Hyperpolarization-activated and cyclic nucleotide-gated cation channel subunit 2 ion channels modulate synaptic transmission from nociceptive primary afferents containing substance P to secondary sensory neurons in laminae I–IIo of the rodent spinal dorsal horn
Abstract
We have previously demonstrated that hyperpolarization-activated and cyclic nucleotide-gated cation channel subunit 2 (HCN2) is expressed by terminals of peptidergic nociceptive primary afferents in laminae I–IIo of the rat spinal dorsal horn. In this study, we investigated the possible neurotransmitters and postsynaptic targets of these HCN2-expressing primary afferent terminals in the superficial spinal dorsal horn by using immunocytochemical methods. We demonstrated that HCN2 widely colocalizes with substance P (SP), and that HCN2-positive terminals that are also immunoreactive for SP form serial close appositions with dendrites and perikarya of neurokinin 1 receptor-immunoreactive neurons. It was also found that HCN2-immunoreactive terminals are frequently apposed to neurons that are immunoreactive for calbindin, µ-opioid receptor and the α-amino-3-hydroxy-5-methylisoxazole-4-propionate receptor subunit GluR2, markers for excitatory interneurons. Investigating HCN2 immunoreactivity in glutamic acid decarboxylase 65-green fluorescent protein transgenic mice, we found that HCN2-positive terminals occasionally also contact cells that contain an isoform of glutamic acid decarboxylase (glutamic acid decarboxylase 65), a marker for GABAergic inhibitory neurons. Application of ZD7288, an antagonist of HCN channels, onto neurons that were recorded in spinal cord slices with whole-cell patch-clamp electrodes reduced the number of monosynaptic excitatory postsynaptic potentials evoked by electrical stimulation of primary afferents at nociceptive intensities. The results suggest that HCN2 may contribute to the modulation of membrane excitability of SP-containing nociceptive primary afferent terminals, may increase the reliability of synaptic transmission from primary afferents to secondary sensory neurons and thus may play a role in the fine-tuning of pain transmission from nociceptive primary afferents to neurons in the spinal dorsal horn.
Introduction
Hyperpolarization-activated cation currents (Ih), mediated by hyperpolarization-activated and cyclic nucleotide-gated (HCN) channels, play major roles in various neural functions. In specific cell types of the nervous system, Ih has been shown to control intrinsic neuronal properties like resting membrane potential, membrane resistance, membrane time and length constants, amplitude and time course of spike afterhyperpolarization (Pape, 1996; Lupica et al., 2001; Surges et al., 2004; Simeone et al., 2005), and thus appears to be an important determinant of the excitability of neurons (Robinson & Siegelbaum, 2003). It has also been demonstrated that Ih impacts on the shape and propagation of subthreshold voltage potentials in dendrites, governing the integration of synaptic inputs (Magee, 1998, 1999; Day et al., 2002; Santoro & Baram, 2003; Poolos, 2004) and attenuating the location dependence of temporal integration of excitatory synaptic potentials (Schwindt & Crill, 1997; Stuart & Spruston, 1998; Magee, 2000; Williams & Stuart, 2000; Berger et al., 2001). It is also well established that Ih contributes to firing properties of nerve cells (Maccaferri & McBain, 1996; Bender et al., 2005), network activity (Accili et al., 2002; Bender et al., 2005), neuronal oscillation and rhythmogenesis (Pape & McCormick, 1989; McCormick & Pape, 1990; Luthi & McCormick, 1998).
In addition to somata and dendrites, HCN channel proteins and Ih are also expressed by axon terminals both in the central and peripheral nervous system, including crayfish neuromuscular junction (Beaumont & Zucker, 2000), presynaptic terminals in chick ciliary ganglion (Fletcher & Chiappinelli, 1992), presynaptic zones of retinal bipolar cells (Kim et al., 2003; Müller et al., 2003), hippocampal mossy fibers (Chevaleyre & Castillo, 2002) and axon terminals of cerebellar basket cells (Santoro et al., 1997; Southan et al., 2000; Luján et al., 2005). It has been proposed that presynaptically expressed Ih may contribute to the modulation of neurotransmitter release and synaptic transmission (Lupica et al., 2001; van Welie et al., 2004). In the crayfish neuromuscular junction, presynaptic Ih has been postulated to enhance transmitter release by increasing the readily releasable vesicle pool by means of cAMP modulation (Beaumont & Zucker, 2000). Moreover, Mellor et al. (2002) put forward the provocative idea that presynaptic Ih channels are necessary for hippocampal mossy fiber long-term potentiation.
Primary viscerosensory and somatosensory neurons in the nodose, trigeminal and spinal dorsal root ganglia also widely express HCN channel proteins and Ih (Mayer & Westbrook, 1983; Grafe et al., 1997; Chaplan et al., 2003; Yao et al., 2003), contributing to setting the resting membrane potential of these neurons (Ingram & Williams, 1996; Doan & Kunze, 1999; Yu et al., 2004). In one of our previous studies, we demonstrated that, in addition to perikarya, HCN2 is also expressed by central terminals of peptidergic nociceptive primary afferents in laminae I–IIo of the rat spinal dorsal horn (Antal et al., 2004). In the present experiment we further characterized the presynaptic properties and putative postsynaptic targets of nociceptive primary afferents expressing HCN2 channel proteins, and investigated the functional significance of HCN2 expression on these axon terminals.
Materials and methods
Animals and preparation of tissue sections
Experiments were carried out on 18 adult rats (Wistar-Kyoto, 250–300 g, Gödöll˝o, Hungary) and three glutamic acid decarboxylase (GAD)65-enhanced green fluorescent protein (eGFP) transgenic adult mice (De Marchis et al., 2004). All animal study protocols were approved by the Animal Care and Protection Committee at the University of Debrecen and were in accordance with the European Community Council Directives. The animals were deeply anesthetized with sodium pentobarbital (50 mg/kg, i.p.) and transcardially perfused with Tyrode's solution, followed by a fixative containing 4% paraformaldehyde in 0.1 m phosphate buffer (pH 7.4). After the transcardial fixation, the lumbar spinal cord was removed, postfixed in the same fixative overnight, and immersed in 10 and 20% sucrose dissolved in 0.1 m phosphate buffer until they sank. To enhance reagent penetration, the removed spinal cords were freeze-thawed in liquid nitrogen, sectioned at 50 µm on a Vibratome and extensively washed in 0.1 m phosphate buffer.
Glutamic acid decarboxylase 65-eGFP mice
A genomic clone, containing a 5.5-kb upstream region and the first six exons of the mouse GAD65 gene, was isolated from a mouse λ phage genomic library, and the eGFP marker gene without its own translation start site was fused in frame to the third exon of the GAD65 gene (De Marchis et al., 2004). Transgenic mice were derived by standard pronuclear injection of CBA/C57B16F2 fertilized eggs. For this study, transgenic mice on the CAB/C57B16 background were used from line GAD65 3e/gfp5.5#35. In this line, a 6.5-kb segment of the GAD65 gene, which includes 5.5 kb of the five upstream regions, the first two exons, and a portion of the third exon and the introns in between, drives the expression of eGFP almost exclusively to the GABAergic neurons in many brain regions, including the spinal cord (Hughes et al., 2005).
Immunohistochemistry
Double-immunostaining protocols were performed in order to study the colocalization of HCN2 immunoreactivity with various presynaptic markers and to identify contacts of HCN2-immunoreactive terminals on spinal postsynaptic neurons. Free-floating sections of the spinal cord were first incubated with a mixture of antibody that contained anti-HCN2 raised in rabbit (diluted 1 : 200, Alomone Laboratories, Jerusalem, Israel) and one of the following antisera: guinea pig anti-vesicular glutamate transporter (VGLUT)1 (diluted 1 : 5000, Chemicon, Hofheim, Germany), guinea pig anti-VGLUT2 (diluted 1 : 2500, Chemicon), guinea pig anti-VGLUT3 (diluted 1 : 5000, Chemicon), mouse anti-substance P (SP) (diluted 1 : 1000, Immundiagnostik AG, Bensheim, Germany), guinea pig anti-neurokinin 1 receptor (NK1R) (diluted 1 : 2000, Affinity Research Products, Exeter, UK), rabbit anti-calbindin D28k (diluted 1 : 500, Santa Cruz Biotechnology, Santa Cruz, CA, USA) and guinea pig anti-µ-opioid receptor (MOR) (diluted 1 : 4000, Chemicon). In the case of GAD65-eGFP mice the anti-HCN2 antibody was mixed with mouse anti-green fluorescent protein (diluted 1 : 2000, Molecular Probes, Eugene, OR, USA). The sections were incubated in the primary antibody solutions for 2 days at 4 °C and were transferred into the appropriate mixtures of secondary antibodies that were selected from the following: goat anti-rabbit IgG conjugated with Alexa Fluor 555 (diluted 1 : 1000, Molecular Probes), goat anti-guinea pig IgG conjugated with Alexa Fluor 488 (diluted 1 : 1000, Molecular Probes), goat anti-mouse IgG conjugated with Alexa Flour 488 (diluted 1 : 500, Molecular Probes), donkey anti-goat IgG conjugated with Cy5 (diluted 1 : 100, Jackson Immunoresearch, West Grove, PA, USA) and donkey anti-rabbit IgG conjugated with Alexa Flour 488 (diluted 1 : 500, Molecular Probes) overnight at 4 °C. Before the antibody treatments the sections were kept in 20% normal goat serum (Vector Laboratories, Burlingame, CA, USA) for 50 min. Antibodies were diluted in 10 mm Tris-phosphate-buffered isotonic saline (pH 7.4) to which 1% normal goat serum (Vector Laboratories) was added. Sections were mounted on glass slides and covered with Vectashield (Vector Laboratories).
The following triple immunostaining protocol was performed to test whether HCN2 terminals that are also positively stained for SP make close appositions with dendrites and perikarya of neurons expressing NK1Rs. The sections were incubated in a mixture of rabbit anti-HCN2 (diluted 1 : 200, Alomone Laboratories), mouse anti-SP (diluted 1 : 1000, Immundiagnostik AG) and guinea pig anti-NK1R (diluted 1 : 2000, Affinity Research Products) for 2 days at 4 °C. The sections were then transferred into a mixture of goat anti-rabbit IgG conjugated with Alexa Fluor 555 (diluted 1 : 1000, Molecular Probes), biotinylated horse anti-mouse IgG (diluted 1 : 200, Molecular Probes) and goat anti-guinea pig IgG conjugated with Alexa Fluor 488 (diluted 1 : 1000, Molecular Probes). The biotinylated secondary antibodies were further reacted with streptavidin conjugated with Alexa Fluor 647 (diluted 1 : 2000, Molecular Probes) for 5–6 h.
The colocalization of HCN2 with VGLUT2 and SP was quantitatively analysed using neurolucida for confocal software (MicroBrightfield, Inc., Colchester, VT, USA). Boutons in laminae I–IIo that showed immunoreactivity for a marker were randomly selected from 1-µm-thick confocal optical sections. The selected boutons were then examined to determine whether they were also immunoreactive for the other marker. The colocalization of both pairs of markers was analysed in three animals. The quantitative measurement was carried out in three confocal sections that were randomly selected from each animal and 100 boutons were investigated in all sections. Thus, the calculation of quantitative figures was based on the investigation of 900 boutons for each pair of markers.
As an extensive colocalization was found between immunoreactivities for HCN2 and SP, the cross-reactivity of the primary antisera was carefully tested on tissue sections by treating the diluted anti-HCN2 and anti-SP with purified HCN2 peptide (Alomone Laboratories) and SP (Immundiagnostik AG). HCN2 peptide or SP was mixed with the antibodies (1 µg HCN2 peptide/1 µg antibody and 1 µg SP/1 µg antibody), left at 4 °C for 16–18 h and then centrifuged. Sections were first incubated with anti-HCN2 raised in rabbit or anti-SP raised in mouse for 2 days at 4 °C. The sections were then transferred into biotinylated goat anti-rabbit IgG (diluted 1 : 200, Vector Laboratories) or biotinylated horse anti-mouse IgG (diluted 1 : 200, Vector Laboratories) overnight. Thereafter, they were treated with an avidin-biotinylated horseradish peroxidase complex (diluted 1 : 100, Vector Laboratories) and the immunoreaction was completed with a diaminobenzidine (Sigma, St Louis, MO, USA) chromogen reaction. Before the antibody treatments the sections were kept in 20% normal goat or horse serum (Vector Laboratories) for 50 min. Antibodies were diluted in 10 mm Tris-phosphate-buffered isotonic saline to which 1% normal goat or horse serum (Vector Laboratories) were added. Sections were mounted on glass slides and covered with Permount neutral medium. The preadsorption of HCN2 peptide to anti-HCN2 and of SP to anti-SP completely abolished the specific immunostaining (Fig. 1b and e). However, the treatment of anti-HCN2 with SP and of anti-SP with HCN2 peptide did not alter the original patterns of immunostaining for HCN2 and SP (Fig. 1a, c, d and f), suggesting that there was no substantial cross-reactivity between anti-HCN2 and anti-SP.
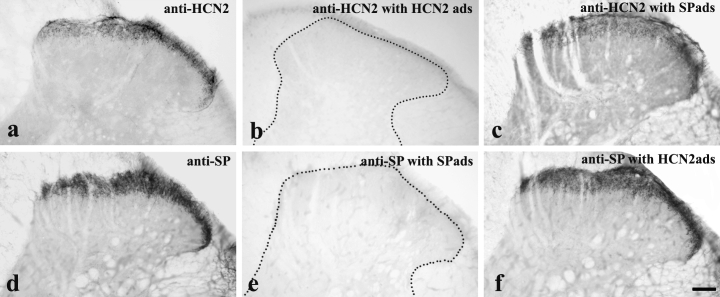
Preadsorption controls testing the cross-reactivity between anti- hyperpolarization-activated and cyclic nucleotide-gated cation channel subunit 2 (HCN2) and anti-substance P (SP). The application of both anti-HCN2 and anti-SP resulted in a strong immunostaining in laminae I–II (a and d). The adsorption of HCN2 peptide to anti-HCN2 (b) and substance P to anti-SP (e) completely abolished the immunostaining. The adsorption of SP to anti-HCN2 (c) and HCN2 peptide to anti-SP (f) did not cause any substantial decline in the strength of the immunostaining. Scale bar, 100 µm.
Electrophysiology
Experiments were carried out on 3-week-old rats (Wistar-Kyoto, Gödöll˝o). Under deep isofluorane anesthesia the animals were decapitated and the lumbar spinal cord was dissected in ice-cold artificial cerebrospinal fluid (pH 7.4) that contained (in mm): NaCl, 130; NaHCO3, 24; KCl, 3.5; NaH2PO4, 1.25; Ca2Cl, 1; MgSO4, 3 and glucose, 10, and was saturated with 95% O2 and 5% CO2. After removing the meninges, blocks of the lumbar spinal cord with dorsal roots attached (length 7–9 mm) were embedded in agar and sectioned at 400–600 µm on a Vibratome. Slices with dorsal roots attached were incubated in artificial cerebrospinal fluid at room temperature (21–23 °C) for at least 1 h prior to recording.
Slices were transferred into a recording chamber, which was constantly perfused with oxygenated artificial cerebrospinal fluid. Neurons located in laminae I–II were visually identified with a Zeiss Axioskop FS microscope equipped with a ×40 water immersion objective, differential interference contrast filter and infrared charge-coupled device camera system (Hamamatsu, Japan). The dorsal root was introduced into a stimulating suction electrode, while single cells in laminae I–II were recorded using conventional whole-cell patch-clamp recordings in a current-clamp mode. Glass pipettes with a resistance of 4–6 MΩ and an Axoclamp ID amplifier (Axon Instruments, Union City, CA, USA) were used for the recording. The electrode filling solution contained (in mm): K-gluconate, 124; NaCl, 14; ATP-Mg, 1; GTP-Na, 0.3 and HEPES, 10.
Dorsal roots were stimulated by a BioSTIM biological stimulator (Supertech, Pécs, Hungary) at 0.2 Hz with 0.1-ms-long current pulses, the amplitude of which varied in the range 0.5–2.0 mA. The conduction velocity of stimulated fibers was calculated from the latency of the intracellular responses and the length of the root. Spontaneous and evoked excitatory postsynaptic potentials (EPSPs) as well as action potentials were recorded from cells that received monosynaptic inputs from C and/or A-delta primary afferents with conduction velocities of 0.3–0.8 and 3.8–15.0 m/s, respectively (Kawasaki et al., 2003). Failure rates of synaptic transmission were calculated for each 10 consecutive dorsal root stimulations by counting the numbers of stimulations that failed to evoke EPSP on the postsynaptic neuron. After recording the spontaneous and evoked EPSPs in control condition, the HCN channel blocker ZD7288 was applied into the bath at a concentration of 10 mm for 10 min (Harris & Constanti, 1995; Pal et al., 2003). Finally, under continuous recording, ZD7288 was washed out of the bath. All recordings were performed at room temperature.
Data were digitized and filtered at 5 kHz (Digidata 1320, Axon Instruments), recorded on an IBM PC and analysed off-line by using pclamp (Axon Instruments), origin (Microcal Software, Northhampton, MA, USA) and whole cell program and electrophysiology data recorder (courtesy of Dr J. Dempster, University of Strathclyde, UK) software packages. Differences among the failure rates of synaptic transmission that were observed during the initial period of recording, early and late phases of ZD7288 application and the wash-out period were statistically evaluated using the paired single-tailed t-test. Significance was assumed at values of P < 0.01 or lower.
Results
Co-localization of hyperpolarization-activated and cyclic nucleotide-gated cation channel subunit 2 with vesicular glutamate transporters 1, 2 and 3 and substance P
In one of our previous studies, we demonstrated that HCN2 is expressed by terminals of peptidergic nociceptive primary afferents in laminae I–IIo of the rat spinal dorsal horn (Antal et al., 2004). To define the major neurotransmitters that may be utilized by HCN2-expressing primary afferents, we studied the colocalization of HCN2 with the vesicular glutamate transporters VGLUT1–3, that are considered to represent the most specific markers so far for glutamatergic axon terminals (Li et al., 2003; Oliveira et al., 2003; Todd et al., 2003; Alvarez et al., 2004; Landry et al., 2004), and SP, a neuropeptide that appears to be crucial in pain transmission from nociceptive primary afferents to secondary sensory neurons of the superficial spinal dorsal horn (for review see Willis & Coggeshall, 2004).
In agreement with previous results, we observed strong punctuate immunostaining for all three VGLUTs in the spinal gray matter in distinctive laminar distribution (Varoqui et al., 2002). In laminae I–IIo, where immunostaining for HCN2 was detected, VGLUT1 immunoreactivity was very sparse (Fig. 2a). In contrast, VGLUT2 immunostaining was particularly dense (Fig. 2b), whereas VGLUT3 labeling appeared to be moderate to weak in lamina I–II (Fig. 2c).
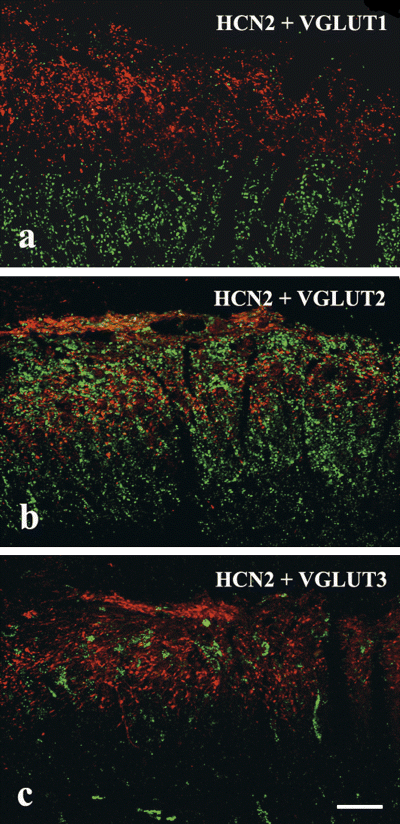
Laser scanning confocal micrographs showing colocalization of hyperpolarization-activated and cyclic nucleotide-gated cation channel subunit 2 (HCN2) with vesicular glutamate transporter (VGLUT)1–3 immunoreactivity in 1-µm-thick single transverse optical sections of the superficial spinal dorsal horn that were double labeled for HCN2 (red) and one of the three VGLUTs (green), VGLUT1 (a), VGLUT2 (b) and VGLUT3 (c). The mixed colors in b indicate that there is a weak colocalization between HCN2 and VGLUT2 but a and c show that VGLUT1 and VGLUT3 immunoreactivities are completely segregated from immunostaining for HCN2. Scale bar, 20 µm.
Investigating the colocalization between VGLUTs and HCN2 immunoreactivity, we found that VGLUT-immunoreactive puncta were almost completely segregated from HCN2-containing terminals. We observed no colocalization between HCN2 and VGLUT1 or VGLUT3 immunoreactivities, and even VGLUT2, despite being the most abundant VGLUT in laminae I–IIo, was detected in only 6% of the investigated HCN2-immunostained terminals.
Immunostaining for SP was very intense in the superficial spinal cord and confined to laminae I–IIo, similar to the distribution of axon terminals immunostained for HCN2 (Fig. 3b and d). Studying the colocalization of the two proteins, we found that 92% of HCN2- and 93% of SP-immunoreactive boutons also showed immunoreactivity for the other marker (Fig. 3b and d).
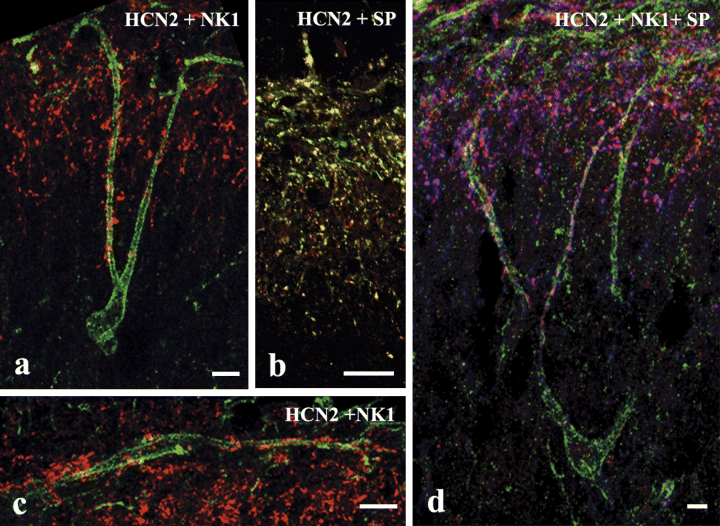
Laser scanning confocal micrographs showing colocalization of hyperpolarization-activated and cyclic nucleotide-gated cation channel subunit 2 (HCN2) with substance P (SP) and neurokinin 1 receptor (NK1R) in the spinal dorsal horn. (a and c) Single, 1-µm-thick transverse optical sections show that HCN2-immunoreactive axon terminals (red) contact dendrites (a and c) and somata (c) of neurons whose perikarya are located in lamina I (c) or laminae III–IV (a) and express strong immunoreactivity for NK1R (green). (b) A single, 1-µm-thick transverse optical section showing the colocalization of HCN2 (red) and SP (green) immunoreactivities. The mixed colors indicate a strong colocalization between HCN2 and SP. (d) Superimposed 6-µm-thick transverse optical section triple immunostained for HCN2 (red), NK1R (green) and SP (blue). The cell body of an NK1R-immunoreactive neuron is located in lamina IV but its dendrites extend to laminae III and II where they receive a number of contacts from axon terminals that are immunoreactive for both HCN2 and SP. Scale bars: 20 µm (a, c and d); 10 µm (b).
Close appositions between hyperpolarization-activated and cyclic nucleotide-gated cation channel subunit 2-immunoreactive primary afferents and spinal neurons immunoreactive for neurokinin 1 receptor, calbindin D28k, GluR2 and µ-opioid receptor or expressing glutamic acid decarboxylase 65
It has been reported that peptidergic nociceptive primary afferents project to both excitatory and inhibitory interneurons in laminae I and II of the superficial spinal and medullary dorsal horn (Carlton & Hayes, 1990; Hayes & Carlton, 1992; Bernardi et al., 1995; Wang et al., 2000). To reveal whether the proportion of peptidergic nociceptive primary afferents that express HCN2 channel protein also contact both excitatory and inhibitory postsynaptic structures we performed double-staining immunocytochemical protocols in which immunostaining for HCN2 was combined with immunostaining for NK1R, calbindin D28k, α-amino-3-hydroxy-5-methylisoxazole-4-propionate receptor subunit GluR2 and MOR, which are known to be markers of various subsets of excitatory neurons in laminae I and II (Antal et al., 1991; Littlewood et al., 1995; Kemp et al., 1996; Spike et al., 1998). HCN2 immunostaining was also carried out in GAD65-eGFP transgenic mice (De Marchis et al., 2004) and this enabled us to investigate close appositions of HCN2-immunoreactive axon terminals on inhibitory spinal neurons.
Confirming results of previous observations we found NK1R-immunoreactive perikarya in laminae I and III–IV in the dorsal horn (Littlewood et al., 1995). NK1R-immunoreactive neurons in lamina I gave rise to dendrites that arborized in lamina I. HCN2-immunoreactive axon terminals formed multiple contacts with the somata and dendrites of these neurons (Fig. 3c). Dendrites arising from NK1R-immunoreactive perikarya in laminae III–IV extended towards the superficial dorsal horn. They traversed lamina III and arborized in laminae I and II, where they received several contacts from HCN2-immunoreactive axon terminals (Fig. 3a and d). Collaterals of HCN2-immunoreactive axons extended down to laminae III and IV along the dendrites of NK1R-immunoreactive neurons, where they made serial contacts with these dendrites (Fig. 3a and d).
Calbindin D28k- and GluR2-immunoreactive neurons were found in large numbers in laminae I and II, whereas neurons stained for MOR were confined to lamina II (Antal et al., 1991; Kemp et al., 1996; Spike et al., 1998). All three sets of neurons were heavily innervated by HCN2-immunoreactive axon terminals (Fig. 4). In the case of calbindin D28k-immunoreactive neurons, HCN2 terminals were distributed all over the entire somato-dendritic membrane compartment, whereas MOR-immunoreactive neurons were contacted mostly on their dendrites (Fig. 4c and d). As GluR2 labeling was largely restricted to somata, in the case of GluR2-immunoreactive neurons the dendritic distribution of HCN2 terminals could not be investigated.
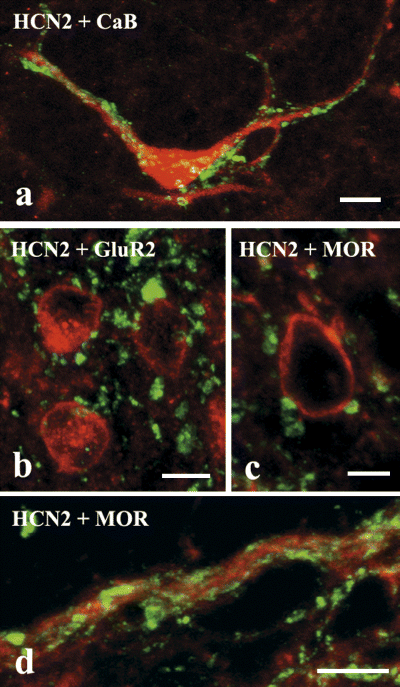
Laser scanning confocal micrographs showing contacts between hyperpolarization-activated and cyclic nucleotide-gated cation channel subunit 2 (HCN2)-immunoreactive axon terminals (green) and dendrites as well as perikarya of neurons immunoreactive for calbindin D28k (CaB) (a, red), GluR2 subunit of α-amino-3-hydroxy-5-methylisoxazole-4-propionate receptors (b, red) and µ-opioid receptor (MOR) (c and d, red). Scale bars, 10 µm.
Although a number of inhibitory neurons were visualized in the zone of HCN2-immunoreactive primary afferents in the GAD65-eGFP transgenic mice, close appositions between green fluorescent protein- and HCN2-immunoreactive neural elements were only occasionally found (Fig. 5). Most of the contacts were revealed on either the somata or primary dendrites of green fluorescent protein-labeled inhibitory neurons (Fig. 5b and c).
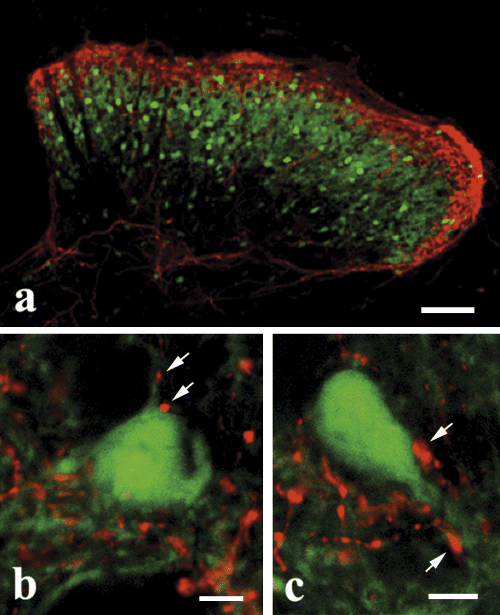
Laser scanning confocal micrographs showing contacts between hyperpolarization-activated and cyclic nucleotide-gated cation channel subunit 2 (HCN2)-immunoreactive axon terminals (red) and green fluorescent protein (GFP)-expressing GABAergic neurons (green) in the spinal dorsal horn of glutamic acid decarboxylase 65-eGFP mice. Arrows in b and c point to HCN2-immunoreactive axon terminals that form close appositions to GFP-expressing GABAergic neurons. Scale bars: 100 µm (a); 10 µm (b and c).
Effect of ZD7288 on synaptic transmission between C/A-delta primary afferents and neurons in laminae I–II of the spinal dorsal horn
It is generally accepted that ZD7288 is the most selective antagonist of HCN channels (Harris & Constanti, 1995; Beaumont & Zucker, 2000; Southan et al., 2000; Pal et al., 2003). It has been demonstrated that Ih can be totally blocked by 10 µm ZD7288 (Harris & Constanti, 1995; Pal et al., 2003). Thus, to investigate whether HCN channels expressed by the central terminals of nociceptive primary afferents are functional, we tested how the efficacy of synaptic transmission between nociceptive primary afferents and secondary sensory neurons can be altered by the application of ZD7288.
We made stable patch-clamp recordings from 30 neurons in laminae I–II of the spinal dorsal horn. Of the 30 recorded neurons, eight received short latency monosynaptic inputs from C and/or A-delta primary afferents (Fig. 6c) and also undoubtedly responded to ZD7288 application (Fig. 6a and d). The present report is based on the investigation of these eight cells. The stimulated primary afferents were identified as C or A-delta fibers on the basis of their conduction velocity (Fig. 6b). Of the eight investigated neurons, six received monosynaptic inputs from C fibers (conduction velocity varied in the range 0.3–0.8 m/s), one was monosynaptically excited by A-delta fibers (conduction velocity 3.8–8 m/s), whereas one received monosynaptic inputs from both C and A-delta fibers.
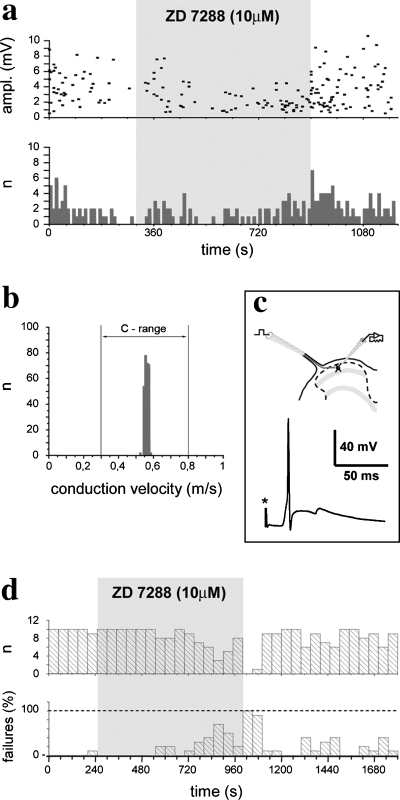
Physiological recordings illustrating that the hyperpolarization-activated and cyclic nucleotide-gated cation channel subunit 2 antagonist ZD7288 reduces the amplitude of excitatory postsynaptic potentials (EPSPs) and increases the failure rate of synaptic transmission. (a) Diagram illustrating the amplitude and numbers of spontaneous EPSPs of a neuron that received monosynaptic inputs from C and A-delta primary afferents. Both the amplitude and numbers of spontaneous EPSPs were slightly reduced during the application of ZD7288. (b) Conduction velocity histogram of primary afferents that evoked monosynaptic action potentials on the neuron illustrated in d. The conduction velocity was calculated from the latency of responses and the length of the stimulated dorsal root. The mean conduction velocity falls in the range of that of C fibers. (c) Schematic illustration of the experimental setup, and a monosynaptic action potential evoked by the stimulation of the dorsal root and recorded from a dorsal horn neuron. (d) Histograms illustrating the numbers of monosynaptic action potentials evoked by stimulation of C primary afferents and the percentage of failures in synaptic transmission. Bins represent 10 consecutive trials in 50-s time frames. Due to the application of ZD7288, the failure rate of synaptic transmission was increased. The effect was reversible.
All of the investigated neurons exhibited spontaneous activities with an amplitude that varied in the range 0.5–10.0 mV. The application of ZD7288 at a concentration of 10 µm caused a marked depression in the amplitude range (0.5–4.0 mV) but only a slight reduction in the numbers of these spontaneous membrane events in the case of all of the investigated neurons (Fig. 6a). After washing out the drug, the control values concerning both the frequency and amplitude recovered without any substantial delay (Fig. 6a).
Electrical stimulation of both C and A-delta fibers evoked monosynaptic postsynaptic potentials on the recorded neurons with varying reliability. In the case of two cells, virtually all stimulation was effective and evoked EPSPs on the postsynaptic neurons in control conditions (Fig. 6d), whereas the other cells responded to C and A-delta fibers with a lower reliability. The average failure rate for all recorded neurons was 34.0 ± 10.1% (± SEM). The application of ZD7288 caused the reliability of synaptic transmission to drop and the number of trials that failed to evoke PSPs on the recorded neurons increased (Fig. 6d), although the effect of ZD7288 developed gradually during drug application. The failure rate increased only slightly in the first 250 s of ZD7288 application (46.0 ± 15.5%, P = 0.2180). After this, however, the reliability of synaptic transmission dropped and the failure rate became significantly higher than during the control period. The average value of the failure rate throughout the second part of ZD7288 application (after the first 250 s) and for all recorded neurons was 74.7 ± 14.5% (P = 0.0051). In the case of one cell the failure rate went up to 100% at the end of drug application (Fig. 6d). The effect of ZD7288 was reversible (Fig. 6d) and the failure rate returned to control values (39.4 ± 9.8%) during the wash-out period. A small gradual decrease in the amplitude of PSPs, presumably due to a slow but steady depletion of synaptic vesicles, was observed throughout the experiments.
Discussion
Here we demonstrated that HCN2 widely colocalizes with SP and that HCN2-positive terminals form serial close appositions with dendrites and perikarya of neurons that are immunoreactive for NK1R, calbindin D28k, MOR and GluR2, markers for excitatory interneurons. We found that HCN2-positive terminals occasionally also contact cells that contain GAD65, a marker for GABAergic inhibitory neurons. Application of ZD7288, an antagonist of HCN channels, onto spinal neurons in laminae I–II reduced the number of monosynaptic EPSPs evoked by electrical stimulation of primary afferents at nociceptive intensities, suggesting that HCN2 may play a role in the fine-tuning of pain transmission from SP-containing nociceptive primary afferents to spinal neurons.
Segregation between hyperpolarization-activated and cyclic nucleotide-gated cation channel subunit 2 and vesicular glutamate transporter immunoreactivity
Glutamate is thought to be used as a neurotransmitter by all primary afferents including nociceptive C and A-delta fibers (De Biasi & Rustioni, 1988; Broman et al., 1993; Levin et al., 1993). Although a long line of evidence has substantiated the role of glutamate as a neurotransmitter, its ubiquitous appearance in neural proteins made the morphological verification of glutamate as a neurotransmitter very difficult. The recent identification of proteins responsible for the vesicular storage of glutamate (VGLUTs) has yielded the first definitive morphological markers of glutamatergic neurons (Bellocchio et al., 2000; Takamori et al., 2000). Until now, three members of the VGLUT family have been cloned (VGLUT1–3) and their distribution has been mapped in various brain areas including the spinal cord (Ni et al., 1994, 1995; Aihara et al., 2000; Fremeau et al., 2001; Herzog et al., 2001; Sakat-Haga et al., 2001; Kaneko et al., 2002; Schäfer et al., 2002; Takamori et al., 2002). A complementary distribution of VGLUT-expressing fibers in the spinal cord, with no or minimal overlapping in nerve endings, has been demonstrated (Todd et al., 2003; Alvarez et al., 2004; Landry et al., 2004). Concerning the superficial spinal dorsal horn, VGLUT1 was found to be sparsely distributed or completely absent in lamiae I–II. In contrast, VGLUT2 and VGLUT3 presented a dense and moderate immunoreactivity in laminae I–II, respectively (Todd et al., 2003; Alvarez et al., 2004; Landry et al., 2004). These previous results are in complete agreement with our present observations.
Given the density of VGLUT2 and VGLUT3 staining in lamina I–II, the very low level of colocalization between VGLUT2 and HCN2, and the complete lack of coexpression between VGLUT3 and HCN2 were unexpected and point out that VGLUT2- and VGLUT3-positive axons do not originate from HCN2-immunoreactive C and A-delta primary afferent fibers. This confirms other thorough evaluations of the origin of VGLUT-containing fibers in the dorsal horn, showing that peptidergic primary afferents as well as non-peptidergic C fibers display low levels of VGLUT2 immunoreactivity or are not immunoreactive with either VGLUT antibody (Todd et al., 2003; Alvarez et al., 2004; Landry et al., 2004). Taken together, these results show that none of the cloned glutamate transporters can be used as a marker of unmyelinated primary afferent fibers, although known to release glutamate upon stimulation (Broman et al., 1993). Our study thus raises the possibility that, besides VGLUT1–3, an additional glutamate vesicular transporter may be expressed in nociceptive afferents.
Co-localization between hyperpolarization-activated and cyclic nucleotide-gated cation channel subunit 2 and substance P immunoreactivity
In one of our previous studies, we demonstrated that HCN2 widely colocalizes with calcitonin gene-related peptide but is almost completely segregated from isolectin-B4 binding in laminae I–II of the spinal dorsal horn, indicating that HCN2 is primarily expressed in peptidergic nociceptive primary afferents (Antal et al., 2004). In addition to calcitonin gene-related peptide, various populations of peptidergic nociceptive primary afferents may express and release other neuropeptides, including SP that is known to play a crucial role in the transmission of nociceptive signals from primary afferents to secondary sensory spinal neurons, thus contributing to pain processing in a substantial manner (Naim et al., 1997; Todd et al., 2002; Willis & Coggeshall, 2004). Thus, we tested SP immunoreactivity of HCN2-containing terminals and found a very substantial colocalization between HCN2 and SP immunoreactivity in laminae I–IIo. This finding indicates that Ih mediated by HCN2 channels may modulate SP-mediated pain processing in the superficial spinal dorsal horn and that this modulation occurs at the level of the first synaptic contact along the pain pathways.
Postsynaptic targets of hyperpolarization-activated and cyclic nucleotide-gated cation channel subunit 2-positive axon terminals in the superficial spinal dorsal horn
It has been well established that nociceptive primary afferents terminate on both excitatory and inhibitory interneurons in the superficial spinal dorsal horn (Carlton & Hayes, 1990; Wang et al., 2000). In monkeys (Macaca fascicularis), 28.1% of dendritic profiles postsynaptic to calcitonin gene-related peptide-immunoreactive peptidergic nociceptive primary afferent terminals turned out to be immunoreactive for GABA (Hayes & Carlton, 1992). Nociceptive primary afferents may form synaptic contacts with GABAergic postsynaptic profiles in much lower numbers in rodents. The predominant type of peptidergic nociceptive primary afferent terminals, the non-glomerular varicosities, have never been found to establish synaptic contacts with GABA-immunoreactive dendrites in the rat spinal cord (Bernardi et al., 1995). However, Bernardi et al. (1995) also reported that 28% of C1 glomerular boutons that constitute the other type of peptidergic nociceptive primary afferent terminals (Ribero-da-Silva & Coimbra, 1982; Ribeiro-da-Silva, 2004) form synaptic contacts with GABAergic dendrites. It is interesting to note that most axon terminals of HCN2-expressing peptidergic nociceptive primary afferents appear as non-glomerular endings and only a small proportion of them can be classified as C1 glomerular boutons (Antal et al., 2004). In the light of these previous reports, it appears to be quite understandable that we only occasionally found contacts between HCN2-immunoreactive terminals and GAD65-eGFP-expressing spinal neurons. Thus, it is likely that peptidergic nociceptive primary afferents that express HCN2 channel protein establish synaptic contacts preferentially with excitatory neurons and contact inhibitory spinal neurons only occasionally in the spinal dorsal horn of rodents.
It has been reported that SP-containing primary afferents establish asymmetric synaptic contacts with neurons possessing NK1Rs and form non-synaptic contacts (close apposition without any synaptic specialization) with MOR-1-immunoreactive neurons in the superficial spinal dorsal horn of rats (Naim et al., 1997; Spike et al., 2002; Todd et al., 2002). Although we did not carry out any electron microscopic analysis in the present study, the high degree of colocalization between HCN2 and SP may suggest that HCN2-containing primary afferents also establish synaptic and non-synaptic contacts with neurons expressing NK1Rs and MORs, respectively. It makes the case even more interesting that NK1-expressing cells have been identified as projection neurons and MOR-immunoreactive neurons are generally regarded as a subpopulation of excitatory interneurons (Kemp et al., 1996; Naim et al., 1997; Spike et al., 2002; Todd et al., 2002). Thus, we may postulate that presynaptic Ih channel mechanisms modulate the transmission of nociceptive nerve impulses from SP-containing primary afferents to both projection neurons and interneurons in the superficial spinal dorsal horn but the characteristics of the postsynaptic effect of Ih activation might be different on the different populations of neurons depending on whether the axon terminal that expresses HCN2 channels establishes synaptic or non-synaptic contact with its postsynaptic target.
Effects of hyperpolarization-activated currents mediated by hyperpolarization-activated and cyclic nucleotide-gated cation channel subunit 2 channels on synaptic transmission between C/A-delta primary afferents and neurons in laminae I–II of the spinal dorsal horn
A remarkable property of many excitatory synapses in the central nervous system is their ability to undergo activity-dependent, long-lasting increases in synaptic strength. This is referred to as long-term potentiation in many brain regions or long-term facilitation (LTF) in the superficial spinal dorsal horn. LTF of synaptic interaction between nociceptive primary afferents and secondary spinal interneurons is believed to play a substantial role in the development of central sensitization, a functional state of nociceptive neural circuits that leads to enhanced pain processing (for review see Ji et al., 2003).
Voltage-dependent ion channels in presynaptic terminals are ideally suited for modulating transmitter release and thus contributing to long-term potentiation and LTF. Among many other voltage-dependent ion channels, presynaptic Ih (HCN) channels have received much attention lately because of the idea that they may also participate in the modulation of synaptic transmission and presynaptic forms of plasticity. It has been reported that, at the crayfish neuromuscular junction, activation of Ih channels induces synaptic facilitation (Beaumont & Zucker, 2000). In hippocampal neurons, the presynaptic mossy fiber long-term potentiation has also been shown to be dependent on Ih activation (Mellor et al., 2002). Our present findings are in agreement with these previous observations. Application of ZD7288, an antagonist of Ih channels, onto neurons that were recorded in spinal cord slices with whole-cell patch-clamp electrodes reduced the number of monosynaptic EPSPs evoked by electrical stimulation of primary afferents at nociceptive intensities, suggesting that Ih may increase the reliability of synaptic transmission from primary afferents to secondary sensory neurons and thus may play a role in the presynaptic modulation of pain transmission from nociceptive primary afferents to neurons in the spinal dorsal horn.
How might activation of Ih increase the reliability of synaptic transmission and lead to LTF? One possibility is that it could enhance Ca2+ influx into the presynaptic terminal by causing depolarization and thus opening voltage-gated Ca2+ channels. Calcium entry into the presynaptic terminal during repetitive stimulation activates calcium–calmodulin-sensitive adenylyl cyclase. The consecutive rise in cAMP further enhances Ih and results in an even stronger depolarization (Cuttle et al., 2001; Mellor et al., 2002) and in slowing calcium channel deactivation during action potential repolarization (Forsythe et al., 1998). If Ih is activated for prolonged periods of time, the enhanced Ca2+ influx can facilitate neurotransmitter release and modulate synaptic short-term plasticity (Yu et al., 2004). Using a combined whole-cell patch-clamp recording and fluorescence Ca2+ imaging method, Yu et al. (2004) showed that Ca2+ might even permeate through the Ih channel itself. Thus, Ca2+ entry through voltage-gated Ca2+ or Ih channels may provide a cellular basis for Ih-mediated events, such as presynaptic plasticity and LTF. Ih is probably important during the after-hyperpolarization phase of the action potential, where Ih opposes the ability of outward potassium currents to drive the membrane potential past the resting membrane potential. The inhibition of the slow after-hyperpolarization may consequently cause an increase in discharge (Weinreich & Wonderlin, 1987). Also, Ih channels might enhance the mobilization of synaptic vesicles to a readily releasable pool without any direct correlation with Ca2+ mobilization, perhaps through some local signaling cascade mediated by the cytoskeleton (Beaumont & Zucker, 2000). All of these possibilities may exist in the spinal dorsal horn.
Acknowledgements
This work was supported by the Hungarian National Science Fund (T 046121) and the European Commission Framework Program 6 (COOP-CT-2004–513190-ION). The authors thank Dr Gábor Veress and Mrs Mária Varga for technical assistance.
Abbreviations
-
- eGFP
-
- enhanced green fluorescent protein
-
- EPSP
-
- excitatory postsynaptic potential
-
- GAD
-
- glutamic acid decarboxylase
-
- HCN
-
- hyperpolarization-activated and cyclic nucleotide-gated
-
- Ih
-
- hyperpolarization-activated current
-
- LTF
-
- long-term facilitation
-
- MOR
-
- µ-opioid receptor
-
- NK1R
-
- neurokinin 1 receptor
-
- SP
-
- substance P
-
- VGLUT
-
- vesicular glutamate transporter