectively).
Statistical method
Results are expressed as mean ± SEM. Differences in body weight, fat and organ masses, plasma hormones, and brain NPY concentration and content were analysed using Student's unpaired t-tests (SPSS 11.5, IL, USA).
Ethics approval
The current study was approved by both the Animal Experimentation Ethics Committee of the University of Melbourne and La Trobe University Animal Ethics Committee.
Results
Body weight and other parameters
Male mice
Male CCK2R KO mice were slightly heavier than wild-type mice (Table 1); however, this failed to reach statistical significance. Tibia length was increased in the male CCK2R KO mice (P < 0.01, Table 1), while N-A length, rib and groin circumferences were similar to the wild-type mice. Liver, kidney, heart and spleen weights were all greater in CCK2R KO mice; however, they had a smaller thymus (P < 0.05). When organ masses were adjusted for body weight, the differences were still evident, with all organs being heavier (liver 5.61 ± 0.16 vs. 5.25 ± 0.11%; kidney 0.70 ± 0.01 vs. 0.56 ± 0.01%; heart 0.52 ± 0.01 vs. 0.45 ± 0.004%; spleen 0.31 ± 0.01 vs. 0.27 ± 0.01%, in CCK2R KO vs. wild-type mice, respectively, P < 0.01). The thymus, however, was the exception to this pattern (0.15 ± 0.01 vs. 0.18 ± 0.01%, in CCK2R KO vs. wild type mice, respectively, P < 0.001).
| Male | Wild-type(n = 15) | CCK2R KO (n = 20) |
|---|---|---|
| Body weight (g) | 25.6 ± 0.6 | 26.4 ± 0.4 |
| N-A length (cm) | 9.31 ± 0.06 | 9.44 ± 0.05 |
| Tibia (cm) | 1.92 ± 0.01 | 1.99 ± 0.02** |
| Rib circumference (cm) | 7.86 ± 0.09 | 7.78 ± 0.10 |
| Groin circumference (cm) | 7.99 ± 0.09 | 8.28 ± 0.12 |
| Liver (mg) | 1347.3 ± 46.8 | 1480.5 ± 31.5* |
| Kidney (mg) | 143.8 ± 4.4 | 183.8 ± 3.3*** |
| Heart (mg) | 116.4 ± 2.7 | 138.3 ± 2.4*** |
| Spleen (mg) | 69.3 ± 3.4 | 83.3 ± 3.9* |
| Thymus (mg) | 45.7 ± 1.8 | 39.6 ± 2.0* |
| BAT (mg) | 52.2 ± 2.3 | 44.2 ± 1.3** |
| Rp WAT (mg) | 57.3 ± 4.9 | 32.5 ± 2.3*** |
| Testicular WAT (mg) | 328.6 ± 26.6 | 170.2 ± 6.6*** |
| Visceral WAT (mg) | 409.0 ± 17.8 | 377.2 ± 9.8 |
| Leg subcutaneous WAT (mg) | 269.3 ± 17.8 | 144.1 ± 7.9*** |
| Total WAT mass (mg) | 1121.5 ± 68.7 | 756.4 ± 24.5*** |
- Results are expressed as mean ± SEM. Data were analysed by Student's unpaired t-test. BAT, brown adipose tissue; N-A, naso-anal; Rp, retroperitoneal; WAT, white adipose tissue. *Significantly different from wild-type animals, P < 0.05. **Significantly different from wild-type animals, P < 0.01. ***Significantly different from wild-type animals, P < 0.001.
However, four of the five body fat depots sampled were significantly smaller in male CCK2R KO mice despite the slightly heavier body weight, and the total body WAT mass sampled was only 68% that of wild-type mice (P < 0.001, Table 1). Furthermore, the percentage of Rp WAT, testicular WAT and leg subcutaneous WAT of total body weight in CCK2R KO mice was almost half that of wild-type animals (Rp WAT 0.12 ± 0.01 vs. 0.22 ± 0.02%; testicular WAT 0.64 ± 0.02 vs. 1.28 ± 0.09%; leg subcutaneous WAT 0.55 ± 0.03 vs. 1.05 ± 0.06%, in CCK2R KO vs. wild-type mice, respectively, P < 0.001).
Female mice
Female mutant mice were 10% heavier than wild-type mice of the same age (Table 2, P < 0.001). CCK2R KO female mice were also bigger than wild-type mice, reflected by greater N-A length and tibia length (P < 0.001). However, rib and groin circumferences were not different between the two strains. Similar to male mice, liver and kidney weights were greater in CCK2R KO female mice (Table 2, P < 0.05); however, only the liver was significantly larger when calculated as a percentage of body weight (5.27 ± 0.05 vs. 4.79 ± 0.17% in CCK2R KO vs. wild-type mice, respectively, P < 0.01).
| Female | Wild-type(n = 19) | CCK2R KO (n = 21) |
|---|---|---|
| Body weight (g) | 19.9 ± 0.4 | 22.0 ± 0.2*** |
| N-A length (cm) | 8.76 ± 0.06 | 9.11 ± 0.05*** |
| Tibia (cm) | 1.86 ± 0.02 | 1.92 ± 0.02* |
| Rib circumference (cm) | 7.17 ± 0.09 | 7.14 ± 0.08 |
| Groin circumference (cm) | 7.32 ± 0.09 | 7.45 ± 0.07 |
| Liver (mg) | 960.8 ± 33.9 | 1155.8 ± 15.7*** |
| Kidney (mg) | 121.2 ± 4.5 | 129.3 ± 1.9 |
| Heart (mg) | 101.6 ± 3.8 | 110.8 ± 2.1* |
| Spleen (mg) | 74.6 ± 2.8 | 80.0 ± 2.3 |
| Thymus (mg) | 61.7 ± 2.7 | 48.5 ± 1.9* |
| BAT (mg) | 45.2 ± 2.3 | 36.8 ± 1.6** |
| Rp WAT (mg) | 37.2 ± 2.4 | 33.1 ± 2.1 |
| Ovarian WAT (mg) | 202.4 ± 14.3 | 135.2 ± 16.3** |
| Visceral WAT (mg) | 357.2 ± 14.3 | 351.8 ± 8.3 |
| Leg subcutaneous WAT (mg) | 251.4 ± 19.7 | 162.1 ± 6.9*** |
| Total WAT mass (mg) | 885.4 ± 42.9 | 715.3 ± 26.5*** |
- Results are expressed as mean ± SEM. Data were analysed by Student's unpaired t-test. BAT, brown adipose tissue; N-A, naso-anal; Rp, retroperitoneal; WAT, white adipose tissue. *Significantly different from wild-type animals, P < 0.05. **Significantly different from wild-type animals, P < 0.01. ***Significantly different from wild-type animals, P < 0.001.
Interestingly, as seen in the male mice, BAT and WAT masses were significantly smaller than wild-type mice in the face of a distinctly greater body weight and larger body size, and the total WAT mass was 20% lower in female CCK2R KO mice compared with wild-type mice (P < 0.01). When fat masses were standardized by body weight, BAT and WAT from all the locations sampled were still significantly lower in CCK2R KO mice (BAT 0.17 ± 0.01 vs. 0.22 ± 0.01%; Rp WAT 0.15 ± 0.01 vs. 0.18 ± 0.01%; ovarian WAT 0.61 ± 0.07 vs. 1.00 ± 0.06%; visceral WAT 1.60 ± 0.03 vs. 1.77 ± 0.05%; leg subcutaneous WAT 0.74 ± 0.03 vs. 1.25 ± 0.10%; total WAT 3.26 ± 0.11 vs. 4.38 ± 0.18%, in CCK2R KO vs. wild-type mice, respectively, P < 0.05).
Plasma leptin, insulin and corticosterone concentrations in wild-type and CCK2R KO mice
Plasma leptin concentration was reduced by more than 50% in CCK2R KO male mice compared with wild-type mice (Fig. 1A,P < 0.001). Although leptin concentration was 19% lower in CCK2R KO female mice, it failed to reach statistical significance (Fig. 1B, P = 0.13). Plasma insulin concentrations were dramatically reduced in CCK2R KO mice of both genders, with only 45% and 15% of the concentration in male and female wild-type mice, respectively (Fig. 1A and B, P < 0.05). Plasma corticosterone concentration was not significantly different between wild-type and CCK2R KO mice of both genders (Fig. 1).
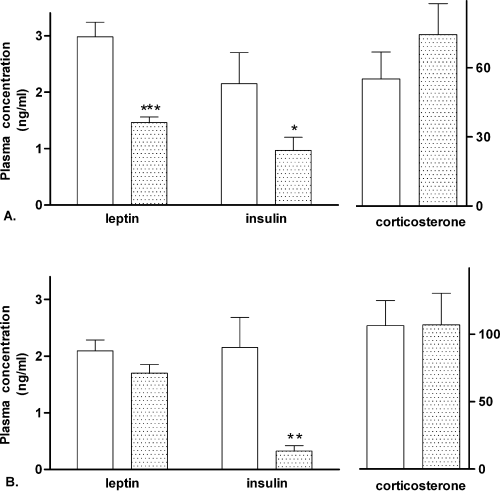
Plasma leptin, insulin and corticosterone concentrations in 10-week-old male (A) wild-type (C57BL/6) (open bar, n = 15) or CCK2R KO (stippled bar, n = 20) mice, and female (B) wild-type (C57BL/6) (stippled bars, n = 19) or CCK2R KO (filled bar, n = 21) mice (for plasma corticosterone concentration n = 8 in each group). Results are expressed as mean ± SEM. Data were analysed by Student's unpaired t-test. *Significantly different from wild-type animals, P < 0.05; **significantly different from wild-type animals, P < 0.01; ***significantly different from wild-type animals, P < 0.001.
Brain NPY content in CCK2R KO and wild-type mice
Male mice
Within the hypothalamus, NPY concentrations were significantly increased in the PVN, Arc and AH by 12, 20 and 25%, respectively, in the CCK2R KO mice compared with wild-type mice (P < 0.05, Fig. 2). No difference was seen in the PH, but NPY concentrations were nearly doubled in the medulla in the CCK2R KO mice (0.52 ± 0.03 vs. 0.29 ± 0.01 ng/mg tissue in the wild-type mice, P < 0.05, Fig. 2). However, cortex NPY concentration remained unchanged by CCK2 receptor deletion. The total hypothalamic NPY content, which is the sum of NPY content in PVN, PH, Arc and AH, was also significantly larger in the CCK2R KO mice (85.6 ± 1.5 ng in the CCK2R KO vs. 79.9 ± 1.3 ng in the wild-type mice, P < 0.01).
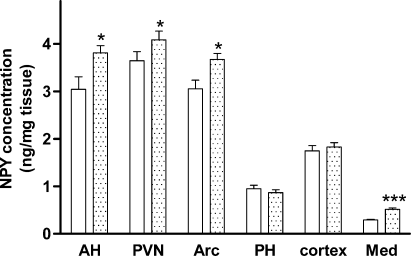
Neuropeptide Y (NPY) concentrations (expressed as ng/mg tissue) in 10-week-old male wild-type (C57BL/6) (open bars, n = 15) or CCK2R KO (stippled bars, n = 20) mice. Areas shown are anterior hypothalamus (AH), paraventricular nucleus (PVN), arcuate nucleus (Arc), posterior hypothalamus (PH), cortex and medulla (Med). Results are expressed as mean ± SEM. Data were analysed by Student's unpaired t-test. *Significantly different from wild-type animals, P < 0.05; ***significantly different from wild-type animals, P < 0.001.
Female mice
Brain NPY concentrations in the female mice were similar to those seen in the male mice. NPY concentrations in the Arc and AH were increased by 20 and 19% in CCK2R KO mice compared with wild-type mice (P < 0.05, Fig. 3). An 8% increase was also seen in the PVN of CCK2R KO mice; however, this failed to reach statistical significance (P = 0.057). No changes were evident in the PH. NPY concentrations in the medulla were higher in CCK2R KO mice compared with wild-type animals, and there was a small increase in the cerebral cortex (P < 0.05, Fig. 3). The total hypothalamic NPY content was also significantly increased by 9% by CCK2 receptor deletion in the female mice (85.9 ± 1.0 ng in the CCK2R KO vs. 78.7 ± 1.2 ng in the wild-type mice, P < 0.001).
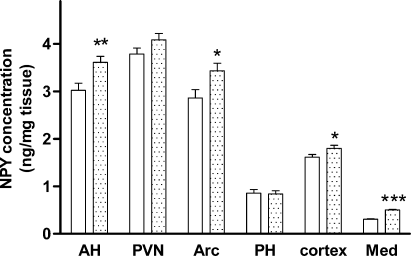
Neuropeptide Y (NPY) concentrations (expressed as ng/mg tissue) in 10-week-old female wild-type (C57BL/6) (stippled bars, n = 19) or CCK2R KO (filled bar, n = 21) mice. Areas shown are anterior hypothalamus (AH), paraventricular nucleus (PVN), arcuate nucleus (Arc), posterior hypothalamus (PH), cortex and medulla (Med). Results are expressed as mean ± SEM. Data were analysed by Student's unpaired t-test. *Significantly different from wild-type animals, P < 0.05; **significantly different from wild-type animals, P < 0.01; ***significantly different from wild-type animals, P < 0.001.
Discussion
The current study, for the first time, systematically characterizes the impact of CCK2 receptor deletion in mice with respect to body weight, organ and adipose tissue parameters, brain NPY content and plasma hormones.
CCK2 receptors have previously been considered to be involved in anxiety rather than appetite and energy metabolism (Miyasaka et al., 2002). Although CCK2 receptor deletion did not modulate the inhibitory effects of CCK8 on food intake in mice (Kopin et al., 1999), central administration of a CCK2 receptor antagonist significantly increased food intake in the rat (Dorre & Smith, 1998), suggesting an involvement of this receptor in appetite or satiety regulation. Furthermore, previous observations from different studies of either food intake or body weight in CCK2R KO animals have yielded conflicting results. Some studies have reported that CCK2 receptor-deficient mice show no obvious abnormalities, with general appearance and body weight similar to their age-matched wild-type littermates (Nagata et al., 1996; Kopin et al., 1999; Noble & Roques, 2002); while other studies, sometimes from the same laboratories, have found increased food and water consumption in CCK2R KO mice, and increased body weight compared with wild-type mice (Miyasaka et al., 2002; Weiland et al., 2004). Miyasaka and colleagues reported that the CCK2R KO mice also had greater energy intake than CCK1 receptor knockout mice (Miyasaka et al., 2002), suggesting that CCK2 receptor may play a greater role in modulating appetite in mice. However, it was postulated that this influence on feeding behaviour only came into play in situations of stress or anxiety. Corticosterone, part of the adaptive physiological response to environmental stressors, is an important appetite stimulator that is activated by food restriction (Dallman et al., 2004). In the current study, no significant change in plasma corticosterone levels was observed due to CCK2 receptor knockout, suggesting the previously observed hyperphagia was not due to anxiety. The higher basal corticosterone levels in female mice are consistent with previous studies on rodents, where females tend to produce more corticosterone than males (Finn et al., 2004; Mantella et al., 2005).
In our study, the body weight of CCK2R KO mice of both genders was greater than age-matched wild-type mice, although significance was only observed in females. Increased tibia length suggested larger body size. Most of the organ weights were also increased in CCK2R KO mice, and remained so when adjusted for body weight. Surprisingly, unlike other obesity models, the larger body weight of CCK2R KO mice did not result in a proportional increase in adipose tissue weight. On the contrary, all the fat pads sampled were markedly reduced in CCK2R KO mice of both genders, either when recorded as net weight or as a percentage of body weight, most likely contributing to the reduced plasma insulin and leptin levels (Bagdade et al., 1967; Maffei et al., 1995). Previously, a CCK2 receptor antagonist was shown to inhibit leptin mRNA expression and secretion, and only CCK2 receptors, not CCK1 receptors, are expressed in adipose tissue, suggesting CCK2 receptors regulate leptin or fat metabolism (Attoub et al., 1999). The larger body weight in the face of reduced fat mass indicates that increased lean body mass, particularly muscle mass, may be a major contributor to the increased body weight.
Conflicting observations also exist on locomotor activity, with enhanced basal activity (Dauge et al., 2001) or decreased locomotor activity (Koks et al., 2001; Weiland et al., 2004) found in different studies. However, energy expenditure and body temperature have both been reported to be increased in CCK2R KO mice (Miyasaka et al., 2002; Weiland et al., 2004), which may have directly contributed to the decreased fat mass observed in these animals. Increased opioid activity in the absence of CCK2 receptor might be one explanation for the enhanced body temperature (Weiland et al., 2004). Furthermore, lean body mass accounts for most of the basal metabolic rate, particularly the thermogenesis in muscle cells, which could augment body temperature (Cunningham, 1991; Dulloo, 2005; Johnstone et al., 2005). Skeletal muscle is an important organ for fatty acid oxidation, whose activity might also help to explain the fat loss in CCK2R KO mice (Kelley, 2005). Our observations are consistent with this early work and suggest that CCK2 receptor may play a role in adipose differentiation. The relative roles of CCK1 and CCK2 receptors in this are unknown.
For the first time, this study reports alterations in brain NPY in CCK2R KO mice. NPY concentrations were increased in most of the hypothalamic regions, including the Arc where NPY is synthesized, the PVN where the orexigenic effects of NPY are exerted, and the AH, which contained the ‘feeding centre’ (the lateral hypothalamic area) and part of the ‘satiety centre’ (the ventromedial hypothalamic area). The increased hypothalamic NPY peptide in CCK2R KO mice is likely to contribute to their hyperphagic state (Weiland et al., 2004), as previous studies have reported increased hypothalamic NPY mRNA expression and peptide where hyperphagia is present, including the ob/ob mice (Wilding et al., 1993), tubby mice (Guan et al., 1998), and type 1 and type 2 diabetic rats (Abe et al., 1991; Morris & Pavia, 2004).
Under normal physiological conditions, Arc NPY gene expression is negatively regulated by leptin signalling (Stephens et al., 1995; Schwartz et al., 1996). The decrease in plasma leptin concentration and WAT mass in the CCK2R KO mice would be expected to stimulate hypothalamic NPY production as observed here. The increased hypothalamic NPY may contribute to the maintenance of a hyperphagic state in the CCK2R KO mice. However, increased NPY levels are reported to reduce thermogenesis and energy expenditure, promote adipose tissue accumulation, and induce obesity (Schwartz et al., 2000; Williams et al., 2001), which was not obvious in our study or previous studies (Miyasaka et al., 2002; Weiland et al., 2004). Consequently, other pathways might override the positive energy balance effects of NPY and drive fat loss in these animals.
Studies on the effects of CCK receptor deletion on hypothalamic NPY synthesis are limited. In the OLETF rat increased NPY was evident in the DMH in 5-week-old pre-obese animals (Bi et al., 2001). DMH-lesioned rats develop hypophagia and show reduced body weight, which was considered to be due to the downregulation of body weight ‘set point’ (Bernardis & Bellinger, 1996). Thus, the elevated levels of NPY expression in the young OLETF rats may initiate the hyperphagia and upregulate body weight ‘set point’, contributing to the subsequent onset of obesity and diabetes. No obvious changes in brain NPY mRNA expression were observed in CCK1 receptor knockout mice (Bi et al., 2001). In the current study, NPY concentration in the PH (containing the DMH), which cooperates with the PVN in initiating and maintaining food intake (Williams et al., 2001), was not significantly altered in CCK2R KO mice. This might be due to the absence of CCK receptors and binding in this area. However, we cannot exclude the possibility of an increase in DMH NPY concentration due to neural input from other hypothalamic regions in younger CCK2R KO mice, which might initiate hyperphagia or alter body weight ‘set point’, as observed in the OLETF rat.
The nucleus tractus solitarius in the medulla receives afferent input from the vagus nerve arising from the gastrointestinal tract, transferring peripheral satiety information, such as CCK levels, and incoming adipose signals, such as leptin and insulin levels (Woods, 2004). Endogenous CCK acts at CCK1 receptors on the vagus in response to small intestinal nutrients (Schwartz, 2000). The gastric overloading due to hyperphagia may increase CCK secretion, which in turn increases the activity of vagal input to the nucleus tractus solitarius, reflected by the increased NPY peptide in the medulla. Furthermore, the hypothalamus receives the input of a small portion of NPY neurons involved in feeding regulation from the brain stem (Grove & Smith, 2003). Increased NPY content in the medulla may also increase NPY release in the hypothalamus, via ascending projections. The NPY receptor subtypes that are engaged in feeding regulation (Y1, Y2 and Y5) have also been demonstrated in the medulla, and exogenous injection of NPY into the hindbrain can increase food intake (Della Zuana et al., 2001). Thus, the increased NPY content in the medulla itself may activate NPY receptors to affect feeding behaviour. Furthermore, direct injection of NPY to the cortex can also stimulate feeding (Della Zuana et al., 2001), suggesting the increased cortex NPY concentration in female CCK2R KO mice may also contribute to the altered food intake.
Other transmitters implicated in appetite control may also be affected in the CCK2R KO mice. The reduction of insulin and leptin in CCK2R KO mice may potentially reduce the activity of anorexigenic proopiomelanocortin neurons, and increase the synthesis and release of the orexigenic peptide agouti-related protein, a melanocortin antagonist (Schwartz et al., 2000). It is likely that in CCK2R KO mice, although caloric intake was increased due to the activation of orexigenic pathways and inactivation of anorexigenic pathways, the energy expenditure process might be triggered to compensate for the excess energy intake, the main contribution of which is fat burning.
In summary, CCK2R KO mice had increased body weight and organ mass, but smaller adipose tissue mass. Hypothalamic and medulla NPY concentrations were significantly increased in the face of reduced plasma leptin levels in the mice with CCK2 receptor deletion. More work needs to be done to study the mechanisms underlying the reduced fat mass in the face of larger body weight and increased hypothalamic NPY peptide, including growth hormone and thyroid hormone profile. Instead of rendering animals prone to obesity, antagonism of CCK2 receptor might offer one pathway towards controlling fat accumulation.
Acknowledgement
This work was funded by the National Health and Medical Research Council of Australia.
Abbreviations
-
- AH
-
- anterior hypothalamus
-
- Arc
-
- arcuate nucleus
-
- BAT
-
- brown adipose tissue
-
- CCK
-
- cholecystokinin
-
- CCK2R KO
-
- CCK2 receptor knockout
-
- DMH
-
- dorsomedial hypothalamus
-
- N-A
-
- naso-anal
-
- NPY
-
- neuropeptide Y
-
- OLETF
-
- Otsuka Long Evans Tokushima Fatty
-
- PH
-
- posterior hypothalamus
-
- PVN
-
- paraventricular nucleus
-
- Rp
-
- retroperitoneal
-
- WAT
-
- white adipose tissue